Abstract
AIMS
To evaluate the effects of therapeutic and supratherapeutic doses of rupatadine on cardiac repolarization in line with a ‘thorough QT/QTc study’ protocol performed according to International Conference on Harmonization guidelines.
METHODS
This was a randomized (gender-balanced), parallel-group study involving 160 healthy volunteers. Rupatadine, 10 and 100 mg day−1, and placebo were administered single-blind for 5 days, whilst moxifloxacin 400 mg day−1 was given on days 1 and 5 in open-label fashion. ECGs were recorded over a 23-h period by continuous Holter monitoring at baseline and on treatment days 1 and 5. Three 10-s ECG samples were downloaded at regular intervals and were analysed independently. The primary analysis of QTc was based on individually corrected QT (QTcI). Treatment effects on QTcI were assessed using the largest time-matched mean difference between the drug and placebo (baseline-subtracted) for the QTcI interval. A negative ‘thorough QT/QTc study’ is one where the main variable is around ≤5 ms, with a one-sided 95% confidence interval that excludes an effect >10 ms.
RESULTS
The validity of the trial was confirmed by the fact that the moxifloxacin-positive control group produced the expected change in QTcI duration (around 5 ms). The ECG data for rupatadine at both 10 and 100 mg showed no signal effects on the ECG, after neither single nor repeated administration. Furthermore, no pharmacokinetic/pharmacodynamic relationship, gender effects or clinically relevant changes in ECG waveform outliers were observed. No deaths or serious or unexpected adverse events were reported.
CONCLUSIONS
This ‘thorough QT/QTc study’ confirmed previous experience with rupatadine and demonstrated that it had no proarrhythmic potential and raised no concerns regarding its cardiac safety.
Keywords: electrocardiographic effects, ICH E14 guideline, QTc interval, rupatadine, thorough QT/QTc study, torsades de pointes
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Drug-induced prolongation of QTc interval on the ECG increases the risk of ventricular tachyarrhythmias.
This problem beset the antihistamine class of drugs in the 1990s and resulted in the withdrawal of two drugs, terfenadine and astemizole.
In 2005 the International Conference on Harmonization approved guideline E14, which has been termed a ‘thorough QT/QTc study’, for the routine clinical testing of the proarrhythmic potential of pharmacotherapy.
WHAT THIS PAPER ADDS
To our knowledge, this is one of the first published trials to evaluate multiple doses of antihistamine therapy using the new criteria and it confirms the cardiac safety of rupatadine, an anti-H1 compound with activity on platelet-activating factor.
Introduction
Cardiac repolarization, as measured by the duration of the QT interval on a standard 12-lead resting electrocardiogram (ECG), is now being used to assess a key aspect of the cardiac safety of pharmacotherapy. However, QT prolongation is recognized as an imperfect biomarker for proarrhythmic risk. Because of its inverse relationship to heart rate, the measured QT interval is routinely corrected by means of various formulae to a less heart rate-dependent value known as the QTc interval. It is well established that prolongation of the QTc interval on the ECG increases the risk of torsades de pointes, which is a polymorphic ventricular tachyarrhythmia that is often asymptomatic, but which can deteriorate into life-threatening ventricular fibrillation [1]. Furthermore, the greater the effect on QTc duration the more likely the risk of developing torsades de pointes [2]. The importance of this adverse effect to clinical pharmacologists is highlighted by the fact that QTc interval prolongation is the commonest cause of delays in registration for noncardiac medicines [1]. One of the first classes of drug to be beset by this problem was the antihistamines in the early 1990s, when terfenadine was initially implicated and then shown to be associated with an increased incidence of serious cardiac events [3]. The phenomenon was identified after many years of uneventful use and generally occurred after overdosage or following concomitant administration of drugs inhibiting one of the key enzymes responsible for the metabolism of terfenadine, cytochrome P450 3A4 [4, 5]. Under normal conditions this enzyme system converts terfenadine to its active metabolite fexofenadine, which does not appear to have any QTc effects [6]. Following terfenadine, astemizole was also found to cause QTc prolongation, and these two drugs have been withdrawn from the market. These events led to a more widely-based concern regarding the cardiotoxic safety of the antihistamine class of drugs [6, 7]. However, as noted in both the ‘Allergic Rhinitis and its Impact on Asthma’ (ARIA) [8] and the ‘Consensus group on new generation antihistamines’ (CONGA) [9] guidelines, the QTc prolongation effect is dependent upon direct blockade of a specific class of potassium channel (the IKr channel) that controls the repolarization of the cardiac action potential and, as such, it is not related to blockade of the H1-receptor. In other words, it is not a class effect. Nevertheless, preclinical and clinical evaluation of possible cardiotoxicity is now a prerequisite for the regulatory assessment of such drugs before they can be approved for human use. This is the basis of the ‘thorough QT/QTc study’ recommendations, which were developed by an Expert Working Group (Efficacy) of the International Conference on Harmonization (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use and which were adopted in October 2005 in the form of the ICH E14 guideline [10].
Rupatadine is a novel second-generation antihistamine that has been shown to inhibit platelet-activating factor (PAF) receptors in addition to H1-receptors [11]. Rupatadine is not a prodrug, and by itself exhibits potent anti-H1 activity. However, a number of its metabolites, including desloratadine and the hydroxylated metabolites, retain antihistaminic activity, which may contribute to the overall efficacy of the drug. It has a rapid onset of action that is sustained over a period of 24 h, possibly as a result of the antihistaminic activity of its major metabolites. It can thus be administered once daily [12]. Recently, the pharmacological properties and clinical efficacy of rupatadine were the subject of a comprehensive review that highlighted the usefulness of this new anti-H1 compound with activity on PAF in allergic rhinitis and chronic urticaria [13]. Furthermore, this review confirmed the safety of rupatadine, including its low potential for central nervous system (CNS) interactions [14–16] and its lack of cardiotoxic potential, based on extensive preclinical and clinical testing. Rupatadine is currently approved in 22 countries in Europe, and in some South American and North and Central African countries.
