Abstract
The beneficial role of statins in primary and secondary prevention of coronary heart disease has resulted in their frequent use in clinical practice. However, safety concerns, especially regarding hepatotoxicity, have driven multiple trials, which have demonstrated the low incidence of statin-related hepatic adverse effects. The most commonly reported hepatic adverse effect is the phenomenon known as transaminitis, in which liver enzyme levels are elevated in the absence of proven hepatotoxicity. This class effect is usually asymptomatic, reversible, and dose-related. However, the increasing incidence of chronic liver diseases, including nonalcoholic fatty liver disease and hepatitis C, has created a new challenge when initiating statin treatment in patients with high cardiovascular risk. These diseases result in abnormally high liver biochemistry values, discouraging statin use by clinicians, fostering treatment discontinuation, and leaving a large number of at-risk patients untreated. A PubMed/MEDLINE search of the literature regarding statin safety (January 1, 1994-December 31, 2008) was performed, using the following search terms: statin safety, statin-related hepatotoxicity, and chronic liver disease and statin use, as well as the specific names of different statins and different liver diseases. Relevant clinical trials, review articles, panel discussions, and guideline recommendations were selected. This review supports the use of statin treatment in patients with high cardiovascular risk whose elevated aminotransferase levels have no clinical relevance or are attributable to known stable chronic liver conditions. For each patient, the decision should be based on an individual assessment of risks and benefits.
ALT = alanine aminotransferase; CHD = coronary heart disease; HCV = hepatitis C virus; HPS = Heart Protection Study; LDL = low-density lipoprotein; NAFLD = nonalcoholic fatty liver disease; PBC = primary biliary cirrhosis; PPP = Prospective Pravastatin Pooling
Coronary heart disease (CHD), the result of coronary atherosclerosis, is the largest single cause of death in the United States and is the leading cause of morbidity and mortality worldwide.1 Central to the pathogenesis of atherosclerosis is the deposition and retention of cholesterol in arterial walls, making lipid modification critical to CHD prevention. Lowering cholesterol levels has been shown unequivocally to reduce cardiovascular events and prevent the development of atherosclerosis.2-4 An estimated 105 million American adults have total blood cholesterol values of 200 mg/dL or higher (to convert to mmol/L, multiply by 0.0259). Of these, about 42 million have values of 240 mg/dL or higher and are considered at high risk. Additionally, high-risk individuals with either preexisting CHD or risk equivalents require more aggressive lipid-lowering intervention.5
The 3-hydroxyl-3-methylglutaryl coenzyme A reductase inhibitors, collectively referred to as statins, have assumed the central role in the treatment of hypercholesterolemia.2-5 Statins have proven to be the most efficacious drug therapy for attaining significant reductions in low-density lipoprotein (LDL) cholesterol levels, and their benefits could extend beyond their plasma LDL–lowering effect.6 Statins have been shown to reduce the incidence of CHD by as much as 21% to 43%, with undeniable effectiveness in both primary and secondary prevention.2,4,5
Despite the well-known importance of statin therapy, the primary care physician faces daily challenges when prescribing statins because of associated illnesses, baseline laboratory abnormalities, and possible adverse effects ascribed to their use. One common challenge is the presence of baseline elevations of serum liver enzyme levels, not infrequently seen in patients at risk of or with established CHD. This abnormality is frequently secondary to associated comorbid conditions, such as obesity, dyslipidemia, prediabetes, and diabetes mellitus, which share features of nonalcoholic fatty liver disease (NAFLD). Other chronic liver diseases, such as viral hepatitis (B or C) and/or cirrhosis, may also coexist in those at high risk of cardiovascular events.
This review surveys the available information regarding the safety of statins in patients at high risk of cardiovascular events who present with or develop abnormal liver transaminase levels. A PubMed/MEDLINE search of the literature (January 1, 1994, to December 31, 2008) was performed using the terms statin safety, statin-related hepatotoxicity, and chronic liver disease and statin use, as well as the specific names of different statins and different liver diseases. Relevant clinical trials, review articles, panel discussions, and guideline recommendations were selected. An evaluation of published studies concerning the hepatic effects of statins in patients with and without known liver disease was performed to develop an evidence-based approach in clinical practice for safe statin use in high-risk populations.
WHAT IS THE EFFECT OF STATINS ON THE LIVER?
