Abstract
BACKGROUND
Differential effects of β-adrenoreceptor antagonists (β-ARB) on central and peripheral blood pressure may relate to change in heart rate and/or vasodilator tone and thus be exaggerated during exercise.
AIMS
To examine acute effects of selective and nonselective β-ARB on central and peripheral blood pressure, cardiac output and peripheral vascular resistance during exercise.
METHODS
Healthy volunteers (n= 20, 18 men, 19–54 years) received propranolol 80 mg, bisoprolol 20 mg, and placebo 1 h before bicycle ergometry (50, 75 and 100 W each for 3 min) in a randomized, cross-over study. Cardiac output was determined by pulmonary uptake of soluble and inert gas tracers (InnoCor, Innovision). Central systolic blood pressure (SBP) was determined from the late systolic shoulder of the digital artery pressure waveform (Finometer, Finopres).
RESULTS
At rest, both β-ARB reduced brachial but not central SBP (compared with placebo). During exercise, β-ARB reduced brachial SBP (reductions of 19.9 ± 4.3 mmHg and 23.2 ± 2.7 mmHg for propranolol and bisoprolol, respectively, at 100 W, each P < 0.0001) but not central SBP. The difference between peripheral and central SBP was reduced, relative to that during placebo, by 21.5 mmHg (95% confidence interval 8.8, 34.1) and 26.4 mmHg (18.1, 34.8) for propranolol and bisoprolol, respectively, at 100 W (each P < 0.01). There was no significant effect of β-ARB on diastolic blood pressure or peripheral vascular resistance.
CONCLUSIONS
Despite reducing brachial blood pressure, acute β-adrenoreceptor blockade in man at rest and during exercise does not reduce central blood pressure.
Keywords: beta-adrenoreceptor antagonists, central blood pressure, exercise, peripheral amplification
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
At rest, β-adrenoreceptor antagonists lower peripheral systolic blood pressure (SBP) but have less effect on central SBP.
Effects of β-adrenoreceptor antagonists on central and peripheral blood pressure during exercise are unknown.
WHAT THIS STUDY ADDS
This study shows that acute β-blockade in healthy normotensive subjects markedly reduces peripheral SBP by ∼ 20 mmHg during moderate exercise but has no significant effect on central SBP.
The differential effect of β-blockade on central and systolic blood pressure is not completely explained by reduction in heart rate. Beta-blockade may blunt dilation of muscular arteries, influencing peripheral amplification during exercise.
Introduction
Beta-adrenoreceptor (β-AR) antagonists (β-ARB) are thought to be less effective at lowering central blood pressure than other antihypertensive drugs [1–3]. This could be due to effects of β-ARB on heart rate or on arterial tone/stiffness and wave reflection [4, 5]. Such effects might be particularly important during exercise when heart rate increases and peripheral arterial tone may be influenced by β-AR vasodilator effects. β-AR vasodilation in man is mediated almost exclusively through β2-AR [6], but effects of selective β2-AR relative to nonselective β-AR blockade on peripheral arterial tone, wave reflection and central arterial pressure, in man, are unknown. However, it is notable that mice lacking a functional β2-AR gene have normal resting blood pressure but exaggerated blood pressure responses to epinephrine and to treadmill exercise [7], suggesting that β2-AR plays a role in determining the haemodynamic responses to exercise through modulation of peripheral vascular resistance. The objective of the present study was to investigate acute effects of the selective β2-ARB bisoprolol and nonselective β-ARB propranolol on central and peripheral blood pressure, cardiac output and peripheral vascular resistance at rest and during low-work load exercise in healthy subjects.
Methods
Subjects
Subjects were normotensive healthy volunteers (n= 20, 18 men). All were asymptomatic with no history of cardiovascular disease and on no regular medication. Subjects were recreationally active but none was an amateur or professional athlete. The study was approved by St Thomas' Hospital Research Ethics Committee, and written informed consent was obtained from all subjects.
Protocol
The study design comprised a randomized three-phase, placebo-controlled double-blind cross-over. Subjects attended on three occasions separated by at least 7 days and received, in random order, placebo, bisoprolol (20 mg) and propranolol (80 mg) 1 h before exercise. These doses and timing of administration were selected because they produce similar effects on heart rate during exercise [8]. Subjects attended in the morning approximately 2 h after a light breakfast having avoided exercise other than walking. After administration of the β-ARB/placebo, subjects remained seated for 60 min. Baseline haemodynamic measurements were made over 12 min. Subjects then performed a period of low-workload exercise on a bicycle ergometer at 50, 75 and 100 W each for 3 min.
