Abstract
Niacin is the most effective lipid-modifying agent for raising high-density lipoprotein cholesterol levels, but it also causes cutaneous vasodilation with flushing. To determine the frequency of flushing in clinical trials, as well as to delineate counseling and treatment approaches to prevent or manage flushing, a MEDLINE search was conducted of English-language literature from January 1, 1985, through April 7, 2009. This search used the title keywords niacin or nicotinic acid crossed with the Medical Subject Headings adverse effects and human. Niacin flushing is a receptor-mediated, mainly prostaglandin D2–driven phenomenon, the frequency, onset, and duration of which are largely determined by the distinct pharmacological and metabolic profiles of different niacin formulations. Subjective assessments include ratings of redness, warmth, itching, and tingling. In clinical trials, most (>60%) niacin users experienced mild or moderate flushing, which tended to decrease in frequency and severity with continued niacin treatment, even with advancing doses. Approximately 5% to 20% of patients discontinued treatment because of flushing. Flushing may be minimized by taking niacin with meals (or at bedtime with a low-fat snack), avoiding exacerbating factors (alcohol or hot beverages), and taking 325 mg of aspirin 30 minutes before niacin dosing. The current review advocates an initially slow niacin dose escalation from 0.5 to 1.0 g/d during 8 weeks and then from 1.0 to 2.0 g in a single titration step (if tolerated). Through effective counseling, treatment prophylaxis with aspirin, and careful dose escalation, adherence to niacin treatment can be improved significantly. Wider implementation of these measures should enable higher proportions of patients to reach sufficient niacin doses over time to prevent cardiovascular events.
GPR = Gi protein–coupled receptor; HDL-C = high-density lipoprotein cholesterol; NSAID = nonsteroidal anti-inflammatory drug; PG = prostaglandin; RCT = randomized controlled trial
Reducing low-density lipoprotein cholesterol levels is the primary treatment for preventing cardiovascular disease, largely because of robust evidence from randomized controlled trials (RCTs) involving statins.1,2 However, many patients optimally treated with statins have residual cardiovascular risk3 associated with low levels of high-density lipoprotein cholesterol (HDL-C), elevated levels of triglycerides, and a preponderance of small, dense (atherogenic) low-density lipoprotein particles.
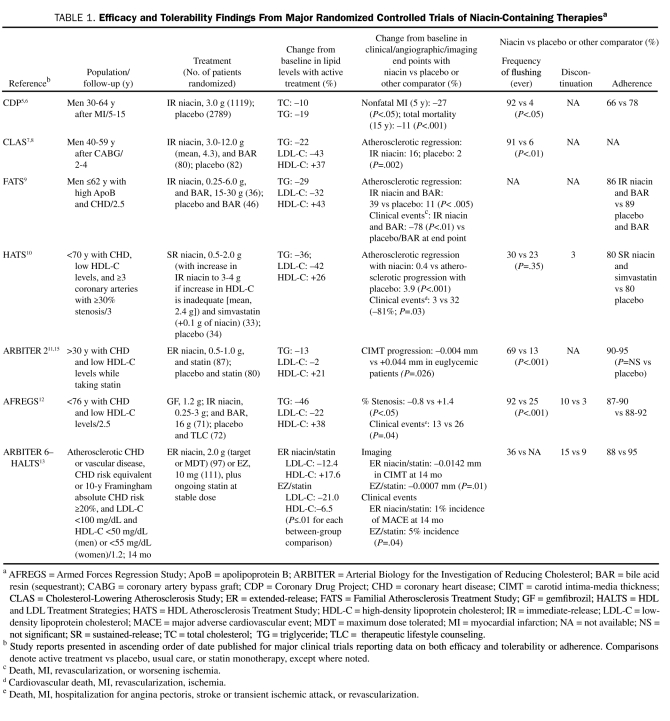
Niacin (3-pyridine-carboxylic acid; C6H5N02) is the most effective medication to raise HDL-C levels. A water-soluble B-complex (B3) vitamin used to treat pellagra, niacin was discovered to lower cholesterol levels at higher (gram) doses in 1955.4 It was also the first lipid-lowering therapy proven in RCTs to significantly lower (by 27%) the risk of myocardial infarction and the risk of all-cause mortality in the long term (by 11%),5,6 as well as confer significant angiographic benefits (Table 1).7,10-15
TABLE 1.
Efficacy and Tolerability Findings From Major Randomized Controlled Trials of Niacin-Containing Therapiesa
Niacin improves the entire lipid panel in patients with dyslipidemia, lowering apolipoprotein B–containing lipoproteins and raising apolipoprotein A–containing lipoproteins (eg, high-density lipoproteins).16 Putative mechanisms for these multidimensional lipid benefits involve interactions of niacin with its Gi protein–coupled receptor (GPR109A) in adipose tissue, reducing free fatty acid release.17,18
One potential obstacle to effective use of lipid therapies is suboptimal treatment adherence and long-term persistence.19-27 In recent studies, approximately 53% of niacin users did not reach recommended daily maintenance doses of 1.0 g or higher, 92% did not reach doses of 2 g, and flushing severity significantly predicted niacin treatment discontinuation.28,29 This systematic review (1) explores the pathophysiology of niacin flushing, (2) characterizes its typical clinical presentation, (3) assesses the approximate frequency of niacin flushing and of discontinuations ascribed to flushing in clinical trials, (4) explores potential strategies to prevent or minimize flushing, and (5) surveys ongoing controversies.
METHODS
An English-language MEDLINE search dating from January 1, 1985 (the year of a key active-comparator trial of immediate- and sustained-release niacin30), to April 7, 2009 (date of searches), was performed. This search used the title keyword niacin or nicotinic acid crossed (using the Boolean operator “AND”) with the Medical Subject Heading adverse effects and the Medical Subject Heading term human, also joined by the Boolean operator “AND.” These sets were crossed with the publication types of correspondence, case reports and series, and comments/editorials using the operator “NOT,” thereby excluding these types of publications. Also excluded using “NOT” were articles related to smoking (NOT: title keyword nicotin*, smok*, cigar*) or schizo* (the normal flushing response is blunted in many patients with schizophrenia). This search returned 205 citations, of which 56 are included in this review. Many of the remaining articles, including reviews and meta-analyses, were subjected to “saturation searches,” which produced additional articles of interest. Other articles were known to the author or identified by using title keywords, including flush*, vaso*, cutan*, prostagl*, Niasp*, and larop*. Other sources of data included full US-prescribing information for niacin-containing treatments and online reports from regulatory agencies.
OVERVIEW OF NIACIN FORMULATIONS
The earliest form of niacin was immediate-release niacin, which is also termed plain or crystalline niacin. Some formulations are available as dietary supplements, whereas others have Food and Drug Administration–approved labeling as lipid-modifying therapies (Niacor; Upsher-Smith, Minneapolis, MN; Nicolar, sanofi-aventis, Bridgewater, NJ).11 Early attempts to reformulate niacin largely to limit flushing resulted in sustained-release niacin, which releases niacin more slowly via combination with an inert (resinous) base.11 Examples include Slo-Niacin (Upsher-Smith) and Nicobid (sanofi-aventis). Endurance Products Company (Tigard, OR) manufactures niacin formulated in a wax matrix (ENDUR-ACIN). Finally, inositol hexani-cotinate (Niacinol; Integrative Therapeutics, Green Bay, WI) is a synthetic prodrug that consists of 6 molecules of niacin esterified to 1 molecule of inositol. Often advertised as “flush-free” niacin, it also appears to be “effect-free” because the esterified niacin is not bioavailable.31
Food and Drug Administration–approved, prescription niacin-containing treatments include extended-release niacin (Niaspan; Abbott Pharmaceuticals, Abbott Park, IL), extended-release niacin and lovastatin (Advicor; Abbott Pharmaceuticals), and extended-release niacin and simvaastatin (Simcor; Abbott Pharmaceuticals).32-34 Although the terms extended-release, sustained-release, slow-release, controlled-release, long-acting, and timed-release niacin all denote different forms of extended-release niacin products, for the purposes of this review, the term extended-release niacin is used only for references specifying any of these terms (or Niaspan, Advicor, or Simcor).
OVERVIEW OF PATHOPHYSIOLOGY OF NIACIN FLUSHING AND PHYSIOLOGY OF TOLERANCE
Interactions between niacin and GPR109A receptors on epidermal Langerhans cells result in increased cytosolic calcium; this increase triggers increases in phospholipase A2 activity and expression of prostaglandin (PG) D2 synthase, with elevated mobilization of arachidonic acid into vasodilator eicosanoids and prostanoids.35-37 Lipid-lowering doses of niacin are associated with 430- to 800-fold increases in circulating levels of a stable PGD2 metabolite (9-α-11-β-PGF2).38 PGD2 binds to DP1 receptors on local capillary smooth muscle cells,37,39 causing capillary vasodilation in the papillae of the upper dermis.40 Acetylsalicylic acid (aspirin) and nonsteroidal anti-inflammatory drugs (NSAIDs) can prevent or blunt flushing by blocking PGD2 synthesis via cyclooxygenase (chiefly, cyclooxygenase 1). However, other PGs (eg, PGE1), other mediators (eg, serotonin), and other signaling pathways may also be involved. (An elaborate consideration of mechanism exceeds the scope of this review, which is clinically focused and hence includes only human studies.)
