Abstract
Many options are available to clinicians for the noninvasive evaluation of the cardiovascular system and patient concerns about chest discomfort. Cardiac computed tomography (CT) is a rapidly advancing field of noninvasive imaging. Computed tomography incorporates coronary artery calcium scoring, coronary angiography, ventricular functional analysis, and information about noncardiac thoracic anatomy. We searched the PubMed database and Google from inception to September 2009 for resources on the accuracy, risk, and predictive capacity of coronary artery calcium scoring and CT coronary angiography and have reviewed them herein. Cardiac CT provides diagnostic information comparable to echocardiography, nuclear myocardial perfusion imaging, positron emission tomography, and magnetic resonance imaging. A cardiac CT study can be completed in minutes. In patients with a nondiagnostic stress test result, cardiac CT can preclude the need for invasive angiography. Prognostic information portends excellent outcomes in patients with normal study results. Use of cardiac CT can reduce health care costs and length of emergency department stays for patients with chest pain. Cardiac CT examination provides clinically relevant information at a radiation dose similar to well-established technologies, such as nuclear myocardial perfusion imaging. Advances in technique can reduce radiation dose by 90%. With appropriate patient selection, cardiac CT can accurately diagnose heart disease, markedly decrease health care costs, and reliably predict clinical outcomes.
CAC = coronary artery calcium; CAD = coronary artery disease; CT = computed tomography; CTA = coronary computed tomographic angiography; EBCT = electron beam CT; ED = emergency department; ICA = invasive coronary angiography; MDCT = multidetector helical CT; MI = myocardial infarction; MPI = myocardial perfusion imaging; NPV = negative predictive value; PPV = positive predictive value
Cardiac computed tomography (CT) is a rapidly evolving technology for the noninvasive evaluation of the cardiovascular system. Numerous potential roles for cardiac CT have been developed recently, such as investigating anomalous coronary arteries, evaluating for pulmonary vein stenoses, and preparing for repeated coronary artery bypass grafting. However, the indication of most interest to the public and physicians is evaluating patients for native vessel coronary artery disease (CAD) using coronary artery calcium (CAC) scoring and coronary computed tomographic angiography (CTA).
We searched the PubMed database and Google, from inception to September 2009, for keywords coronary artery calcium, coronary CT angiography, and radiation risk to identify information sources of interest. We also searched references in other review articles. From Google, we selected publications from trusted sources, such as the Food and Drug Administration and the National Academy of Sciences. From PubMed, we selected articles about test performance characteristics based on the quality of their methods, preferentially using randomized controlled trial data. We selected articles about clinical outcomes from randomized trials when available and from large cohorts as secondary sources. The purpose of this review is to summarize the recent data regarding accuracy, sensitivity, and specificity of CTA and the responsible use of cardiac CT.
CT TECHNOLOGY
Two types of CT scanners are available for imaging the heart. The first is electron beam CT (EBCT), which is an older technology infrequently used today. The second is multidetector helical CT (MDCT), which represents most CT use. Electron beam CT does not use a mechanical rotating gantry. Instead, an electron gun generates electrons, which are then electromagnetically steered across a stationary tungsten anode.1 Although EBCT has excellent temporal resolution,2 its spatial resolution is markedly less than that of MDCT, resulting in a notable decrease in the use of EBCT. The newest MDCT scanner section thickness is 0.4 mm, allowing reliable imaging of small structures, such as coronary arteries. For comparison, the crystal thickness of nuclear myocardial single-photon emission CT is 4 to 10 mm.
For editorial comment, see page 309
State-of-the-art MDCT scanners use slip-ring rotating gantries that can revolve around the patient in 350 milliseconds or less. Only half rotation is required to generate tomograms; therefore, the heart can be imaged in 175 milliseconds or less. Another solution to the temporal resolution problem is the development of dual-source CT scanners. These instruments use 2 radiation sources and detectors at 90°, reducing the temporal resolution further, to approximately 83 milliseconds or less.3 These times are sufficiently short to image the heart during diastole for most patients. β-Blockade (typically with β1-selective agents) can be used to decrease the heart rate when necessary. Dual-source CT configurations and other high-temporal-resolution CT software configurations make it feasible to even perform imaging on some patients with dysrhythmias, such as atrial fibrillation.4
To image the heart, it is necessary to synchronize the imaging process with the cardiac cycle. Two general techniques to accomplish this are prospective triggering and retrospective gating, which use an electrocardiogram for reference. Diastole is the optimal time to acquire images of the coronary arteries because the heart is nearly motionless. For information about ventricular function, the entire cardiac cycle must be imaged.
