Abstract
Background
Attentional control of executive cognitive function (ECF) decreases in older individuals with Alzheimer Disease (AD). In order to examine early AD-related changes in the neural substrates of ECF attentional control, we measured activation dorsolateral prefrontal (dLPFC), posterior parietal (PPC), and anterior cingulate cortex (ACC) in adults with mild cognitively impairment (MCI) and in cognitively normal (CN) adults.
Methods
Functional magnetic resonance imaging analysis of brain activation in MCI (n = 8, mean age 79.5) and CN (n = 8 mean age 81.5) during increasing loads of attentional demands.
Results
MCI and CN older adults performed with similar accuracy and reaction time. MCI had greater activation than CN in PPC (right p = .03 and left p = .05) and dlPFC areas (right p = .002 and left p = .004), while activation in ACC was similar in the two groups. Response to increasing loads of the task differed by group: MCI selectively engaged bilateral PPC (right p = .03, left p = .04), while CN subjects increased bilateral dlPFC activation (right p = .005 and left p = .02) and ACC activation (p = .04). Among MCI, greater load-related changes in PPC activity were associated with smaller load-related changes in accuracy rates (r = −.85, p = .07) and greater increases in reaction times (r = .97, p = .01). In CN subjects, load-related change in PPC activation was associated with load-related change in reaction time (r = .76, p = .02) but not with changes in accuracy rates.
Conclusions
PPC and dlPFC may show early functional changes associated with MCI.
Keywords: Executive cognitive function, dementia, functional MRI
Early diagnosis of Alzheimer Disease (AD) is complicated by the similarities between mild cognitive impairment and normal cognitive aging – both on brain MRI and on neurological examination.
Functional MRI (fMRI) of the brain offers the possibility to discriminate cognitively normal older adults from individuals with mild cognitive impairment (MCI) by detecting patterns of brain activation in response to simple tasks. Neuroimaging studies of early Alzheimer Disease (AD) have shown increased prefrontal and parietal activity in patients compared with older controls executing a variety of executive cognitive tasks (Woodard et al 1998; Backman et al 1999; Saykin et al 1999; Bookheimer et al 2000; Corbetta et al 2000; Thulborn et al 2000; Grady et al 2003). Neuropsychological evidence of impaired Executive Cognitive Function (ECF)(Ready et al 2003) and evidence of disturbed metabolism (Piert et al 1996), blood flow (Encinas et al 2003) and functional connectivity (Elgh et al 2003) in neocortical systems subserving ECF in early AD patients, suggests that ECF impairment may precede Alzheimer Disease (Royall et al 2002; Baddeley et al 2001). Posterior parietal cortex (PPC), dorsolateral prefrontal cortex (dlPFC) and Anterior Cingulate Cortex (ACC) are key areas for the implementation of attentional control during the execution of ECF tasks (Royall et al 2002; Milham et al 2002). PPC in particular seems to play a crucial role in attentional control and in tasks requiring orienting and shifting of attention both in young and older individuals (Thiel et al 2004). Although increases in prefrontal and posterior parietal activity in early AD have been interpreted as compensatory reallocation or recruitment of cognitive resource, there is still a lack of direct evidence linking ability to perform ECF tasks and function of specific cortical systems in very old adults at risk for Alzheimer Disease.
In the present study, we adopted a cuing paradigm similar to that one developed by Rosenbaum (Rosenbaum et al 1983) to control and manipulate attentional processes. The Preparing to Overcome Prepotency (POP) task consists of three phases, each one involving different components of executive processes: 1) The pre-cue phase requires active maintenance and representation of two candidate responses based on two simple and over-learnt task rules (green cue = direction of motor response is congruent with probe direction; red cue = direction of motor response is opposite to probe direction); 2) The cue - or preparation- phase anticipates information about the decision to be taken, thereby allowing selection of one out of two competing responses; 3) The probe -or decision-phase, requires ultimate monitoring of the conflicting situation and rapid motor execution. As in the switching Stroop task (MacDonald et al 2000), the preparation phase involves an executive maintenance and selection components with engagement of dlPFC and PPC, while the response phase engages dorsal ACC for conflict monitoring. We hypothesized that in MCI older adults, functional activation of dlPFC, PPC and ACC during the POP task would be greater compared to cognitively normal adults of similar age. We also hypothesized that activation in these areas would correlate with behavioral performance in both groups. Specifically, we looked for evidence that those patients who were able to recruit parietal and prefrontal areas to a greater degree during these tasks would perform more accurately.
Methods and Materials
Study Participants
Nondemented older individuals participating in the Cardiovascular Health Study (CHS)(Fried et al 1991) or the Gingko in Evaluation of Memory (GEM) study at the field site of Pittsburgh, Pennsylvania were recruited for our study from April 2002 to April 2003. The GEM study was designed as a 5 year, double blind, placebo controlled trial of Gingko Biloba in the prevention of dementia and was developed upon the infrastructure of the CHS study. Participants were included to the GEM study if they had no significant neurological or neurodegenerative diseases that would affect cognitive function. Those who were taking cognitive enhancers or were at risk for bleeding disorders were excluded. The clinical coordinators of the GEM or CHS studies contacted those participants who had received a neuropsychological evaluation within the previous 3 months and those who were interested in the study were then contacted and explained the study in greater detail. Of 30 participants who agreed to participate, we included those individuals who were eligible for fMRI scanning (no questionable history of metallic fragments, cardiac pacemaker, aneurism clip, cochlear implants, weight of more than 250 lbs or claustrophobia), did not have history of other neurological disease, brain injury, psychotic mood, substance abuse disorders, eye movement abnormalities, did not have any clinically relevant event (depression, stroke or other medical problem requiring hospitalization) occurring between the last neuropsychological examination and the fMRI scan and did not start the treatment as part of the GEM protocol. Of 20 participants thus recruited, 1 interrupted the fMRI scanning procedure for discomfort and 3 could not arrange to come to the MR center. Of the 16 participants included, 8 were MCI and 8 were cognitively normal. The study was approved by the University of Pittsburgh Institutional Review Board and all subjects gave written informed consent.