NIACIN ABSORPTION KINETICS AND THE RISK OF FLUSHING
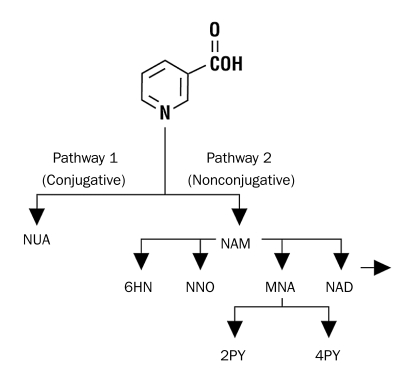
The pharmacodynamic effects of niacin are linked to its hepatic metabolism via 1 of 2 saturable pathways41,42 (Figure 1): a low-affinity, high-capacity pathway, in which niacin is conjugated with glycine to form nicotinuric acid, and a high-affinity, low-capacity amidative pathway, in which niacin forms nicotinamide. It was once thought that the conjugative pathway, and particularly formation of nicotinuric acid, was associated with flushing and that the amidation pathway, and particularly formation of nicotinamide, was associated with hepatotoxicity; the metabolic fates of niacin formulations via either of these pathways are in turn influenced by these formulations' dissolution and pharmacokinetic profiles. However, a more recent consideration of the evidence by Stern43 emphasized that the parent compound nicotinic acid, through its interaction with the GPR109A receptor, is the chief mediator of flushing and not any particular metabolite, such as nicotinuric acid, formed via the conjugative pathway. This review also concluded that “unlike flushing, the mechanism of nicotinic acid hepatotoxicity has not been determined. Specifically, there is no evidence that a metabolite is responsible … .”43
FIGURE 1.
Niacin metabolism. Niacin is metabolized via 2 pathways. In the first low-affinity, high-capacity pathway (pathway 1, left), niacin is conjugated with glycine to form nicotinuric acid (NUA). The second high-affinity, low-capacity pathway (pathway 2, right) involves a number of oxidation-reduction reactions that produce nicotinamide (NAM) and ultimately pyrimidine metabolites. 6HN = 6-hydroxy-nicotinamide; COOH = carboxylic acid; MNA = N-methylnicotinamide; N = nitrogen; NAD = nicotinamide adenine dinucleotide; NNO = nicotinamide-N-oxide; 2PY = N-methyl-2-pyridone-5-carboxamide; 4PY = N-methyl-4-pyridone-5-carboxamide.
From Am J Cardiol,42 with permission from Elsevier.
Because immediate-release niacin is completely absorbed from the gastrointestinal tract in 1 to 2 hours, immediate-release niacin rapidly saturates the low-capacity amidation pathway, such that most of the drug is metabolized via the conjugative pathway.11 The dissolution and gastrointestinal absorption of sustained-release niacin are not complete until after more than 12 hours. Sustained-release niacin is chiefly metabolized by the amidation pathway and has been associated with hepatic dysfunction (and potentially related gastrointestinal effects) in up to 52% of patients.44,45 With an intermediate dissolution and absorption profile (complete in 8-12 hours), extended-release niacin has a better balanced hepatic metabolism across the conjugative and amidative pathways.19,41
TOLERANCE
Although flushing typically occurs as levels of circulating niacin increase, long-term tolerance to flushing results not from decreased niacin levels per se but rather from reduced output of PGD2 over time with continued dosing.46
TYPICAL CLINICAL PRESENTATION OF NIACIN FLUSHING
Virtually all niacin users initially experience flushing,19 typically commencing 15 to 30 minutes after ingesting immediate-release niacin, 30 to 120 minutes after ingesting extended-release niacin, or at more variable times after sustained-release niacin ingestion. Niacin users often experience “prickly heat” or a sense of warmth in the face, neck, ears, trunk, and, less frequently, the upper or lower extremities.47 Other common features include erythema, itching, and tingling. Symptoms typically last for less than 1 hour to 2.5 hours.48,49 A meta-analysis demonstrated that the overall incidence of flushing with extended-release niacin was similar in women (79%) and men (82%), but women were significantly less likely to complete extended-release niacin studies (68% vs 79%; P=.014) and spent significantly less time using these medications (mean, 15.5 vs 17.3 weeks; P<.001).50
ASSESSMENT
Flushing Assessment Tool
A self-administered questionnaire with up to 22 items, the Flushing Assessment Tool evaluates the severity and troublesomeness of cutaneous warmth, redness, itching, and/or tingling. Individual and overall symptoms are rated on a scale of 1 to 10, in which 1 through 3 signify mild flushing and 10 indicates very severe flushing.51 An 8-week validation trial was conducted in 276 adults with dyslipidemia.51 The overall troublesomeness of flushing was significantly correlated with treatment dissatisfaction. However, changes in most Flushing Assessment Tool measures from treatment week 1 to 6 suggested minimal effects of flushing on quality of life.51
Flushing Symptom Questionnaire
The Flushing Symptom Questionnaire comprises 11 items, each of which is scored as 0 (no symptom) to 10 (extreme symptoms). Item 3 concerning the severity of overall flushing symptoms is also termed the Global Flushing Severity Score. A similar 8-week RCT involving 175 candidates for niacin therapy was conducted to determine the validity of the Flushing Symptom Questionnaire.52,53 During the first niacin treatment week, 47% to 58% of patients experienced daily flushing episodes, most of which were mild and minimally bothersome, and 16% had sleep disturbances. In all, 43% to 49% of those taking 1.0 g of niacin had moderate or greater mean flushing (severity score of ≥4) compared with less than 15% of placebo recipients.52 The mean proportion of days with moderate or greater flushing decreased from 34% in treatment week 1 to 18% at week 2 or afterward.53 Fourteen extended-release niacin users (8%) discontinued treatment because of flushing, including 11 (79%) in the first treatment week.
FREQUENCIES OF FLUSHING AND RELATED DISCONTINUATIONS WITH NIACIN-CONTAINING REGIMENS
Landmark Trials
Most of these studies evaluated the effects of immediate-release niacin, which in some cases was combined with bile acid resins and/or diet, on clinical, angiographic, and/or imaging end points (Table 1).5-10,12,15 In these studies, flushing was reported by up to 92% of patients receiving niacin-containing regimens but caused treatment discontinuation in smaller proportions (2.5%-10%).
Other Clinical Trials Involving Niacin-Containing Regimens: 2000-2009
Studies summarized in Table 254-64 evaluated the effects of a combination regimen of extended-release niacin and a statin as well as of other combination regimens on surrogate (lipid or lipoprotein) outcome measures. These studies demonstrated that many patients (29%-74%) in these trials experienced at least 1 flushing episode, 75% to 92% of episodes were rated as mild or moderate, flushing frequencies declined over time, and approximately 4% to 12% of patients discontinued treatment because of flushing.54-60 The long-term OCEANS (Open-Label Evaluation of the Safety and Efficacy of a Combination of Niacin Extended-Release and Simvastatin in Patients with Dyslipidemia) study showed that less than 40% of patients who experienced flushing in the first 12 weeks of treatment, when extended-release niacin/simvastatin doses were being escalated from 0.5 g/40 mg to 2.0 g/40 mg, did so during the following 12 weeks.57
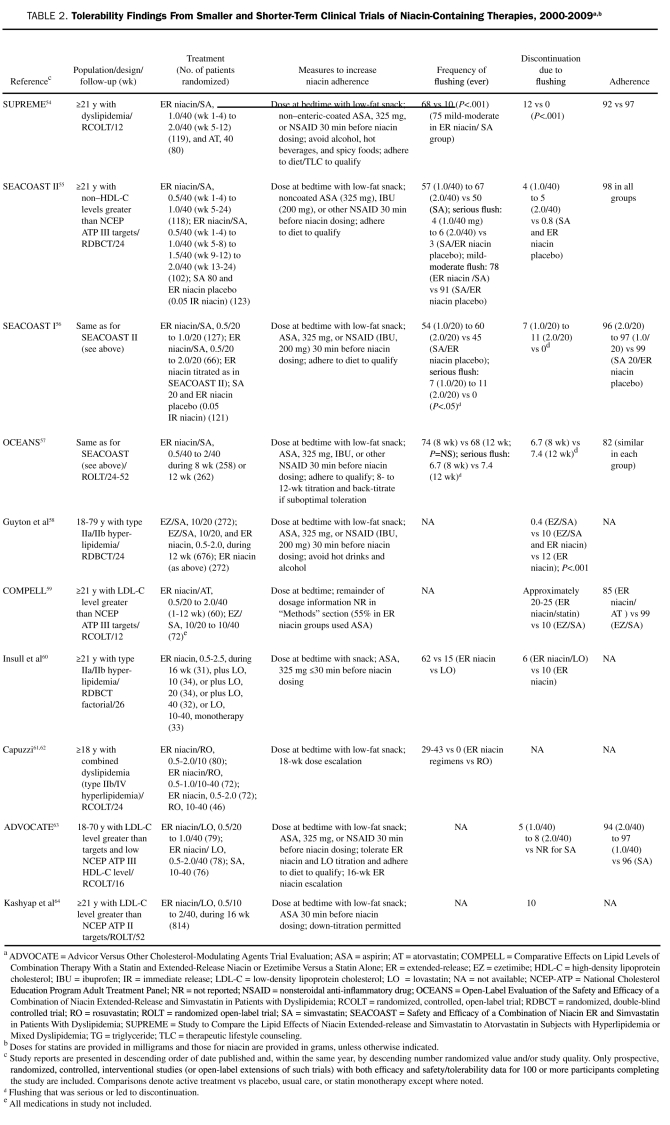
TABLE 2.