With prospective gating, MDCT predicts when diastole will occur and turns on the x-ray source after a preselected delay. The x-ray source remains on for only a brief period and is turned off before the next QRS complex. This brief x-ray pulse is sufficient to image the entire heart during a single heartbeat using the newest MDCT scanners. Prospective gating can reduce the radiation exposure by up to 90% compared with retrospective gating. With retrospective gating, the x-ray tube remains on throughout the entire cardiac cycle and for as many heartbeats as is required to image the entire heart. After data acquisition, images are reconstructed to represent any desired part of the cardiac cycle.
CLINICAL USES OF CARDIAC CT
Cardiac CT is a term encompassing several tests, including CAC scoring, CTA, ventricular function analysis, and structural analysis of masses, congenital defects, bypass grafts, and reconstructive procedures. Our review focuses on the assessment of CAD with CAC and CTA.
CAC Scoring
Calcium is frequently present in coronary atherosclerotic lesions and can be detected by any radiographic study; however, CT is the most sensitive. Hounsfield units are the standard measure of density in CT. Hounsfield units range from −1000 (air) to well over 1000 (cortical bone), and they are an indication of the density of tissues. Lesions greater than 130 Hounsfield units are attributed to coronary calcium. Agatston scoring is the most widely used method for quantifying coronary artery calcifications as seen on CT.5 The score is derived by examining 3-mm-thick axial tomograms, identifying coronary plaques with calcium, and multiplying the plaque area by a weighting factor, which is determined by the maximum calcium lesion density. The other method commonly used is the volume method, which has been described as having better reproducibility and less variability.6 One of the original uses of EBCT was for CAC scoring, although modern MDCT scanners are comparable to EBCT for CAC scoring.7,8
Overall, the presence of CAC is highly sensitive and moderately specific for detection of CAD. The negative predictive value (NPV) of a CAC score of 0 can be as high as 99% and is associated with a 0.1% annual risk of cardiovascular events9,10 and 99.4% survival for 10 years.11 However, because of CAC's low specificity, its overall diagnostic accuracy is approximately 70%, and the test is inadequate as a single assessment for establishing the diagnosis of CAD.12
Scoring of CAC has been most thoroughly evaluated in asymptomatic patients with intermediate risk of major adverse cardiovascular events as predicted by the Framingham risk score. In this population, the risk of coronary heart disease death or myocardial infarction (MI) is 0.4% with a CAC score of 0 through 99, 1.3% with a CAC score of 100 through 399, and 2.4% with a CAC score of 400 or greater.13 In addition to predicting the risk of a major adverse cardiovascular event, CAC scoring improves the area under the receiver operating characteristic curve for predicting major adverse cardiovascular events and is positively correlated with increasing hazard ratios with increasing tertiles of CAC score.14-18 The CAC scores predict risk in all ethnic groups19 despite the fact that the absolute CAC scores for the 90th percentile are different.20 A study encompassing 20,000 patient-years of follow-up showed that all patients with an Agatston score greater than 100 had a relative risk increase of 9.2 for nonfatal MI or death and those with a score higher than 400 had a relative risk increase of 26.2. Agatston scores of 0, 1 through 99, 100 through 399, and 400 or higher correlated with total event (coronary death, nonfatal MI, bypass surgery, or angioplasty) rates of 0.54%, 1%, 5.5%, and 14%, respectively.17
Coronary Computed Tomographic Angiography
Coronary computed tomographic angiography is a noninvasive anatomic assessment of CAD. In determining its optimal role in cardiac imaging and risk assessment, experts have compared it with other noninvasive tests, with anatomic tests, and in unique clinical environments. As with other diagnostic tests, CTA has also been studied for its capacity to predict future cardiovascular events.
Noninvasive testing is widely used for diagnosing CAD, and clinicians have several options to select from. Although CTA investigates for CAD based on anatomy rather than function, such as with dobutamine stress echocardiography, nuclear myocardial perfusion imaging (MPI), and positron emission tomography, the overall performance of these tests at accurately diagnosing the presence of CAD is similar. Nixdorff et al21 studied 71 patients with dobutamine stress echocardiography and CTA. The positive and negative likelihood ratios for dobutamine stress echocardiography were 4.37 and 0.36 compared with 3.50 and 0.11 for CTA, respectively. Budoff et al22 studied 30 patients, and the sensitivity and specificity of CTA (94% and 96%, respectively) were higher than for nuclear MPI (81% and 78%, respectively). Gaemperli et al23 studied 78 patients (24% with known CAD) referred for invasive coronary angiography (ICA) with CTA and MPI. Both CTA and ICA performed similarly at predicting reversible ischemia detected on nuclear MPI. The area under the receiver operating characteristic curve was 0.88 for CTA and 0.87 for ICA. Chow et al24 found that CTA was more sensitive and specific than rubidium 82 positron emission tomography. Sato et al25 studied 104 patients with CTA and thallium MPI. As with other studies, the NPV of CTA was excellent (99% at >60% stenosis and 96% at >70% stenosis). Ravipati et al26 studied 47 patients with CTA and MPI. The positive predictive value (PPV) and NPV of CTA were 92% and 100%, respectively, in this population. The MPI performed markedly worse, with a PPV of 78% and an NPV of 28%.