Statins exert a potent inhibition of hepatic 3-hydroxyl-3-methylglutaryl coenzyme A reductase, which accounts for the reduction in LDL cholesterol observed with these drugs. Because of their powerful effects on this liver enzyme, concern about hepatotoxicity has been raised, and multiple medical trials have been performed to assess their safety.7
Statin treatment has been associated with a broad spectrum of hepatic adverse effects. The most common is an asymptomatic and usually transient elevation of serum aminotransferase levels that often occurs in the first 12 weeks of therapy. Most of the time, this biochemical finding is not correlated with histopathological changes and therefore does not meet criteria as a true indicator of liver injury.6-9 Although the underlying mechanism remains unclear, it may result from changes in the lipid components of the hepatocyte membrane, leading to an increase in its permeability with a subsequent “leakage” of liver enzymes. This is supported by the observation that elevations in aminotransferase levels (alanine aminotransferase [ALT] being a more reliable indicator than aspartate aminotranserase) occur with the use of structurally unrelated statins, as well as with other effective lipid-lowering drugs.8,10,11 Thus, the term transaminitis has been adopted to best define this phenomenon of liver enzyme abnormalities in the absence of proven hepatotoxicity.11
Clinically important statin-related hepatotoxicity is an extremely rare adverse effect; thus, when suspected, the first step should be to rule out other causes.8 Patients treated with statins who present with persistent elevations in ALT of greater than 10 times the upper limit of normal often have associated comorbid conditions and/or concomitant use of medications known to interact with statins or that could induce hepatotoxicity per se.12 Medication history, blood tests, and liver imaging to exclude other causes of liver disease are essential to help establish the diagnosis.7,8,13 Because most statins are metabolized via the cytochrome system, toxicity induced by interacting drugs often contributes to hepatotoxicity. Furthermore, undiagnosed liver diseases should be investigated when large increases in liver enzyme levels develop once statin therapy is initiated.14
In very rare cases in which true statin-related hepatotoxicity has been demonstrated, no characteristic biochemical or histologic pattern of liver injury has been established. Hepatocellular, cholestatic, or even mixed histologic patterns have been reported, and the proposed mechanism is either an idiosyncratic or an immunoallergic reaction.8,9 Isolated cases of autoimmune hepatitis revealed by statin treatment have been described with variable degrees of severity.15,16 This entity should be considered in the setting of persistent elevated aminotransferase levels after discontinuation of statins and with associated autoimmune features, such as increased autoantibodies and immunoglobulin levels.16 Statin-related acute liver failure is extremely unusual, and the incidence reported with lovastatin is 1 per 114,000 patient-years, almost similar to that of idiopathic acute liver failure in the general population (1 per 130,000 patient-years).11,17 In this context, statins were associated with fulminant liver failure in 3 of 51,741 cases of liver transplants in the United States from 1990 to 2002.18
INCIDENCE OF ELEVATIONS OF AMINOTRANSFERASE LEVELS DURING STATIN TREATMENT
No unifying criterion defines the incidence of drug-related liver test abnormalities and their clinical relevance. A post-marketing drug-induced hepatotoxicity white paper defined drug-induced liver injury by the presence of increases in ALT level of more than 2 to 3 times the upper limit of normal or in conjugated bilirubin level of more than 2 times the upper limit of normal. However, it has been proposed that increases in ALT level of more than 10 times the upper limit of normal should be used to differentiate true hepatotoxicity from transaminitis.19 Moreover, other authors have only accepted abnormal findings on liver biochemistries as clinically relevant if they meet Hy Rule criteria for monitoring drug hepatotoxicity. Those criteria imply an elevation of ALT level of more than 3 times the upper limit of normal in combination with elevated total bilirubin levels (>2 times the upper limit of normal) at any time after starting a new drug.14,19-22
Data obtained from several clinical trials had important implications for statin-associated elevation in aminotransferase levels.
First, the incidence of elevated aminotransferase levels across multiple studies performed with different types of statins generally did not exceed 3% of the studied patients' sample.23-27 In most trials, no significant differences were observed when the statin was compared with placebo, as was the case in SSSS (Scandinavian Simvastatin Survival Study), HPS (Heart Protection Study), JUPITER (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin), and the PPP (Prospective Pravastatin Pooling) project.4,9,19,26,28,29 In the PPP study, the percentage of either mild to moderate (>3 but <5 times the upper limit of normal) or severe (>10 times the upper limit of normal) elevations in aminotransferase levels were not significantly different between the statin-treated and the placebo group (0.9% vs 1% and 0.2% vs 0.1%, respectively).29
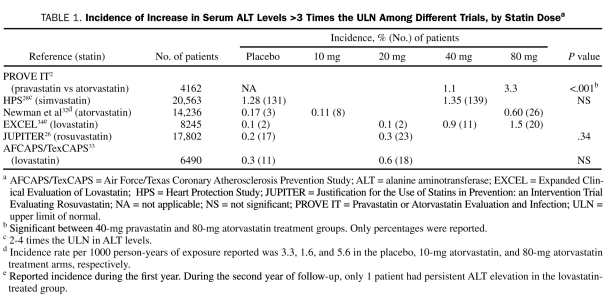
Second, there seems to be a direct relationship between the statin dose and the incidence of transaminitis.30-32 In some trials, a higher incidence of transaminitis was more commonly observed with higher doses of atorvastatin (Table 1).31 Indeed, the reported average incidence of elevations in serum aminotransferase levels to more than 3 times the upper limit of normal was less than 1% in patients receiving low to moderate doses, which has been proven by a meta-analysis to be no different from placebo.30 Similarly, the incidence of transaminitis increases up to 2% to 3% in those receiving high doses of statins.32-34 In either case, the increase was related to the dose and not to the level of LDL cholesterol reduction.35,36 Of note, the incidence of transaminase elevations is similar among all statins, despite their different pharmacokinetic characteristics.