Cognitive Function Evaluation
The participants of the GEM and CHS study are screened with the same battery of neuropsychiatric tests to identify dementia, mild cognitive impairment (MCI) and normal cognitive status (Lopez et al 2003c). Previously published guidelines for adjudication of MCI status utilized within the CHS cohort (Lopez et al 2003b, 2003c) were applied to both GEM and CHS participants of our study. Table 1 shows neuropsychological test scores for MCI and cognitively normal individuals. Components of the neuropsychological battery included the following tests: the America version of the National Reading test (Nelson 1982), Raven’s Colored Progressive Matrices (Raven 1956): California Verbal Learning Test (Delis et al 1987), Rey-Osterreith figure (Rey 1941; Osterreith 1944), Immediate and Delayed Recall, modified Boston Naming test (Huff et al 1986), Verbal fluency test (Benton 1968), Block design (modified from the Wechsler Adult Intelligence Scale-revised; Wechsler 1981), Stroop Neuropsychological Screening Test (Trenerry et al 1989), Trail Making (Reitan 1958) and the Digit Spans (Wechsler 1981). If a participant failed tests for memory or in more than one other cognitive domain (premorbid intelligence, language, visuoperceptual/visuoconstructional, executive function or motor), a neurologist completed a neurologic exam which included assessment of cranial nerves, motor tone, abnormal movements, strength, deep tendon reflexes, release signs, plantar response and clonus, cerebellar testing, primary sensory testing (including graphesthesia and stereognosis), gait, and postural stability. The MCI cases presented memory deficits, defined as performance >1.5 SD below that of individuals of comparable age and education, OR presented deterioration in other cognitive domain (e.g., language, executive functions, visuo-constructional abilities), OR presented one abnormal test in at least two cognitive domains, without sufficiently severe cognitive function impairment, or loss of instrumental activities of daily living (IADLs) to constitute dementia.
Table 1.
Neuropsychological Test Scores in Older Adults with Normal CN vs. Those with MCI
| Median Score (Range) | |||
|---|---|---|---|
| Neuropsychological Tests | CN (n = 8) | MCI (n = 8) | |
| Premorbid | AMNART IQa | 127 (111–129) | 116 (107–130) |
| Raven’s | 28 (23–34) | 27 (15–35) | |
| Memory | California Verbal Testa | 9 (7–10) | 6 (3–9) |
| Rey figure delayed recalla | 15.5 (9–23) | 12 (5–16) | |
| Construction | Rey figure copy | 21 (17–23) | 18 (14–22) |
| Block design | 11 (8–28) | 10 (2–21) | |
| Language | Naming | 27 (20–28) | 26.5 (20–29) |
| Word generation (letters)a | 24 (16–34) | 18 (11–27) | |
| Word generation (categories)a | 16 (13–24) | 12.5 (7–16) | |
| Psychomotor Speed | Trails A | 55 (32–82) | 39 (26–62) |
| Executive Functions | Trails B/Aa | 1.8 (1–3) | 2.3 (2–5) |
| Stroop color word | 78 (53–120) | 78 (39–104) | |
AMNART IQ, American version National Reading Test-Intelligence Quotient; CN, cognitively normal subjects; MCI, mild cognitive impairment.
Indicates significant mean difference at p <.05 (independent sample t-test).
Preparing to Overcome Prepotency (POP) Task
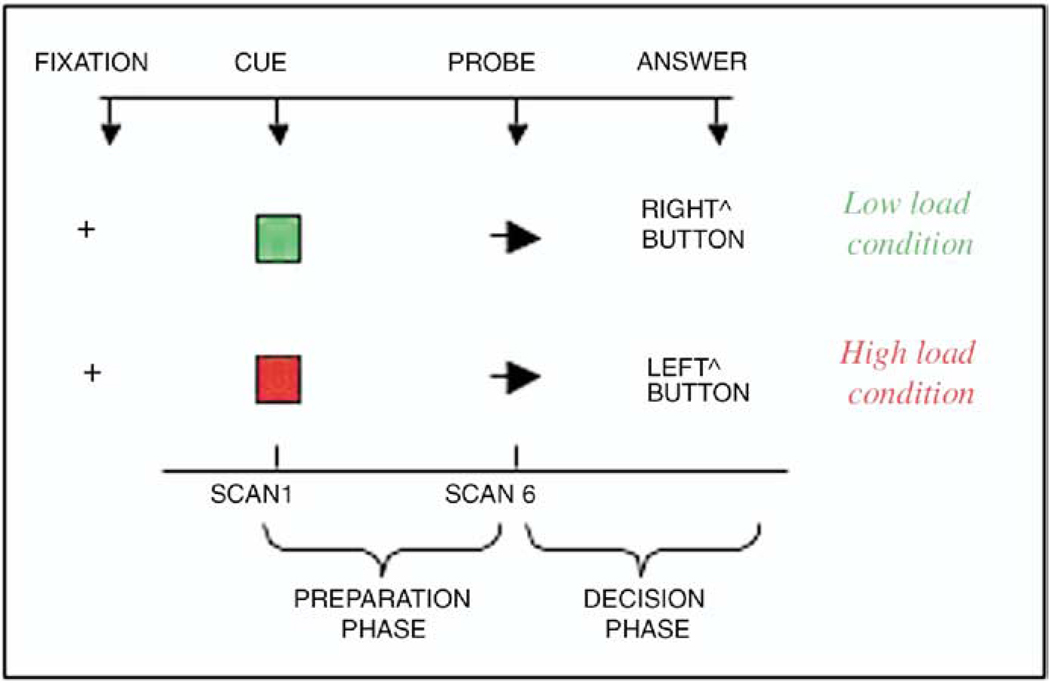
After signing informed consent, participants were instructed on the POP task outside the magnet for as long as needed to familiarize with the task (5–10 minutes). The POP task (Figure 1) involves presentation of cues (green/red square) during the preparation phase of the task, indicating whether response to an upcoming probe (arrow on right/left) during the decision phase of the task should be congruent (low-load condition) or incongruent (high-load condition). During congruent conditions, the individual has to push a button underneath his left index finger if the arrow is pointing left, while he has to push a button placed underneath his right index finger if the arrow is pointing right. During incongruent tasks, the individual has to invert this order (e.g. push the button underneath his left index if the arrow is pointing right). Red and green cues alternated randomly during the task and 25% of the trials contained a red cue. Parameters of behavioral performance included accuracy rates (AR, number of correct answers/ number of total answers), reaction time (RT, msec) calculated on correct trials only. Reliability on the POP task has been verified in a sample of 16 mid-life control subjects (mean age = 23.4 SD = 4.9) who performed the task on two separate occasions 4 weeks apart. Their test-retest