Peripheral blood pressure measurements
Brachial systolic (bSBP) and diastolic (bDBP) blood pressure were measured in the left arm, using an appropriately sized cuff at the level of the heart by a single trained observer using mercury sphygmomanometry with diastolic measurements taken at phase IV. Mercury sphygmomanometry, when measured at phase IV by an experienced observer, is more reproducible than automated methods [9] and agrees with intra-arterial measurements during exercise [10]. Mean arterial blood pressure (MAP) was estimated from bDBP plus one-third of the brachial pulse pressure.
Cardiac output
Cardiac output (CO) was determined by pulmonary uptake of soluble and inert gas tracers (InnoCor; Innovision, Odense, Denmark). Subjects re-breathed a gas mixture (1% SF6, 5% N2O and 94% O2) for periods of 20 s. Expired gases were sampled continuously and analysed by an infrared photoacoustic analyser [11]. Stroke volume (SV) was calculated from CO and heart rate. Systemic vascular resistance (SVR) was calculated from MAP and CO and expressed in units of mmHg l−1 min−1.
Central blood pressure
Digital arterial pressure waveforms were obtained from the index finger of the left arm using a servo-controlled finger cuff (Finometer; Finapres, Amsterdam, the Netherlands). Central systolic pressure was estimated from the late systolic shoulder of the pressure waveform (SBP2) [12]. Digital pressure waveforms were obtained over a minimum of 10 cycles (from which mean values of SBP2 were calculated) before brachial cuff inflation. The analysis was repeated with pressure waveforms calibrated directly by the Finometer system and with waveforms calibrated from brachial systolic and diastolic values of blood pressure. SBP2 obtained from the Finometer closely approximates central aortic pressure at rest and during haemodynamic changes similar to those that occur during exercise such as vasodilation induced by nitroglycerin and during increases in heart rate induced by atrial pacing [13]. The Finometer system was used in preference to the commercially available SphygmoCor system (Atcor, West Ryde, Australia), because the latter employs a hand-held tonometer that cannot be used during exercise. Digital artery pressure waveforms obtained using a servo-controlled finger cuff are similar to radial artery pressure waveforms obtained using the SphygoCor system [14].
Statistical analysis
Subject characteristics (Table 1) are presented as means (SD), other results are presented as means ± SE. Comparisons between treatments were made by analysis of variance (anova) for repeated measurements with drug class (propranolol, bisoprolol, placebo) as a within-subject factor. A categorical variable representing order of treatment phase was used to test for any carry-over effects. A prespecified contrast between each treatment group was used to test for differences between β-ARB and placebo and between bisoprolol and propranolol. Multiple regression analysis was used to examine the relation between peripheral amplification (bSBP–cSBP) and heart rate. Dummy variables for treatment group were incorporated to test the interaction of this relationship with presence or absence of β-ARB. SPSS version 13.0 was used for all analysis (SPSS Inc., Chicago, IL, USA). All tests were two-tailed and P < 0.05 was taken as significant.
Table 1.
Subject characteristics (n= 20)
| Variable | Mean (SD) |
|---|---|
| Age, years | 31 (10) |
| Height, cm | 178 (8) |
| Weight, kg | 75 (12) |
| Body mass index | 23 (4) |
| SBP, mmHg | 117 (8) |
| DBP, mmHg | 74 (7) |
| MAP, mmHg | 89 (6) |
| Total cholesterol, mmol−1 | 4.6 (1.0) |
| HDL-cholesterol, mmol−1 | 1.3 (0.3) |
| Triglycerides, mmol−1 | 1.1 (0.6) |
| Blood glucose, mmol−1 | 4.9 (0.8) |
Results
Subject characteristics are shown in Table 1. All subjects completed the exercise regime with a mean increase in heart rate (on the placebo day) at 100 W of 46.3 ± 3.1 min−1. Mean values of blood pressure and other haemodynamic measurements at rest, during exercise and during recovery are shown in Figure 1.