Coronary computed tomographic angiography has been compared directly to ICA, which is the most commonly used anatomic test for CAD. In 4 studies of patients referred for ICA, CTA demonstrated an NPV of 95% to 100%, even in patients with markedly elevated CAC scores.27-30 In the CORE-64 (Coronary Artery Evaluation Using 64-Row Multidetector Computed Tomography Angiography) study, 266 patients from 9 centers worldwide underwent CTA before ICA. Patients with Agatston scores higher than 600 were excluded. The NPV of CTA was less than that reported in other studies (83%); however, CTA performed the same as quantitative coronary angiography for predicting the need for percutaneous coronary intervention or coronary artery bypass grafting in the subsequent 30 days (area under the receiver operating characteristic curve of 0.84 and 0.82, respectively).31 In summary, in patients with high pretest likelihood of CAD, such as those referred for ICA, the predictive value of a negative test result is excellent. The specificity of CTA (90% in CORE-64 study) is inadequate to replace diagnostic ICA at this time.
In addition to these comparisons to existing imaging modalities, CTA has distinct advantages in some unique clinical situations. Coronary anomalies can be diagnosed with ICA; however, volume scanning with CT allows reproduction of an image from camera angles not permitted by the limits of ICA, making CT highly accurate for this diagnosis.32 Identification of noncalcified plaque, vessel remodeling, and spotty calcification has been demonstrated with CTA.33 Low-attenuation plaque has been shown to be predictive of future acute coronary syndromes, with 76% specificity and a hazard ratio of 22.8 at 24 months of follow-up.34
The performance of CTA has been studied in specific patient environments, such as the emergency department (ED). In the ED, CT can differentiate patients with acute chest pain with and without CAD. Gallagher et al35 performed a prospective evaluation of 85 patients with CTA and MPI. In these patients, the NPV was 99% with CTA and 97% with MPI. No major adverse cardiac events occurred in 30 days of follow-up in patients who had been evaluated in the ED. Goldstein et al36 analyzed the cost-benefit of using 64-section MDCT in the ED; 197 patients were randomized to undergo CTA or nuclear MPI in addition to standard care. Mean duration of stay in the ED was 3.4 hours for the CTA group and 15 hours for the MPI group, with an average savings of nearly $300 per patient by using CTA. No adverse cardiovascular events occurred in either group after 6 months of follow-up.
Data demonstrate that CTA can predict patient outcomes. Gopal et al37 described 493 patients who underwent CTA with 100% follow-up during a mean ± SD of 40±9 months. No MI, stroke, cardiac hospitalization, or death occurred in patients whose CTA demonstrated absent or nonobstructive CAD (no lesion >50% stenotic). Survival was 79% in patients with obstructive CAD. Hadamitzky et al38 studied 1256 consecutive patients prospectively for 18 months. They found that the presence of obstructive CAD on CTA was associated with an odds ratio of 17.3 of severe cardiac events (cardiac death, MI, or unstable angina). In patients without CAD, the rate of cardiac events was markedly lower than was predicted by Framingham risk estimation.
In summary, CTA provides useful diagnostic and prognostic information in a variety of patient populations. The accuracy of CTA is similar to many other diagnostic studies, and CT can be used as a tool to avoid unnecessary risks of invasive tests, such as ICA.
RADIATION RISK ASSOCIATED WITH CARDIAC CT
Perhaps the most often discussed drawback of cardiac CT imaging modalities is concern about radiation exposure. Discussion of this topic is difficult because of confusing terms, imprecise measures of radiation dose, and limited evidence to accurately estimate future risk from exposure. The most commonly used term in the medical imaging literature to reflect the biological risk associated with exposure to ionizing radiation is the effective dose, which is expressed in units of millisieverts (equivalent to millijoules absorbed per kilogram of tissue).39 The effective dose is a mathematical concept used to describe how the energy delivered to an organ can be extrapolated to a whole-body estimate of harm. The effective dose was originally intended as a measure of how to estimate risk from radiation exposure in people who have occupational exposure to radiation sources. It has been adopted by researchers to compare radiation doses from various medical imaging modalities. Despite its widespread use, the effective dose should be considered applicable only for aggregate comparisons, not as risk attributable to a single patient undergoing a single scan.