Pearson r on reaction time change between high- and low-load trials was .64 (p = .01, 1-tailed). Significant correlations were also found for the other parameters of performance (i.e., reaction time and error rates in both high and low-load conditions). We computed means and percent change in reaction time ([mean reaction time in high-load minus low-load conditions]/ mean reaction time in low-load conditions) and in accuracy rates (mean accuracy rates in high-load minus low-load conditions]/ mean accuracy rates in low-load conditions). The computation of percent change requires normalization of the arithmetical difference of load-related measurements (e.g., reaction time during high-load minus reaction time during low-load) by baseline performance (e.g., reaction time during low-load condition). This approach therefore allows comparisons across individuals and across groups. Greater percent changes in reaction time or accuracy rates were considered a measure of poorer performance, in that it indicated a slower or less accurate response during high-load tasks compared to low-load.
Figure 1.
One example for each of two conditions is illustrated. ^Indicates button placed underneath the right or left finger.
Imaging Analysis
Protocol
Subsequent to the POP practice sessions, participants underwent a 60 min fMRI scanning protocol in a 3.0T GE scanner (GE Medical Systems, Fremont, California). All participants were offered to be acclimated in a “simulation scanner” prior to the fMRI session. We used a gradient echo acquisition (GRE) spiral acquisition (1-shot, repetition time [TR] = 1500 msec, echo time [TE] = 18 msec) where the MR scanning was synchronized with individual trials and 27 slices, 3.2mm thick, were collected parallel to the AC/PC line, every 1.5 seconds. We used a slow event-related design with five blocks and 24-trial/blocks of the task. A volumetric Spoiled Gradient Recalled Acquisition (SPGR) three-dimensional (3D SPGR) sequence was acquired, TR = 25 msec, TE = 5, Nutation angle = 40, 124 slices. Slices with 0 mm gap will generate a 256×256×192 matrix with a 24×18cm field of view (FOV).
Data Preprocessing
fMRI data were corrected for movement, using a 6-parameter linear algorithm (Woods et al 1998). A linear detrending algorithm was also performed, using only data within 3 standard deviations of the mean to estimate the linear trend. An outlier correction algorithm was performed to remove data that was more than 7 standard deviations from the mean. Global normalization was performed multiplicatively to give each subject a mean intensity of 3000. All analyses were conducted on a single-subject basis.
Automated Parcellation
This method has been described in detail elsewhere (Rosano et al, in press; Aizenstein et al 2004). Regions of interest (ROIs) were defined on the Montreal Neurological Institute (MNI) labeled single-subject high-resolution anatomical brain (Tzourio-Mazoyer et al 2002). Regions from the aal map corresponding to dlPFC (Brodmann areas 9, 45 and 46) and PPC (Brodmann areas 7 and 44, respectively) were obtained of the MRIcro software package (GE Medical Systems) (Tzourio-Mazoyer et al 2002). ACC was identified based on our previous functional study (Carter et al 2000). Each subject’s in plane anatomical image was cross- registered to his/her high-resolution anatomical image using a 6-parameter linear algorithm. Then the MNI single-subject high-resolution anatomical image was aligned with each subject’s high-resolution anatomical image using a 2nd order nonlinear warping algorithm with 30 parameters (Woods et al 1998). The ROIs areas were then mapped onto each subject fMRI data using a 30-parameter nonlinear registration (AIR). The parcellation of each subject was visually inspected to assure an accurate parcellation. The nonlinear warping accounted for the nonlinear differences between the template and target MRI (i.e., individual subject). The registration of the MNI to the individual’s brain does not transform (i.e., stretch or warp) the individual’s brain itself, rather the labels from the MNI brain are transformed to fit the specific shape and size of the individuals’ anatomy.
Statistical Analyses
Performance was compared within group for increasing difficulty of the task (e.g. from low- to high-load condition) and across groups for each condition. Because of the skewed distribution of reaction time (kurtosis: −.8 and 2.2 for low and high load conditions, respectively), Wilcoxon ranks test and Mann-Whitney test were used for paired and independent samples analysis.