TABLE 1.
Incidence of Increase in Serum ALT Levels >3 Times the uLN Among Different Trials, by Statin Dosea
Finally, most cases of transaminitis exhibit spontaneous improvement without the need for drug discontinuation, probably a result of the development of adaptation or tolerance. This was demonstrated in several trials, in which nearly 70% of cases with increased aminotransferase levels after initiation of statin therapy resolved spontaneously.13,35 This fact has led many clinicians to consider the concept of “rechallenge”—a retrial of the same or a different statin after resolution of abnormal aminotransferase levels—with follow-up liver biochemistries to ensure continued normalization of their values.12
Should statins be used in patients with baseline elevation of liver aminotransferase levels? Are patients with elevated baseline aspartate aminotransferase/ALT levels at greater risk of developing statin-related hepatotoxicity?
The Third National Health and Nutrition Examination Survey (1988-1994) revealed that asymptomatic elevations in serum aminotranferase levels are found in 7.9% of the US population, and it was suggested that this may mark chronic liver disease.37 The most common etiologies were NAFLD and hepatitis C, followed by alcoholic liver disease, hepatitis B, and hemochromatosis. Given this prevalence, the clinician frequently faces this scenario in daily practice.
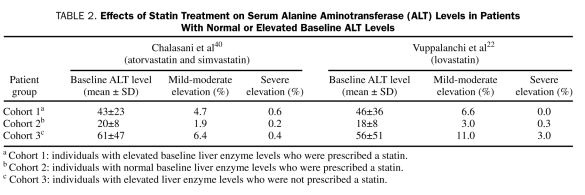
Nonalcoholic fatty liver disease includes a broad spectrum of disorders ranging from isolated fatty liver to nonalcoholic steatohepatitis. This entity is commonly associated with components of the metabolic syndrome and with a higher mortality from cardiovascular disease.38 However, physicians are often reluctant to prescribe statins in this population for several reasons. One is the concern about statin safety in patients with chronic liver disease, given the Food and Drug Administration's labeling for statins. Also, statins increase hepatic LDL receptor expression and hepatic lipogenesis, raising concerns about possible worsening of hepatic fatty infiltration.7,39 However, studies in individuals with suspected NAFLD and elevated liver enzyme levels revealed that the incidence and magnitude of elevations in aminotransferases in patients receiving statins were not significantly different from those not taking statins (Table 2).22,40 Also, in a subgroup of patients with preexisting liver abnormalities who participated in the PPP trial, no significant differences were found between the simvastatin-treated and the placebo group in terms of increases in aminotransferase levels, which ranged between 1.5 and 3.0 times the upper limit of normal (40.1% vs 38.5%, respectively).29 These results support the hypothesis that fluctuations in liver enzyme values may represent natural disease progression rather than a statin-related effect.41
TABLE 2.
Effects of Statin Treatment on Serum Alanine Aminotransferase (ALT) Levels in Patients With Normal or Elevated Baseline ALT Levels
This conclusion is further supported by the results of the Dallas Heart Study, in which the relationships between statin use, ALT elevations, and hepatic steatosis were evaluated. Hepatic triglyceride content was measured, and participants were divided into 2 groups: one with and the other without statin treatment. The study revealed a lack of relationship between statin use and more severe worsening of hepatic steatosis (40% vs 38%; P=.89) or elevated ALT values (13% vs 15%; P=.58).39
Recent studies suggest that statin treatment may in fact improve hepatic steatosis. In a small uncontrolled study in patients with increased levels of baseline liver enzymes (<3 times the upper limit of normal) and biopsy-proven NAFLD, a 6-month treatment course with pravastatin improved liver histology and failed to cause further increases in serum aminotransferase levels.42 Furthermore, a controlled longitudinal histopathological study showed that long-term statin treatment improved liver histology with regression of steatosis over time without significant changes in liver tests.38 In summary, these results suggest that statins can be safely used in patients with NAFLD, with appropriate monitoring.
