Abstract
OBJECTIVE: To determine the long-term outcome of computed tomographic (CT) quantification of coronary artery calcium (CAC) used as a triage tool for patients presenting with chest pain to an emergency department (ED).
PATIENTS AND METHODS: Patients (men aged 30-62 years and women aged 30-65 years) with chest pain and low-to-moderate probability of coronary artery disease underwent both conventional ED chest pain evaluation and CT CAC assessment prospectively. Patients' physicians were blinded to the CAC results. The results of the conventional evaluation were compared with CAC findings on CT, and the long-term outcome in patients undergoing CT CAC assessment was established. Primary end points (acute coronary syndrome, death, fatal or nonfatal non–ST-segment elevation myocardial infarction, fatal or nonfatal ST-segment elevation myocardial infarction) and secondary outcomes (coronary artery bypass grafting, percutaneous transluminal coronary angioplasty, coronary stenting, or a combination thereof) were obtained when the patient was dismissed from the ED or hospital and then at 30 days, 1 year, and 5 years.
RESULTS: Of the 263 study patients, 133 (51%) had a CAC score of zero. This absence of CAC correlated strongly with the likelihood of noncardiac chest pain. Among 133 patients with a CAC score of zero, only 1 (<1%) had cardiac chest pain. Conversely, of the 31 patients shown to have cardiac chest pain, 30 (97%) had evidence of CAC on CT. When a CAC cutoff score of 36 was used, as suggested by receiver operating characteristic analysis, sensitivity was 90%; specificity, 85%; positive predictive value, 44%; and negative predictive value, 99%. During long-term follow-up, patients without CAC experienced no cardiac events at 30 days, 1 year, and 5 years.
CONCLUSION: Findings suggest that CT CAC assessment is a powerful adjunct in chest pain evaluation for the population at low-to-intermediate risk. Absent or minimal CAC in this population makes cardiac chest pain extremely unlikely. The absence of CAC suggests an excellent long-term (5-year) prognosis, with no primary or secondary cardiac outcomes ocurring in study patients at 5-year follow-up.
Computed tomographic coronary artery calcium assessment is a powerful adjunct in evaluation of chest pain for the population at low-to-intermediate risk; the absence of coronary artery calcium in this population makes cardiac chest pain extremely unlikely and suggests an excellent 5-year prognosis.
ACS = acute coronary syndrome; CAC = coronary artery calcium; CAD = coronary artery disease; CI = confidence interval; CT = computed tomography; CTA = CT angiography; ECG = electrocardiography; ED = emergency department; IQR = interquartile range; MDCT = multidetector CT; MI = myocardial infarction; ROC = receiver operating characteristic
Chest pain is the presenting concern for more than 8 million emergency department (ED) visits annually.1 The ED evaluation of chest pain is resource-intense and has inherent limitations. Medical history and physical examination frequently yield little insight into the source of the chest pain. More than one-third of patients with documented acute myocardial infarctions (MIs) lack chest pain on presentation.2 Resting 12-lead electrocardiography (ECG) has limited value in chest pain cases and provides a specific diagnosis of acute cardiac injury in only 5% of patients with chest pain.1,3 Most initial ECG findings for patients with an acute coronary syndrome (ACS) are nondiagnostic for ischemia.1,4 Determination of the level of a single cardiac marker (eg, creatine kinase, creatine kinase–MB, troponin) during ED evaluation may be inadequate to exclude an ACS because of the delay between myocardial damage and serum marker level elevation.5 These limitations result in hospitalization of more than 50% of patients with chest pain,6 yet only one-third of these hospitalized patients are eventually shown to have cardiac chest pain.7
For editorial comment, see page 309
Coronary artery calcium (CAC) correlates directly with coronary atherosclerosis and is a marker of plaque burden.8-11 Quantification of CAC with cardiac computed tomography (CT) is sensitive, accurate, and reproducible.10-12 Positive findings for CAC on cardiac CT are nearly 100% specific for atheromatous plaque.13 Negative cardiac CT findings (CAC score, 0) have a negative predictive value of greater than 96%.13 Studies have shown that CAC predicts coronary death, nonfatal MI, and the need for coronary revascularization in asymptomatic patients.14 Prior studies also suggest that CAC detection by cardiac CT is useful in the evaluation of chest pain in the ED.10,15 Our study evaluates the integration of cardiac CT for CAC detection into the assessment and triage of patients with chest pain in the ED and examines its value in long-term outcome in patients.
PATIENTS AND METHODS
This prospective study was approved by the Mayo Clinic Institutional Review Board. Funding was provided by a Mayo Foundation grant. Patients presenting to the ED of Saint Marys Hospital in Rochester, MN, with chest pain meeting the criteria for unstable angina were placed into low-, intermediate-, and high-risk groups on the basis of Agency for Healthcare Research and Quality guidelines.16-18 Enrollment was open to men (age, 30-62 years) and women (age, 30-65 years) in the low- or intermediate-risk groups. Enrollment was limited to these age groups because prior studies suggested that CAC prevalence increases with age and that greater than 60% of women and 80% of men older than 60 years have detectable CAC on electron beam CT.8,19 Patient enrollment was conducted by our study coordinator and the investigators based in the ED (L.F.V. and D.A.L.). Consecutive patients meeting the eligibility criteria on their initial presentation and before any further cardiac testing were enrolled Monday through Friday. Enrollment was limited to Monday through Friday because of the availability of the cardiac CT scanner. Patient enrollment was contingent on an initial cardiac marker value within reference range and nondiagnostic findings on initial ECG. Patients were excluded if they had findings of acute ischemia, elevated cardiac marker levels, hemodynamic instability, pregnancy, or atrial fibrillation; were unable to give informed consent; or had a previous diagnosis of coronary artery disease (CAD). All patients underwent cardiac CT for CAC detection.