All fMRI data analyses were performed on scan data collected from correct trials. Relative increase in average fMRI signal change (average signal change measured during high-load minus low-load) was calculated during the preparation phase for PPC and dlPFC and during the decision phase for ACC. For each subject and each region of interest (ROI), a mean time series was generated using the voxels from the entire ROI. We calculated the mean MR signal bilaterally for the regions of interest at each of the 13 scans. The resulting values were transformed into a % change from baseline and the max value of each time series during each phase of the task was extracted for each individual and each ROI. We have previously shown that high-load condition of a variant of the POP task is sensitive to the functioning of dlPFC during the preparation phase and of ACC during the decision phase (MacDonald et al 2000). Because PPC plays a relevant role for the processing of visual spatial information and is also associated to reduced attention in dementia (Thiel et al 2004), task-related activation in PPC was compared between groups during the preparation phase. We hypothesized that activation of dlPFC and PPC would be greater in MCI vs. cognitively normal subjects during the preparation phase of the task, and in ACC during the decision phases of the task. Peak activation of the time series of each ROI was modeled as outcome in linear regression models. Years of education (more or less than 13 years), Amnart score, cognitive status (cognitively normal/ MCI), load of the task (high/low) and interaction between cognitive status and load of the task entered the model in stepwise fashion. Peak activation of the time series of each ROI was compared within group and across condition using paired t-tests (e.g.: low vs. high load condition in MCI), and across group within condition using independent sample t tests (e.g.: MCI vs. cognitively normal subjects during red trials).
To test the association between performance and brain activation, we calculated coefficients of correlation between relative increase in brain fMRI signal change and percent increase in reaction time. Because we observed a decline in accuracy rates with greater percent changes in reaction time (r = −.7), we also tested for partial correlation coefficients between brain fMRI signal change and percent increase in reaction time after adjusting for accuracy rates.
Results
Of 16 older adults recruited for this study, 8 were MCI (mean age: 79.5, 4 women, 7 caucasian) and 8 were cognitively normal (mean age 79.5, 3 women, all Caucasian). All participants were right handed, and all but two had more than 16 years of education. Table 1 shows that MCI scored consistently worse than cognitively normal adults in each of the neuropsychological tests used to complete diagnosis of cognitive status. All patients were classified as multiple cognitive domain MCI by one of us (OL). All patients had 1 abnormal test result in at least two cognitive (not memory) domains. Six out of 8 patients also had abnormal results in at least 1 test of the memory domain.
Behavioral Response to the POP Task
Recording of behavioral responses was not completed for 2 individuals, and this precluded analysis of their reaction time or accuracy. However, for these two individuals the order of trial types, i.e., which trials were high- versus low-load, was available and allowed for functional MRI analysis.
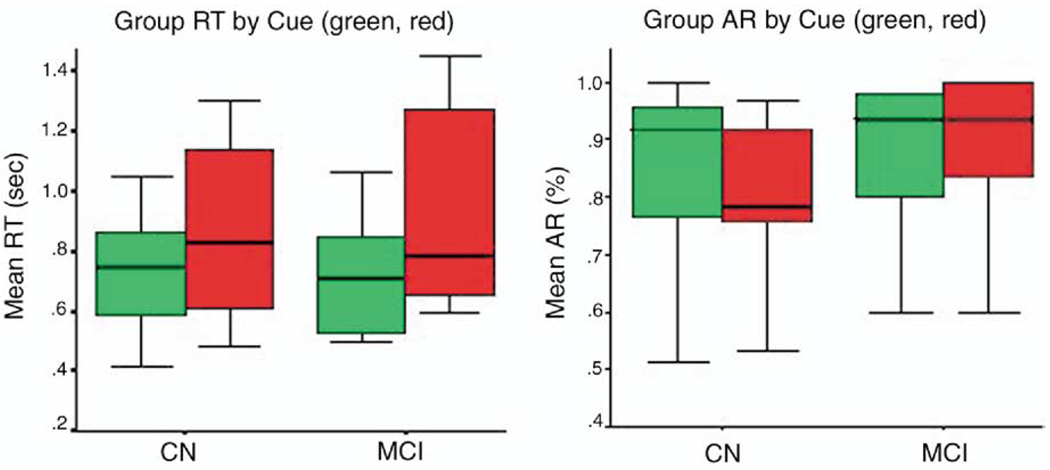
In both groups, reaction times increased and accuracy rates decreased going from low-load to high-load conditions (Figure 2). When going from low-to high-load condition, reaction time significantly increased in MCI (from 797.4 to 938.4 msec, z = −2.4, p = .01) and in cognitively normal subjects (from 697.4 to 817.2 msec, z= −2.02, p = .04) Small but consistent decrease in average accuracy rates was observed in MCI (98.6 to 95.8%, z = −.7, p = .4) and in cognitively normal subjects (97.8 to 92.1%, z = −2.1, p = .03) in response to task-load. Differences between groups were not significant for reaction times (low load: z = −.73, p = .5; high load: z = −.9, p = .4) nor for accuracy rates (low load: z = −.7, p = .5; high load: z = −1.1, p = .3).
Figure 2.
Group Reaction time (RT) and Accuracy Rate (AR) during POP task in Cognitively Normal (CN) and MCI older adults (n = 8 and n = 6, respectively). Green and red indicate low load and high load condition, respectively. Boxplots indicate interquartile range (box), median (line across the box) and extreme values (whiskers). POP, Preparing to Overcome Prepotency task; MCI, mild cognitive impairment.