The aim of this clinical trial was to investigate further the electrocardiographic properties of rupatadine and in particular its effects on QT/QTc interval as a surrogate for proarrhythmic potential. To our knowledge, this is one of the first published trials to evaluate multiple doses of antihistamine therapy using the criteria adopted in the ICH E14 guideline in what has been termed a ‘thorough QT/QTc study’[10].
Methods and subjects
Study participants
Eligible subjects included healthy men and women between the ages of 18 and 45 years, with a body mass index between 19 and 27 kg m−2, who were nonsmokers (for at least the past 6 months) and who did not have any specific ECG abnormalities on the 12-lead surface ECG, in particular PR > 240 ms, QRS > 110 ms or QTc > 430 ms in men and QTc > 450 ms in women, bradychardia (<50 bpm) or clinically significant minor ST wave changes on the screening ECG.
The study protocol was approved by an investigational independent review board (Ethical Committee Santa Creu i Sant Pau Hospital, Barcelona, Spain) and the Spanish Drug Agency (Ministerio de Sanidad y Consumo) and was conducted in accordance with the European Legislation, the Declaration of Helsinki (as amended in Edinburgh, 2000 and subsequent updates) and Good Clinical Practice guidelines. All subjects were required to provide written informed consent before inclusion in the study.
Study design
This was a randomized, double-blind for ECG evaluators, single- and multiple-dose, parallel-group study designed to assess the electrocardiographic effects of therapeutic and supratherapeutic doses of rupatadine in healthy volunteers using criteria outlined in ‘a thorough QT/QTc study’[10]. The trial was placebo-controlled and included a moxifloxacin-treated group as an internal positive control since this quinolone antibacterial agent is known to increase QTc duration by between 5 and 10 ms [1, 17, 18].
Subjects were randomly allocated (stratified to ensure that they were gender-balanced) to a 5-day course of treatment with one of the following regimens: rupatadine 10 mg once daily (therapeutic dosage); rupatadine 100 mg once daily (supratherapeutic dosage); placebo once daily; and moxifloxacin 400 mg on days 1 and 5 with placebo taken on days 2, 3 and 4. This moxifloxacin dosage was considered to be suitable to assess the sensitivity of the QT assay while not causing unnecessary risk in terms of inducing torsades de pointes. The choice of supratherapeutic dose of rupatadine was defined as a 10-fold increase above the usual clinical dosage (the target for most drugs is three to five times, but antihistamines are well tolerated and so a 10-fold increase was chosen). Medication was always taken in the presence of a member of the research team, thus ensuring 100% compliance with therapy.
The study procedure was as follows: 4 weeks prior to starting the trial all candidates were screened for suitability with respect to inclusion/exclusion criteria, a physical examination was performed including ECG, and blood and urine samples were taken for routine haematology and biochemistry laboratory tests. All treatments included a 24-h baseline period (day 0), when all subjects received a placebo dose, immediately followed by 5 days of treatment (days 1–5). Subjects were randomized to one of the four treatment groups on day 0.
An electronic ECG recording device (digital flashcard; H-1 Mortara Instruments, Milwaukee, WI, USA) registered 23-h continuous 12-lead ECGs for each volunteer on day 0 (baseline), day 1(after single dose) and day 5 (in steady state). The ECGs were stored on separate flashcards and these were sent to a central ECG laboratory (eResearch Technology, Philadelphia, PA, USA) for high-resolution measurement of cardiac intervals, and morphological assessment.
A blood sample profile was taken on the same days for pharmacokinetic analysis. At discharge, a complete physical examination was performed, including ECG and routine laboratory tests.
ECG assessments
Three ECGs each lasting about 10 s were downloaded from the electronic ECG recording devices at the scheduled time points (30 min, 1, 1.5, 2, 3, 4, 6, 8, 12, 14, 16, 20 and 23 h) and were analysed using digital techniques by a validated central laboratory using three complexes from lead II, as recommended by regulatory guidance. All ECG assessments were performed by analysts and cardiologists from the central laboratory who were blinded to the treatment and the timing of the recording being evaluated.
Manual measurement of the R-R interval, P-R interval, QRS duration and QT interval (from lead II in three ECGs at each time point) was performed using a high-resolution on-screen calliper method at the central laboratory.
From these data the primary ECG efficacy variable, individual QTc corrected for heart rate (QTcI), was calculated using the formula QTcI = QT/(RR)b, where b is the estimated slope of the linear regression model of log(QT) = a + b × log RR [17]. In addition, corrections for heart rate were also made using Fridericia's formula (QTcF = QT/RR0.33) [19] and Bazett's formula (QTcB = QT/RR0.5) [20].
Statistical analyses
The primary evaluable population for the QT/QTc analysis included all subjects who completed the baseline day and the 5-day course of treatment. The safety population included all subjects who received at least one treatment administration.
The sample size chosen for this study was based on precedents set by similar ECG safety studies [1] and was based on formal power calculations. The null hypothesis assumes that there is no relationship in the QTcI change from baseline (day 0) vs. dose of rupatadine in relation to baseline (day 0) vs. placebo. The positive control was used to determine ‘assay sensitivity’. This study was planned to detect a small positive change in QTcI duration from baseline (day 0) using an agent, moxifloxacin, of about 5–10 ms. The analysis should provide at least an 80% power to show that the upper limit of the 90% confidence interval (CI) for the comparison of rupatadine vs. placebo would fall below 10 ms, if the true mean difference between treatment and placebo groups is not more than 3 ms.
The ICH E14 guideline recommends a time-matched analysis, which is based on the ‘change from baseline in QTc interval’. For this analysis, 90% CIs based upon a comparison of the baseline time point (day 0) with the corresponding time points on day 1 and then again on day 5 including confidence limits. Each treatment group was compared with the placebo group. A 90% CI was displayed and the upper bound of this CI was compared with the 10 ms bound as the guideline specifies.