Attending physicians, blinded to the CT CAC results, determined the extent of the patient's cardiac evaluation. Determination of cardiogenic chest pain was made using one of several cardiac confirmatory tests, including exercise treadmill testing (n=220), myocardial perfusion imaging (n=31), stress echocardiography (n=24), and cardiac angiography (n=50). During the immediate evaluation period, 56 patients (21%) underwent multiple tests. These conventional cardiac tests were considered the standard to which CAC scores were compared.
For CT CAC assessment, a standard clinical protocol20,21 was followed. With use of an Imatron C150 or C300 electron beam CT scanner (GE Medical Imaging Systems, Fairfield, CT), consecutive CT slices spanning the entire heart (slice thickness, 3 mm) were obtained. Computed tomography was performed without intravenous administration of contrast medium and with ECG triggering at 80% of the cardiac cycle (diastolic diastasis) during 100-ms exposures. Phantom calibration was performed for each scan, with dual scans obtained for quality assurance and reproducibility measurements. Scans were interpreted by a cardiovascular radiologist (J.F.B.). CAC scoring was performed using the standard Agatston algorithm20,21; we used a calcium threshold of 130 HUs and less than 1 mm of contiguous calcification (≥4 pixels). This method of determining CAC has highly reproducible results.12 Both CAC scores and percentage scores referenced to age and sex were calculated.
Patients were contacted and their medical records reviewed to obtain follow-up information. Primary outcomes (ACS, death, fatal or nonfatal non–ST-segment elevation MI, fatal or nonfatal ST-segment elevation MI) and secondary outcomes (coronary artery bypass grafting, percutaneous transluminal coronary angioplasty, coronary stenting, or a combination thereof) were obtained when the patient was dismissed from the ED or hospital and then at 30 days, 1 year, and 5 years after study enrollment.
Statistical Analyses
Variables were described using mean ± SD if distributed normally or median and interquartile range (IQR) otherwise. Dichotomous variables were described using frequency counts and percentages. Comparisons of variables between cardiac and noncardiac sources of chest pain (ascertained using the criterion standard) were made using the Wilcoxon rank sum test, Fisher exact test, or χ2 test as necessary. CAC score cutoff values were investigated using logistic regression, receiver operating characteristic (ROC) curves, area under the curve, and negative and positive predictive values. The rate of the composite event (the first occurrence of any ACS, fatal or nonfatal non–ST-segment elevation MI, fatal or nonfatal ST-segment elevation MI, or death) was visually compared using Kaplan-Meier curves, stratified by the CAC score category.
RESULTS
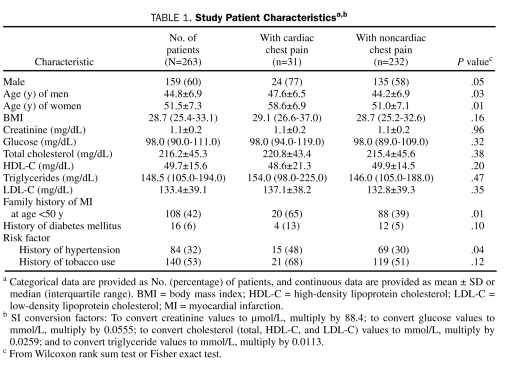
A total of 270 patients were enrolled. Seven patients with CAC scores of 0 had their CAC scans unblinded by the attending physicians and were subsequently excluded from all analyses. Of the 263 remaining patients, 159 (60%) were men (mean age, 44.8±6.9 years) and 104 (40%) were women (mean age, 51.5±7.3 years). The mean body mass index was 29.0 (min-max, 18.7-50.5), and 95% of the patients were white (Table 1). A statistically significant difference (in both sexes) was noted in age ± SD between patients with cardiac chest pain (men, 47.6±6.5 years; women, 58.6±6.9 years) and those without cardiac chest pain (men, 44.2±6.9 years; women, 51.0±7.1 years) (Table 1).
TABLE 1.
Study Patient Characteristicsa,b
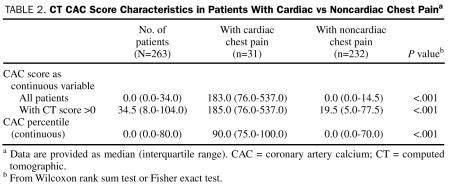
CAC scores were calculated for all patients and ranged from 0 to 1420 (median, 0; mean, 66) (Table 2). Of the 263 patients, 133 (51%) had no CAC (CAC score, 0). CAC was detected (CAC score, >0) on CT in the remaining 130 patients (49%) (median, 35; mean, 133). The CAC percentiles ranged from 0% to 100% (median, 0%; IQR, 0%-80%; mean, 38%). Four groups were defined on the basis of commonly used CAC score ranges (ie, 0, 1-10, 11-50, and ≥51). A trend was observed showing a direct correlation between the percentage of patients who had a cardiac source of their pain in these groups (1%, 2%, 13%, and 44%, respectively; P<.001) and the CAC score groups. This trend remained significant even when restricted to patients with a CAC score of less than 51 (P=.004 for trend test for first 3 groups).
TABLE 2.