Patterns of Brain Activation
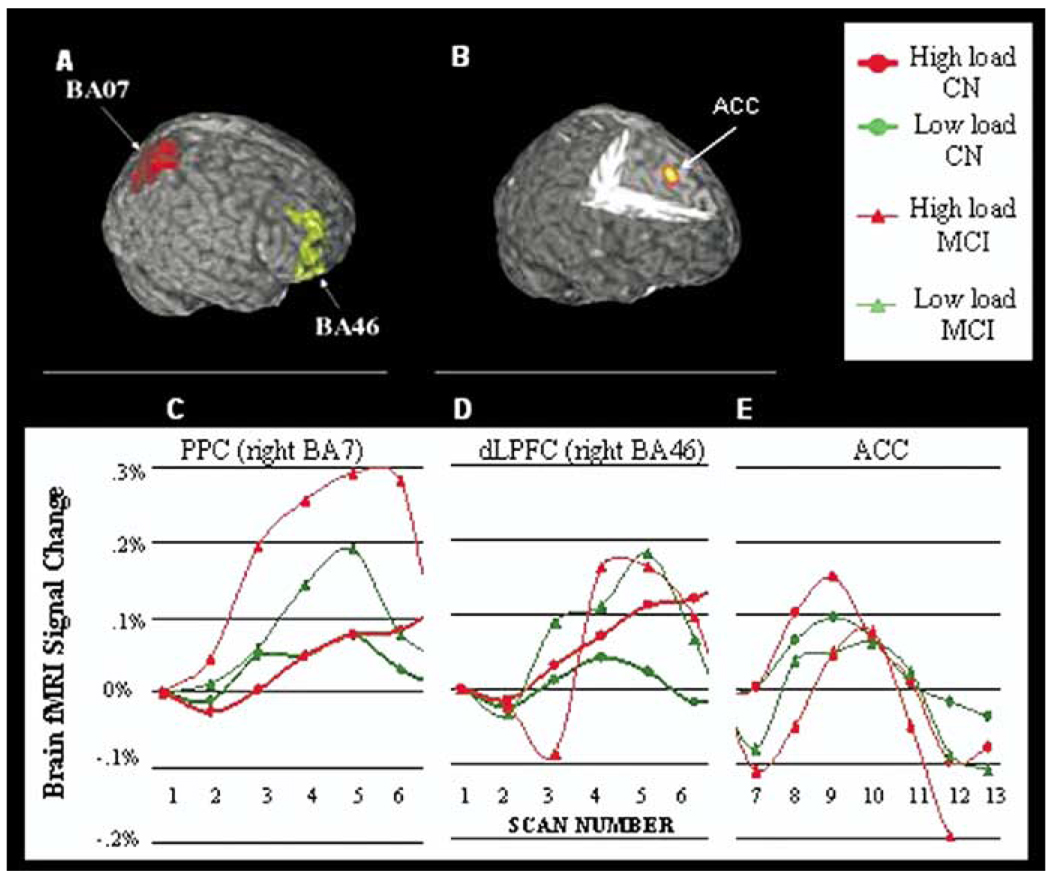
Activation in MCI and cognitively normal subjects was measured in PPC, dlPFC and ACC (Figure 3 A–B). Brain activation in response to high loads of the task substantially differed in MCI vs. Normal subjects in PPC and dlPFC (interaction of cognitive status by load: PPC: p = .001, dlPFC: p = .002), but not in ACC (interaction of cognitive status by load: p = .72). In MCI adults, PPC activation was significantly greater in high vs. low load (right: t7 = 3.5, p = .01; t7 = 2.7, left: .03), while in cognitively normal adults PPC-related activation did not substantially change with task- load (Figure 3C; right: t7 = 1.96, p = .09; t7 = 1.35, left: .22). In contrast, cognitively normal subjects responded to high load conditions by increasing dlPFC-related activation (Figure 3D; right hemisphere: t7 = 5.5, p = .0009; left hemisphere: t7 = 2.7, p = .03), while MCI did not (right: t7 = 1.8, p = .12, left: t7 = 1.24, p = .24). MCI showed significantly more activation compared to cognitively normal subjects both in PPC (low-load: t16 = 2.4, p = .03 and t16 = 2.2, p = .05 for right and left hemisphere; high-load: t16 = 3.1, p = .007 and t16 = 2.2, p = .04, for right and left hemisphere) and dlPFC (low-load condition: t16 = 3.7, p = .002 and t16 = 3.6, .004 for right and left hemisphere; high load condition: t16 = 2.1, p = .049 and t16 = 1.9, .08 for right and left hemisphere). Similar results were observed bilaterally for BA45, while activation was small or not significant in Brodmann area 9. In MCI adults, load related change in PPC activation was associated with worse performance on Amnart test (r = −.85, p = .007), and worse performance on Rey delayed recalled test (r = −.785, p = .02), word generation categories (r = −.8, p = .02) and Stroop color word test (r = .83, p = .01). Associations were not significant for cognitively normal subjects. In stepwise linear regression models, cognitive status alone explained 40% of the variance of PPC activation (right BA7). Cognitive status remained a significant and independent predictor of PPC activation after adjusting for Amnart score and education (t = 2.7, p = .02). Amnart score or education did not substantially change regression coefficients for cognitive status and dlPFC cortex. A modest load effect was observed in ACC (Figure 3E) for cognitively normal subjects (p = .03), but not for MCI (p = .12). We did not detect a significant cognitive status effect (p = .8) nor a cognitive status by load interaction effect (p = .72) in this region.
Figure 3.
Regions of Interest [upper row: (A– B)] included Brodmann area 7 (BA7), Brodmann area 46 (BA46) and Anterior Cingulate Cortex (ACC) as defined from our previous study (Carter et al 2000). Time Series activation (C– E) are illustrated for MCI (n = 8) and cognitively normal older adults (CN, n = 8) during low-load and high-load condition. Brain fMRI signal change was measured in PPC and dlPFC during the preparation phase and in ACC during the decision phase of the task dlPFC, dorsolateral prefrontal cortex; PPC, posterior parietal cortex; ACC, anterior cingulate cortex; fMRI, functional magnetic resonance imaging.