Figure 1.
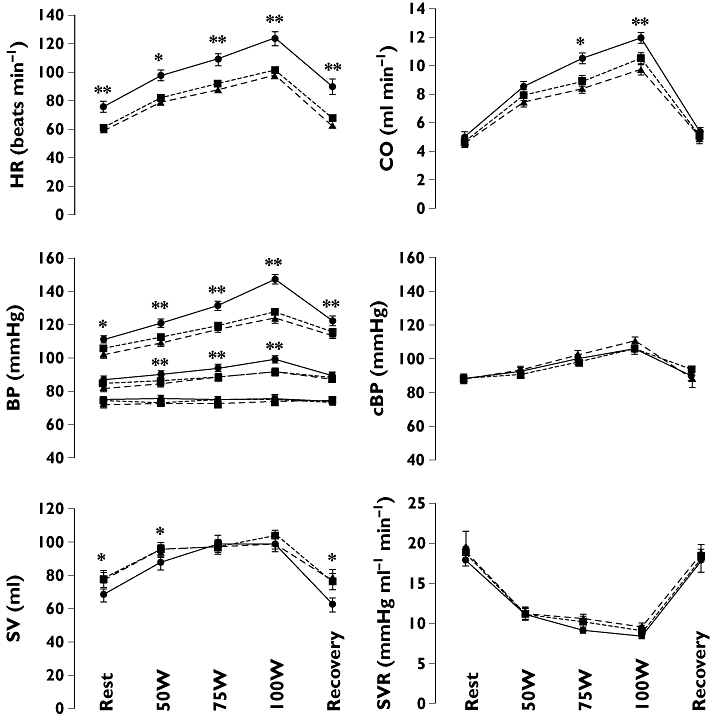
Cardiac output (CO), heart rate (HR), brachial blood pressure (BP, systolic, mean and diastolic), central systolic blood pressure (cSBP), stroke volume (SV) and systemic vascular resistance (SVR) at rest, during exercise and during recovery after placebo (•, solid line), propranolol (▪, dotted line) and bisoprolol (▴, dashed line). *Propranolol and bisoprolol significantly different from placebo, P < 0.05. **Propranolol and bisoprolol significantly different from placebo, P < 0.01
Haemodynamics at rest
Compared with placebo, propranolol and bisoprolol reduced heart rate by a similar amount (by 15.7 ± 2.5 and 17.9 ± 1.9 min−1 for propranolol and bisoprolol, respectively, each P < 0.0001). Despite the reduction in heart rate, CO at rest was maintained after β-ARB due to an increase in SV (by 9.1 ± 3.1 ml and 10.4 ± 4.1 ml relative to placebo for propranolol and bisoprolol, respectively, each P < 0.05). Diastolic blood pressure and systemic vascular resistance were similar after β-ARB and placebo. bSBP was lower after β-ARB compared with placebo (by 6.4 ± 2.4 and 9.9 ± 1.5 mmHg for propranolol and bisoprolol, respectively, each P < 0.01) but central systolic blood pressure (cSBP) was not significantly different after β-ARB and placebo. There were no significant differences between effects of bisoprolol relative to propranolol on any of the haemodynamic measurements.
Haemodynamics during exercise
Propranolol and bisoprolol reduced heart rate during exercise by a similar amount (by 22 ± 4 and 26 ± 3 min−1 relative to placebo at 100 W for propranolol and bisoprolol, respectively, each P < 0.0001). At 50 W CO was maintained after β-ARB, but at higher work loads CO was lower after β-ARB (by 1.4 ± 0.4 and 2.0 ± 0.3 l min−1 at 100 W relative to placebo for propranolol and bisoprolol, respectively, each P < 0.01, P < 0.001 for interaction of β-ARB/placebo with work load across all work loads). This was mainly due to a reduction in heart rate since, at this work load, SV was similar after β-ARB and placebo. Diastolic blood pressure and systemic vascular resistance were similar after β-ARB and placebo. bSBP was lower after β-ARB compared with placebo (P < 0.0001) and the effect of β-ARB to lower bSBP increased with increasing work load (reductions in bSBP of 9.6 ± 3.7 and 11.4 ± 2.7 mmHg at 50 W and of 19.9 ± 4.3 and 23.2 ± 2.7 mmHg at 100 W for propranolol and bisoprolol, respectively, P < 0.001 for interaction of β-ARB/placebo with work load across all work loads). MAP was also significantly lower after β-ARB compared with placebo. By contrast to bSBP, cSBP was not significantly reduced by β-ARB and effects of β-ARB on brachial compared with central SBP were significant: the difference between peripheral and central systolic blood pressure (bSBP–cSBP) was reduced, relative to that during placebo, by 21.5 mmHg (95% confidence interval 8.8, 34.1) and 26.4 mmHg (18.1, 34.8) for propranolol and bisoprolol, respectively, at 100 W (each P < 0.01). There were no significant differences between effects of bisoprolol relative to propranolol on any of the haemodynamic measurements.