Moreover, an additional time-averaged analysis was also performed as recommended in former guidelines. It is based on the time-averaged mean change from baseline using the average of all time points at baseline (day 0) compared with all time points on treatment for each drug (separately for day 1 and day 5). Thus, for each subject the mean overall baseline time points were subtracted from the mean of all time points on treatment for each drug on days 1 and 5. Each treatment group was compared with the placebo group and the statistical analysis was based upon the summary measure. A 90% CI was displayed and the upper bound of this CI was compared with the 10 ms bound.
In addition, a categorical (outlier) analysis was performed to summarize the total number (and %) of outlier subjects in the active treatment groups for the various ECG intervals. For each ECG interval, the difference between the maximum value on treatment from the mean baseline (day 0) value was recorded. A subject was considered as an outlier if the following criteria were met:
QT: maximum value of >500 ms when not present at baseline (new onset)
QTc: maximum value of >500 ms when not present at baseline (new onset)
QTc: maximum change from baseline between 30 and 60 ms
QTc: maximum change from baseline of >60 ms
PR: maximum value of >200 ms and >25% compared with baseline
QRS: change from baseline: >25% increase when QRS > 100 ms
HR: treatment value reflecting a 25% decrease from baseline to a HR < 50 beats min−1 or a 25% increase from baseline reflecting a HR > 100 beats min−1.
If a subject experienced more than one episode of a particular outlier event during the study, they were counted only once for that event. However, an additional analysis was performed where all outlier episodes were considered.
Although the predictive value of the changes in ECG morphology has not been established, these data are typically obtained as a part of a ‘thorough QT/QTc study’. Morphological analysis was performed with regard to the ECG waveform interpretation as defined by the cardiologist ECG central laboratory. ‘New changes’ were defined as ‘not present on any baseline ECG, but present on any on-treatment ECG’ and included abnormal T or U waves, second-degree/third-degree/left bundle branch heart block, ST segment changes or evidence of myocardial infarction pattern. Data were evaluated in terms of the number and percentage of subjects in each treatment group.
Statistical analysis was also performed to evaluate possible gender differences. An analysis of covariance (ancova) of change from baseline ECG interval, with treatment, gender and treatment by gender interaction as model terms, and baseline value as a model covariate were performed for each ECG interval. The test was made at the 0.10 significance level. The relationship between the change in QTcI and plasma rupatadine concentrations, as well as its main active metabolites, was investigated using a linear regression model.
Results
One hundred and sixty-eight volunteers fulfilled the entry criteria of the study and were randomized to treatment. The demographic and baseline data for these subjects, collated according to the treatment that they received, are summarized in Table 1. There were no statistically or clinically significant differences between the four groups. Eight subjects were withdrawn from the analysis, seven as a result of incomplete ECG recordings and one due to a major protocol violation (mistake with the drug administration). A total of 160 volunteers were evaluable and analysed.
Table 1.
Demographic and baseline characteristics of the four treatment groups
| Rupatadine10 mg | Rupatadine100 mg | Moxifloxacin400 mg | Placebo | |
|---|---|---|---|---|
| No. of patients | 45 | 41 | 41 | 41 |
| Male/female | 23/22 | 21/20 | 20/21 | 21/20 |
| Age (years) | 27.0 ± 5.1 | 26.7 ± 6.1 | 25.9 ± 5.4 | 25.7 ± 5.3 |
| Body mass index (kg m−2) | 23.4 ± 2.1 | 23.1 ± 1.9 | 22.3 ± 2.2 | 22.9 ± 2.2 |
| Systolic BP (mmHg) | 119 ± 10 | 123 ± 10 | 118 ± 9 | 120 ± 11 |
| Diastolic BP (mmHg) | 60 ± 8 | 61 ± 8 | 61 ± 9 | 60 ± 8 |
| Heart rate (beats min−1) | 67 ± 8 | 67 ± 10 | 68 ± 9 | 68 ± 9 |
| QRS interval (ms) | 86.2 ± 8.3 | 87.9 ± 8.6 | 87.8 ± 9.7 | 87.8 ± 9.7 |
| PR interval (ms) | 154.1 ± 16.0 | 155.1 ± 15.9 | 155.9 ± 14.9 | 155.9 ± 14.9 |
| QT interval (ms) | 370.2 ± 25.6 | 370.8 ± 23.8 | 367.5 ± 24.2 | 367.5 ± 24.2 |
| QTc interval (ms) | 388.7 ± 21.4 | 387.5 ± 17.6 | 388.3 ± 19.7 | 388.3 ± 19.7 |
In total, three ECGs were downloaded at 13 time points on three separate days for 160 subjects, which means that almost 19 000 ECGs were analysed during this study. Overall, rupatadine (even at doses 10 times higher than normal) did not affect QT interval and, as a consequence, cardiac repolarization, as evidenced by no changes in ECG waveform patterns, or dose-related or gender-related effects. As expected, moxifloxacin, which was used as an internal positive control, produced the expected increases in QTc interval.
Time-matched analysis of change in QTcI
Results pertaining to the placebo-corrected mean change (baseline controlled) in QTcI interval (and 90% CI) over a 24-h period on days 1 and 5 of therapy are shown in Figure 1a and b, respectively. Moxifloxacin showed the expected increases in the change in mean QTcI values at most time points (18 of the 26 time points) on days 1 and 5, with maximum values of 17 ms on day 1 and 14 ms on day 5.
Figure 1.