CT CAC Score Characteristics in Patients With Cardiac vs Noncardiac Chest Paina
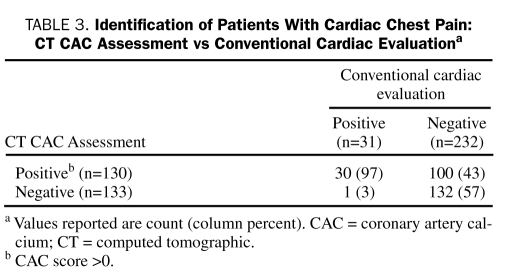
Cardiogenic chest pain was strongly related to the presence of CAC. Of 31 patients (12%) shown to have cardiac chest pain on the basis of conventional cardiac evaluation, 30 (97%) had CAC (Table 3). Median CAC score in those with a CAC score greater than 0 was 185 (IQR, 76.0-537.0), with a median calcium percentile of 90% (IQR, 75%-100%). Thirteen patients (5% of total patients, 42% of patients with cardiac chest pain) sustained an MI during hospitalization, although they had normal initial cardiac marker levels and nondiagnostic findings on ECG. All 13 patients had CAC (min-max of CAC score, 37-1368; mean, 321). Of the 20 patients (8% of total patients) who underwent revascularization during initial hospitalization, 19 (95%) had CAC (min-max of CAC score, 6-1368; mean, 355).
TABLE 3.
Identification of Patients With Cardiac Chest Pain: CT CAC Assessment vs Conventional Cardiac Evaluationa
Similarly, absence of CAC was strongly associated with the finding of noncardiac chest pain. No CAC was detected in 133 patients (51% of total patients), and only 1 (<1%) had cardiogenic chest pain (Table 3). A 43-year-old male smoker without diabetes who underwent angiography after an indeterminate treadmill test had 3 stenoses that required stenting. Compared with patients with cardiogenic chest pain, patients with noncardiac pain had a median CAC score of 0 (IQR, 0-14.5; P<.001) and a median calcium percentile of 0% (IQR, 0%-70%; P<.001; Wilcoxon rank sum test).
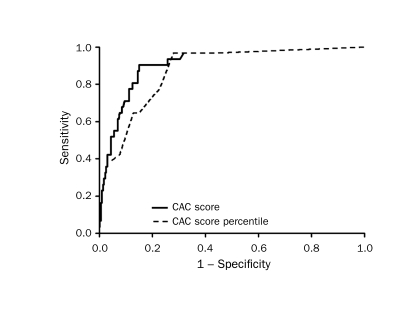
A ROC curve predicting conventional test outcomes by using CAC score was constructed. The area under the curve was 0.91 (Figure 1). When age and sex were added to the CAC score in a multiple logistic regression to predict cardiac source, there was no improvement in either model likelihood, nor in the ROC area (decrease in the ROC curve area from 0.91 to 0.86; P=.065). A CAC score greater than 0 defining a postive test has a sensitivity of 97% (95% confidence interval [CI], 83%-100%), a specificity of 57% (95% CI, 50%-63%), a positive predictive value of 23% (95% CI, 16%-31%), and a negative predictive value of 99% (95% CI, 96%-100%) for cardiac chest pain (Table 4) when compared with conventional cardiac testing. Raw CAC scores were compared with age- and sex-based calcium percentiles. ROC analysis yielded an area under the curve of 0.87, indicating that, in this setting, the actual CAC score was more helpful than calcium percentiles.
FIGURE 1.
Receiver operating characteristic curve comparing the coronary artery calcium (CAC) score with the CAC score percentile.
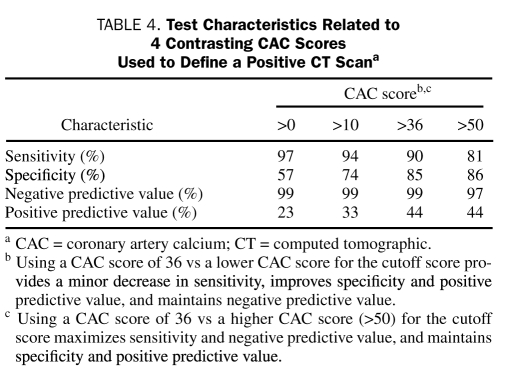
TABLE 4.
Test Characteristics Related to 4 Contrasting CAC Scores used to Define a Positive CT Scana
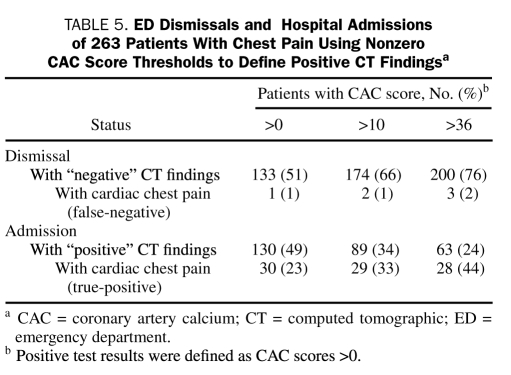
A CAC score of 36 was suggested by ROC analysis as a threshold value (positive score defined as ≥37). This threshold achieves improved specificity with only a slight reduction in sensitivity for the detection of cardiac chest pain. Indeed, it yields the point on the ROC curve closest to the upper left corner and maximizes the sum of sensitivity and specificity. The threshold score of 36 yielded a sensitivity of 90% (95% CI, 74%-98%), a specificity of 85% (95% CI, 80%-89%), and a negative predictive value of 99% (95% CI, 96%-100%) (Table 4). With this threshold score, 63 patients (24%) had positive CAC results, and 28 (44%) of these (positive predicted value, 44%) had a cardiac source of chest pain (Table 5). In the group with negative results (CAC score, ≤36), 197 (99%) of 200 patients had noncardiac chest pain, and 3 (2%) would have been classified as having cardiac chest pain (Table 5). These 3 patients included a 43-year-old man (CAC score, 0) with positive findings on angiography, a 48-year-old man (CAC score, 12) with positive findings on myocardial perfusion imaging who remained event-free at 1 year, and a 47-year-old woman (CAC score, 6) who underwent angiography and stenting of an 80% stenosis after an indeterminate treadmill test.