Recovery
At 3 min into recovery, mean values of heart rate returned to within approximately 10 min−1 of baseline but remained lower after β-ARB compared with placebo (by 22.6 ± 3.8 and 26.2 ± 3.6 min−1 for propranolol and bisoprolol, respectively, P < 0.0001). bSBP was lower (by 8.0 ± 4.2 and 8.8 ± 3.1 mmHg after propranolol and bisoprolol, P < 0.05 for β-ARB vs. placebo), but CO, systemic vascular resistance and cSBP were similar after β-ARB and placebo.
Relationship between peripheral amplification and heart rate
The difference between peripheral and central blood pressure (bSBP–SBP2) at rest and during exercise was closely related to heart rate (R= 0.39, P < 0.001, Figure 2). However, there was a significant interaction (P < 0.05) between this relationship and presence or absence of β-ARB. When considering measurements after β-ARB alone the relation between bSBP–SBP2 and heart rate was not significant (R= 0.12, P= NS).
Figure 2.
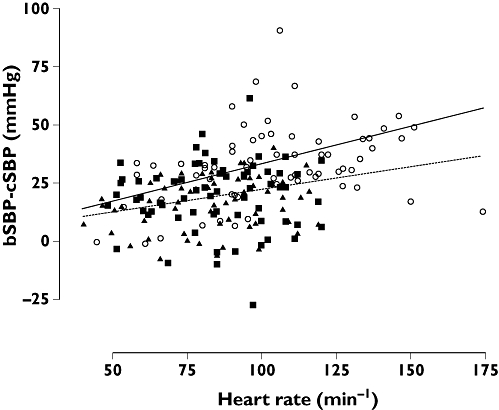
Relationship between the difference between brachial and central systolic blood pressure (bSBP–cSBP) and heart rate during rest and exercise for the three treatment groups (○: placebo; ▪: propranolol; ▴: bispoprolol). The solid line is the regression line for placebo (R= 0.46, P < 0.001) and the dotted line that for the combined β-blocker groups (R= 0.12, P= NS)
Effects of calibration of peripheral blood pressure waveforms
Calibration of peripheral blood pressure waveforms using sphygmomanometric measures of brachial systolic and diastolic pressure instead of using the Finometer calibration had little effect on estimates of cSBP (mean differences <4 mmHg) and made no difference to results of the statistical analysis.
Discussion
Previous studies have demonstrated that, for the same level of peripheral systolic blood pressure reduction, β-ARB-based antihypertensive regimes are associated with less effect on central blood pressure [2, 4, 15, 16]. This differential effect on peripheral and central blood pressure has been attributed to β-AR blockade and could contribute to less favourable effects of β-ARB-based antihypertensive regimes compared with other antihypertensive regimes on outcome [17]. In the present study, β-AR blockade reduced heart rate and peripheral blood pressure at rest but had no significant effect on central blood pressure. Furthermore, as noted in previous studies [18], β-AR blockade at rest was associated with no change in CO because of opposing changes in SV and heart rate and no significant change in systemic vascular resistance. The reduction in peripheral systolic blood pressure could, therefore, be regarded as purely due to decreased peripheral amplification and to be without physiological benefit in terms of reduction in cardiac load.