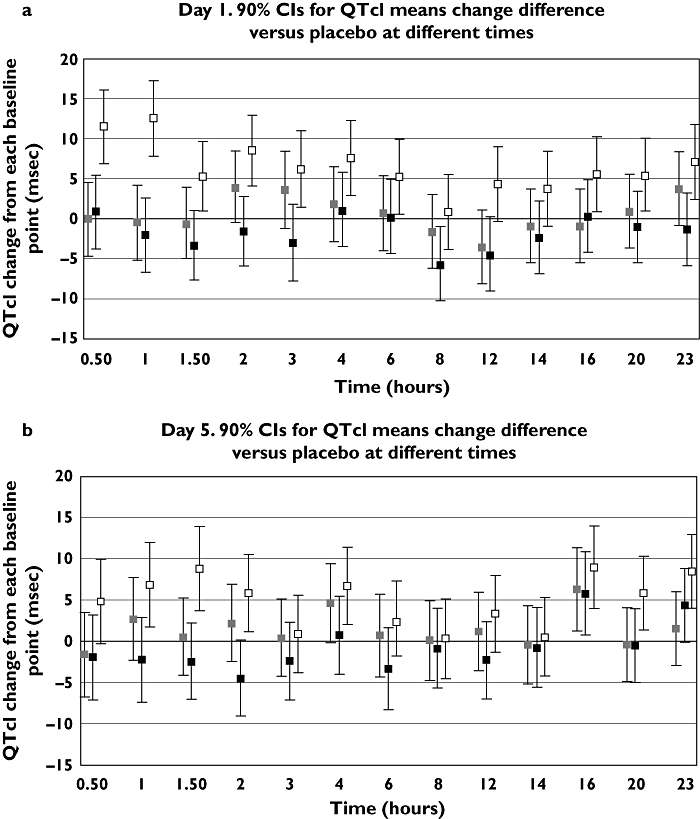
Mean change in placebo-corrected QTcI [90% CI] (baseline substracted) over a 23-h period in healthy volunteers (40/group) treated with rupatadine 10 or 100 mg day−1 for 5 days, or moxifloxacin 400 mg on days 1 and 5 (time-matched analysis). (a) Day 1; (b) Day 5. Moxi 400 – Placebo ( ); Rup 100 – Placebo (
); Rup 100 – Placebo ( ); Rup 10 – Placebo (
); Rup 10 – Placebo ( )
)
The end-point of the study showed that the values for the upper bound of the 90% two-sided CI (equivalent to a 95% one-sided CI) after the first dose for rupatadine 10 and 100 mg (day 1) were 8 and 6 ms, respectively (Table 2). These values were obtained 2 and 3 h after administration of rupatadine 10 mg and 4 h after administration of rupatadine 100 mg. Likewise on day 5, at all time points up to 14 h the QTcI response was <10 ms for the 10-mg dose and <6 ms for the 100-mg dose. The only positive result occurred at 16 h on day 5, when a value of 11 ms was recorded for both dosages of the antihistamine. This result is almost certainly spurious, since it coincided with the largest negative change in QTcI value for placebo.
Table 2.
Results from a time-matched analysis relating to the upper bound of the 90% CI (two-sided) for the change in QTcI during treatment with rupatadine and moxifloxacin (placebo-corrected)
| Upper bound of the 90% confidence interval | ||||||
|---|---|---|---|---|---|---|
| Day 1 | Day 5 | |||||
| Rupatadine | Moxifloxacin | Rupatadine | Moxifloacin | |||
| Time (h) | 10 mg day−1 | 100 mg day−1 | 400 mg day−1 | 10 mg day−1 | 100 mg day−1 | 400 mg day−1 |
| 0.5 | 5 | 5 | 16 | 3 | 3 | 10 |
| 1 | 4 | 3 | 17 | 8 | 3 | 12 |
| 1.5 | 4 | 1 | 10 | 5 | 2 | 14 |
| 2 | 8 | 3 | 13 | 7 | 0 | 10 |
| 3 | 8 | 2 | 11 | 5 | 2 | 6 |
| 4 | 6 | 6 | 12 | 9 | 5 | 11 |
| 6 | 5 | 5 | 10 | 6 | 2 | 7 |
| 8 | 3 | 1 | 5 | 5 | 4 | 5 |
| 12 | 1 | 0 | 9 | 6 | 2 | 8 |
| 14 | 4 | 2 | 8 | 4 | 4 | 5 |
| 16 | 4 | 5 | 10 | 11 | 11 | 14 |
| 20 | 5 | 3 | 10 | 4 | 4 | 10 |
| 23 | 8 | 3 | 12 | 9 | 9 | 13 |
Time-averaged analysis of change in QTcI
As expected, moxifloxacin produced increases in the mean change in QTcI duration in this time-averaged analysis on days 1 (6 ms) and 5 (4 ms).
There was no signal for a clinically relevant change in placebo-corrected QTcI values from mean baseline with either rupatadine 10 mg day−1 (upper CI 0 ms both, on days 1 and 5) or rupatadine 100 mg day−1 (upper CI −2 ms and −1 ms on days 1 and 5, respectively) (Figure 2).
Figure 2.
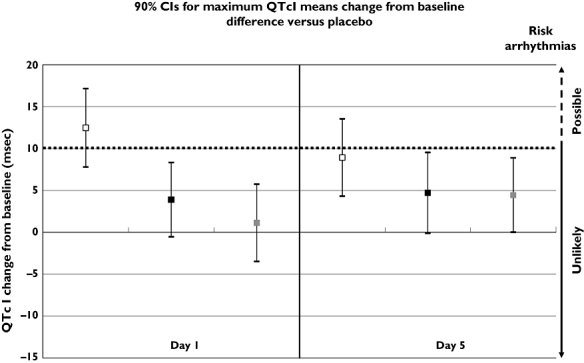
90% CIs for maximum QTcI mean change difference vs. placebo for rupatadine 10 and 100 mg, and moxifloxacin 400 mg (time-average analysis). Mox. 400 mg – Placebo ( ); Rup. 100 mg – Placebo (
); Rup. 100 mg – Placebo ( ); Rup. 10 mg – Placebo (
); Rup. 10 mg – Placebo ( )
)
Categorical (outlier) analyses
Table 3 provides details of all outliers recorded during the study in the four treatment groups. No values >500 ms for QT or QTcI were recorded for any of the treatments and there were no cases in which the increase in QTcI from baseline exceeded 60 ms. The recorded outliers were generally isolated events with no apparent treatment-related trends. Overall, there were more reported outliers for moxifloxacin (especially in the criterion of QTcI changes between 30 and 60 ms).