Although the effective dose is a relatively interchangeable value for measuring radiation dose, the dose in similar CTAs varies considerably. A recent analysis of radiation dose from cardiac CT in 1965 patients from 50 medical centers demonstrated a 6-fold variation in median dose among sites. Marked variability existed even when performed on the same scanner in the same institution.40 Most patients in that study (94%) underwent a retrospectively gated CT scan. Use of a prospective gating, dose-limiting scanning protocol was observed to markedly reduce radiation dose by 76%. In conjunction with other previously noted dose reduction strategies, radiation can be reduced to the range of diagnostic ICA.41 Other reasons for the observed variation include patient characteristics, such as weight and heart rate.
Even with reduced doses, the ultimate question is assessing the risk associated with small doses of radiation. Data exist on large single doses from a nuclear explosion or accident, but these data are difficult to extrapolate to the small repeated doses used in medical imaging. The largest epidemiological study to date, with more than 5,192,710 person-years of follow-up in people exposed to workplace radiation, concluded that a dose-response relationship exists between radiation exposure and some types of cancer. However, the authors further concluded that the study could not assess the effect of very low doses (<10 mSv).42 An extensive review of the literature along with point estimates of risk for various types of cancers has been published by the National Academy of Sciences.39 A similar review intended for a general public audience is hosted on the Food and Drug Administration Web site.43
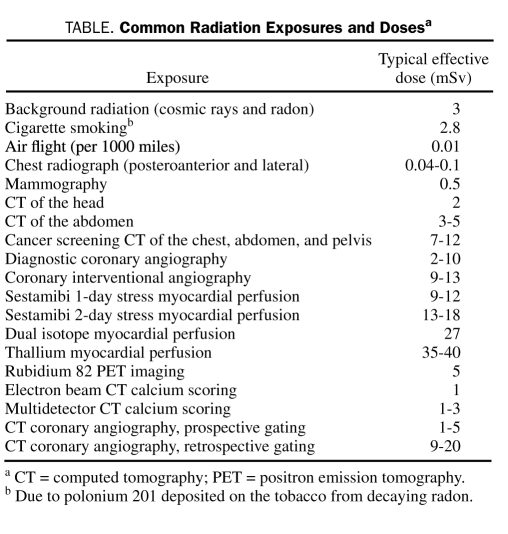
All this information about the risk of medical imaging must be considered in the context of other radiation exposures. All people experience an average exposure of 3 mSv per year from cosmic radiation, radon, and other naturally occurring radiation sources (range, 1-10 mSv).44 Background radiation is just one of many environmental and medical sources of radiation exposure41,45-55 (Table). However, little objection is raised regarding the use of technetium-based nuclear MPI, despite the fact that the radiation dose from MPI is similar to or greater than the dose from a newer-generation cardiac CT study. If proper radiation control measures are used, a prospectively gated CTA causes less radiation than some people receive annually from the background and is one-tenth the dose from a thallium-based MPI study.
TABLE.
Common Radiation Exposures and Dosesa
Finally, although radiation from medical imaging introduces certain risks, the failure to diagnose important disease introduces separate and distinct risks for patients. Balancing these risks is the key to responsible use of all diagnostic tests, including cardiac CT.
SELECTING THE APPROPRIATE PATIENT FOR CTA
The American College of Cardiology, American College of Radiology, and several other subspecialty societies have outlined criteria for appropriate use of cardiac CT.56 Physicians from the participating groups reviewed potential indications for cardiac CT and rated the indications based on a balance between evidence of and experience with cardiac CT; these indications were based on the Delphi method from the RAND corporation.57 The 2006 appropriateness criteria did not recommend routine use of CTA for the investigation of CAD; however, the clinical experience and use of CTA have markedly expanded since that time, and the criteria are currently being revised.
ILLUSTRATIVE CASES
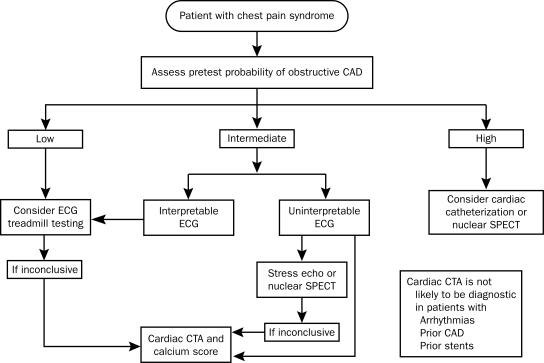
To illustrate how CTA might be used for patients encountered in a general medical setting, we describe 3 patients who may warrant evaluation for cardiovascular disease. Key to deciding whether CTA is appropriate is to know whether the patient has already been diagnosed as having CAD and determining the pretest probability of making the diagnosis of CAD. The authors of the appropriateness criteria used the quality of patients' symptoms, their age, and their sex to determine their pretest probability, as described in the American College of Cardiology/American Heart Association Guideline Update on Exercise Stress Testing58 (Figure).
FIGURE.