Tolerability Findings From Smaller and Shorter-Term Clinical Trials of Niacin-Containing Therapies, 2000-2009a,b
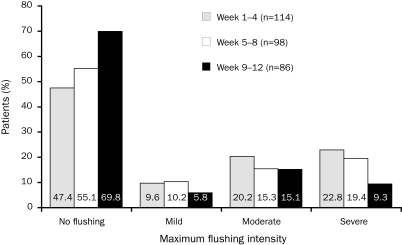
In the SEACOAST (Safety and Efficacy of a Combination of Niacin ER and Simvastatin in Patients With Dyslipidemia) trial, 27% of patients who experienced flushing while taking 1.0 g/20 mg of extended-release niacin/simvastatin (and 30% of patients taking 2.0 g/20 mg of extended-release niacin/simvastatin) during the first 12 weeks but who completed the study did not flush during the second 12 weeks.56 In the recent SUPREME (Study to Compare the Lipid Effects of Niacin Extended-Release and Simvastatin to Atorvastatin in Subjects With Hyperlipidemia or Mixed Dyslipidemia) trial, extended-release niacin was up-titrated more aggressively, from 1.0 g for treatment weeks 1 to 4 to 2.0 g for weeks 5 to 1254; despite this, approximately 47% of patients experienced no flushing during weeks 1 to 4, 55% experienced no flushing during weeks 5 to 8, and 70% experienced no flushing during weeks 9 to 12 (Figure 2).54 The overall incidence of flushing was approximately 39% during treatment week 1 and 26% at week 5 (when the dose was advanced).
FIGURE 2.
Decrease in the proportion of patients receiving extended-release niacin/simvastatin who experienced flushing and decrease in flushing intensity with increased duration of treatment in the Study to Compare the Lipid Effects of Niacin Extended-Release and Simvastatin to Atorvastatin in Subjects With Hyperlipidemia or Mixed Dyslipidemia (SUPREME). Bars depict maximum intensity of flushing experienced in each 4-wk period. No increase in flushing intensity was observed during weeks 5 to 8, when the daily dose of extended-release niacin/simvastatin was increased from 1 g/40 mg to 2 g/40 mg.
From J Clin Lipidol,54 with permission from Elsevier.
Clinical Trials of Niacin Monotherapy: 1985–1999
Studies summarized in eTable 165-73 (available online, linked to this article) evaluated the effects of niacin monotherapy on surrogate (lipid or lipoprotein) end points and patient-related flushing per se (ie, flush provocation studies). In 1 long-term study, more than 40% of patients using extended-release niacin experienced flushing at treatment week 4 compared with less than 20% at week 96.65 The mean per-patient incidence of flushing per month decreased approximately 10-fold: from 1.9 episodes to 0.19 episode.
In a meta-analysis74 of HDL-C–raising therapies involving more than 4000 patients using immediate-release niacin (n=3062),75-83 sustained-release niacin (n=536),66,67,77,84-86 or extended-release niacin (n=648),68-71,87,88 some of the results of which are summarized in eTable 1, most patients (70%) receiving niacin experienced flushing (vs 4% with placebo; P<.001). This proportion included 85% of patients receiving immediate-release niacin, 66% receiving extended-release niacin, and 26% receiving sustained-release niacin.74 Approximately 13% of niacin users discontinued treatment (vs 6% of those receiving a placebo).
Extended- or Prolonged-Release Niacin. Eleven additional published reports provided data on flushing in more than 7000 patients.89-99 A particularly low frequency of flushing was observed in the IMPACT (Impact of Medical Specialty on Patient Compliance to Treatment) study, a 12-week, open-label trial involving 4499 patients with dyslipidemia receiving combination therapy with extended-release niacin and lovastatin (0.5 g/20 mg to 1.0 g/40 mg) once nightly.90 Before beginning therapy, patients received a recorded message about controlling cholesterol, dietary and physical activity counseling, and extended-release niacin's safety profile, with a focus on flushing. Patients also received written reminders to take their medications. At treatment week 8, patients were reevaluated, with laboratory monitoring, queries about adverse events, and adherence reminders, including dosing at bedtime with a low-fat snack and taking aspirin or an NSAID 30 minutes before niacin dosing. Only 18% of extended-release niacin users reported daily flushing, and 6% of patients discontinued niacin because of flushing.
Sustained-Release Niacin. Three prospective trials44,100,101 and 2 retrospective studies102,103 of patients receiving sustained-release niacin were identified. In a study by McKenney et al,44 higher proportions of patients receiving immediate-release niacin (22%-53%) than sustained-release niacin (13%-22%) at daily doses of 0.25 to 3.0 g experienced vasodilator symptoms. In the other prospective studies, 0% to approximately 7% of patients discontinued use of sustained-release niacin because of flushing.100,101 Other studies did not report flushing data specifically by niacin formulation and were excluded.104-107
Immediate-Release Niacin. Nine smaller studies assessed the effects of immediate-release niacin, alone or in combination, on more than 600 patients receiving immediate-release niacin-containing regimens in prospective108-115 and retrospective116 studies. Many involved high starting doses of immediate-release niacin or rapid titration schedules. The percentage of patients discontinuing use of immediate-release niacin because of flushing ranged from 0% to 21%. Other studies without specific flushing data were excluded.104,117-119
STRATEGIES TO PREVENT OR MANAGE NIACIN FLUSHING
Counseling
According to the Health Belief Model,120-123 patients are more likely to adhere to their treatments if they understand that they are susceptible to a disease, that treatment will reduce this susceptibility (without causing offsetting untoward effects), and if they are personally able to adopt the treatment. Given that dyslipidemia is a largely asymptomatic condition, it can be difficult to persuade a patient with no observable symptoms to take a medication that initially causes discomfort. Accordingly, it is important to explain the potential benefits of niacin therapy; the typically transitory, reversible, and harmless nature of flushing; and, in contrast, the stable and lasting coronary preventive benefits of continued niacin administration (Table 3). Patients need to know that niacin for dyslipidemia is essentially a “lifetime drug.”
TABLE 3.
Educational Messages to Help Patients Prevent or Minimize Niacin Flushing
Engaging the patient in this sort of dialogue can help to cement a stronger physician-patient bond. Patients are advised to take niacin with a low-fat snack (eg, applesauce) at bedtime or with a meal, which can improve both vasocutaneous and gastrointestinal tolerability. Taking niacin with food may also increase niacin's bioavailability,124 whereas taking it at bedtime allows many patients to sleep through any flushing and may blunt nocturnal release of free fatty acids.
Counseling approaches recommended by the National Lipid Association to prevent or minimize flushing are presented in Table 4.125 As suggested by the IMPACT study,90 ongoing patient education and reminders may assist in promoting niacin adherence. Patients are asked to describe the symptoms of flushing by formulation; for instance, flushing with immediate-release niacin is probably the shortest lived and may be a more consistent experience compared with other formulations.126 Immediate-release niacin should be administered in divided doses, typically in the middle of breakfast and dinner, and after taking 325 mg of aspirin on awakening (at least during the early weeks of treatment).126 One approach is to initiate immediate-release niacin treatment at 0.05 to 0.10 g (50-100 mg) twice daily for the first week, then double the daily dose each week until the daily dosage is raised to 1.0 to 2.0 g.127
TABLE 4.
Educational Messages to Help Physicians Prevent or Minimize Niacin Flushing and Enhance Tolerability
Patients should be advised that flushing is a nonallergic, typically time-limited phenomenon; although uncomfortable, it is not usually harmful. Flushing will not occur with each dose and should subside during a period of weeks if not days with continued dosing. Failure of flushing to abate with time may reflect inconsistent dosing. If the patient has not taken niacin in 3 or more days, it may be necessary to restart treatment at a low dose and reescalate. To improve patient expectations of flushing, it may be useful to administer the starting niacin dose in the office. In a number of clinical trials, patients took either 325 mg of a non–enteric-coated aspirin or an NSAID (eg, 200 mg of ibuprofen) 30 to 60 minutes before (but not 15 or 120 minutes before) niacin use, and this approach has been endorsed by the National Lipid Association.125 Some authorities recommend taking 325 mg of aspirin the day before the first niacin dose and continuing its use for the first month of treatment.126 Given the perception of heat associated with flushing, patients may wish to take their niacin with a cold (non-alcoholic) beverage. They should avoid alcohol, spicy foods, hot beverages, and hot showers or baths around the time of dosing. Patients should also be advised to avoid missing niacin doses and to report any such lapses to their clinicians.
In contrast to certain RCTs that did not allow back-titration (down-titration), the physician and patient have wide latitude to individualize the schedule of niacin dose escalation. Some sensitive patients will need to “start lower and go slower” or even “start low, go slow, and go back.” To assist in this process, it is important to schedule a follow-up visit with laboratory monitoring approximately 4 to 8 weeks after initiating niacin treatment and to identify other patient concerns about flushing. Doing so enables the physician to assess patient safety and toleration of niacin, make any needed medication adjustments, and review key counseling messages. The follow-up visit also offers an occasion to revisit the lipid and coronary preventive benefits of niacin, focus patients on attainable intermediate objectives (eg, achievement of cholesterol level goals), and hence promote adherence.128
Pharmacotherapeutic Strategies
Optimized Niacin Formulation. Extended-release niacin was reformulated with a different coating to optimize its dissolution, absorption, and metabolic profiles. In a flush provocation study, 53% of patients receiving 650 mg of aspirin before treatment and 61% receiving 650 mg of concomitant aspirin experienced flushing with 2.0 g of reformulated extended-release niacin.129 Aspirin before treatment reduced the median duration of the first flush by approximately 43% (37 vs 65 minutes for extended-release niacin alone; P=.008), the mean intensity by 34% (P<.001), and the proportion of patients with moderate or greater flushing by 62% (14% vs 37%; P<.001).