TABLE 5.
ED Dismissals and Hospital Admissions of 263 Patients With Chest Pain using Nonzero CAC Score Thresholds to Define Positive CT Findingsa
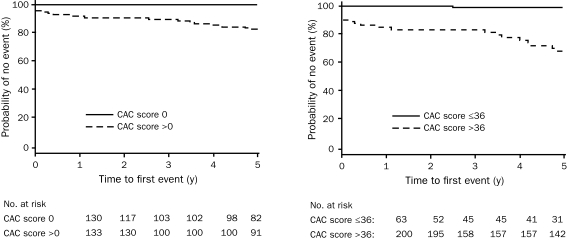
Follow-up information was obtained from patients at 30 days, 1 year, and 5 years. Thirty-day data were obtained from 261 patients (99%), with no additional cardiac outcomes noted. At 1 year, data were obtained from 258 patients (98%), with 4 patients (2%) having undergone additional testing. All 4 underwent angiography; 2 had CAD with subsequent stenting (CAC scores, 1420 and 5 at study enrollment), and 2 had CAD that required no intervention (CAC scores, 633 and 90 at study enrollment). At 5 years, 212 patients (81%) were available for follow-up. During this period, 9 patients (3%) underwent additional cardiac testing. All 9 had positive findings on cardiac angiography that required intervention, and all had CAC on their initial evaluation (range of CAC scores, 65-650). Two of these patients presented during follow-up with acute MIs. The cumulative rate of occurrence of the composite end point (ACS, fatal or nonfatal non–ST-segment elevation MI, fatal or nonfatal ST-segment elevation MI, or death) is shown in Figure 2.
FIGURE 2.
Kaplan-Meier curves of event-free survival from major cardiac event (acute coronary syndrome, death, fatal or nonfatal non–ST-segment myocardial infarction, fatal or nonfatal ST-segment elevation myocardial infarction) among those with a coronary artery calcium (CAC) score of 0 vs >0 (left) and among those with a receiver operating characteristic–optimized CAC score ≤36 vs >36 (right).
DISCUSSION
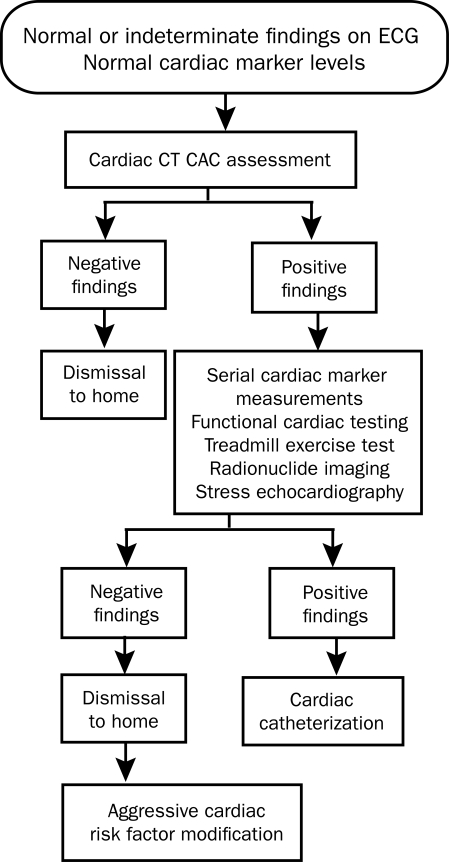
This prospective blinded study addresses the utility of cardiac CT CAC assessment in the evaluation of patients presenting to the ED with chest pain. Cardiac CT CAC assessment can be easily integrated early into the ED chest pain evaluation algorithm (Figure 3). Electron beam CT scanners are no longer manufactured because of limitations in noncardiac image quality and the equipment cost compared with conventional CT scanners.22 Multidetector CT (MDCT) scanners have evolved to the point that cardiac image acquisition is comparable to that of electron beam CT scanners. Studies have showed CAC detection equivalency between electron beam CT and multislice CT.23,24 With MDCT, accurate CAC detection is now widely available to EDs across the United States.
FIGURE 3.
Algorithm of evaluation in patients who present with chest pain to the emergency department, with integration of cardiac computed tomographic (CT) coronary artery calcium (CAC) determination. ECG = electrocardiography.
Detection of CAC by CT is noninvasive, easily tolerated, and rapid, and it requires no intravenous contrast medium. It is not limited by a patient's concurrent medication use, ability to exercise, or baseline ECG abnormalities. CAC correlates directly with coronary atherosclerosis and is a marker of plaque burden.8-11 Our study results confirm earlier findings that the actual CAC score is more helpful than calcium percentiles in this ED patient population.25 Coronary artery stenoses are rare in patients without CAC in most studies.15,26 However, Rubinshtein et al27 found that CAD was evident on cardiac CT angiography (CTA) in 7% of patients with CAC scores of 0. These data are not directly comparable to the findings of our study because of the differences in study populations and end points. Rubinshtein's study patients were older and of different ethnic groups; more than half had chronic chest pain and had undergone previous evaluation (referral population). In addition, Rubinshtein's findings were based on the degree of visually assessed stenoses by CTA, not the presence of ischemia or the incidence of hard coronary events as in our study.