Association Between Brain Activation and Performance
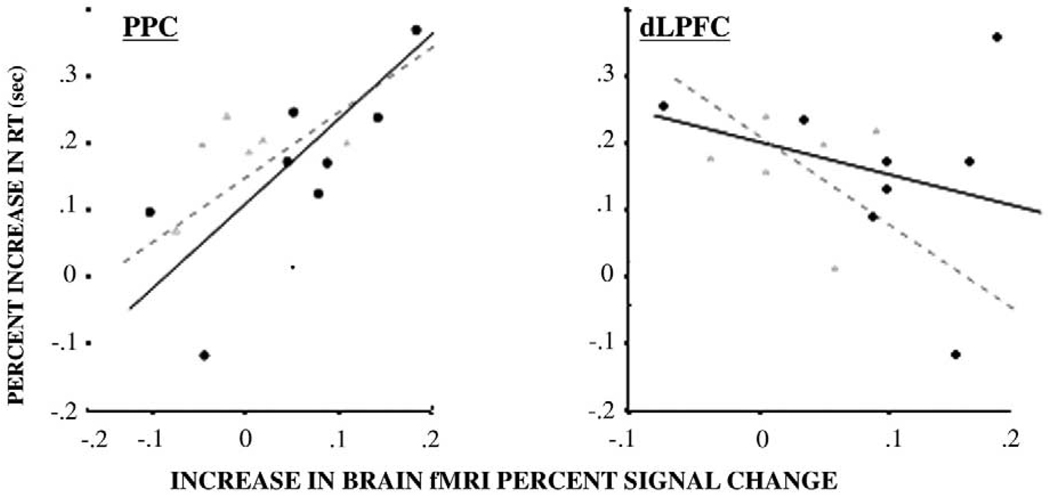
Among older individuals with MCI, those with greater relative increase in PPC-derived signal (high- minus low-load related activation) also had smaller load-related changes in accuracy rates (partial correlation coefficients after adjustment for reaction time [p value]: r = −.85 [p = .07]) and greater relative increase in reaction time (partial correlation coefficients after adjustment for accuracy rates: r = .97, p = .01, Figure 4A). In cognitively normal subjects, load-related change in PPC activation was associated with load-related change in reaction time (partial correlation coefficients after adjustment for accuracy rates [p value]: r = .76, p = .02, Figure 4A) but not with accuracy rates (partial correlation coefficients after adjustment for reaction time [p value]: r = .09, [p = .4]), Greater load-related changes in dlPFC-derived signal was associated with smaller changes in reaction time (Figure 4B) although associations were not statistically significant for MCI or for cognitively normal subjects (partial correlation coefficients = −.7 [p = .1] and −.5 [p = .2] after adjusting for accuracy rates). Associations between load-related change in dlPFC and accuracy rates were also not significant (MCI: r = .6, p = .2; cognitively normal subjects: r = .3 [p = .3]). Associations between relative increase in ACC activation and percent increase in reaction time or accuracy rates were not significant (partial correlation coefficient [p value]: r = .48 [p = .3], and r = −.6 [p = .8]).
Figure 4.
Scatterplots of the association between increase in reaction time [RT] (high load – low load/low load) and increase in average fMRI signal change (high-load –low load) in PPC (right BA7) and dlPFC (right BA46). Gray triangles refer to MCI (n = 6) and black circles indicate cognitively normal (CN) adults (n = 8). Regression lines are shown for cognitively normals (black) and MCI (gray). fMRI, functional magnetic resonance imaging; PPC, posterior parietal cortex; dlPFC, dorsolateral prefrontal cortex; MCI, mild cognitive impairment.
Discussion
Compared to cognitively normal older adults, MCI showed greater dlPFC and PPC activation while maintaining ACC activity and performance in the same range as the control group. When challenged by higher load/ incongruent conditions, these older adults showed different preparation-related patterns of activation based on their cognitive status: while cognitively normal subjects showed increased level of activation in dlPFC, MCI adults showed increased PPC activity. Among MCI, those who showed greater load-related changes in PPC activation also maintained a successful performance even in high challenging conditions (e.g. smaller load-related changes in accuracy rates), while responding with longer reaction times. The finding that MCI with greater load-related PPC had lower scores on cognitive function tests suggests that greater PPC activation may be an indicator of mild cognitive impairment.
Our results of increased brain activation in those individuals at high risk of dementia are consistent with previous neuroimaging studies that reported greater prefrontal and parietal brain activity in individuals with early Alzheimer’s disease (AD) compared with healthy age-matched controls (review by Petrella et al 2003). This increased regional brain activation has been interpreted as a compensatory reallocation of cognitive resources, but direct evidence for a facilitating effect on performance in older individuals at high risk for AD has been lacking.
Increased activation in PPC regions has been reported in individuals at risk for AD during working memory tasks (Bookheimer et al 2000; Saykin et al 1999) and in particular in tasks requiring attention or spatial orientation (Thulborn et al 2000). A similar recruitment of parietal regions has been reported during tasks of shifting of attention in healthy older adults compared with younger adults (Corbetta et al 2000), indicating that increased PPC activation might be a common response to functional loss resulting from various causes. Increased recruitment of PPC in MCI may indicate an underlying decrease in the brain’s ability to implement attentional control early in the course of the disease. PPC may be responding to increased attentional demands/generalized impairment by increasing activation. Alternatively, it is possible that cognitive processes associated with PPC activation interfered with MCI ability to quickly perform the task.
In this study, MCI appeared to redistribute activation in prefrontal and parietal areas while achieving successful performance during complex decision-making tasks. There may be a trade-off between increased activation in PPC and reduced activation in dlPFC in response to high loads of the task in MCI. The structural and functional connectivity between posterior parietal and prefrontal cortex (Cavada and Goldman-Rakic 1989a, 1989b; Cabeza et al 1997) suggests that these regions may work together to accomplish attentional control.