Table 3.
Mean change in ECG variables from baseline and categorical (outlier) analysis in healthy volunteers administered 5-day regimens involving rupatadine 10 mg once daily, rupatadine 100 mg once daily, moxifloxacin 400 mg once daily (on days 1 and 5) and placebo
| Parameter | Rupatadine10 mg | Rupatadine100 mg | Moxifloxacin400 mg | Placebo | ||||
|---|---|---|---|---|---|---|---|---|
| Study day | 1 | 5 | 1 | 5 | 1 | 5 | 1 | 5 |
| Number of subjects | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 |
| Heart rate (bpm) [HR; beats min−1] | 2 | 2 | 8 | 10 | 3 | 3 | 2 | 3 |
| Tachycardic HR outliers (n[%]) | 6 [15%] | 3 [7.5%] | 17 [42.5%] | 18 [45%] | 7 [17.5%] | 5 [12.5%] | 4 [10%] | 7 [17.5%] |
| Bradycardic HR outliers | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PR interval (ms) | −2 | −1 | −2 | −1 | −3 | 0 | −2 | −1 |
| PR outliers (n[%]) | 0 | 0 | 0 | 0 | 0 | 1 [2.5%] | 0 | 0 |
| QRS duration (ms) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| QRS outliers (n) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| QT interval (ms) | −6 | −2 | −17 | −16 | −2 | 0 | −5 | −4 |
| QT new > 500 ms (n) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| QT new > 470 ms (n[%]) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 [2.5%] |
| QTcI interval (ms) | −2 | 1 | −4 | 0 | 4 | 6 | −2 | 1 |
| QTcI 90% CI minimum | −4 | 0 | −5 | −2 | 3 | 4 | −4 | −1 |
| QTcI 90% CI maximum | −1 | 3 | −3 | 2 | 5 | 7 | −1 | 3 |
| QTcI new > 500 ms (n) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| QTcI new > 470 ms (n) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| QTcI 30–60 ms (n[%]) | 2 [5%] | 2 [5%] | 3 [7.5%] | 4 [10%] | 5 [12.5%] | 11 [27.5%] | 4 [10%] | 4 [10%] |
| QTcI > 60 ms (n) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| QTcF interval (ms) | −2 | 2 | −4 | 0 | 3 | 5 | −2 | 1 |
| QTcF 90% CI minimum | −3 | 0 | −5 | −2 | 2 | 3 | −3 | −1 |
| QTcF 90% CI maximum | −1 | 4 | −3 | 2 | 5 | 7 | −1 | 2 |
| QTcF new > 500 ms (n) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| QTcF new > 470 ms (n) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| QTcF 30–60 ms (n[%]) | 2 [5%} | 2 [5%} | 3 {7.5%] | 3 {7.5%] | 4 [10%] | 11 [27.5%] | 2 [5%] | 5 [12.5%] |
| QTcF > 60 ms (n) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| QTcB interval (ms) | 0 | 4 | 3 | 9 | 6 | 8 | −1 | 3 |
| QTcB new > 500 ms (n) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| QTcB new > 470 ms (n) | 0 | 2 [5%} | 1 [2.5%] | 0 | 0 | 1 [2.5%] | 1 [2.5%] | 1 [2.5%] |
| QTcB 30–60 ms (n[%]) | 10 [25%] | 16 [40%] | 12 [30%] | 15 [37.5%] | 17 [42.5%] | 20 [50%] | 9 [22.5%] | 17 [42.5%] |
| QTcB > 60 ms (n) | 1 [2.5%] | 0 | 0 | 0 | 0 | 0 | 0 | 1 [2.5%] |
| New abnormal U waves, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| New ST segment depression, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| New T wave inversion n (%) | 1 (2.5%) | 1 (2.5%) | 0 | 0 | 1 (2.5%) | 0 | 0 | 0 |
| New heart block | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
ECG morphology
No consistent morphological changes in the ECG were observed for any of the treatments. Isolated T-wave inversions were noted, but these were not considered to be clinically important in this setting. Furthermore, there were no abnormal U waves observed on the ECG recordings.
Gender analysis
There was no difference between the QTcI results when comparing men and women, again providing no evidence that rupatadine affects cardiac repolarization.
Pharmacokinetic/pharmacodynamic relationship
A plot of the baseline controlled QTcI at regular time points on days 1 and 5 with the plasma concentrations of rupatadine and its main active metabolites at these time points revealed no pharmacokinetic/pharmacodynamic interaction (Figure 3).
Figure 3.
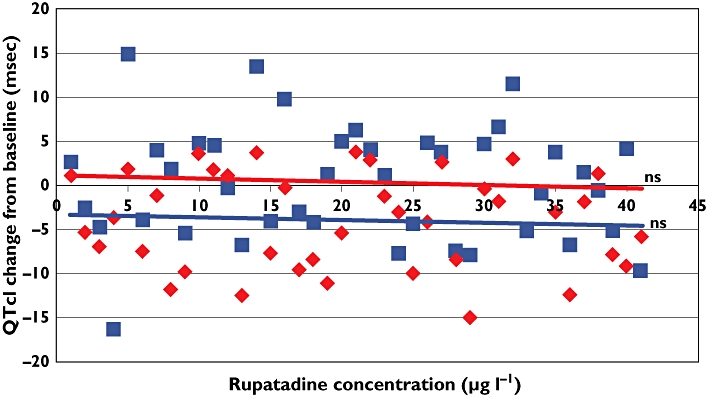
Maximum QTcI change from baseline (with linear regression analysis). Day 1 ( ); Day 5 (
); Day 5 ( )
)
Adverse events
A total of 102 patients (60.7%) reported at least one adverse event and the relative incidences were as follows: placebo 39%, rupatadine 10 mg 60%, rupatadine 100 mg 88% and moxifloxacin 56%. The most frequently related adverse event reported was somnolence (33.3% and 53.7% with rupatadine 10 mg and 100 mg, respectively). After moxifloxacin the most frequently reported events were nausea (19.5%) and somnolence (14.6%). The majority were classed as mild, with only 8.8% (rupatadine 10 mg), 19.5% (rupatadine 100 mg) and 12.2% (moxifloxacin 400 mg) considered to be of moderate intensity. Neither deaths nor serious or unexpected adverse events were recorded during the trial.