Responsible use of cardiac computed tomography in evaluating chest pain. This flow diagram incorporates coronary computed tomographic angiography (CTA) in a suggested framework for evaluating a patient with acute chest pain. See the American Heart Association criteria for further detail on the assessment of pretest probability.58 CAD = coronary artery disease; ECG = electrocardiogram; echo = echocardiography; SPECT = single-photon emission computed tomography.
Data from J Am Coll Radiol.56
Case 1
This patient is a 45-year-old male advertising executive whose father died of an MI at the age of 72 years. His mother has diabetes mellitus and stage 3 chronic kidney disease. The patient has hypertension, which is currently being treated with 25 mg of hydrochlorothiazide. He has experienced no chest pain and would like to know about his risk of cardiovascular disease.
It is not uncommon for the “worried well” to read about new medical technology or new laboratory tests and believe that additional testing is superior to less testing. However, we know that, in patient populations with very low pretest probability, the likelihood of a false-positive test result can be a serious concern if it then leads to further, sometimes invasive, testing, which unnecessarily exposes patients to more risk. Because this patient has no symptoms, his pretest probability of CAD is 5% to 10%.58 Because his pretest probability is so low, CTA would be inappropriate; however, CAC scoring may be appropriate. If the patient's total CAC score is 0, his annual risk of a cardiovascular event would be approximately 0.1%, whereas his risk of death is less than 0.4% throughout the following 10 years. The patient can simply be reassured of his favorable risk profile. However, if his total CAC score were elevated (any value greater than zero), it would be appropriate to consider primary prevention with aggressive medical therapy. Further review of this practice has been published.59
Case 2
This patient is a 63-year-old man who continues to smoke despite a history of MI treated with a drug-eluting stent 2 years ago. During the past 3 months, he has had a progressive increase in dyspnea when working on his farm.
We know that this patient already had CAD, and his symptoms are concerning for progressive angina. The details of his symptoms would suggest that cardiac catheterization or a noninvasive analysis of myocardial perfusion is necessary. As noted previously, CTA compares favorably with nuclear MPI; however, those studies were conducted in patients without known CAD. Because CTA is an anatomic evaluation and not a functional one, its role in this patient is limited. Studies of patients with prior coronary artery stenting have concluded that coronary segments with stents cannot be reliably imaged with current technology. Current CT technology does not allow accurate evaluation of myocardial perfusion or perfusion reserve, and so CTA would be inappropriate for this patient.
Case 3
This patient is a 52-year-old woman who has diabetes mellitus managed with diet and glyburide. Both her parents had MIs at unknown ages. She has no personal history of CAD but is concerned about recent exertional arm discomfort, which is relieved with rest. Painful osteoarthritis makes walking difficult.
This patient has symptoms of atypical angina (arm pain provoked with exertion and relieved by rest). Her age and sex predict an intermediate (10%-90%) pretest probability of CAD based on American College of Cardiology/American Heart Association guidelines.58 Her osteoarthritis limits her ability to exercise for stress testing. Coronary computed tomographic angiography would be an excellent noninvasive evaluation.
CONCLUSION
Cardiac CT, and particularly CTA, is a rapidly improving noninvasive imaging modality for assessment of many types of cardiac disease, especially CAD. The technology has limitations, particularly in patients with arrhythmias or prior CAD and stenting.
Coronary artery calcium scoring has excellent NPV, which is additive beyond traditional risk factor assessment, such as Framingham risk. Extensive data have demonstrated the prognostic value of CAC scoring. These data suggest that aggressive medical therapy for patients with high CAC scores (>400) is beneficial.60 Because the data are not conclusive, this treatment strategy is not part of current guidelines. If concern about CAD persists, other appropriate tests should be considered.
Coronary computed tomographic angiography has excellent NPV. Currently, it is not an adequate replacement for diagnostic catheter-based coronary angiography, and it does not reliably predict ischemia. Performance of CTA for diagnosing CAD is comparable, if not superior, to other noninvasive and functional imaging modalities. In addition, CTA can provide excellent diagnostic information for a reasonable radiation dose, although dose varies considerably in different centers with different equipment and protocols.
Coronary computed tomographic angiography has been shown to be a useful test in assessment of a variety of patients in multiple clinical settings. For the primary care physician and general medical physician, it can be a valuable test for evaluation of acute and chronic chest pain syndromes in patients with an intermediate likelihood of CAD.