Safety concerns also encompass patients with hepatitis C; its prevalence in the United States is 1.6%.43 Given the relatively high prevalence and chronicity of hepatitis C virus (HCV) infection, its presence in patients at high cardiovascular risk is not infrequent. However, physicians are often hesitant to prescribe statins in this population because of their uncertainty as to whether the benefits outweigh the risks. Safety of statins in patients with HCV infection was evaluated in a controlled cohort study conducted at the Stanford Veterans Administration Hospital. This study compared the incidence of mild to moderate and severe elevations in aminotransferase levels between patients with positive HCV infection with and without statin therapy and anti-HCV–negative patients taking statins. Although baseline liver enzymes were higher in patients with hepatitis C, there was no significant difference in the incidence of mild-moderate (P=.94) to severe (P=.87) increases in liver enzyme levels between statin-treated groups with or without HCV infection. Of note, severe increases in transaminase levels were more associated with the HCV-infected cohort not treated with statins. These findings suggest that there is not a higher risk of alterations in liver biochemistry values in patients with HCV infection.44,45 Also, the recent description of a HCV-associated dysmetabolic syndrome (which includes the triad of insulin resistance, hypocholesterolemia, and steatosis) raises the possibility that there might be valid reasons to consider statin therapy in these patients, given their possible increased risk of cardiovascular disease.7 Because statins up-regulate LDL receptors, which are deemed to be the port of entrance for the HCV to the hepatocytes, statin use might exacerbate HCV infection.46 However, this has not been demonstrated, and indeed some in vitro as well as in vivo studies revealed that statins were not only safe but also beneficial in these patients as a potential adjuvant therapy for HCV infection.47,48
Evidence regarding statin safety in patients with less frequent chronic liver diseases is scarce. The usefulness of lipid-lowering agents in primary biliary cirrhosis (PBC), a condition that is widely associated with hyperlipidemia in its first stages, has been debated.49 It has been held that, despite its prevalent hyperlipidemia, there is no association with atherosclerosis and increased cardiovascular risk, with multiple theories supporting a cardioprotective effect of lipoprotein X, high-density lipoprotein, and other markers that are increased in PBC. However, a meta-analysis performed by Sorokin et al50 did not find enough statistical power in the supportive trials and suggests that physicians customize an approach according to patients' associated comorbid conditions. In a small trial in patients with PBC, simvastatin did not induce significant changes in aminotransferase levels, and, aside from its LDL cholesterol– and total cholesterol–lowering effect, there was statistically significant improvement in disease markers.49 In addition, the interactions between alcohol intake and statin treatment have been poorly studied because most randomized trials have excluded patients with excessive alcohol intake. Of note, occasional findings from the HPS revealed that, in patients with a baseline alcohol intake of more than 21 units per week, there was no evidence of a higher risk of statin-related myopathy or elevation in liver enzyme levels.28,31 A recent study in a large cohort of patients with diabetes suggested that statins might hold a preventive effect in the development of hepatocellular carcinoma in patients at risk.51
The assessment of statin safety in the post–liver transplant population is critically important, especially given the fact that dyslipidemia is observed in 20% to 60% of cases. In this context, several important issues should be considered. First, the most commonly used immunosuppressive agents, such as tacrolimus and cyclosporine A, are metabolized via the cytochrome P450 system, which increases the risk of adverse effects due to drug interactions. This kind of interaction is uncommon with pravastatin because it is not significantly metabolized through cytochrome P450. The second issue to consider is that these immunosuppressive agents also affect plasma lipid levels, resulting in hypercholesterolemia. However, in a small controlled cross-over study conducted by Zachoval et al,52 a 6-week period of treatment with pravastatin or cerivastatin was well tolerated in patients undergoing liver transplant, in terms of liver function and immunosuppression. However, evidence of long-term safety in such patients is still lacking.17
The use of statins has been precluded in patients with acute viral or alcohol-associated liver disease, as well as in patients with advanced liver disease. The rationale behind this contraindication is that the primary goal during acute injury is to avoid any potential risk of further liver injury until the patient has totally recovered.24 In cases of severely decompensated chronic liver disease, drug metabolism may be impaired.53-55 This was illustrated in a small nonrandomized parallel group trial, in which the safety of rosuvastatin was compared in patients with normal liver function, cirrhosis Child-Pugh class A (mild impairment), and cirrhosis Child-Pugh class B (moderate impairment). Rosuvastatin steady-state and peak serum concentrations were increased in the Child-Pugh class B group.55 Although statins seem to be safe in patients with compensated liver disease, these results imply that more extensive hepatic impairment may alter their pharmacokinetics and metabolism, leading to abnormally high levels of the drug. Patients with decompensated cirrhosis usually present with low cholesterol levels because of their impaired synthesis of cholesterol in this state of the disease. Therefore, the use of statins would not be indicated in this population.
In an effort to better examine the potential hepatotoxicity of statins in patients with underlying liver disease, Lewis et al56 performed a multicenter controlled prospective study in patients with well-compensated chronic liver disease of any etiology. The patients were divided into 2 groups: one group received placebo and the other received treatment with 80 mg of pravastatin. This dose was intended to capture as many dose-related effects as possible. From a sample of 326 patients, 64% had documented NAFLD, 23% were affected by HCV infection, and the rest had other causes of chronic liver disease (hepatitis B, hemochromatosis, autoimmune hepatitis, past alcohol intake, cirrhosis due to PBC, or cryptogenic cirrhosis). The results showed no significant differences in ALT elevation rates between the statin- and the placebo-treated groups (P=.23). Furthermore, there was no difference in event rates among liver disease groups, despite the fact that some patients had up to 5 times the upper limit of normal elevations in baseline transaminase levels. These findings favor the safe use of pravastatin in patients with well-compensated chronic liver disease and hypercholesterolemia. The results of the study, as well as the evidence heretofore discussed, are consistent with the conclusions drawn by the Liver Expert Panel. The panel proposed that compensated cirrhosis and chronic liver disease should not be considered contraindications to statin therapy and that statins could be used safely in patients with NAFLD. Moreover, it held that the latter population should be considered important candidates for statin therapy because of their significantly increased cardiovascular risk.24
HOW SHOULD PATIENTS BE MONITORED AND EVALUATED FOR POTENTIAL HEPATIC ADVERSE EFFECTS?