Discussion
The antihistamines are some of the most widely used drugs in the world today, and they are first-line treatment options in common ailments such as allergic rhinitis and chronic urticaria. As a class the development of the antihistamines has been a steady evolution based on classical drug-receptor theory. The goal of developing more effective and/or safer antihistamines has been driven by the prominent role played by histamine in the pathophysiology of common allergic/inflammatory disorders. The first products to reach the marketplace included drugs such as chlorphenamine (chlorpheniramine) and promethazine. These agents were very effective and potent antihistamines, but were associated with significant adverse CNS effects, most notably sedation [21]. As a result of the detrimental effects on performance and psychomotor activity, the focus turned to the development of ‘nonsedating’ antihistamines. This research led to the introduction of drugs such as astemizole, cetirizine, ebastine, loratadine, mizolastine and terfenadine – drugs which had significantly reduced effects on the CNS. These agents were widely used in the treatment of rhinoconjunctivitis and urticaria. However, as noted earlier, in the 1990s two of the second-generation antihistamines (astemizole and terfenadine) were associated with QTc prolongation and occasional episodes of the life-threatening torsades de pointes. Today, the metabolites or enantiomers of some of the original second-generation products have been introduced into the marketplace (e.g. desloratadine, fexofenadine and levocetirizine), since they have proven to be safer than the parent compounds (loratadine, terfenadine and cetirizine, respectively) [22, 23]. These have been joined by newer antihistamines such as rupatadine, which was developed with the goal of creating a powerful anti-H1 compound with activity on PAF, but without CNS or cardiotoxic side-effects.
The cardiac safety of rupatadine has been extensively assessed as part of its preclinical and clinical evaluation programme. A total of 6450 ECGs from 4000 healthy volunteers and 2450 adult patients with allergy have been evaluated as part of this analysis [11]. Rupatadine dosages in these studies ranged from 2.5 to 80 mg either as single doses or once daily for 2–4 weeks and were tested under a range of conditions: with or without food [24]; administered alone or concomitantly with alcohol, erythromycin or ketoconazole; and in young and elderly healthy volunteers of both sexes. In this evaluation rupatadine produced no clinically relevant changes in QT/QTc intervals despite the fact that drugs that increase the systemic exposure of the antihistamine (erythromycin and ketoconazole are potent cytochrome P450 3A4 isoenzyme inhibitors) were co-administered [11–13].
Preclinical evaluation of the cardiovascular effects of rupatadine has been widely reviewed elsewhere [11–13]. These reviews highlighted the fact that it has no clinically relevant effects on the cardiovascular system, as shown by the following findings: doses of > 100 times that recommended in humans had no effect on ECG parameters (QTc, PR or QRS intervals), mean blood pressure or heart rate in rats, guinea pigs and dogs [11–13, 25]; it was not associated with arrhythmias or an increased rate of cardiovascular mortality in these animal models; neither rupatadine nor its metabolite (desloratadine) affected the cardiac action potential in in vitro isolated dog Purkinje fibres at concentrations at least 2000 times greater than the Cmax reached after administration of a 10-mg dose in humans [25]; and, finally, in vitro, rupatadine concentrations required to block the HERG (human ether-a-gogo related gene) potassium channel or the human cloned hKv1.5 potassium channel expressed in a mouse L-cell line were almost 2000-fold greater than serum concentrations determined after administering rupatadine 10 mg to human volunteers [11, 13, 26].
There are two recent reports on the cardiac effects of rupatadine [27, 28]. In the first, the authors state that data from Spain show a statistical association between rupatadine use and heart rhythm disturbances. This assertion seems confusing, since the lower limit of the 95% CI for rupatadine reporting odds ratio (ROR) contains the value 1. Furthermore, the 95% CI for rupatadine ROR was recalculated and it yielded values of 0.97–10.48. The ROR for levocetirizine, desloratadine, loratadine and ebastine were also statistically significant, the first two drugs producing values higher than those for rupatadine. In addition, the ROR, as a disproportionality measure, is simply one way of identifying drug–adverse drug reaction associations that may be interesting for a clinical review, but, more importantly, ROR does not ensure a causal relationship. The second report was a brief letter to the editor in which the authors refer a case of torsade de pointes and its association with rupatadine. However, a response by Fité-Mora [29] to the Editor of the journal provided clarification regarding the original letter. Perhaps most importantly, the patient had been receiving sertraline therapy for the previous 6 months and he had already been assessed for an episode of syncope. Furthermore, the patient had experienced prolonged QTc on the ECG in 2001 and 2003.
In summary, the possible relationship between cardiac effects and rupatadine has not been accurately demonstrated in these two papers. Analysis of the cases was partial and incomplete, given that other aspects were involved, such as concomitant treatment, previous history of prolonged QTc, etc. Nevertheless, a continued and rigorous postmarketing pharmacovigilance system is mandatory for all authorized drugs due to the relevance of the data obtained in everyday clinical practice.