REFERENCES
- 1.Gerber TC, Kuzo RS, Karstaedt N, et al. Current results and new developments of coronary angiography with use of contrast-enhanced computed tomography of the heart. Mayo Clin Proc. 2002;77(1):55-71 [DOI] [PubMed] [Google Scholar]
- 2.Lanzer P, Garrett J, Lipton MJ, et al. Quantitation of regional myocardial function by cine computed tomography: pharmacologic changes in wall thickness. J Am Coll Cardiol. 1986;8(3):682-692 [DOI] [PubMed] [Google Scholar]
- 3.Miller JC, Abbara S, Mamuya WS, Thrall JH, Uppot RN. Dual-source CT for cardiac imaging. J Am Coll Radiol. 2009;6(1):65-68 [DOI] [PubMed] [Google Scholar]
- 4.Oncel D, Oncel G, Tastan A. Effectiveness of dual-source CT coronary angiography for the evaluation of coronary artery disease in patients with atrial fibrillation: initial experience. Radiology 2007;245(3):703-711 [DOI] [PubMed] [Google Scholar]
- 5.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827-832 [DOI] [PubMed] [Google Scholar]
- 6.Callister TQ, Cooil B, Raya SP, Lippolis NJ, Russo DJ, Raggi P. Coronary artery disease: improved reproducibility of calcium scoring with an electron-beam CT volumetric method. Radiology 1998;208(3):807-814 [DOI] [PubMed] [Google Scholar]
- 7.Horiguchi J, Yamamoto H, Akiyama Y, Marukawa K, Hirai N, Ito K. Coronary artery calcium scoring using 16-MDCT and a retrospective ECG-gating reconstruction algorithm. AJR Am J Roentgenol. 2004;183(1):103-108 [DOI] [PubMed] [Google Scholar]
- 8.Goldin JG, Yoon HC, Greaser LE, III, et al. Spiral versus electron-beam CT for coronary artery calcium scoring. Radiology 2001;221(1):213-221 [DOI] [PubMed] [Google Scholar]
- 9.Haberl R, Becker A, Leber A, et al. Correlation of coronary calcification and angiographically documented stenoses in patients with suspected coronary artery disease: results of 1,764 patients. J Am Coll Cardiol. 2001;37(2):451-457 [DOI] [PubMed] [Google Scholar]
- 10.Sarwar A, Shaw LJ, Shapiro MD, et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging 2009;2(6):675-688 [DOI] [PubMed] [Google Scholar]
- 11.Budoff MJ, Shaw LJ, Liu ST, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49(18):1860-1870 [DOI] [PubMed] [Google Scholar]
- 12.O'Rourke RA, Brundage BH, Froelicher VF, et al. American College of Cardiology/American Heart Association expert consensus document on electron-beam computed tomography for the diagnosis and prognosis of coronary artery disease. Circulation 2000;102(1):126-140 [DOI] [PubMed] [Google Scholar]
- 13.Greenland P, Bonow RO, Brundage BH, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA writing committee to update the 2000 expert consensus document on electron beam computed tomography). Circulation 2007;115(3):402-426 [DOI] [PubMed] [Google Scholar]
- 14.Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA 2004;291(2):210-215 [DOI] [PubMed] [Google Scholar]
- 15.Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O'Malley PG. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol. 2005;46(5):807-814 [DOI] [PubMed] [Google Scholar]
- 16.Kondos GT, Hoff JA, Sevrukov A, et al. Electron-beam tomography coronary artery calcium and cardiac events: a 37-month follow-up of 5635 initially asymptomatic low- to intermediate-risk adults. Circulation 2003;107(20):2571-2576 [DOI] [PubMed] [Google Scholar]
- 17.Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis heart study. J Am Coll Cardiol. 2005;46(1):158-165 [DOI] [PubMed] [Google Scholar]
- 18.Budoff MJ, Nasir K, McClelland RL, et al. Coronary calcium predicts events better with absolute calcium scores than age-sex-race/ethnicity percentiles: MESA (multi-ethnic study of atherosclerosis). J Am Coll Cardiol. 2009;53(4):345-352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336-1345 [DOI] [PubMed] [Google Scholar]
- 20.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the multiethnic study of atherosclerosis (MESA). Circulation 2006;113(1):30-37 [DOI] [PubMed] [Google Scholar]
- 21.Nixdorff U, Kufner C, Achenbach S, et al. Head-to-head comparison of dobutamine stress echocardiography and cardiac computed tomography for the detection of significant coronary artery disease. Cardiology 2008;110(2):81-86 [DOI] [PubMed] [Google Scholar]
- 22.Budoff MJ, Rasouli ML, Shavelle DM, et al. Cardiac CT angiography (CTA) and nuclear myocardial perfusion imaging (MPI): a comparison in detecting significant coronary artery disease. Acad Radiol. 2007;14(3):252-257 [DOI] [PubMed] [Google Scholar]
- 23.Gaemperli O, Schepis T, Valenta I, et al. Functionally relevant coronary artery disease: comparison of 64-section CT angiography with myocardial perfusion SPECT. Radiology 2008;248(2):414-423 [DOI] [PubMed] [Google Scholar]
- 24.Chow BJ, Dennie C, Hoffmann U, et al. Comparison of computed tomographic angiography versus rubidium-82 positron emission tomography for the detection of patients with anatomical coronary artery disease. Can J Cardiol. 2007;23(10):801-807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato A, Hiroe M, Tamura M, et al. Quantitative measures of coronary stenosis severity by 64-slice CT angiography and relation to physiologic significance of perfusion in nonobese patients: comparison with stress myocardial perfusion imaging. J Nucl Med. 2008;49(4):564-572 [DOI] [PubMed] [Google Scholar]