In MCI patients of this study, retrieving instructions following the cue-prompt appeared to be an actual challenge, regardless of the nature of the cue itself (e.g. green or red). This is consistent with our findings of increased dlPFC and PPC activation in MCI compared to cognitively normal adults, during the low-load condition. Association between activation in the prefrontal cortex and learning recall has also been observed by others (Gabrieli et al 1998; Buckner et al 1999). Working memory deficits have been observed in individuals at risk for AD (Grady et al 2003; Bookheimer et al 2000; Petrella et al 2003) and it is possible that our patients increased dlPFC activation above cognitively normal subjects’ levels during low loads of the task to compensate for this deficit. For greater levels of conflict, such as those experienced during both the preparation and decision phase of the task after the red cue, the cognitive demands might have exceeded the MCI’s compensatory reserve, thus yielding a next to null load-related response in dlPFC. The weak response of dlPFC to the load of the task observed in MCI may reflect frontal lobe neural degeneration (Encinas et al 2003; Nagahama et al 2003) in MCI.
A focal degeneration of the ACC area may also be the reason why MCI patients did not increase activation in ACC while they were engaged in the decision phase of higher load tasks. We are not aware of volumetric analysis of ACC in association with cognitive impairment in older adults, therefore it is hard to draw any conclusion. Models of attentional controls have proposed that ACC plays a role in the evaluation and monitoring of conditions that can lead to erroneous responses, such as circumstances with high levels of conflict (Carter et al 2000; MacDonald et al 2000). From our findings, it appears that in MCI the role of the ACC in this process is relatively intact (e.g. accuracy rates were similar across groups), and that the difficulties arise in mechanisms responsible for modulating attentional control rather than in detecting such demands.
It is striking that these very old individuals maintained a very high level of performance, which was only marginally affected by their cognitive status and approximated performance observed in young adults (Rosano et al, unpublished data). Stronger associations between performance and brain activation could have been found for more complex task conditions. However, one of the strengths of the POP task consists in fact in its simplicity. When dealing with very old populations, it is of crucial importance to use a simple task that does not require complex reasoning or instructions and whose execution is unlikely to be affected by the level of education of the participant. Another strength of this study is that accuracy and reaction time of the participants were measured while recording their actual brain signal, thus allowing correlation of performance with brain activation. The relatively small number of individuals of our study may limit the statistical validity of the association between brain activation and performance, especially in light of missing behavioral data from 2 subjects. Another limitation was that the number of individuals with less than 13 years of education or of African-American race was too small to test the relative contribution of education and race to brain activation or behavioral performance.
In conclusion, our results suggest that PPC activation may represent an early sign of conversion to cognitive impairment in very high functioning individuals. Activation in PPC and dlPFC may identify individuals with incipient cognitive impairment. Future studies will need to address whether changes in the pattern of brain activation in older adults may identify the prodromal stages of AD-related cognitive changes.
Acknowledgments
This research was supported by National Institute on Aging (NIA) Training Grants T32 AG00181-11, RO1 HL-64587, NO1 HC-85079, NO1 HC-85086, AG05133 and U01 AT00162.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aizenstein HJ, Clark KA, Butters MA, Cochran JL, Stenger VA, Meltzer CC, et al. The BOLD hemodynamic response in healthy aging. Journal of Cognitive Neuroscience. 2004;16:786–793. doi: 10.1162/089892904970681. [DOI] [PubMed] [Google Scholar]
- Backman L, Andersson JLR, Nyberg L, Winblad B, Nordberg A, Almkvist O. Brain regions associated with episodic retrieval in normal aging and Alzheimer’s disease. Neurology. 1999;52:1861–1870. doi: 10.1212/wnl.52.9.1861. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Baddeley HA, Bucks RS, Wilcock GK. Attentional control in Alzheimer’s disease. Brain. 2001;124(Pt 8):1492–1508. doi: 10.1093/brain/124.8.1492. [DOI] [PubMed] [Google Scholar]
- Benton AL. Differential behavioral effects in frontal lobe disease. Neuropsychologia. 1968;6:53–60. [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, et al. Patterns of brain activation in people at risk for Alzheimer’s disease. N Engl J Med. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Kelley WM, Petersen SE. Frontal cortex contributes to human memory formation. Nat Neurosci. 1999;2(4):311–314. doi: 10.1038/7221. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, et al. Age-related differences in neural activity during memory encoding and retrieval: a positron emission tomography study. J Neurosci. 1997;17:391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, Cohen JD. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2000;97(4):1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J Comp Neurol. 1989a;287(4):393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol. 1989b;287(4):422–445. doi: 10.1002/cne.902870403. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci. 2000;(3):292–297. doi: 10.1038/73009. Erratum in: Nat Neurosci 2000 May;3(5):521. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober B. The California Verbal Learning Test. New York: Psychological Corporation; 1987. [Google Scholar]
- Elgh E, Larsson A, Eriksson S, Nyberg L. Altered prefrontal brain activity in persons at risk for Alzheimer’s disease: an fMRI study. Int Psychogeriatr. 2003;15(2):121–133. doi: 10.1017/s1041610203008810. [DOI] [PubMed] [Google Scholar]