The current study, based on the most up-to-date requirements of the regulatory authorities, has shown that rupatadine at therapeutic and supratherapeutic dosages did not demonstrate any effects on QTc duration that would indicate that it has proarrhythmic potential. In the updated ICH E14 guideline a negative result ‘is one in which the upper bound of the 95% one-sided CI for the largest time-matched mean effect of the drug on QTc interval excludes 10 ms’ In this clinical trial 25 of 26 time points for both rupatadine 10 mg day−1 and 100 mg day−1 after either a single dose or following 5 days' treatment met this criteria, while 18 of 26 time points for moxifloxacin (a positive control known to increase QTc duration) failed this test. Furthermore, the one time-point on which both doses of rupatadine increased the upper bound 95% CI for the change in QTcI by >10 ms was almost certainly spurious since it coincided with the largest negative change in QTcI value for the placebo group, while the rupatadine QTcI value per se was lower than those recorded at baseline (day 0). Placebo spontaneous variation in QTc is a common feature and may simply be accounted for by environmental conditions such as food, activity or emotional levels. Furthermore, at 20 h, when the QTcI value for placebo returned to usual levels, the upper bound 90% CI for rupatadine was 4 for both the therapeutic and supratherapeutic doses. In addition to relatively small changes in placebo-corrected QTcI interval values and negative ‘thorough QT/QTc study’ results, no pharmacokinetic/pharmacodynamic relationship with either rupatadine or its main metabolites was observed, no gender effects occurred, and there was no clinically relevant imbalance of ECG waveform outliers during the study.
Similar results were recently published for levocetirizine in a trial designed to comply with ‘thorough QT/QTc study’ criteria [30]. This trial differed from the present one in that it employed a crossover design and assessed only single doses of the antihistamine in a relatively smaller number of healthy volunteers (n= 52), and employed only a supratherapeutic dose that was six times higher than the therapeutic one. Nevertheless, the consistency between clinical data and the results of the two ‘human pharmacology laboratory’ studies provides a good level of comfort regarding the lack of cardiotoxicity associated with the newer second-generation antihistamines.
Thus, the findings of the ‘thorough QT/QTc study’ with rupatadine at therapeutic and supratherapeutic dosages administered for 5 days are in accordance with its reported extensive preclinical and clinical experience, and indicate that it has no proarrhythmic potential and hence no cardiac safety concerns.
Competing interests
E.D. has been employed in J Uriach y Compañía, S.A. I.I. is an employee of Uriach-Pharma, Uriach Group. I.P. was an employee of Uriach-Pharma, Uriach Group. A.S. is an employee of Uriach Group. M.J.B. has been paid by Laboratorios Uriach for speaking and consulting and has received funds for research.
The authors would like to thank Steve Clissold, PhD (ContentEdNet, Madrid, Spain) for editorial assistance and all the personnel of Hospital Santa Creu i Sant Pau and volunteers involved in the study. This study was funded by Grupo Uriach S.A. (Barcelona, Spain), and partially supported by the National Scientific Research Programme of the Spanish Ministry of Science and Technology (Madrid, Spain).
REFERENCES
- 1.Morganroth J. A definitive or thorough phase1 QT ECG trial as a requirement for drug safety assessment. J Electrocardiol. 2004;37:25–9. doi: 10.1016/j.jelectrocard.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Haverkamp W, Breithardt G, Camm AJ, Janse MJ, Rosen MR, Antzelevitch C, Escande D, Franz M, Malik M, Moss A, Shah R. The potential for QT prolongation and proarrhythmia by non-antiarrhythnic drugs: clinical and regulatory implications. Report on a policy conference of the European Society of cardiology. Eur Heart J. 2000;21:1216–31. doi: 10.1053/euhj.2000.2249. [DOI] [PubMed] [Google Scholar]
- 3.Woosley RL, Chen Y, Freiman JP, Gillis RA. Mechanisms of the cardiotoxic actions of terfenadine. JAMA. 1993;268:1532–6. [PubMed] [Google Scholar]
- 4.Leurs R, Church MK, Taglialatela M. H1-antihistamines: inverse agonism, anti-inflammatory actions and cardiac effects. Clin Exp Allergy. 2002;32:489–98. doi: 10.1046/j.0954-7894.2002.01314.x. [DOI] [PubMed] [Google Scholar]
- 5.Yap YG, Camm AJ. Drug induced QT prolongation and Torsades de Pointes. Heart. 2003;89:1363–72. doi: 10.1136/heart.89.11.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dávila I, Sastre J, Bartra J, del Cuvillo A, Jáuregui I, Montoro J, Mullol J, Valero AL. Effect of H1 antihistamines upon the cardiovascular system. J Invest Allergol Clin Immunol. 2006;16(Suppl. 1):13–23. [PubMed] [Google Scholar]
- 7.Hove-Madsen L, Llach A, Molina CE, Prat-Vidal C, Farré J, Roura S, Cinca J. The proarrhythmic antihistaminic drug terfenadine increases spontaneous calcium release in human atrial myocytes. Eur J Pharmacol. 2006;553:215–21. doi: 10.1016/j.ejphar.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 8.Bousquet J, Van Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108(Suppl. 5):S147–334. doi: 10.1067/mai.2001.118891. [DOI] [PubMed] [Google Scholar]