Aspirin and NSAIDs. Findings from studies specifically evaluating adjunctive or prophylactic aspirin or NSAIDs to attenuate niacin flushing are summarized in eTable 2 (available online, linked to this article).129-134 Of note, 325 mg of aspirin significantly (P<.001 vs placebo) attenuated flushing symptoms when administered 30 to 60 but not 15 or 120 minutes before immediate-release niacin, and an aspirin dose of 650 mg was not superior to 325 mg.132 In another study, pretreatment with 325 mg of aspirin 30 minutes before niacin was significantly superior to 200 mg of ibuprofen in blunting flushing and itching in patients experiencing these manifestations.133 In a previous study, 325 mg of aspirin was superior to 80 mg of aspirin in mitigating flushing with 0.5 g of immediate-release niacin.134
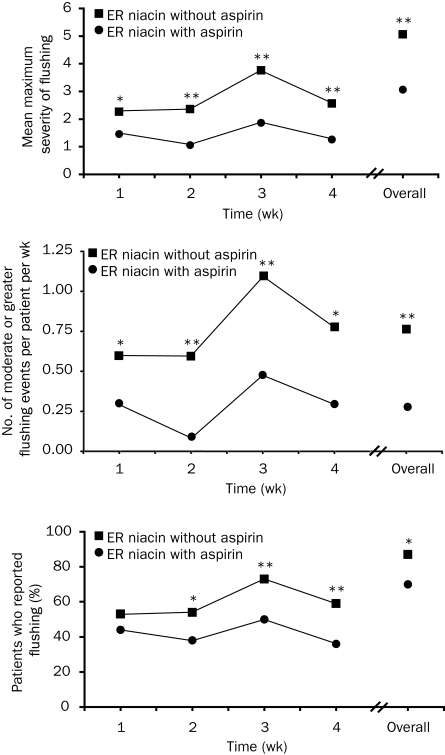
The flushing-specific discontinuation rate in a recent 4-week study was 1.8% among patients randomized to receive 325 mg of aspirin before extended-release niacin treatment compared with 9.4% among those receiving extended-release niacin without aspirin (P=.007).130 In this study, 15% of patients receiving aspirin prophylaxis with a 0.5- or 1.0-g starting dose of extended-release niacin experienced moderate or greater flushing during the first treatment week (vs 29% not receiving aspirin; P=.01). Pretreatment with aspirin significantly reduced mean maximum flushing severity, number of moderate or greater flushing episodes, and the incidence of flushing by 18% (P=.014; Figure 3).130 The incidence of flushing was also significantly lower among patients receiving an extended-release niacin starting dose of 0.5 g (32%) compared with 1.0 g (53%; P=.05).
FIGURE 3.
Severity of flushing events with and without aspirin (top and middle) and flushing incidence (bottom) in a study evaluating the effects of 1 wk of prophylaxis with 325 mg of aspirin, followed by ongoing treatment as an adjunct for flushing associated with extended-release niacin, 0.5 to 2.0 g, during treatment weeks 1 through 4. Top and Middle, Flushing events were graded on a 10-point scale ranging from mild (1-3) to very severe (10). Top, Mean maximum flushing severity scores are presented over time among patients who received aspirin treatment at any time (arms 1, 2, 4, and 5) vs patients who did not receive aspirin treatment (arms 3 and 6). Middle, The number of moderate or greater flushing events recorded per patient per week are presented over time among patients receiving aspirin treatment at any time (arms 1, 2, 4, and 5) vs patients who did not receive aspirin treatment (arms 3 and 6). *P<.05; **P<.001 vs extended-release niacin with aspirin.
From Am J Cardiovasc Drugs,130 with permission from Wolters Kluwer Health.
A literature review135 demonstrated that the mean rate of treatment discontinuation because of niacin flushing was approximately 6.5% in studies in which 325 mg of aspirin was recommended as needed or mandatory to prevent or minimize flushing: 8% in studies using extended-release niacin vs 18% in those using immediate-release niacin. The mean incidence of niacin flushing across the studies was 29% (range, 18%-100%). In a recent study, 23% of patients receiving extended-release niacin and aspirin experienced flushing during the first treatment month compared with 32% of those without prior aspirin therapy (P=.02).89
Laropiprant. A selective PGD2 receptor (DP1) antagonist approved as a treatment adjunct to mitigate niacin flushing in certain non-US markets, laropiprant has been combined with extended-release niacin (modified- or prolonged-release niacin; Tredaptive; Merck, Whitehouse Station, NJ). The proprietary extended-release niacin formulation in Tredaptive is not identical to the proprietary formulation in Niaspan, Advicor, and Simcor.
In healthy volunteers, laropiprant is rapidly absorbed (median time to maximum drug concentration, 0.8-2.0 hours), with no effects of food on absorption, and the apparent terminal elimination half-life is 10 to 18 hours.136
In a single-dose study, adjunctive (concomitant) laropiprant significantly reduced flushing associated with 1.5 g of extended-release niacin: by 47% for a 30-mg dose of laropiprant, by 67% for a 100-mg dose, and by 74% for a 300-mg dose.137 Coadministration of extended-release niacin with either 30 mg or 100 mg of laropiprant decreased peak malar (cheek) skin blood flow (objective measure of vasodilation) by 50% and 70%, respectively. Reductions in patient-rated flushing correlated significantly with decreases in malar blood flow (r=0.64-0.68; P<.001).137 In multiple-dose studies, 200 mg of adjunctive laropiprant reduced time-weighted average flushing scores by 65% (vs 1.5 g of extended-release niacin alone) on day 1 and by 52% on day 3.137 In a phase 1 study (study 044), coadministration of 325 mg of aspirin did not confer significant incremental benefits in reducing the incidence and severity of residual flushing associated with 40 mg of laropiprant and 2.0 g of extended-release niacin.138
In a phase 2, dose-ranging study, adjunctive laropiprant (18.75 to 150 mg [pooled]) resulted in lower proportions of patients reporting moderate or greater flushing during the first week of treatment with 1.0 g of extended-release niacin (vs 1.0 g of extended-release niacin alone).139 In a pivotal phase 3 study (study 020), approximately 31% of patients who were using adjunctive laropiprant and extended-release niacin (1.0 g) experienced moderate or greater flushing during treatment week 1 compared with 56% receiving monotherapy with 1.0 g of extended-release niacin (P<.001) and 6% taking placebo.140 Corresponding proportions of patients with severe or extreme flushing were 14% in the laropiprant and extended-release niacin group and 33% in the extended-release niacin group (P<.001).140
During treatment weeks 2 to 24 in study 020, 14% of patients taking laropiprant and extended-release niacin experienced 1 day per week or more with moderate or greater flushing compared with 29% of those taking extended-release niacin monotherapy (P<.001) and approximately 2% of those taking placebo.140 During the maintenance phase, patients in the laropiprant and extended-release niacin group experienced approximately 1 day of moderate or greater flushing per patient-month compared with 1 day per patient-week in the extended-release niacin group. Commenting on these data, the authors contended that “tolerance to this level of flushing is incomplete during at least a 6-month period.”141 Ten percent of the extended-release niacin and laropiprant group discontinued treatment because of flushing compared with 22% of the extended-release niacin group (P<.001) and less than 1.0% of the placebo group. The combination of laropiprant and extended-release niacin is being evaluated in the ongoing phase 3 HPS2-THRIVE (Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events).
DISCUSSION
The Way Forward
One ongoing controversy concerns schedules for extended-release niacin dose escalation. For many years, the adage about niacin dosing has been to “start low and go slow[ly].” More recently, however, studies have suggested that a shorter titration interval, on the order of 8 or 12 weeks (vs 16 weeks), to increase the niacin daily dose from 0.5 to 2.0 g may be associated with comparable or even superior flushing data. In the OCEANS study, 68% of patients using an 8-week extended-release niacin and simvastatin titration schedule experienced flushing compared with approximately 74% of those using a 12-week titration.57 Regimens of patients receiving the PGD2 antagonist laropiprant along with extended-release niacin have consisted of a 2-step schedule: initiating niacin treatment at the relatively high daily dose of 1.0 g, which was maintained for 4 weeks, and then escalating to 2 g at treatment week 4 and maintaining this dose for 12 weeks.142 In my practice, we use a combination of these 2 approaches, starting low (0.5 g/d) and going slowly (for a total of 8 weeks) until a stable dose of 1.0 g has been reached. At this juncture, the daily niacin dose is increased directly from 1.0 g to the maintenance daily dose of 2.0 g (if tolerated).