Current recommendations from the National Cholesterol Education Program Adult Treatment Panel III advise liver chemistry monitoring on initiation of statin treatment, 12 weeks after initiation, and then yearly or even before if indicated (eg, when changing dose).3 However, evidence obtained from several clinical trials has caused controversy about this recommendation. As previously stated, the incidence of statin-related acute liver failure is almost the same as that in the general population, and an idiosyncratic reaction is the most often suggested mechanism.10 Large randomized trials have proven the safety of low to moderate doses of lovastatin, pravastatin, simvastatin, atorvastatin, and rosuvastatin, showing no significantly increased risk of liver biochemistry abnormalities.26,30 Finally, in most trials (ie, AFCAPS/TexCAPS [Air Force/Texas Coronary Atherosclerosis Prevention Study], SSSS), persistently elevated liver enzyme levels turned out to be false positives. Moreover, very few (1 of 24) of those with suspected statin-related liver failure had their condition detected by subsequent monitoring.10,33 These facts question the usefulness and cost-effectiveness of this routine monitoring, given the infrequency of statin-related liver failure cases, the proven safety of most of the statins, and the lack of evidence that routine liver biochemistry assessment would prevent idiosyncratic or serious liver disease.9,28,30,31 In fact, because of the initial transaminitis phenomenon, this monitoring can result in premature discontinuation by primary care physicians of a potentially life-saving drug therapy.
In this context, the National Lipid Association and the Liver Expert Panel suggested that no evidence exists to support continued monitoring with liver tests. However, they still consider it prudent for clinicians to perform pertinent laboratory testing during the patient's routine medical assessment.23-25 Even though it has already been proven that elevated baseline aminotransferase levels are not associated with a higher risk of hepatotoxicity, baseline measurements may be beneficial for future diagnostic and comparative purposes.35 If elevated aminotransferase levels are discovered, clinical correlation and further medical evaluation are necessary.24
HOW SHOULD DYSLIPIDEMIC PATIENTS WITH ELEVATED LIVER ENZYME LEVELS BE MANAGED?
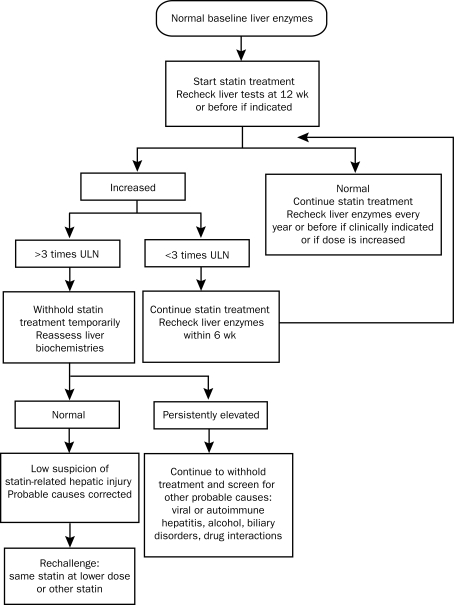
Management of dyslipidemic patients with high cardiovascular risk and increased liver enzyme levels represents a challenge. In more than one clinical scenario, the physician is confronted with the dilemma of whether to treat with statins, and in every case it is important to weigh the benefits and risks of treatment (Figure).
FIGURE.
Algorithm for management of abnormal liver enzymes before and during statin treatment. ULN = upper limit of normal.
One case scenario involves patients with normal baseline transaminase levels who develop asymptomatic isolated elevation of liver transaminase levels during follow-up. If the increment is below the threshold of 3 times the upper limit of normal in the setting of transaminitis, statin treatment can be continued with monitoring of liver biochemistries.23 No consensus exists about the best time to recheck liver biochemistry values. In the HPS and in the AFCAPS/TexCAPS trial, the liver profile was rechecked after 3 and 2 weeks, respectively, with normalization of the values in more than 70% of the cases. Other authors suggest repeating the test after 6 weeks.53 In contrast, in cases in which the increment in aminotransferase levels is beyond the 3 times the upper limit of normal threshold, it is recommended that the treatment be stopped and the levels be reassesed (again no consensus about the timing of this reassessment was found). When the elevation in transaminase levels is persistent, statins should be with-held and further screening, including exclusion of the 2 hepatic avidin-biotin complexes and drug interactions (eg, the use of acetaminophen, over-the-counter medications, and herbal preparations), should be performed if clinically indicated.23,24 However, if clinical suspicion for statin-related liver injury is low, a rechallenge with either the same drug and dose, a different statin, or the same statin at a lower dose should be attempted.3,12 The ideal period after the normalization of the liver enzyme levels when the rechallenge can be done is not clearly stated.