On the basis of our study results, negative findings on cardiac CT CAC assessment permit the immediate and safe dismissal from the ED of patients with normal findings on an initial ED evaluation (nondiagnostic findings on ECG and initial cardiac marker determination within reference range). This low risk endures for several years in our study; for a patient returning to the ED months or years later with chest pain, the prior cardiac CT CAC findings may allow more directed testing and possibly reduce resource utilization. It is also evident that a nonnegative CAC score can be used as a dismissal threshold value, allowing for improved specificity with only a slight reduction in sensitivity for the detection of cardiac chest pain (Table 4). A CAC score of 36 was suggested by ROC analysis as the threshold value (positive score defined as ≥37). Using this ROC-suggested score maximizes the number of patients who can be dismissed safely and immediately (76%, using the threshold CAC score of 36 vs 51% using a CAC score of 0), while minimizing the number of patients with cardiac chest pain who are erroneously dismissed (2%) (Table 5). These results should reduce the current 4% rate of undiagnosed acute MI and unstable angina in the ED.28
In addition to the immediate dismissal permitted by negative findings on CT CAC assessment, positive findings on CT CAC assessment provide important new information that is unavailable from the current cardiac testing algorithm. The detection of subclinical atherosclerosis in patients with positive findings on cardiac CT CAC assessment who are not currently experiencing cardiac chest pain (positive predictive value, 44%) will allow early risk factor modification, thereby potentially preventing future cardiac events.
The current ED chest pain evaluation algorithm uses functional cardiac stress testing (exercise ECG testing, stress echocardiography, and myocardial perfusion imaging) as the determinant of disposition in patients with normal or indeterminate findings on ECG and serial cardiac maker determinations. These test results stratify risk, allowing patients at low risk to be dismissed. Patients who are not at low risk undergo additional testing. These functional tests often do not detect coronary atherosclerosis in patients with mild to moderate coronary artery luminal obstruction because of their dependence on reduced coronary flow under stress conditions.29 Among patients classified as being at low risk on the basis of negative findings on a treadmill test (Duke treadmill score, <5), 40% had mild to moderate coronary artery stenosis (luminal stenosis, <75%) on cardiac angiography.30 The rupture and subsequent thrombosis of these nonobstructing lesions (stenosis, <50% of luminal diameter) account for nearly two-thirds of ACS cases.31 Cardiac CT CAC assessment (sensitivity, 90%; specificity, 85%) compares favorably with exercise ECG testing (sensitivity, 68%; specificity, 77%), single-photo emission CT myocardial perfusion imaging (sensitivity, 88%; specificity, 77%), and stress echocardiography (sensitivity, 76%; specificity, 88%).32
Because of the limitations with cardiac stress testing, chest pain assessment in the ED is not targeted toward CAD detection, although that is the common perception. Instead, the assessment is focused on the risk stratification of patients. This distinction is important. For primary care physicians providing ED follow-up care, a negative finding on conventional cardiac stress test gives no indication as to the presence of underlying subclinical atherosclerosis or the need for further evaluation or treatment. For emergency medicine physicians evaluating patients with recurrent chest pain, the length of time for which a recent negative conventional stress test finding confers low-risk status is unknown. For patients, a negative finding on functional stress test with its low-risk stratification allows a safe ED dismissal, but it does not address their primary concern—the presence or absence of CAD. The integration of cardiac CT CAC assessment into the ED evaluation of patients with chest pain addresses these concerns, refocuses the process on CAD detection, and provides patients with knowledge about the presence or absence of atherosclerosis, allowing them to focus their health concerns beyond the scope of their ED visit.
The use of noninvasive cardiac CTA for patients with chest pain has shown promise.33-35 Many authors are calling for its use in this chest pain population with low-to-moderate probability of CAD.36-38 A recent study using cardiac CTA in patients with chest pain found that it allows an immediate diagnosis in 75% of cases, facilitating more rapid ED dismissal.34 More careful analysis shows that 68% of the patients had normal results on cardiac CTA, which could have been detected with less risk and cost by a cardiac CT CAC assessment. The other 32% required further testing because of inconclusive cardiac CTAs or to further evaluate coronary artery stenoses found on CTA. One-third of this group required subsequent conventional cardiac angiography, exposing these patients to a second dose of iodinated contrast medium and radiation. Our findings suggest that CT CAC assessment alone has a similar accuracy. Cardiac CTA provides little additive benefit over the considerable improvement in sensitivity and specificity provided by cardiac CT CAC assessment in the differentiation of cardiac chest pain from noncardiac chest pain in this group of patients. The costs, technical requirements, and risks associated with cardiac CTA will likely limit its role in the initial evaluation of patients in this age and risk group presenting to the ED with chest pain. An American Heart Association scientific statement on noninvasive coronary artery imaging suggests that, in a population at low risk of CAD, cardiac CTA is inappropriate, given the risk associated with the radiation exposure vs the possible benefit.39
Recently, a number of articles in the medical and nonmedical press have highlighted concerns about radiation exposure during medical testing.40-43 Cardiac testing can expose the patient to substantial amounts of radiation. Stress/rest technetium Tc 99m sestamibi myocardial perfusion imaging exposes the patient to 9 mSv; diagnostic invasive cardiac angiography, to 7 mSv; conventional chest CT, to 7 mSv; and chest radiography, to 0.1 mSv.44 A cardiac CT CAC assessment performed by MDCT exposes the patient to an effective radiation dose of 1 to 3 mSv, depending on the type of gating used.44,45 A CAC determination by electron beam CT exposes the patient to 1 mSv.45 Current standard cardiac CTA protocols expose women to an effective dose of 21 mSv and men to 15 mSv.46 Radiation exposure increases the patient's future risk of cancer. It has been estimated that the lifetime attributable risk of cancer associated with radiation exposure from a single cardiac CTA of a 40-year-old patient is 0.35% (1 in 284) for a woman and 0.10% (1 in 1007) for a man.46 In a 60-year-old patient, the lifetime attributable risk is 0.22% (1 in 466) for a woman, and 0.08% (1 in 1241) for a man.46 The radiation risks from numerous CTAs are additive over a patient's lifetime.47 If cardiac CTA becomes accepted as an ED imaging tool for patients with acute chest pain, the frequency with which these patients undergo cardiac CTA will likely increase.