Although neither aspirin nor laropiprant influences the lipid-modifying effects of niacin, neither agent completely abrogates the flushing response. Aspirin can also saturate the glycine elimination (conjugative) pathway shared with niacin and, hence, in theory, actually increase plasma niacin levels. This could, in turn, contribute to increased GPR109A receptor–mediated, PGD2-driven flushing.134,143 However, this probably has never been observed because the net effect of 325 mg of aspirin is to reduce PGD2 levels and hence contribute to the development of tachyphylaxis.134 Furthermore, aspirin can increase the risk of gastrointestinal events and/or bleeding. Niacin is often associated with gastrointestinal effects, and both active peptic ulcer disease and arterial bleeding contraindicate treatment with niacin-containing regimens.33-35
In a clinical pharmacology study, laropiprant at supratherapeutic doses had no significant effect on bleeding time, whereas 325 mg of aspirin prolonged bleeding time by 44% (P=.004 vs placebo) 24 hours after administration.136 However, laropiprant also causes adverse gastrointestinal effects. In the pivotal laropiprant phase 3 study (study 020), 2.9% of the laropiprant and extended-release niacin group experienced nausea (vs 2.0% with extended-release niacin alone) and 2.6% experienced diarrhea (vs 1.8%).140 Across laropiprant studies 020, 022, and 054, 16.5% of the laropiprant and extended-release niacin groups experienced gastrointestinal adverse events (vs 11.7% of the extended-release niacin group).144
Despite a lower rate of discontinuation because of flushing in the laropiprant and extended-release niacin groups (approximately 4% vs 9% with extended-release niacin), a higher proportion of the laropiprant and extended-release niacin group discontinued treatment because of gastrointestinal (2.5% vs 1.5%) or laboratory adverse events (1.3% vs 0.7%), particularly abnormal liver function test results. In its Committee for Medicinal Products for Human Use assessment, the European Medicines Agency concluded that “the lower discontinuation rate due to flushing [with laropiprant and extended-release niacin vs extended-release niacin alone] is partly offset by other side effects, in particular the occurrence of gastrointestinal symptoms.”144 In a recent study (reported after the literature search had been conducted), frequencies of gastrointestinal adverse events were comparable in patients randomized to laropiprant and extended-release niacin (1.0 g for 4 weeks followed by 2.0 g for 12 weeks) or extended-release niacin monotherapy (0.5 g titrated to 2.0 g at 0.5-g monthly increments) when data were normalized by time of exposure to extended-release niacin at 2.0 g (1.7% vs 1.8%).142 In this study, approximately 7% of patients in the laropiprant plus extended-release niacin group discontinued treatment because of flushing, compared with 12% in the extended-release niacin group (P=.002).142
In contrast, patients receiving laropiprant and extended-release niacin may have increased frequencies of niacin-related, nonflushing adverse events (vs extended-release niacin monotherapy) because of dose inequities; recipients of laropiprant and extended-release niacin might experience a somewhat higher frequency of nonvasodilatory untoward effects because they are able to achieve higher niacin doses without flushing. Treatment adjuncts such as laropiprant and aspirin typically need to be used for a relatively short period to tide patients over the early niacin dosing period, hence limiting overall exposure to these adjuncts. Given a low percentage of patients discontinuing laropiprant and extended-release niacin therapy after treatment week 16 and the dubious effects of long-term withdrawal of laropiprant on extended-release niacin flushing, the European Medicines Agency also concluded that the efficacy of laropiprant had not been demonstrated for more than 24 weeks.144
Perhaps the most appropriate use of aspirin and laropiprant, along with counseling and other measures, should be as temporary “bridges” that are targeted to patients during the first several weeks of treatment, when niacin doses are being advanced, as well as when restarting niacin treatment after a lapse in dosing. Studies are needed to predict which patients are most likely to experience poor vasocutaneous toleration of niacin and target the most intensive counseling and pharmacological support to these individuals. However, no study to my knowledge has yet demonstrated an “at-risk genotype” for flushing. Studies are also needed to compare the effects of aspirin prophylaxis and adjunctive laropiprant on flushing, as well as to establish an optimal dosing paradigm (eg, via studies comparing extended-release niacin dosing with dinner or at bedtime).
Other potential treatment adjuncts to mitigate niacin flushing include agents targeting the serotonergic component of the vasocutaneous reaction (eg, cyproheptadine [off-label indication]). Other candidates include flavonoids (eg, quercetin [Algonot Plus; Algonot LLC, Sarasota, FL] and luteolin [Luti-Max; SYNORx Inc, San Clemente, CA]), which also inhibit mast cell PGD2 secretion.145,146 Another possible approach is to develop new niacin receptor (GPR109A) agonists with a superior therapeutic index for inhibiting lipolysis (and hence improving lipid levels) over vasodilation, even though both of these processes are receptor-mediated.147
Potential Limitations
Limitations of this review include potential publication bias; the decision not to include case reports and case series; a focus on data from RCTs, in which patients are often highly selected for superior treatment adherence (potential selection bias); and inclusion of healthy volunteers in some of the studies. Many studies reviewed reported frequencies of flushing separately from those of the individual symptoms (eg, pruritus [itching] or paresthesia [tingling]). These factors may have caused an underestimation of the true incidence and severity of flushing associated with niacin compared with more naturalistic clinical settings.
Many RCTs in this review did not determine whether decreases in mean incidences of flushing over time with niacin truly resulted from tachyphylaxis in most patients or from a survivor effect in which patients with poor toleration were more likely to discontinue treatment in the early weeks, leaving a more flushing-resistant phenotype in the later weeks. In future trials, conducting an analysis of “time to first flush” or “time [or cumulative niacin dose] to flushing-related discontinuation” might help to adjust for this potential statistical artifact.142,148,149
Data on available niacin receptor agonists (Acipimox [Pfizer]) or from proprietary databases (eg, EMBASE) were not reviewed. Many reports providing data on flushing did not explicitly state whether the aspirin administered was enteric-coated. Combined with cellulose and/or other inert ingredients that resist gastric disintegration, coated aspirin is absorbed more slowly than other forms, with a time to peak concentration of approximately 3 to 4 hours compared with 0.25 hour to 2 hours for uncoated aspirin, which is closer to the time at onset of flushing with many niacin formulations. Hence, coated aspirin may need to be administered more than 30 minutes before niacin, although no clinical study has evaluated the comparative antiflushing efficacy of coated (vs uncoated) aspirin.150
CONCLUSION
Niacin is the most effective agent for raising HDL-C levels, but its use has been constrained by potentially dose-limiting, receptor-mediated, mainly PG-driven cutaneous vasodilation with flushing. Although typically mild or moderate and reversible with continued dosing, niacin flushing warrants effective counseling, treatment adjuncts or prophylaxis, careful dose escalation with follow-up monitoring, input from the patient, and regimen adjustments as needed. Wider implementation of these measures should enable higher proportions of patients to reach and persist with sufficient niacin doses over time to prevent cardiovascular events.
Supplementary Material
Acknowledgments
Assistance in research and editorial support were provided by Stephen W. Gutkin, BA, Rete Biomedical Communications Corp, Wyckoff, NJ.
REFERENCES
- 1.Adult Treatment Panel Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106(25):3143-3421 [PubMed] [Google Scholar]
- 2.Graham I, Atar D, Borch-Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice: full text. Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). Eur J Cardiovasc Prev Rehabil. 2007;14(Suppl 2):S1-S113 [DOI] [PubMed] [Google Scholar]
- 3.Hausenloy DJ, Yellon DM. Targeting residual cardiovascular risk: raising high-density lipoprotein cholesterol levels. Heart 2008;94(6):706-714 [DOI] [PubMed] [Google Scholar]
- 4.Altschul R, Hoffer A, Stephen JD. Influence of nicotinic acid on serum cholesterol in man. Arch Biochem. 1955;54(2):558-559 [DOI] [PubMed] [Google Scholar]