Consensus about the best statin therapeutic algorithm in patients with an established and compensated chronic liver disease is hard to reach because of the absence of evidence in this area. Although the Food and Drug Administration continues to recommend that statins be contraindicated in patients with chronic liver disease, several authors have recommended starting low-dose statin treatment (because of the possible greater incidence of liver enzyme elevations with higher doses) and having levels rechecked 2 weeks later.53,54 They also propose that liver biochemistry monitoring should be performed every month for the first 3 to 4 months and 4 times a year thereafter. If the levels of transaminases increase to more than 3 times baseline values, discontinuation of the drug should be considered. Clinical correlation with worsening of underlying disease, as well as exclusion of alcohol abuse and drug interactions, should be done before attempting permanent discontinuation of the drug. Once levels return to baseline, rechallenge can be considered. Even less intensive monitoring may be adequate in these patients; however, until there are data indicating that such an approach would be safe and until greater consensus has been reached, a cautious attitude in this setting should be adopted.
CONCLUSION
Faced with the dilemma of managing the care of patients who have multiple comorbid conditions and who are receiving multidrug therapy, the primary care physician must customize treatment to each patient. Statin use and the associated decrease in levels of LDL cholesterol are of extreme importance for the primary and secondary prevention of CHD. In light of the significant reduction in the risk of life-threatening cardiovascular events that statins provide, primary care physicians should not withhold statin therapy from patients whose transaminase elevations have no clinical relevance or are attributable to known stable chronic conditions. This practice is supported by increasing evidence that attests not only to the safety but also to the additional benefits of statin therapy for these population groups.
Acknowledgments
The first author thanks Joshua Trabin, MD, for editorial assistance in the preparation of the submitted manuscript.
REFERENCES
- 1.Centers for Disease Control and Prevention (CDC) Leading causes of death. FastStats Web site. http://www.cdc.gov/nchs/FASTATS/lcod.htm. http://www.cdc.gov/nchs/FASTATS/lcod.htm Accessed February 2, 2010.
- 2.Grundy SM, Cleeman JI, Bairey CN, et al. Coordinating Committee of the National Cholesterol Education Program, Endorsed by the National Heart, Lung, and Blood Institute, American College of Cardiology Foundation, and American Heart Association Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines [published correction appears in Circulation. 2004;110(6):763] Circulation 2004;110(2):227-239 [DOI] [PubMed] [Google Scholar]
- 3.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285(19):2486-2497 [DOI] [PubMed] [Google Scholar]
- 4.Pedersen TR, Kieksus J, Berg K, et al. Scandinavian Simvastatin Survival Study Group Randomized trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344(8934):1383-1389 [PubMed] [Google Scholar]
- 5.Stein EA. The power of statins: aggressive lipid lowering. Clin Cardiol. 2003;26(4)(suppl 3):III25-III31 [DOI] [PubMed] [Google Scholar]
- 6.Forrester JS, Libby P. The inflammation hypothesis and its potential relevance to statin therapy. Am J Cardiol. 2007;99(5):732-738 [DOI] [PubMed] [Google Scholar]
- 7.Argo CK, Loria P, Caldwell SH, Lonardo A. Statins in liver disease: a molehill, an iceberg, or neither? Hepatology 2008;48(2):662-669 [DOI] [PubMed] [Google Scholar]
- 8.Bhardwaj SS, Chalasani N. Lipid lowering agents that cause drug induced hepatotoxicity. Clin Liver Dis. 2007;11(3):597-613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang CY, Schiano TD. Drug hepatotoxicity. Aliment Pharmacol Ther. 2007;25(10):1135-1151 [DOI] [PubMed] [Google Scholar]
- 10.Tolman KG. The liver and lovastatin. Am J Cardiol. 2002;89(12):1374-1380 [DOI] [PubMed] [Google Scholar]
- 11.Dujovne CA. Side effects of statins: hepatitis versus “transaminitis”–myositis versus CPKitis. Am J Cardiol. 2002;89(12):1411-1413 [DOI] [PubMed] [Google Scholar]
- 12.Charles EC, Olson KL, Sandhoff BJ, McClure DL, Merenich JA. Evaluation of cases of severe statin–related transaminitis within a large health maintenance organization. Am J Med. 2005;118(6):618-624 [DOI] [PubMed] [Google Scholar]
- 13.Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97(8A):52C-60C [DOI] [PubMed] [Google Scholar]
- 14.Chalasani N. Statins and hepatotoxicity: focus on patients with fatty liver. Hepatology 2005;41(4):690-695 [DOI] [PubMed] [Google Scholar]
- 15.Alla V, Abraham J, Siddiqui J, et al. Autoimmune hepatitis triggered by statins. J Clin Gastroenterol. 2006;40(8):757-761 [DOI] [PubMed] [Google Scholar]
- 16.Pelli N, Setti M, Ceppa P, Toncini C, Indiveri F. Autoimmune hepatitis revealed by atorvastatin. Eur J Gastroenterol Hepatol. 2003;15(8):921-924 [DOI] [PubMed] [Google Scholar]
- 17.Onofrei MD, Butler KL, Fuke DC, Miller HB. Safety of statin therapy in patients with preexisting liver disease. Pharmacotherapy 2008;28(4):522-529 [DOI] [PubMed] [Google Scholar]
- 18.Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug induced liver injury in the United States. Liver Transpl. 2004;10(8):1018-1023 [DOI] [PubMed] [Google Scholar]
- 19.US Food and Drug Administration (FDA) PhRMA/FDA/ASSLD drug induced hepatotoxicity white paper post marketing considerations: November 2000. http://www.fda.gov/downloads/Drugs/ScienceResearch/ResearchAreas/ucm091462.pdf. http://www.fda.gov/downloads/Drugs/ScienceResearch/ResearchAreas/ucm091462.pdf Accessed February 2, 2010.