- 26.Ravipati G, Aronow WS, Lai H, et al. Comparison of sensitivity, specificity, positive predictive value, and negative predictive value of stress testing versus 64-multislice coronary computed tomography angiography in predicting obstructive coronary artery disease diagnosed by coronary angiography. Am J Cardiol. 2008;101(6):774-775 [DOI] [PubMed] [Google Scholar]
- 27.Mollet NR, Cademartiri F, van Mieghem CA, et al. High-resolution spiral computed tomography coronary angiography in patients referred for diagnostic conventional coronary angiography. Circulation 2005;112(15):2318-2323 [DOI] [PubMed] [Google Scholar]
- 28.Pugliese F, Mollet NR, Runza G, et al. Diagnostic accuracy of non-invasive 64-slice CT coronary angiography in patients with stable angina pectoris. Eur Radiol. 2006;16(3):575-582 [DOI] [PubMed] [Google Scholar]
- 29.Ehara M, Surmely JF, Kawai M, et al. Diagnostic accuracy of 64-slice computed tomography for detecting angiographically significant coronary artery stenosis in an unselected consecutive patient population: comparison with conventional invasive angiography. Circ J. 2006;70(5):564-571 [DOI] [PubMed] [Google Scholar]
- 30.Ropers D, Rixe J, Anders K, et al. Usefulness of multidetector row spiral computed tomography with 64- x 0.6-mm collimation and 330-ms rotation for the noninvasive detection of significant coronary artery stenoses. Am J Cardiol. 2006;97(3):343-348 [DOI] [PubMed] [Google Scholar]
- 31.Miller JM, Rochitte CE, Dewey M, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008;359(22):2324-2336 [DOI] [PubMed] [Google Scholar]
- 32.Bluemke DA, Achenbach S, Budoff M, et al. Noninvasive coronary artery imaging: magnetic resonance angiography and multidetector computed tomography angiography: a scientific statement from the American Heart Association committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention, and the Councils on Clinical Cardiology and Cardiovascular Disease in the young. Circulation 2008;118(5):586-606 [DOI] [PubMed] [Google Scholar]
- 33.Kitagawa T, Yamamoto H, Horiguchi J, et al. Characterization of noncalcified coronary plaques and identification of culprit lesions in patients with acute coronary syndrome by 64-slice computed tomography. JACC Cardiovasc Imaging 2009;2(2):153-160 [DOI] [PubMed] [Google Scholar]
- 34.Motoyama S, Sarai M, Harigaya H, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009;54(1):49-57 [DOI] [PubMed] [Google Scholar]
- 35.Gallagher MJ, Ross MA, Raff GL, Goldstein JA, O'Neill WW, O'Neil B. The diagnostic accuracy of 64-slice computed tomography coronary angiography compared with stress nuclear imaging in emergency department low-risk chest pain patients. Ann Emerg Med. 2007;49(2):125-136 [DOI] [PubMed] [Google Scholar]
- 36.Goldstein JA, Gallagher MJ, O'Neill WW, Ross MA, O'Neil BJ, Raff GL. A randomized controlled trial of multi-slice coronary computed tomography for evaluation of acute chest pain. J Am Coll Cardiol. 2007;49(8):863-871 [DOI] [PubMed] [Google Scholar]
- 37.Gopal A, Nasir K, Ahmadi N, et al. Cardiac computed tomographic angiography in an outpatient setting: an analysis of clinical outcomes over a 40-month period. J Cardiovasc Comput Tomogr. 2009;3(2):90-95 [DOI] [PubMed] [Google Scholar]
- 38.Hadamitzky M, Freissmuth B, Meyer T, et al. Prognostic value of coronary computed tomographic angiography for prediction of cardiac events in patients with suspected coronary artery disease. JACC Cardiovasc Imaging 2009;2(4):404-411 [DOI] [PubMed] [Google Scholar]
- 39.Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation. Board on Radiation Effects Research. Division on Earth and Life Studies. National Research Council of the National Academies Health Risks From Exposure to Low Levels of Ionizing Radiation: BEIR VII-Phase 2 Washington, DC: National Academies Press; 2006. [PubMed] [Google Scholar]
- 40.Hausleiter J, Meyer T, Hermann F, et al. Estimated radiation dose associated with cardiac CT angiography. JAMA 2009;301(5):500-507 [DOI] [PubMed] [Google Scholar]
- 41.McCollough C, Cody D, Edyvean S, et al. American Association of Physicists in Medicine (AAPM) The measurement, reporting, and management of radiation dose in CT: report of AAPM task group 23 of the Diagnostic Imaging Council CT Committee 2008. No. 96 http://www.aapm.org/pubs/reports/rpt_96.pdf Accessed January 29, 2010
- 42.Cardis E, Vrijheid M, Blettner M, et al. The 15-country collaborative study of cancer risk among radiation workers in the nuclear industry: estimates of radiation-related cancer risks. Radiat Res. 2007;167(4):396-416 [DOI] [PubMed] [Google Scholar]
- 43.US Food and Drug Administration Radiation-emitting products: computed tomography (CT) http://www.fda.gov/cdrh/ct/risks.html. http://www.fda.gov/cdrh/ct/risks.html Accessed January 29, 2010.