- 9.Holgate ST, Canonica GW, Simons FER, Taglialatela M, Tharp M, Timmerman H, Yanai K. Consensus group on new-generation antihistamines (CONGA): present status and recommendations. Clin Exp Allergy. 2003;33:1305–24. doi: 10.1046/j.1365-2222.2003.01769.x. [DOI] [PubMed] [Google Scholar]
- 10.ICH Guidance. Clinical Evaluation of QT/Qtc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs. London, UK: ICH Steering Committee; 2005. E14, Step 5 Available at http://www.ich.org/cache/compo/475-272-1.html#E14 (last accessed December 2009. [Google Scholar]
- 11.Izquierdo I, Merlos M, Garcia-Rafanell J. Rupatadine, a new selective histamine HI receptor and platelet-activating factor (PAF) antagonist: a review of pharmacological profile and clinical management of allergic rhinitis. Drugs Today (Barc) 2003;39:451–68. doi: 10.1358/dot.2003.39.6.799450. [DOI] [PubMed] [Google Scholar]
- 12.Picado C. Rupatadine: pharmacological profile and its use in the treatment of allergic disorders. Expert Opin Pharmacother. 2006;7:1989–2001. doi: 10.1517/14656566.7.14.1989. [DOI] [PubMed] [Google Scholar]
- 13.Mullol J, Bousquet J, Bachert C, Canonica WG, Gimenez-Arnau A, Kowalski ML, Marti-Guadaño E, Maurer M, Picado C, Scadding G, Van Cauwenberge P. Rupatadine in allergic rhinitis and chronic urticaria. Allergy. 2008;63(Suppl. 87):5–28. doi: 10.1111/j.1398-9995.2008.01640.x. [DOI] [PubMed] [Google Scholar]
- 14.Barbanoj MJ, García-Gea C, Antonijoan R, Izquierdo I, Donado E, Pérez I, Solans A, Jané F. Evaluation of the cognitive, psychomotor and pharmacokinetic profiles of rupatadine, hydroxyzine and cetirizine, in combination with alcohol, in healthy volunteers. Hum Psychopharmacol. 2006;21:13–26. doi: 10.1002/hup.741. [DOI] [PubMed] [Google Scholar]
- 15.Barbanoj MJ, García-Gea C, Morte A, Izquierdo I, Pérez I, Jane F. Central and peripheral evaluation of rupatadine, a new antihistamine/platelet-activating factor antagonist, at different doses in healthy volunteers. Neuropsychobiology. 2004;50:311–21. doi: 10.1159/000080959. [DOI] [PubMed] [Google Scholar]
- 16.Vuurman E, Theunissen E, van Oers A, van Leeuwen C, Jolles J. Lack of effects between rupatadine 10 mg and placebo on actual driving performance of healthy volunteers. Hum Psychopharmacol. 2007;22:289–97. doi: 10.1002/hup.856. [DOI] [PubMed] [Google Scholar]
- 17.Morganroth J, Ilson BE, Shaddinger BC, Dabiri GA, Patel BR, Boyle DA, Sethuraman VS, Montague TH. Evaluation of vardenafil and sildenafil on cardiac repolarization. Am J Cardiol. 2004;93:1378–83. doi: 10.1016/j.amjcard.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 18.Summary of product characteristics of moxifloxacin. Available at http://www.emc.medicines.org.uk/medicine/11841/SPC/Avelox+400+Tablets/ (last accessed 18 December 2009.
- 19.Fridericia LS. Die Systolendauer im Elektrokardiogramm bei normalen Menschen und bei Herzkranken. Acta Med Scand. 1920;53:469–86. [Google Scholar]
- 20.Bazett HC. An analysis of the time-relations of the electrocardiogram. Heart. 1920;7:353–70. [Google Scholar]
- 21.Tillement J-P. Pharmacological profile of the new antihistamines. Clin Exp All Rev. 2005;5:7–11. [Google Scholar]
- 22.Walsh GM. Anti-inflammatory properties of antihistamines: an update. Clin Exp All Rev. 2005;5:21–5. [Google Scholar]
- 23.Devillier P. Comparing the new antihistamines: the role of pharmacological parameters. Clin Exp Allergy. 2006;36:5–7. doi: 10.1111/j.1365-2222.2006.02421.x. [DOI] [PubMed] [Google Scholar]
- 24.Solans A, Carbó M, Peña J, Nadal T, Izquierdo I, Merlos M. Influence of food on the oral bioavailability of rupatadine tablets in healthy volunteers: a single-dose, randomized, open-label, two-way crossover study. Clin Ther. 2007;29:900–8. doi: 10.1016/j.clinthera.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Izquierdo I, Pérez I, Villa M, Giral M, Merlos M, Forn J. Lack of electrocardiographic effects of rupatadine, new nonsedating selective histamine H1-receptor and PAF antagonist. Allergy. 2001;56(Suppl. 68):212. [Google Scholar]
- 26.Caballero R, Valenzuela C, Longobardo M, Tamargo J, Delpón E. Effects of rupatadine, a new dual antagonist of histamine and platelet-activating factor receptors, on human cardiac Kv1.5 channels. Br J Pharmacol. 1999;128:1071–81. doi: 10.1038/sj.bjp.0702890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carvajal A, Macias D, Salado I, Sainz M, Ortega S, Campo C, Garcia del Pozo J, Martin Arias L, Velasco A, Gonçalves S, Pombal R, Carmona R. Heart rhythm disturbances associated with rupatadine: a case series from the Spanish and Portuguese pharmacovigilance systems. Clin Pharmacol Ther. 2009;85:481–4. doi: 10.1038/clpt.2008.269. [DOI] [PubMed] [Google Scholar]
- 28.Nombela-Franco L, Ruiz-Antoran B, Toquero-Ramos J, Silva-Melchor L. Torsade de pointes associated with rupatadine. Rev Esp Cardiol. 2008;61:328–9. [PubMed] [Google Scholar]
- 29.Fité-Mora R. Torsade de pointes associated with rupatadine. Rev Esp Cardiol. 2009;62:330–1. doi: 10.1016/s1885-5857(09)71568-3. author reply 332. [DOI] [PubMed] [Google Scholar]
- 30.Hulhoven R, Rosillon D, Letiexhe M, Meeus M-A, Daoust A, Stockis A. Levocetirizine does not prolong the QT/QTc interval in healthy subjects: results from a thorough QT study. Eur J Clin Pharmacol. 2007;63:1011–7. doi: 10.1007/s00228-007-0366-5. [DOI] [PubMed] [Google Scholar]