- 20.Senior JR. Monitoring for hepatotoxicity: what is the predictive value of liver function tests? Clin Pharmacol Ther. 2009;85(3):331-334 [DOI] [PubMed] [Google Scholar]
- 21.US Department of Health and Human Services. Food and Drug Administration (FDA) Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) Guidance for industry: drug-induced liver injury: premarketing clinical evaluation Published July2009. http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-drugs-gen/documents/document/ucm174090.pdf Accessed February 2, 2010
- 22.Vuppalanchi R, Teal E, Chalasani N. Patients with elevated baseline liver enzymes do not have higher frequency of hepatotoxicity from lovastatin than those with normal baseline liver enzymes. Am J Med Sci. 2005;329(2):62-65 [DOI] [PubMed] [Google Scholar]
- 23.McKenney JM, Davidson MH, Jacobson TA, Guyton JR. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97(8A):89C-94C [DOI] [PubMed] [Google Scholar]
- 24.Cohen DE, Anania FA, Chalasani N, National Lipid Association Statin Safety Task Force. Liver Expert Panel An assessment of statin safety by hepatologists. Am J Cardiol. 2006;97(8A):77C-81C [DOI] [PubMed] [Google Scholar]
- 25.Pasternak RC, Smith SC, Jr, Bairey-Merz CN, Grundy SM, Cleeman JI, Lenfant C. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. Circulation 2002;106(8):1024-1028 [DOI] [PubMed] [Google Scholar]
- 26.Ridker PM, Danielson E, Fonseca FA, et al. JUPITER Study Group Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195-2207 [DOI] [PubMed] [Google Scholar]
- 27.Stein EA, Amerena J, Ballantyne CM, et al. Long term efficacy and safety of rosuvastatin 40 mg in patients with severe hypercholesterolemia. Am J Cardiol. 2007;100(9):1387-1396 [DOI] [PubMed] [Google Scholar]
- 28.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high risk individuals: a randomized placebo-controlled trial. Lancet 2002;360(9326):7-22 [DOI] [PubMed] [Google Scholar]
- 29.Pfeffer MA, Keech A, Sacks FM, et al. Safety and tolerability of pravastatin in long-term clinical trials: Prospective Pravastatin Pooling (PPP) Project. Circulation 2002;105(20):2341-2346 [DOI] [PubMed] [Google Scholar]
- 30.De Denus S, Spinler SA, Miller K, Peterson AM. Statins and liver toxicity: a meta-analysis. Pharmacotherapy 2004;24(5):584-591 [DOI] [PubMed] [Google Scholar]
- 31.Armitage J. The safety of statins in clinical practice. Lancet 2007;370(9601):1781-1790 [DOI] [PubMed] [Google Scholar]
- 32.Newman C, Tsai J, Szarek M, Luo D, Gibson E. Comparative safety of atorvastatin 80 mg versus 10 mg derived from analysis of 49 complete trials in 14,236 patients. Am J Cardiol. 2006;97:61-67 [DOI] [PubMed] [Google Scholar]
- 33.Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. JAMA 1998;279(20):1615-1622 [DOI] [PubMed] [Google Scholar]
- 34.Bradford RH, Shear CL, Chremos AN, et al. Expanded Clinical Evaluation of Lovastatin (EXCEL) Study results: two year efficacy and safety follow-up. Am J Cardiol. 1994;74(7):667-673 [DOI] [PubMed] [Google Scholar]
- 35.Bays H. Statin safety: an overview and assessment of the data–2005. Am J Cardiol. 2006;97(8A):6C-26C [DOI] [PubMed] [Google Scholar]
- 36.Jacobson TA. Statin safety: lessons from new drug applications for marketed statins. Am J Cardiol. 2006;97(8A):44C-51C [DOI] [PubMed] [Google Scholar]
- 37.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98(5):960-967 [DOI] [PubMed] [Google Scholar]
- 38.Ekstedt M, Franzen L, Mathiesen UL, Holmgvist M, Bodemar G, Kechagias S. Statins in non-alcoholic fatty liver disease and chronically elevated liver enzymes: a hystopathological follow-up study. J Hepatol. 2007;47(1):135-141 [DOI] [PubMed] [Google Scholar]