- Encinas M, De Juan R, Marcos A, Gil P, Barabash A, Fernandez C, et al. Regional cerebral blood flow assessed with 99mTc-ECD SPET as a marker of progression of mild cognitive impairment to Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2003;30:1473–1480. doi: 10.1007/s00259-003-1277-z. [DOI] [PubMed] [Google Scholar]
- Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, et al. The Cardiovascular Health Study: Design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD, Poldrack RA, Desmond JE. The role of left prefrontal cortex in language and memory. Proc Natl Acad Sci U S A. 1998;95:906–913. doi: 10.1073/pnas.95.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Beig S, Keightley ML, Burian H, Black S. Evidence from Functional Neuroimaging of a Compensatory Prefrontal Network in Alzheimer’s Disease. Journal of Neuroscience. 2003;23(3):986. doi: 10.1523/JNEUROSCI.23-03-00986.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff FJ, Collins C, Corkin S, Rosen TJ. Equivalent forms of the Boston Naming Test. J Clin Exp Neuropsychol. 1986;8:556–562. doi: 10.1080/01688638608405175. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Jagust WJ, DeKosky ST, Becker JT, Fitzpatrick A, Dulberg C, et al. Prevalence and Classification of Mild Cognitive Impairment in the Cardiovascular Health Study Cognition Study: Part 1. Arch Neurology. 2003a;60(10):1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Jagust WJ, Dulberg C, Becker JT, DeKosky ST, Fitzpatrick A, et al. Risk Factors for Mild Cognitive Impairment in the Cardiovascular Health Study Cognition Study: Part 2. Arch Neurology. 2003b;60(10):1394–1399. doi: 10.1001/archneur.60.10.1394. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Kuller LH, Fitzpatrick A, Ives D, Becker JT, Beauchamp N. Evaluation of Dementia in the Cardiovascular Health Cognition Study. Neuroepidemiology. 2003c;22:1–12. doi: 10.1159/000067110. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Milham MP, Erickson KI, Banich MT, Kramer AF, Webb A, Wszalek T, Cohen NJ. Attentional control in the aging brain: insights from an fMRI study of the stroop task. Brain Cogn. 2002;49(3):277–296. doi: 10.1006/brcg.2001.1501. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Nabatame H, Okina T, Yamauchi H, Narita M, Fujimoto N, et al. Cerebral correlates of the progression rate of the cognitive decline in probable Alzheimer’s disease. Eur Neurol. 2003;50(1):1–9. doi: 10.1159/000070851. [DOI] [PubMed] [Google Scholar]
- Nelson HE. National Adult Reading Test. Windsor, England: NFER-Nelson; 1982. [Google Scholar]
- Osterreith PA. Le test de copie d’un figure complexe. Arch Psychologie. 1944;30:206–356. [Google Scholar]
- Petrella JR, Coleman RE, Doraiswam PM. State of the Art Neuroimaging and Early Diagnosis of Alzheimer Disease: A Look to the Future. Radiology. 2003;226:315–336. doi: 10.1148/radiol.2262011600. [DOI] [PubMed] [Google Scholar]
- Piert M, Koeppe RA, Giordani B, Berent S, Kuhl DE. Diminished glucose transport and phosphorylation in Alzheimer’s disease determined by dynamic FDG-PET. J Nucl Med. 1996;37(2):201–208. [PubMed] [Google Scholar]
- Raven JC. Coloured Progressive Matrices. Los Angeles, CA: Western Psychological Services; 1956. [Google Scholar]
- Ready RE, Ott BR, Grace J, Cahn-Weiner DA. Apathy and executive dysfunction in mild cognitive impairment and Alzheimer disease. Am J Geriatr Psychiatry. 2003;11(2):222–228. [PubMed] [Google Scholar]
- Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percep Mot Skills. 1958;8:271–276. [Google Scholar]
- Rey A. L’examinen psychologie dans les cas d’encephalopathie traumatique. Arch Psychologie. 1941;30:286–340. [Google Scholar]
- Rosano C, Newman AB, Kuller L, Carter C, Lopez O, Becker J, Aizenstein H. Morphometric analysis of gray matter volume in demented older adults: exploratory analysis of the cardiovascular health study brain MRI database. Neuroepidemiology. doi: 10.1159/000085140. (in press) [DOI] [PubMed] [Google Scholar]
- Rosenbaum DA. The movement precuing technique: assumptions, applications, and extensions. In: Magill RA, editor. Memory and Control of Action. North-Holland: Amsterdam; 1983. pp. 231–274. [Google Scholar]
- Royall DR, Lauterbach EC, Cummings JL, Reeve A, Rummans TA, Kaufer DI, et al. Executive control function: a review of its promise and challenges for clinical research. A report from the Committee on Research of the American Neuropsychiatric Association. J Neuropsychiatry Clin Neurosci. 2002;14(4):377–405. doi: 10.1176/jnp.14.4.377. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Flashman LA, Frutiger SA, Johnson SC, Mamourian AC, Moritz CH, et al. Neuroanatomic substrates of semantic memory impairment in Alzheimer’s disease: patterns of functional MRI activation. J Int Neuropsychol Soc. 1999;5:377–392. doi: 10.1017/s135561779955501x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel CM, Zilles K, Fink GR. Cerebral correlates of alerting, orienting and reorienting of visuospatial attention: an event-related fMRI study. Neuroimage. 2004;21(1):318–328. doi: 10.1016/j.neuroimage.2003.08.044. [DOI] [PubMed] [Google Scholar]
- Thulborn KR, Martin C, Voyvodic JT. Functional MR imaging using a visually guided saccade paradigm for comparing activation patterns in patients with probable Alzheimer’s disease and in cognitively able elderly volunteers. AJNR Am J Neuroradiol. 2000;21:524–531. [PMC free article] [PubMed] [Google Scholar]
- Trenerry MR, Crosson B, DeBoe J, Leber WR. STROOP Neuropsychological Screening Test. Odessa, FL: Psychological Assessment Resources; 1989. [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale, Revised. New York: The Psychological Corporation; 1981. [Google Scholar]
- Woodard JL, Grafton ST, Votaw JR, Green RC, Dobraski ME, Hoffman JM. Compensatory recruitment of neural resources during overt rehearsal of word lists in Alzheimer’s disease. Neuropsychology. 1998;12:491–504. doi: 10.1037//0894-4105.12.4.491. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC. Automated image registration: II. Intersubject validation of linear and nonlinear models. J Computer Assisted Tomography. 1998;22(1):153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]