Our study has some limitations. First, the method of patient selection raises the potential for selection bias. Consecutive patients meeting the eligibility criteria on their initial presentation and before any further cardiac testing were enrolled Monday through Friday because of cardiac CT scanner availability. Patients who met the initial eligibility criteria but underwent additional cardiac testing before enrollment were ineligible for the study. Second, the composition of the study group was predominantly white, which raises concern about the generalization of our conclusions across races. Evidence exists that atherosclerosis in ethnic minority populations manifests with less calcification than has been reported in the white population.48,49 Also of concern are recent publications that suggested that CAD in women manifests differently than in men.50,51 Although limited, our data suggest that CAC detection is still useful in women presenting with chest pain. To determine whether our conclusions hold across race and sex, a multicenter study is needed to address the use of cardiac CT CAC assessment in persons of ethnic minority groups and in women presenting with chest pain. Third, our study focused on middle-aged patients because the clinical application of CT CAC scanning is suited in that age group. CAC scores increase dramatically as age increases, and the test's negative predictive value declines correspondingly.52 Among asymptomatic men older than 50 years and women older than 65 years, 50% of the population had detectable CAC.52 It is unclear whether our study results can be extended to a higher age range, whether the absolute CAC score or CAC percentiles are preferable, and whether the ROC threshold needs to be adjusted for a higher age range.
CONCLUSION
Most patients with chest pain in the ED do not have a cardiac source of their pain, yet most undergo extensive testing and many are admitted to the hospital because the current ED evaluation process is unable to determine the pain source. Despite a recent consensus statement that relegates the use of cardiac CT CAC assessment to cases for which conventional functional testing is unavailable or has indeterminate findings,53 our study suggests that CAC detection by CT is a powerful adjunct in the initial evaluation of chest pain. Cardiac CT CAC detection provides a substantial improvement in sensitivity and specificity compared with conventional cardiac stress testing. It provides similar, if not improved, sensitivity and specificity compared with cardiac CTA, but with much less cost and radiation exposure.
Early integration of cardiac CT CAC assessment into the chest pain evaluation algorithm represents a major change in the practice of emergency medicine. Early assessment of coronary plaque burden by cardiac CT expedites the differentiation between cardiac and noncardiac chest pain, allowing most patients with noncardiac chest pain to be immediately dismissed. Additionally, the improved sensitivity of cardiac CT CAC scanning detects subclinical atherosclerosis, allowing early risk factor modification and thus potentially preventing future cardiac events. Early integration of cardiac CT CAC assessment in the ED allows resources to be focused on patients with CAD, providing cost-effective, expeditious, and directed additional testing.
Acknowledgments
The authors are indebted to Rebecca Nelson, CCRC, for her work as study coordinator and to Bonnie L. Espy for her help with manuscript preparation.
Footnotes
Funding was provided by an intramural grant from Mayo Foundation.
REFERENCES
- 1.Storrow AB, Gibler WB. Chest pain centers: diagnosis of acute coronary syndromes. Ann Emerg Med. 2000;35(5):449-461 [PubMed] [Google Scholar]
- 2.Canto JG, Shlipak MG, Rogers WJ, et al. Prevalence, clinical characteristics, and mortality among patients with myocardial infarction presenting without chest pain. JAMA 2000;283(24):3223-3229 [DOI] [PubMed] [Google Scholar]
- 3.Lee TH, Rouan GW, Weisberg MC, et al. Sensitivity of routine clinical criteria for diagnosing myocardial infarction within 24 hours of hospitalization. Ann Intern Med. 1987;106(2):181-186 [DOI] [PubMed] [Google Scholar]
- 4.Gibler WB, Lewis LM, Erb RE, et al. Early detection of acute myocardial infarction in patients presenting with chest pain and nondiagnostic ECGs: serial CK-MB sampling in the emergency department [published correction appears in Ann Emerg Med. 1991;20(4):420] Ann Emerg Med. 1990;19(12):1359-1366 [DOI] [PubMed] [Google Scholar]
- 5.Karras DJ, Kane DL. Serum markers in the emergency department diagnosis of acute myocardial infarction. Emerg Med Clin North Am. 2001;19(2):321-337 [DOI] [PubMed] [Google Scholar]
- 6.Klocke FJ, Baird MG, Lorell BH, et al. American College of Cardiology. American Heart Association Task Force on Practice Guidelines. American Society for Nuclear Cardiology ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging). Circulation 2003;108(11):1404-1418 [DOI] [PubMed] [Google Scholar]
- 7.Miller CD, Lindsell CJ, Khandelwal S, et al. Is the initial diagnostic impression of “noncardiac chest pain” adequate to exclude cardiac disease [published correction appears in Ann Emerg Med. 2005;45(1):87]?Ann Emerg Med. 2004;44(6):565-574 [DOI] [PubMed] [Google Scholar]