Our study is the first, as far as we are aware, to examine effects of β-AR blockade on central and peripheral BP during exercise. The influence of β-AR blockade on exercise haemodynamics is of importance, since β-ARB have previously been recommended in and are widely used in young subjects [19, 20]. The efficacy of β-ARB to limit the SBP response to exercise may be particularly important, because this determines cardiac load during exercise and is an independent predictor of mortality [21, 22]. At low work load, CO after β-AR blockade was maintained despite a reduction in heart rate due to an increase in SV. The observation of an increase in SV at moderate levels of exercise has been reported in a previous study [18], although an invasive study in hypertensive subjects reported a decrease in SV [23] and it is possible that the effect of β-AR blockade on SV depends on subject age and diastolic/systolic function. At higher levels of exercise, β-AR blockade reduced CO due to a reduction in heart rate (with no significant effect on SV). β-AR blockade markedly reduced bSBP but was without effect on cSBP.
Differential effects of β-AR blockade on central and peripheral BP during exercise could relate to altered ventricular ejection and or arterial tone/stiffness influencing pressure wave reflection and hence central blood pressure. However, β-AR blockade was without effect on SV at higher work loads when differential effects on central and peripheral blood pressure were most marked. β-AR blockade did not significantly modulate arterial tone in resistance vasculature. Previous studies have shown no effect of β-AR blockade or β-AR-based antihypertensive regimes on large artery (aortic and carotid-femoral) pulse wave velocity [4, 15, 16, 24]. Thus an effect on stiffness of large elastic arteries is unlikely. An alternative explanation for the differential effects of β-AR blockade on central and peripheral BP relates to heart rate. Reduction in heart rate prolongs systolic ejection time, delays the peak of the outgoing pressure wave, thus increasing the tendency of reflected pressure waves to augment outgoing pressure during systole. Peripheral amplification (the difference between peripheral and central blood pressure) is known to depend upon heart rate [25] and a reduction in heart rate may be one mechanism underlying the differential effects of β-AR blockade on central and peripheral blood pressure [4, 15, 16].
In the present study a close correlation between peripheral amplification and heart rate was observed in the absence of β-AR blockade. However, in the presence of β-AR blockade, this relationship was not significant and there was a significant interaction between the peripheral amplification–heart rate relation and presence or absence of β-AR blockade. Thus the present study suggests that a reduction in heart rate alone is insufficient to explain the differential effects of β-AR blockade on central and peripheral SBP. It is possible that β-AR blockade has an additional mechanism to prevent dilation of muscular conduit arteries that influence pressure amplification during exercise [26].
An additional finding in the present study was the lack of effect of both nonselective and selective β-AR blockade on systemic vascular resistance during exercise. Vasodilation of resistance vasculature is mediated through β2-AR [6], and activation of β2-AR by circulating catecholamines and/or by spill-over of norepinephrine from sympathetic nerves could modulate peripheral resistance. Indeed, in β2-AR gene knock-out mice exercise but not resting MAP is elevated compared with wild-type mice, suggesting that activation of β2-AR reduces systemic vascular resistance during exercise [7].The lack of effect of nonselective and selective β-AR on systemic vascular resistance suggests that, in man, the influence of β2-AR activation on peripheral vascular resistance during exercise is of relatively minor importance, at least at the moderate levels of exercise used in our study. However, it is possible that β2-AR responses were not completely blocked by the dose of propranolol. Furthermore, effects of β-AR on the arterial pressure waveform will inevitably influence the accuracy of the estimation of MAP and hence peripheral vascular resistance. Further studies are thus required to confirm lack of effect of β2-ARB on peripheral vascular resistance during exercise.
Our study had a number of other important limitations. Although our method for estimating central blood pressure from SBP2 has been validated by comparison with measured aortic pressure during interventions that produce similar haemodynamic changes to exercise [13], we cannot be certain that it provides an accurate measure of cSBP during exercise. As with other methods for estimating central from peripheral blood pressure, the method we used is subject to error from the non-invasive calibration of peripheral blood pressure [27]. However, this would not affect changes in cSBP relative to those in peripheral blood pressure [13], nor the effects of β-AR blockade relative to placebo on cSBP. We also obtained similar results using two independent methods of calibration. It is likely that, at the dose used, bisoprolol produced some nonspecific blockade of β2-AB. However, since neither bisoprolol nor propranolol produced a rise in peripheral resistance (the hypothesized effect of β2-AR blockade), this would not affect the interpretation of the present results. Our study examined acute effects of β-AR blockade, and does not therefore address longer term effects of β-AR blockade. Furthermore, our study was performed in healthy normotensive subjects and therefore the findings must be extrapolated to hypertensive subjects with caution.