- 39.Browning JD. Statins and hepatic steatosis: perspectives from the Dallas Heart Study. Hepatology 2006;44(2):466-471 [DOI] [PubMed] [Google Scholar]
- 40.Chalasani N, Aljadhey H, Kesterson J, Murray MD, Hall SD. Patients with elevated liver enzymes are not at higher risk from statin hepatotoxicity. Gastroenterology 2004;126(5):1287-1292 [DOI] [PubMed] [Google Scholar]
- 41.Vuppalanchi R, Chalasani N. Statins for hyperlipidemia in patients with chronic liver disease: are they safe? Clin Gastroenterol Hepatol. 2006;4(7):838-839 [DOI] [PubMed] [Google Scholar]
- 42.Rallidis LS, Drakoulis CK, Parasi AS. Pravastatin in patients with nonalcoholic steatohepatitis: results of a pilot study. Atherosclerosis 2004;174(1):193-196 [DOI] [PubMed] [Google Scholar]
- 43.Centers for Disease Control and Prevention (CDC) Viral hepatitis topics: statistics and surveillance. http://www.cdc.gov/hepatitis/Statistics.htm#section1. http://www.cdc.gov/hepatitis/Statistics.htm#section1 Accessed February 2, 2010.
- 44.Khorashadi S, Hasson NK, Cheung RC. Incidence of statin hepatotoxicity in patients with hepatitis C. Clin Gastroenterol Hepatol. 2006;4(7):902-907 [DOI] [PubMed] [Google Scholar]
- 45.Gibson K, Rindone JP. Experience with statin use in patients with chronic hepatitis C infection. Am J Cardiol. 2005;96(9):1278-1279 [DOI] [PubMed] [Google Scholar]
- 46.Lonardo A, Loria P, Carulli N. Dysmetabolic changes associated with HCV: a distinct syndrome? Intern Emerg Med. 2008;3(2):99-108 [DOI] [PubMed] [Google Scholar]
- 47.Bader T, Fazili J, Madhoun M, et al. Fluvastatin inhibits hepatitis C replication in humans. Am J Gastroenterol. 2008;103(6):1383-1389 [DOI] [PubMed] [Google Scholar]
- 48.Ye J, Wang C, Sumpter R, Jr, Brown MS, Goldstein JL, Gale M., Jr Disruption of hepatitis C virus RNA replication through inhibition of host protein geranylgeranylation. Proc Natl Acad Sci U S A 2003;100(26):15865-15870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ritzel U, Leonhardt U, Nather M, Schafer G, Armstrong VW, Ramadori G. Simvastatin in primary biliary cirrhosis: effects on serum lipids and distinct disease markers. J Hepatol. 2002;36(4):454-458 [DOI] [PubMed] [Google Scholar]
- 50.Sorokin A, Brown JL, Thompson PD. Primary biliary cirrhosis, hyperlipidemia, and atherosclerotic risk: a systematic review. Atherosclerosis 2007;194(2):293-299 [DOI] [PubMed] [Google Scholar]
- 51.El-Serag HB, Johnson ML, Hachem C, Morgana RO. Statins are associated with a reduced risk of hepatocellular carcinoma in a large cohort of patients with diabetes. Gastroenterology 2009;136(5):1601-1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zachoval R, Gerbes AL, Schwandt P, Parhofer KG. Short-term effects of statin therapy in patients with hyperlipoproteinemia after liver transplantation: results of a randomized cross-over trial. J Hepatol. 2001;35(1):86-91 [DOI] [PubMed] [Google Scholar]
- 53.Anfossi G, Massucco P, Bonomo K, Trovati M. Prescription of statins to dyslipidemic patients affected by liver diseases: a subtle balance between risks and benefits. Nutr Metab Cardiovasc Dis. 2004;14(4):215-224 [DOI] [PubMed] [Google Scholar]
- 54.Russo MW, Jacobson IM. How to use statins in patients with chronic liver disease. Cleve Clin J Med. 2004;71(1):58-62 [DOI] [PubMed] [Google Scholar]
- 55.Simonson SG, Martin PD, Mitchell P, Schneck DW, Lasseter KC, Warwick MJ. Pharmacokinetics and pharmacodynamics of rosuvastatin in subjects with hepatic impairment. Eur J Clin Pharmacol. 2003;58(10):669-675 [DOI] [PubMed] [Google Scholar]
- 56.Lewis JH, Mortensen ME, Zweig S, Fusco MJ, Medoff JR, Belder R, Pravastatin in Chronic Liver Disease Study Investigators Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology 2007;46(5):1456-1463 [DOI] [PubMed] [Google Scholar]