- 8.Janowitz WR, Agatston AS, Kaplan G, Viamonte M., Jr Differences in prevalence and extent of coronary artery calcium detected by ultrafast computed tomography in asymptomatic men and women. Am J Cardiol. 1993;72(3):247-254 [DOI] [PubMed] [Google Scholar]
- 9.Stanford W, Thompson BH, Weiss RM. Coronary artery calcification: clinical significance and current methods of detection. AJR Am J Roentgenol. 1993;161(6):1139-1146 [DOI] [PubMed] [Google Scholar]
- 10.Georgiou D, Budoff MJ, Kaufer E, Kennedy JM, Lu B, Brundage BH. Screening patients with chest pain in the emergency department using electron beam tomography: a follow-up study. J Am Coll Cardiol. 2001;38(1):105-110 [DOI] [PubMed] [Google Scholar]
- 11.Simons DB, Schwartz RS, Edwards WD, Sheedy PF, Breen JF, Rumberger JA. Noninvasive definition of anatomic coronary artery disease by ultrafast computed tomographic scanning: a quantitative pathologic comparison study. J Am Coll Cardiol. 1992;20(5):1118-1126 [DOI] [PubMed] [Google Scholar]
- 12.Kaufmann RB, Sheedy PF, II, Breen JF, et al. Detection of heart calcification with electron beam CT: interobserver and intraobserver reliability for scoring quantification. Radiology 1994;190(1):347-352 [DOI] [PubMed] [Google Scholar]
- 13.Budoff MJ, Achenbach S, Blumenthal RS, et al. American Heart Association Committee on Cardiovascular Imaging and Intervention. American Heart Association Council on Cardiovascular Radiology and Intervention. American Heart Association Committee on Cardiac Imaging. Council on Clinical Cardiology Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation 2006;114(16):1761-1791 [DOI] [PubMed] [Google Scholar]
- 14.Arad Y, Spadaro LA, Goodman K, Newstein D, Guerci AD. Prediction of coronary events with electron beam computed tomography. J Am Coll Cardiol. 2000;36:1253-1260 [DOI] [PubMed] [Google Scholar]
- 15.Laudon DA, Vukov LF, Breen JF, Rumberger JA, Wollan PC, Sheedy PF. Use of electron-beam computed tomography in the evaluation of chest pain patients in the emergency department. Ann Emerg Med. 1999;33:15-21 [DOI] [PubMed] [Google Scholar]
- 16.Braunwald E, Mark DB, Jones RH, et al. Agency for Health Care Policy and Research. National Heart, Lung, and Blood Institute, Public Health Service. US Department of Health and Human Services Unstable angina: diagnosis and management: clinical practice guideline number 10. http://www.medana.unibas.ch/eng/internt/angc1.htm. http://www.medana.unibas.ch/eng/internt/angc1.htm AHCPR Publication No. 94-0602. Amended May 1994. Accessed March 3, 2010.
- 17.Braunwald E, Antman EM, Beasley JW, et al. ACC/AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Unstable Angina) [published correction appears in J Am Coll Cardiol. 2001;38(1):294-295] J Am Coll Cardiol. 2000;36(3):970-1062 [DOI] [PubMed] [Google Scholar]
- 18.Braunwald E, Antman EM, Beasley JW, et al. American College of Cardiology. American Heart Association. Committee on the Management of Patients With Unstable Angina ACC/AHA 2002 guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Unstable Angina). J Am Coll Cardiol. 2002;40(7):1366-1374 [DOI] [PubMed] [Google Scholar]
- 19.Goel M, Wong ND, Eisenberg H, et al. Risk factor correlates of coronary calcium as evaluated by ultrafast computed tomography. Am J Cardiol. 1992;70:977-980 [DOI] [PubMed] [Google Scholar]
- 20.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827-832 [DOI] [PubMed] [Google Scholar]
- 21.Girshman J, Wolff SD. Techniques for quantifying coronary artery calcification. Radiol Clin North Am. 2004July;42(4):767-771 [DOI] [PubMed] [Google Scholar]
- 22.Obuchowski NA, Modic MT. Calcium scoring: criteria for evaluating its effectiveness. Radiol Clin North Am. 2004July;42(4):773-778 [DOI] [PubMed] [Google Scholar]
- 23.Stanford W, Thompson BH, Burns TL, Heery SD, Burr MC. Coronary artery calcium quantification at multi-detector row helical CT versus electron-beam CT. Radiology 2004;230(2):397-402 [DOI] [PubMed] [Google Scholar]
- 24.Carr JJ, Crouse JR, III, Goff DC, Jr, D'Agostino RB, Jr, Peterson NP, Burke GL. Evaluation of subsecond gated helical CT for quantification of coronary artery calcium and comparison with electron beam CT. AJR Am J Roentgenol. 2000;174(4):915-921 [DOI] [PubMed] [Google Scholar]
- 25.Budoff MJ, Nasir K, McClelland RL, et al. Coronary calcium predicts events better with absolute calcium scores than age-sex-race/ethnicity percentiles: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2009;53:345-352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho JS, Fitzgerald SJ, Stolfus LL, et al. Relation of a coronary artery calcium score higher than 400 to coronary stenoses detected using multidetector computed tomography and to traditional cardiovascular risk factors. Am J Cardiol. 2008;101(10):1444-1447 [DOI] [PubMed] [Google Scholar]
- 27.Rubinshtein R, Gaspar T, Halon DA, Goldstein J, Peled N, Lewis BS. Prevalence and extent of obstructive coronary artery disease in patients with zero or low calcium score undergoing 64-slice cardiac multidetector computed tomography for evaluation of a chest pain syndrome. Am J Cardiol. 2007;99(4):472-475 [DOI] [PubMed] [Google Scholar]
- 28.Pope JH, Selker HP. Acute coronary syndromes in the emergency department: diagnostic characteristics, tests, and challenges. Cardiol Clin. 2005;23(4):423-451 [DOI] [PubMed] [Google Scholar]