- 44.United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) Sources and effects of ionizing radiation, volume 1: sources New York, NY: United Nations; 2000. http://www.unscear.org/unscear/en/publications/2000_1.html Accessed January 29, 2010 [Google Scholar]
- 45.Earls JP, Schrack EC. Prospectively gated low-dose CCTA: 24 months experience in more than 2,000 clinical cases. Int J Cardiovasc Imaging 2008;25(suppl 2):177-187 [Google Scholar]
- 46.Ware DE, Huda W, Mergo PJ, Litwiller AL. Radiation effective doses to patients undergoing abdominal CT examinations. Radiology 1999;210(3):645-650 [DOI] [PubMed] [Google Scholar]
- 47.Shrimpton PC, Hillier MC, Lewis MA, Dunn M. National survey of doses from CT in the UK: 2003. Br J Radiol. 2006;79(948):968-980 [DOI] [PubMed] [Google Scholar]
- 48.Liu H, Zhuo W, Chen B, Yi Y, Li D. Patient doses in different projections of conventional diagnostic X-ray examinations. Radiat Prot Dosimetry 2008;132(3):334-338 [DOI] [PubMed] [Google Scholar]
- 49.Thompson RC, Cullom SJ. Issues regarding radiation dosage of cardiac nuclear and radiography procedures. J Nucl Cardiol. 2006;13(1):19-23 [DOI] [PubMed] [Google Scholar]
- 50.Radiological Society of North America (RSNA) Safety: radiation exposure in X-ray examinations. RadiologyInfo Web site. http://www.radiologyinfo.org/en/safety/ http://www.radiologyinfo.org/en/safety/ Accessed February 2, 2010.
- 51.Betsou S, Efstathopoulos E, Katritsis D, Faulkner K, Panayiotakis G. Patient radiation doses during cardiac catheterization procedures. Br J Radiol. 1998;71(846):634-639 [DOI] [PubMed] [Google Scholar]
- 52.Mettler FA, Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 2008;248(1):254-263 [DOI] [PubMed] [Google Scholar]
- 53.Morin RL, Gerber TC, McCollough CH. Radiation dose in computed tomography of the heart. Circulation 2003;107(6):917-922 [DOI] [PubMed] [Google Scholar]
- 54.Gerber TC, Carr JJ, Arai AE, et al. Ionizing radiation in cardiac imaging: a science advisory from the American Heart Association Committee on Cardiac Imaging of the Council on Clinical Cardiology and Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention. Circulation 2009;119(7):1056-1065 [DOI] [PubMed] [Google Scholar]
- 55.Budoff MJ, Achenbach S, Blumenthal RS, et al. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation 2006;114(16):1761-1791 [DOI] [PubMed] [Google Scholar]
- 56.ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 Appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group. J Am Coll Radiol. 2006;3(10):751-771 [DOI] [PubMed] [Google Scholar]
- 57.Patel MR, Spertus JA, Brindis RG, et al. ACCF proposed method for evaluating the appropriateness of cardiovascular imaging. J Am Coll Cardiol. 2005;46(8):1606-1613 [DOI] [PubMed] [Google Scholar]
- 58.Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to update the 1997 exercise testing guidelines). J Am Coll Cardiol. 2002;40(8):1531-1540 [DOI] [PubMed] [Google Scholar]
- 59.Bonow RO. Clinical practice: should coronary calcium screening be used in cardiovascular prevention strategies? N Engl J Med. 2009;361(10):990-997 [DOI] [PubMed] [Google Scholar]
- 60.Arad Y, Spadaro LA, Roth M, Newstein D, Guerci AD. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: the St. Francis heart study randomized clinical trial. J Am Coll Cardiol. 2005;46(1):166-172 [DOI] [PubMed] [Google Scholar]