- 5.Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol. 986;8(6):1245-1255 [DOI] [PubMed] [Google Scholar]
- 6.Coronary Drug Project Research Group Clofibrate and niacin in coronary heart disease. JAMA 1975;231(4):360-381 [PubMed] [Google Scholar]
- 7.Blankenhorn DH, Selzer RH, Crawford DW, et al. Beneficial effects of colestipol-niacin therapy on the common carotid artery: two- and four-year reduction of intima-media thickness measured by ultrasound. Circulation 1993;88(1):20-28 [DOI] [PubMed] [Google Scholar]
- 8.Blankenhorn DH, Nessim SA, Johnson RL, Sanmarco ME, Azen SP, Cashin-Hemphill L. Beneficial effects of combined colestipol-niacin therapy on coronary atherosclerosis and coronary venous bypass grafts. JAMA 1987;257(23):3233-3240 [PubMed] [Google Scholar]
- 9.Brown G, Albers JJ, Fisher LD, et al. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N Engl J Med. 1990;323(19):1289-1298 [DOI] [PubMed] [Google Scholar]
- 10.Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345(22):1583-1592 [DOI] [PubMed] [Google Scholar]
- 11.McKenney J. Niacin for dyslipidemia: considerations in product selection. Am J Health Syst Pharm. 2003;60(10):995-1005 [DOI] [PubMed] [Google Scholar]
- 12.Whitney EJ, Krasuski RA, Personius BE, et al. A randomized trial of a strategy for increasing high-density lipoprotein cholesterol levels: effects on progression of coronary heart disease and clinical events. Ann Intern Med. 2005;142(2):95-104 [DOI] [PubMed] [Google Scholar]
- 13.Taylor AJ, Villines TC, Stanek EJ, et al. Extended-release niacin or ezetimibe and carotid intima-media thickness. N Engl J Med. 2009;361(22):2113-2122 [DOI] [PubMed] [Google Scholar]
- 14.Taylor AJ, Lee HJ, Sullenberger LE. The effect of 24 months of combination statin and extended-release niacin on carotid intima-media thickness: ARBITER 3. Curr Med Res Opin. 2006;22(11):2243-2250 [DOI] [PubMed] [Google Scholar]
- 15.Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 2004;110(23):3512-3517 [DOI] [PubMed] [Google Scholar]
- 16.Knopp RH. Evaluating niacin in its various forms. Am J Cardiol. 2000;86(12A):51L-56L [DOI] [PubMed] [Google Scholar]
- 17.Kamanna VS, Kashyap ML. Mechanism of action of niacin. Am J Cardiol. 2008;101(8A):20B-26B [DOI] [PubMed] [Google Scholar]
- 18.Knowles HJ, te Poele RH, Workman P, Harris AL. Niacin induces PPARgamma expression and transcriptional activation in macrophages via HM74 and HM74a-mediated induction of prostaglandin synthesis pathways. Biochem Pharmacol. 2006;71(5):646-656 [DOI] [PubMed] [Google Scholar]
- 19.McKenney J. New perspectives on the use of niacin in the treatment of lipid disorders. Arch Intern Med. 2004;164(7):697-705 [DOI] [PubMed] [Google Scholar]
- 20.Simons LA. Treatment of lipids: implications for the general practitioner. Aust Fam Physician 1996;25(7):1053-1059 [PubMed] [Google Scholar]
- 21.Abughosh SM, Kogut SJ, Andrade SE, Larrat P, Gurwitz JH. Persistence with lipid-lowering therapy: influence of the type of lipid-lowering agent and drug benefit plan option in elderly patients. J Manag Care Pharm. 2004;10(5):404-411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA 2002;288(4):462-467 [DOI] [PubMed] [Google Scholar]
- 23.Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. JAMA 2002;288(4):455-461 [DOI] [PubMed] [Google Scholar]
- 24.Avorn J, Monette J, Lacour A, et al. Persistence of use of lipid-lowering medications: a cross-national study. JAMA 1998;279(18):1458-1462 [DOI] [PubMed] [Google Scholar]
- 25.Andrade SE, Walker AM, Gottlieb LK, et al. Discontinuation of antihyperlipidemic drugs--do rates reported in clinical trials reflect rates in primary care settings? N Engl J Med. 1995;332(17):1125-1131 [DOI] [PubMed] [Google Scholar]
- 26.Chodick G, Shalev V, Gerber Y, et al. Long-term persistence with statin treatment in a not-for-profit health maintenance organization: a population-based retrospective cohort study in Israel. Clin Ther. 2008;30(11):2167-2179 [DOI] [PubMed] [Google Scholar]
- 27.LaFleur J, Thompson CJ, Joish VN, Charland SL, Oderda GM, Brixner DI. Adherence and persistence with single-dosage form extended-release niacin/lovastatin compared with statins alone or in combination with extended-release niacin. Ann PharmacoTher. 2006;40(7-8):1274-1279 [DOI] [PubMed] [Google Scholar]
- 28.Kamal-Bahl S, Watson DJ, Ambegaonkar BM. Patients' experiences of niacin-induced flushing in clinical practice: a structured telephone interview. Clin Ther. 2009;31(1):130-140 [DOI] [PubMed] [Google Scholar]
- 29.Kamal-Bahl SJ, Burke TA, Watson DJ, Wentworth CE. Dosage, titration, and gaps in treatment with extended release niacin in clinical practice. Curr Med Res Opin. 2008;24(6):1817-1821 [DOI] [PubMed] [Google Scholar]
- 30.Knopp RH, Ginsberg J, Albers JJ, et al. Contrasting effects of unmodified and time-release forms of niacin on lipoproteins in hyperlipidemic subjects: clues to mechanism of action of niacin. Metabolism 1985;34(7):642-650 [DOI] [PubMed] [Google Scholar]
- 31.Meyers CD, Carr MC, Park S, Brunzell JD. Varying cost and free nicotinic acid content in over-the-counter niacin preparations for dyslipidemia. Ann Intern Med. 2003;139(12):996-1002 [DOI] [PubMed] [Google Scholar]
- 32.AbbottNiaspan® (niacin extended-release). US full prescribing information. http://www.rxabbott.com/pdf/niaspan.pdf. http://www.rxabbott.com/pdf/niaspan.pdf Accessed May 18, 2009.
- 33.AbbottSimcor® (niacin extended-release/simvastatin). US full prescribing information. http://www.rxabbott.com/pdf/simcor_pi.pdf. http://www.rxabbott.com/pdf/simcor_pi.pdf Accessed May 18, 2009.
- 34.Abbott Advicor® (niacin extended-release/lovastatin tablets). US full prescribing information Available at http://www.rxabbott.com/pdf/advicor.pdf Accessed May 18, 2009
- 35.Maciejewski-Lenoir D, Richman JG, Hakak Y, Gaidarov I, Behan DP, Connolly DT. Langerhans cells release prostaglandin D2 in response to nicotinic acid. J Invest Dermatol. 2006;126(12):2637-2646 [DOI] [PubMed] [Google Scholar]
- 36.Benyó Z, Gille A, Kero J, et al. GPR109A (PUMA-G/HM74A) mediates nicotinic acid-induced flushing. J Clin Invest. 2005;115(12):3634-3640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pike NB. Flushing out the role of GPR109A (HM74A) in the clinical efficacy of nicotinic acid. J Clin Invest. 2005;115(12):3400-3403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morrow JD, Parsons WG, III, Roberts LJ. Release of markedly increased quantities of prostaglandin D2 in vivo in humans following the administration of nicotinic acid. Prostaglandins 1989;38(2):263-274 [DOI] [PubMed] [Google Scholar]
- 39.Cheng K, Wu TJ, Wu KK, et al. Antagonism of the prostaglandin D2 receptor 1 suppresses nicotinic acid-induced vasodilation in mice and humans. Proc Natl Acad Sci U S A 2006;103(17):6682-6687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benyó Z, Gille A, Bennett CL, Clausen BE, Offermanns S. Nicotinic acid-induced flushing is mediated by activation of epidermal Langerhans cells. Mol Pharmacol. 2006;70(6):1844-1849 [DOI] [PubMed] [Google Scholar]
- 41.Guyton JR. Extended-release niacin for modifying the lipoprotein profile. Expert Opin PharmacoTher. 2004;5(6):1385-1398 [DOI] [PubMed] [Google Scholar]
- 42.Piepho RW. The pharmacokinetics and pharmacodynamics of agents proven to raise high-density lipoprotein cholesterol. Am J Cardiol. 2000;86(12A):35L-40L [DOI] [PubMed] [Google Scholar]
- 43.Stern RH. The role of nicotinic acid metabolites in flushing and hepatotoxicity. J Clin Lipidol. 2007;1:191-193 [DOI] [PubMed] [Google Scholar]
- 44.McKenney JM, Proctor JD, Harris S, Chinchili VM. A comparison of the efficacy and toxic effects of sustained- vs immediate-release niacin in hypercholesterolemic patients. JAMA 1994;271(9):672-677 [PubMed] [Google Scholar]
- 45.Henkin Y, Oberman A, Hurst DC, Segrest JP. Niacin revisited: clinical observations on an important but underutilized drug. Am J Med. 1991;91(3):239-246 [DOI] [PubMed] [Google Scholar]
- 46.Stern RH, Spence JD, Freeman DJ, Parbtani A. Tolerance to nicotinic acid flushing. Clin Pharmacol Ther. 1991;50(1):66-70 [DOI] [PubMed] [Google Scholar]
- 47.Carlson LA. Nicotinic acid: the broad-spectrum lipid drug. A 50th anniversary review. J Intern Med. 2005;258(2):94-114 [DOI] [PubMed] [Google Scholar]
- 48.Guyton JR, Simmons PD. Flushing and other dermatologic adverse events associated with extended-release niacin therapy. J Clin Lipidol. 2009;3:101-108 [DOI] [PubMed] [Google Scholar]
- 49.Davidson MH. Niacin use and cutaneous flushing: mechanisms and strategies for prevention. Am J Cardiol. 2008;101(8A):14B-19B [DOI] [PubMed] [Google Scholar]
- 50.Goldberg AC. A meta-analysis of randomized controlled studies on the effects of extended-release niacin in women. Am J Cardiol. 2004;94(1):121-124 [DOI] [PubMed] [Google Scholar]
- 51.Kawata AK, Revicki DA, Thakkar R, et al. Flushing ASsessment Tool (FAST): psychometric properties of a new measure assessing flushing symptoms and clinical impact of niacin therapy. Clin Drug Investig. 2009;29(4):215-229 [DOI] [PubMed] [Google Scholar]