- 29.Gibbons RJ, Balady GJ, Bricker JT, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Committee to Update the 1997 Exercise Testing Guidelines ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines) [published correction appears in J Am Coll Cardiol. 2006;48(8):1731] J Am Coll Cardiol. 2002;40(8):1531-1540 [DOI] [PubMed] [Google Scholar]
- 30.Shaw LJ, Peterson ED, Shaw LK, et al. Use of a prognostic treadmill score in identifying diagnostic coronary disease subgroups. Circulation 1998;98(16):1622-1630 [DOI] [PubMed] [Google Scholar]
- 31.Shah PK. Pathophysiology of plaque rupture and the concept of plaque stabilization. Cardiol Clin. 2003;21(3):303-314 [DOI] [PubMed] [Google Scholar]
- 32.Garber AM, Solomon NA. Cost-effectiveness of alternative test strategies for the diagnosis of coronary artery disease. Ann Intern Med. 1999;130:719-728 [DOI] [PubMed] [Google Scholar]
- 33.Hoffmann U, Nagurney JT, Moselewski F, et al. Coronary multidetector computed tomography in the assessment of patients with acute chest pain [published correction appears in Circulation. 2006;114(25):e651] Circulation 2006;114(21):2251-2260 [DOI] [PubMed] [Google Scholar]
- 34.Goldstein JA, Gallagher MJ, O'Neill WW, Ross MA, O'Neil BJ, Raff GL. A randomized controlled trial of multi-slice coronary computed tomography for evaluation of acute chest pain. J Am Coll Cardiol. 2007;49(8):863-871 [DOI] [PubMed] [Google Scholar]
- 35.Gallagher MJ, Ross MA, Raff GL, Goldstein JA, O'Neill WW, O'Neil B. The diagnostic accuracy of 64-slice computed tomography coronary angiography compared with stress nuclear imaging in emergency department low-risk chest pain patients. Ann Emerg Med. 2007;49(2):125-136 [DOI] [PubMed] [Google Scholar]
- 36.Hollander JE, Chang AM, Shofer FS, McCusker CM, Baxt WG, Litt HI. Coronary computed tomographic angiography for rapid discharge of low-risk patients with potential acute coronary syndromes. Ann Emerg Med. 2009;53(3):295-304 [DOI] [PubMed] [Google Scholar]
- 37.Takakuwa KM, Hallpern EJ. Evaluation of a “triple rule-out” coronary CT angiography protocol: use of 64-section CT in low-to-moderate risk emergency department patients suspected of having acute coronary syndrome. Radiology 2008;248:438-446 [DOI] [PubMed] [Google Scholar]
- 38.Halpern EJ. Triple-rule-out CT angiography for evaluation of acute chest pain and possible acute coronary syndrome. Radiology 2009;252:332-345 [DOI] [PubMed] [Google Scholar]
- 39.Bluemke DA, Achenbach S, Budoff M, et al. Noninvasive coronary artery imaging: magnetic resonance angiography and multidetector computed tomography angiography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention, and the Councils on Clinical Cardiology and Cardiovascular Disease in the Young. Circulation 2008;118(5):586-606 [DOI] [PubMed] [Google Scholar]
- 40.Fazel R, Krumholz HM, Wang Y, et al. Exposure to low-dose ionizing radiation from medical imaging procedures. N Engl J Med. 2009;361:849-857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Redberg RF. Cancer risks and radiation exposure from computed tomographic scans. Arch Intern Med. 2009;169(22):2049-2050 [DOI] [PubMed] [Google Scholar]
- 43.Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 2009;169(22):2078-2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gerber TC, Kantor B, McCollough GH. Radiation dose and safety in cardiac computed tomography. Cardiol Clin. 2009;27:665-677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morin RL, Gerber TC, McCollough CH. Radiation dose in computed tomography of the heart. Circulation 2003;107(6):917-922 [DOI] [PubMed] [Google Scholar]
- 46.Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA 2007;298(3):317-323 [DOI] [PubMed] [Google Scholar]
- 47.Coles DR, Smail MA, Negus IS, et al. Comparison of radiation doses from multislice computed tomography coronary angiography and conventional diagnostic angiography. J Am Coll Cardiol. 2006;47(9):1840-1845 [DOI] [PubMed] [Google Scholar]
- 48.Bild DE, Detrano R, Peterson D, et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2005;111(10):1313-1320 [DOI] [PubMed] [Google Scholar]
- 49.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2006;113(1):30-73 [DOI] [PubMed] [Google Scholar]
- 50.Bairey Merz N, Bonow RO, Sopko G, et al. National Heart, Lung and Blood Institute. American College of Cardiology Foundation Women's ischemic syndrome evaluation: current status and future research directions: report of the National Heart, Lung and Blood Institute workshop: October 2-4, 2002: executive summary. Circulation 2004;109(6):805-807 [DOI] [PubMed] [Google Scholar]
- 51.Milner KA, Funk M, Richards S, Wilmes RM, Vaccarino V, Krumholz HM. Gender differences in symptom presentation associated with coronary heart disease. Am J Cardiol. 1999;84(4):396-399 [DOI] [PubMed] [Google Scholar]
- 52.Mitchell TL, Pippin JJ, Devers SM, et al. Age- and sex-based nomograms from coronary artery calcium scores as determined by electron beam computed tomography. Am J Cardiol. 2001;87(4):453-456 [DOI] [PubMed] [Google Scholar]
- 53.Greenland P, Bonow RO, Brundage BH, et al. American College of Cardiology Foundation Clinical Expert Consensus Task Force. (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) Society of Atherosclerosis Imaging and Prevention. Society of Cardiovascular Computed Tomography ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2007;49(3):378-402 [DOI] [PubMed] [Google Scholar]