In conclusion, despite reducing brachial blood pressure, acute β-AR blockade in man at rest and during exercise does not reduce central blood pressure. This differential effect on central and peripheral SBP is not completely explained by reduction in heart rate. Beta-blockade may blunt dilation of muscular arteries, influencing peripheral amplification during exercise.
Competing interests
None to declare.
This work was supported by British Heart Foundation Project Grant PG/04/119/11870. The authors acknowledge financial support from the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy's & St Thomas' NHS Foundation Trust in partnership with King's College London and King's College Hospital NHS Foundation Trust
REFERENCES
- 1.Asmar RG, London GM, O'Rourke ME, Safar ME. Improvement in blood pressure, arterial stiffness and wave reflections with a very-low-dose perindopril/indapamide combination in hypertensive patient: a comparison with atenolol. Hypertension. 2001;38:922–6. doi: 10.1161/hy1001.095774. [DOI] [PubMed] [Google Scholar]
- 2.Morgan T, Lauri J, Bertram D, Anderson A. Effect of different antihypertensive drug classes on central aortic pressure. Am J Hypertens. 2004;17:118–23. doi: 10.1016/j.amjhyper.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, Hughes AD, Thurston H, O'Rourke M. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes. Principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–25. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- 4.London GM, Asmar RG, O'Rourke MF, Safar ME. Mechanism(s) of selective systolic blood pressure reduction after a low-dose combination of perindopril/indapamide in hypertensive subjects: comparison with atenolol. J Am Coll Cardiol. 2004;43:92–9. doi: 10.1016/j.jacc.2003.07.039. [DOI] [PubMed] [Google Scholar]
- 5.Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol. 2000;525:263–70. doi: 10.1111/j.1469-7793.2000.t01-1-00263.x. Pt 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawes M, Chowienczyk PJ, Ritter JM. Effects of inhibition of the l-arginine/nitric oxide pathway on vasodilation caused by beta-adrenergic agonists in human forearm. Circulation. 1997;95:2293–7. doi: 10.1161/01.cir.95.9.2293. [DOI] [PubMed] [Google Scholar]
- 7.Chruscinski AJ, Rohrer DK, Schauble E, Desai KH, Bernstein D, Kobilka BK. Targeted disruption of the beta2 adrenergic receptor gene. J Biol Chem. 1999;274:16694–700. doi: 10.1074/jbc.274.24.16694. [DOI] [PubMed] [Google Scholar]
- 8.Leopold G, Ungethum W, Pabst J, Simane Z, Buhring KU, Wiemann H. Pharmacodynamic profile of bisoprolol, a new beta 1-selective adrenoceptor antagonist. Br J Clin Pharmacol. 1986;22:293–300. doi: 10.1111/j.1365-2125.1986.tb02890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brett SE. 2001. Influence of cardiovascular risk factors on exercise blood pressure. PhD thesis. University of London. [Google Scholar]
- 10.Brett SE, Ritter JM, Chowienczyk PJ. Diastolic blood pressure changes during exercise positively correlate with serum cholesterol and insulin resistance. Circulation. 2000;101:611–5. doi: 10.1161/01.cir.101.6.611. [DOI] [PubMed] [Google Scholar]
- 11.Clemensen P, Christensen P, Norsk P, Gronlund J. A modified photo- and magnetoacoustic multigas analyzer applied in gas exchange measurements. J Appl Physiol. 1994;76:2832–9. doi: 10.1152/jappl.1994.76.6.2832. [DOI] [PubMed] [Google Scholar]
- 12.Pauca AL, Kon ND, O'Rourke MF. The second peak of the radial artery pressure wave represents aortic systolic pressure in hypertensive and elderly patients. Br J Anaesth. 2004;92:651–7. doi: 10.1093/bja/aeh121. [DOI] [PubMed] [Google Scholar]