- 52.Norquist JM, Watson DJ, Yu Q, Paolini JF, McQuarrie K, Santanello NC. Validation of a questionnaire to assess niacin-induced cutaneous flushing. Curr Med Res Opin. 2007;23(7):1549-1560 [DOI] [PubMed] [Google Scholar]
- 53.Paolini JF, Mitchel YB, Reyes R, et al. Measuring flushing symptoms with extended-release niacin using the flushing symptom questionnaire: results from a randomised placebo-controlled clinical trial. Int J Clin Pract. 2008;62(6):896-904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Insull W, Jr, Basile JN, Vo AN, Jiang P, Thakkar R, Padley RJ. Efficacy and safety of combination therapy with niacin extended-release and simvastatin versus atorvastatin in patients with dyslipidemia: the SUPREME study. J Clin Lipidol. 2009;3:109-118 [DOI] [PubMed] [Google Scholar]
- 55.Ballantyne CM, Davidson M, McKenney JM, Keller LH, Bajorunas DR, Karas RH. Comparison of the efficacy and safety of a combination tablet of niacin extended-release and simvastatin with simvastatin 80 mg monotherapy: the SEACOAST II (high-dose) study. J Clin Lipidol. 2008;2:79-90 [DOI] [PubMed] [Google Scholar]
- 56.Ballantyne CM, Davidson MH, McKenney J, Keller LH, Bajorunas DR, Karas RH. Comparison of the safety and efficacy of a combination tablet of niacin extended release and simvastatin vs simvastatin monotherapy in patients with increased non-HDL cholesterol (from the SEACOAST I study). Am J Cardiol. 2008;101(10):1428-1436 [DOI] [PubMed] [Google Scholar]
- 57.Karas RH, Kashyap ML, Knopp RH, Keller LH, Bajorunas DR, Davidson MH. Long-term safety and efficacy of a combination of niacin extended release and simvastatin in patients with dyslipidemia: the OCEANS study. Am J Cardiovasc Drugs 2008;8(2):69-81 [DOI] [PubMed] [Google Scholar]
- 58.Guyton JR, Brown BG, Fazio S, Polis A, Tomassini JE, Tershakovec AM. Lipid-altering efficacy and safety of ezetimibe/simvastatin coadministered with extended-release niacin in patients with type IIa or type IIb hyperlipidemia. J Am Coll Cardiol. 2008;51(16):1564-1572 [DOI] [PubMed] [Google Scholar]
- 59.McKenney JM, Jones PH, Bays HE, et al. Comparative effects on lipid levels of combination therapy with a statin and extended-release niacin or ezetimibe versus a statin alone (the COMPELL study). Atherosclerosis 2007;192(2):432-437 [DOI] [PubMed] [Google Scholar]
- 60.Insull W, Jr, McGovern ME, Schrott H, et al. Efficacy of extended-release niacin with lovastatin for hypercholesterolemia: assessing all reasonable doses with innovative surface graph analysis. Arch Intern Med. 2004;164(10):1121-1127 [DOI] [PubMed] [Google Scholar]
- 61.Capuzzi DM, Morgan JM, Carey CM, et al. Rosuvastatin alone or with extended-release niacin: a new therapeutic option for patients with combined hyperlipidemia. Prev Cardiol. 2004;7(4):176-181 [DOI] [PubMed] [Google Scholar]
- 62.Capuzzi DM, Morgan JM, Weiss RJ, Chitra RR, Hutchinson HG, Cressman MD. Beneficial effects of rosuvastatin alone and in combination with extended-release niacin in patients with a combined hyperlipidemia and low high-density lipoprotein cholesterol levels. Am J Cardiol. 2003;91(11):1304-1310 [DOI] [PubMed] [Google Scholar]
- 63.Bays HE, Dujovne CA, McGovern ME, et al. Comparison of once-daily, niacin extended-release/lovastatin with standard doses of atorvastatin and simvastatin (the ADvicor Versus Other Cholesterol-Modulating Agents Trial Evaluation [ADVOCATE]). Am J Cardiol. 2003;91(6):667-672 [DOI] [PubMed] [Google Scholar]
- 64.Kashyap ML, McGovern ME, Berra K, et al. Long-term safety and efficacy of a once-daily niacin/lovastatin formulation for patients with dyslipidemia. Am J Cardiol. 2002;89(6):672-678 [DOI] [PubMed] [Google Scholar]
- 65.Capuzzi DM, Guyton JR, Morgan JM, et al. Efficacy and safety of an extended-release niacin (Niaspan): a long-term study. Am J Cardiol. 1998;82(12A):74U-81U [DOI] [PubMed] [Google Scholar]
- 66.Keenan JM, Bae CY, Fontaine PL, et al. Treatment of hypercholesterolemia: comparison of younger versus older patients using wax-matrix sustained-release niacin. J Am Geriatr Soc. 1992;40(1):12-18 [DOI] [PubMed] [Google Scholar]
- 67.Keenan JM, Fontaine PL, Wenz JB, Myers S, Huang ZQ, Ripsin CM. Niacin revisited: a randomized, controlled trial of wax-matrix sustained-release niacin in hypercholesterolemia. Arch Intern Med. 1991;151(7):1424-1432 [DOI] [PubMed] [Google Scholar]
- 68.Grundy SM, Vega GL, McGovern ME, et al. Efficacy, safety, and tolerability of once-daily niacin for the treatment of dyslipidemia associated with type 2 diabetes: results of the assessment of diabetes control and evaluation of the efficacy of Niaspan trial. Arch Intern Med. 2002;162(14):1568-1576 [DOI] [PubMed] [Google Scholar]
- 69.Goldberg AC. Clinical trial experience with extended-release niacin (Niaspan): dose-escalation study. Am J Cardiol. 1998;82(12A):35U-38U [DOI] [PubMed] [Google Scholar]
- 70.Knopp RH, Alagona P, Davidson M, et al. Equivalent efficacy of a time-release form of niacin (Niaspan) given once-a-night versus plain niacin in the management of hyperlipidemia. Metabolism 1998;47(9):1097-1104 [DOI] [PubMed] [Google Scholar]
- 71.Morgan JM, Capuzzi DM, Guyton JR. A new extended-release niacin (Niaspan): efficacy, tolerability, and safety in hypercholesterolemic patients. Am J Cardiol. 1998;82(suppl 12A):29U-34U [DOI] [PubMed] [Google Scholar]
- 72.Cefali EA, Simmons PD, Stanek EJ, Shamp TR. Improved control of niacin-induced flushing using an optimized once-daily, extended-release niacin formulation. Int J Clin Pharmacol Ther. 2006;44(12):633-640 [DOI] [PubMed] [Google Scholar]
- 73.Guyton JR, Goldberg AC, Kreisberg RA, Sprecher DL, Superko HR, O'Connor CM. Effectiveness of once-nightly dosing of extended-release niacin alone and in combination for hypercholesterolemia. Am J Cardiol. 1998;82(6):737-743 [DOI] [PubMed] [Google Scholar]
- 74.Birjmohun RS, Hutten BA, Kastelein JJ, Stroes ES. Efficacy and safety of high-density lipoprotein cholesterol-increasing compounds: a meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2005;45(2):185-197 [DOI] [PubMed] [Google Scholar]
- 75.Wink J, Giacoppe G, King J. Effect of very-low-dose niacin on high-density lipoprotein in patients undergoing long-term statin therapy. Am Heart J. 2002;143(3):514-518 [DOI] [PubMed] [Google Scholar]
- 76.Elam MB, Hunninghake DB, Davis KB, et al. Effect of niacin on lipid and lipoprotein levels and glycemic control in patients with diabetes and peripheral arterial disease: the ADMIT study: a randomized trial. JAMA 2000;284(10):1263-1270 [DOI] [PubMed] [Google Scholar]
- 77.Brown BG, Bardsley J, Poulin D, et al. Moderate dose, three-drug therapy with niacin, lovastatin, and colestipol to reduce low-density lipoprotein cholesterol <100 mg/dl in patients with hyperlipidemia and coronary artery disease. Am J Cardiol. 1997;80(2):111-115 [DOI] [PubMed] [Google Scholar]
- 78.Mostaza JM, Schulz I, Vega GL, Grundy SM. Comparison of pravastatin with crystalline nicotinic acid monotherapy in treatment of combined hyperlipidemia. Am J Cardiol. 1997;79(9):1298-1301 [DOI] [PubMed] [Google Scholar]
- 79.O'Keefe JH, Jr, Harris WS, Nelson J, Windsor SL. Effects of pravastatin with niacin or magnesium on lipid levels and postprandial lipemia. Am J Cardiol. 1995;76(7):480-484 [DOI] [PubMed] [Google Scholar]
- 80.Vega GL, Grundy SM. Lipoprotein responses to treatment with lovastatin, gemfibrozil, and nicotinic acid in normolipidemic patients with hypoalphalipoproteinemia. Arch Intern Med. 1994;154(1):73-82 [PubMed] [Google Scholar]
- 81.King JM, Crouse JR, Terry JG, Morgan TM, Spray BJ, Miller NE. Evaluation of effects of unmodified niacin on fasting and postprandial plasma lipids in normolipidemic men with hypoalphalipoproteinemia. Am J Med. 1994;97(4):323-331 [DOI] [PubMed] [Google Scholar]
- 82.O'Kane MJ, Trinick TR, Tynan MB, Trimble ER, Nicholls DP. A comparison of acipimox and nicotinic acid in type 2b hyperlipidaemia. Br J Clin Pharmacol. 1992;33(4):451-453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Garg A, Grundy SM. Nicotinic acid as therapy for dyslipidemia in noninsulin-dependent diabetes mellitus. JAMA 1990;264(6):723-726 [PubMed] [Google Scholar]
- 84.Aronov DM, Keenan JM, Akhmedzhanov NM, Perova NV, Oganov RY, Kiseleva NY. Clinical trial of wax-matrix sustained-release niacin in a Russian population with hypercholesterolemia. Arch Fam Med. 1996;5(10):567-575 [DOI] [PubMed] [Google Scholar]
- 85.Vacek JL, Dittmeier G, Chiarelli T, White J, Bell HH. Comparison of lovastatin (20 mg) and nicotinic acid (1.2 g) with either drug alone for type II hyperlipoproteinemia. Am J Cardiol. 1995;76(3):182-184 [DOI] [PubMed] [Google Scholar]
- 86.Lavie CJ, Mailander L, Milani RV. Marked benefit with sustained-release niacin therapy in patients with “isolated” very low levels of high-density lipoprotein cholesterol and coronary artery disease. Am J Cardiol. 1992;69(12):1083-1085 [DOI] [PubMed] [Google Scholar]