- 13.Munir S, Guilcher A, Kamalesh T, Clapp B, Redwood S, Marber M, Chowienczyk P. Peripheral augmentation index defines the relationship between central and peripheral pulse pressure. Hypertension. 2008;51:112–8. doi: 10.1161/HYPERTENSIONAHA.107.096016. [DOI] [PubMed] [Google Scholar]
- 14.Millasseau SC, Guigui FG, Kelly RP, Prasad K, Cockcroft JR, Ritter JM, Chowienczyk PJ. Non-invasive assessment of the digital volume pulse: comparison with the peripheral pressure pulse. Hypertension. 2000;36:952–6. doi: 10.1161/01.hyp.36.6.952. [DOI] [PubMed] [Google Scholar]
- 15.Asmar RG, London GM, O'Rourke ME, Safar ME. Improvement in blood pressure, arterial stiffness and wave reflections with a very-low-dose perindopril/indapamide combination in hypertensive patient: a comparison with atenolol. Hypertension. 2001;38:922–6. doi: 10.1161/hy1001.095774. [DOI] [PubMed] [Google Scholar]
- 16.Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, Hughes AD, Thurston H, O'Rourke M. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–25. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- 17.Lindholm LH, Carlberg B, Samuelsson O. Should beta blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet. 2005;366:1545–53. doi: 10.1016/S0140-6736(05)67573-3. [DOI] [PubMed] [Google Scholar]
- 18.Van Baak MA, Koene FM, Verstappen FT. Exercise haemodynamics and maximal exercise capacity during beta-adrenoceptor blockade in normotensive and hypertensive subjects. Br J Clin Pharmacol. 1988;25:169–77. doi: 10.1111/j.1365-2125.1988.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams B, Poulter NR, Brown MJ, Davis M, McInnes GT, Potter JF, Sever PS, Thom SM. British Hypertension Society guidelines for hypertension management 2004 (BHS-IV): summary. BMJ. 2004;328:634–40. doi: 10.1136/bmj.328.7440.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown MJ, Cruickshank JK, Dominiczak AF, MacGregor GA, Poulter NR, Russell GI, Thom S, Williams B. Better blood pressure control: how to combine drugs. J Hum Hypertens. 2003;17:81–6. doi: 10.1038/sj.jhh.1001511. [DOI] [PubMed] [Google Scholar]
- 21.Kjeldsen SE, Mundal R, Sandvik L, Erikssen G, Thaulow E, Erikssen J. Supine and exercise systolic blood pressure predict cardiovascular death in middle-aged men. J Hypertens. 2001;19:1343–8. doi: 10.1097/00004872-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Mundal R, Kjeldsen S, Sandvik L, Erikssen G, Thaulow E, Erikssen J. Exercise blood pressure predicts cardiovascular mortality in middle-aged med. Hypertension. 1994;24:56–62. doi: 10.1161/01.hyp.24.1.56. [DOI] [PubMed] [Google Scholar]
- 23.Isbary J, Greding H, Nechwatal W, Konig E. [Hemodynamic changes in patients with arterial hypertension after beta-receptor blockade with propranolol and metoprolol (author's transl)] Z Kardiol. 1978;67:857–62. [PubMed] [Google Scholar]
- 24.Ting CT, Chou CY, Chang MS, Wang SP, Chiang BN, Yin FC. Arterial hemodynamics in human hypertension. Effects of adrenergic blockade. Circulation. 1991;84:1049–57. doi: 10.1161/01.cir.84.3.1049. [DOI] [PubMed] [Google Scholar]
- 25.Wilkinson IB, Mohammad NH, Tyrrell S, Hall IR, Webb DJ, Paul VE, Levy T, Cockcroft JR. Heart rate dependency of pulse pressure amplification and arterial stiffness. Am J Hypertens. 2002;15:24–30. doi: 10.1016/s0895-7061(01)02252-x. [DOI] [PubMed] [Google Scholar]
- 26.Munir S, Jiang B, Guilcher A, Brett S, Redwood S, Marber M, Chowienczyk P. Exercise reduces arterial pressure augmentation through vasodilation of muscular arteries in humans. Am J Physiol Heart Circ Physiol. 2008;294:H1645–50. doi: 10.1152/ajpheart.01171.2007. [DOI] [PubMed] [Google Scholar]
- 27.O'Rourke MF, Adji A. An updated clinical primer on large artery mechanics: implications of pulse waveform analysis and arterial tonometry. Curr Opin Cardiol. 2005;20:275–81. doi: 10.1097/01.hco.0000166595.44711.6f. [DOI] [PubMed] [Google Scholar]