- 87.Kuvin JT, Ramet ME, Patel AR, Pandian NG, Mendelsohn ME, Karas RH. A novel mechanism for the beneficial vascular effects of high-density lipoprotein cholesterol: enhanced vasorelaxation and increased endothelial nitric oxide synthase expression. Am Heart J. 2002;144(1):165-172 [DOI] [PubMed] [Google Scholar]
- 88.Davignon J, Roederer G, Montigny M, et al. Comparative efficacy and safety of pravastatin, nicotinic acid and the two combined in patients with hypercholesterolemia. Am J Cardiol. 1994;73(5):339-345 [DOI] [PubMed] [Google Scholar]
- 89.Poldermans D, Dunkelgrun M, Schouten O, Hostalek U. Prolonged-release nicotinic acid in patients with atherosclerotic disease in the Netherlands. Eur Surg Res. 2008;41(4):313-318 [DOI] [PubMed] [Google Scholar]
- 90.Rubenfire M. Safety and compliance with once-daily niacin extended-release/lovastatin as initial therapy in the Impact of Medical Subspecialty on Patient Compliance to Treatment (IMPACT) study. Am J Cardiol. 2004;94(3):306-311 [DOI] [PubMed] [Google Scholar]
- 91.Lin TH, Voon WC, Yen HW, et al. Randomized comparative study of the effects of treatment with once-daily, niacin extended-release/lovastatin and with simvastatin on lipid profile and fibrinolytic parameters in Taiwan. Kaohsiung J Med Sci. 2006;22(6):257-265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sharma M, Sharma DR, Singh V, et al. Evaluation of efficacy and safety of fixed dose lovastatin and niacin(ER) combination in Asian Indian dyslipidemic patients: a multicentric study. Vasc Health Risk Manag. 2006;2(1):87-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dubé MP, Wu JW, Aberg JA, et al. Safety and efficacy of extended-release niacin for the treatment of dyslipidaemia in patients with HIV infection: AIDS Clinical Trials Group Study A5148. Antivir Ther. 2006;11(8):1081-1089 [PMC free article] [PubMed] [Google Scholar]
- 94.Pan J, Van JT, Chan E, Kesala RL, Lin M, Charles MA. Extended-release niacin treatment of the atherogenic lipid profile and lipoprotein(a) in diabetes. Metabolism 2002;51(9):1120-1127 [DOI] [PubMed] [Google Scholar]
- 95.Kane MP, Hamilton RA, Addesse E, Busch RS, Bakst G. Cholesterol and glycemic effects of Niaspan in patients with type 2 diabetes. Pharmacotherapy 2001;21(12):1473-1478 [DOI] [PubMed] [Google Scholar]
- 96.Harikrishnan S, Rajeev E, Tharakan JA, et al. Efficacy and safety of combination of extended release niacin and atorvastatin in patients with low levels of high density lipoprotein cholesterol. Indian Heart J 2008;60:215-222 [PubMed] [Google Scholar]
- 97.Birjmohun RS, Kastelein JJ, Poldermans D, Stroes ES, Hostalek U, Assmann G. Safety and tolerability of prolonged-release nicotinic acid in statin-treated patients. Curr Med Res Opin. 2007;23(7):1707-1713 [DOI] [PubMed] [Google Scholar]
- 98.Vogt A, Kassner U, Hostalek U, Steinhagen-Thiessen E. Evaluation of the safety and tolerability of prolonged-release nicotinic acid in a usual care setting: the NAUTILUS study. Curr Med Res Opin. 2006;22(2):417-425 [DOI] [PubMed] [Google Scholar]
- 99.Guyton JR, Blazing MA, Hagar J, et al. Niaspan-Gemfibrozil Study Group Extended-release niacin vs gemfibrozil for the treatment of low levels of high-density lipoprotein cholesterol. Arch Intern Med. 2000;160(8):1177-1184 [DOI] [PubMed] [Google Scholar]
- 100.Chojnowska-Jezierska J, Adamska-Dyniewska H. Efficacy and safety of one-year treatment with slow-release nicotinic acid:onitoring of drug concentration in serum. Int J Clin Pharmacol Ther. 1998;36(6):326-332 [PubMed] [Google Scholar]
- 101.Luria MH. Effect of low-dose niacin on high-density lipoprotein cholesterol and total cholesterol/high-density lipoprotein cholesterol ratio. Arch Intern Med. 1988;148(11):2493-2495 [PubMed] [Google Scholar]
- 102.Gray DR, Morgan T, Chretien SD, Kashyap ML. Efficacy and safety of controlled-release niacin in dyslipoproteinemic veterans. Ann Intern Med. 1994;121(4):252-258 [DOI] [PubMed] [Google Scholar]
- 103.Squires RW, Allison TG, Gau GT, Miller TD, Kottke BA. Low-dose, time-release nicotinic acid: effects in selected patients with low concentrations of high-density lipoprotein cholesterol. Mayo Clin Proc. 1992;67(9):855-860 [DOI] [PubMed] [Google Scholar]
- 104.McKenney JM, McCormick LS, Weiss S, Koren M, Kafonek S, Black DM, Collaborative Atorvastatin Study Group A randomized trial of the effects of atorvastatin and niacin in patients with combined hyperlipidemia or isolated hypertriglyceridemia. Am J Med. 1998;104(2):137-143 [DOI] [PubMed] [Google Scholar]
- 105.Alderman JD, Pasternak RC, Sacks FM, Smith HS, Monrad ES, Grossman W. Effect of a modified, well-tolerated niacin regimen on serum total cholesterol, high density lipoprotein cholesterol and the cholesterol to high density lipoprotein ratio. Am J Cardiol. 1989;64(12):725-729 [DOI] [PubMed] [Google Scholar]
- 106.Jelesoff NE, Ballantyne CM, Xydakis AM, Chiou P, Jones PH, Guyton JR. Effectiveness and tolerability of adding ezetimibe to niacin-based regimens for treatment of primary hyperlipidemia. Endocr Pract. 2006;12(2):159-164 [DOI] [PubMed] [Google Scholar]
- 107.Gibbons LW, Gonzalez V, Gordon N, Grundy S. The prevalence of side effects with regular and sustained-release nicotinic acid. Am J Med. 1995;99(4):378-385 [DOI] [PubMed] [Google Scholar]
- 108.Mills E, Prousky J, Raskin G, et al. The safety of over-the-counter niacin: a randomized placebo-controlled trial. BMC Clin Pharmacol. 2003;3:4-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Martin-Jadraque R, Tato F, Mostaza JM, Vega GL, Grundy SM. Effectiveness of low-dose crystalline nicotinic acid in men with low high-density lipoprotein cholesterol levels. Arch Intern Med. 1996;156(10):1081-1088 [PubMed] [Google Scholar]
- 110.Lal SM, Hewett JE, Petroski GF, Van Stone JC, Ross G., Jr Effects of nicotinic acid and lovastatin in renal transplant patients: a prospective, randomized, open-labeled crossover trial. Am J Kidney Dis. 1995;25(4):616-622 [DOI] [PubMed] [Google Scholar]
- 111.Tsalamandris C, Panagiotopoulos S, Sinha A, Cooper ME, Jerums G. Complementary effects of pravastatin and nicotinic acid in the treatment of combined hyperlipidaemia in diabetic and non-diabetic patients. J Cardiovasc Risk 1994;1(3):231-239 [DOI] [PubMed] [Google Scholar]
- 112.Jacobson TA, Chin MM, Fromell GJ, Jokubaitis LA, Amorosa LF. Fluvastatin with and without niacin for hypercholesterolemia. Am J Cardiol. 1994;74(2):149-154 [DOI] [PubMed] [Google Scholar]
- 113.Jacobson TA, Amorosa LF. Combination therapy with fluvastatin and niacin in hypercholesterolemia: a preliminary report on safety. Am J Cardiol. 1994;73(14):25D-29D [DOI] [PubMed] [Google Scholar]
- 114.Schectman G, Hiatt J, Hartz A. Evaluation of the effectiveness of lipid-lowering therapy (bile acid sequestrants, niacin, psyllium and lovastatin) for treating hypercholesterolemia in veterans. Am J Cardiol. 1993;71(10):759-765 [DOI] [PubMed] [Google Scholar]
- 115.Kane JP, Malloy MJ, Ports TA, Phillips NR, Diehl JC, Havel RJ. Regression of coronary atherosclerosis during treatment of familial hypercholesterolemia with combined drug regimens. JAMA 1990;264(23):3007-3012 [PubMed] [Google Scholar]
- 116.Rindone JP, Arriola OG. Experience with crystalline niacin as the preferred drug for dyslipidemia in a specialty clinic. Pharmacotherapy 1997;17(6):1296-1299 [PubMed] [Google Scholar]
- 117.Gardner SF, Marx MA, White LM, Granberry MC, Skelton DR, Fonseca VA. Combination of low-dose niacin and pravastatin improves the lipid profile in diabetic patients without compromising glycemic control. Ann PharmacoTher. 1997;31(6):677-682 [DOI] [PubMed] [Google Scholar]
- 118.Duvall WL, Blazing MA, Saxena S, Guyton JR. Targeting cardiovascular risk associated with both low density and high density lipoproteins using statin-niacin combination therapy. J Cardiovasc Risk 2002;9(6):339-347 [DOI] [PubMed] [Google Scholar]
- 119.Taher TH, Dzavik V, Reteff EM, Pearson GJ, Woloschuk BL, Francis GA. Tolerability of statin-fibrate and statin-niacin combination therapy in dyslipidemic patients at high risk for cardiovascular events. Am J Cardiol. 2002;89(4):390-394 [DOI] [PubMed] [Google Scholar]
- 120.Olsen S, Smith S, Oei T, Douglas J. Health belief model predicts adherence to CPAP before experience with CPAP. Eur Respir J. 2008;32(3):710-717 [DOI] [PubMed] [Google Scholar]
- 121.Wai CT, Wong ML, Ng S, et al. Utility of the Health Belief Model in predicting compliance of screening in patients with chronic hepatitis B. Aliment Pharmacol Ther. 2005;21(10):1255-1262 [DOI] [PubMed] [Google Scholar]
- 122.Rosenstock IM, Strecher VJ, Becker MH. Social learning theory and the Health Belief Model. Health Educ Q. 1988;15(2):175-183 [DOI] [PubMed] [Google Scholar]
- 123.Becker MH, Radius SM, Rosenstock IM, Drachman RH, Schuberth KC, Teets KC. Compliance with a medical regimen for asthma: a test of the health belief model. Public Health Rep. 1978;93(3):268-277 [PMC free article] [PubMed] [Google Scholar]
- 124.Moon YS, Kashyap ML. Niacin extended-release/lovastatin: combination therapy for lipid disorders. Expert Opin PharmacoTher. 2002;3(12):1763-1771 [DOI] [PubMed] [Google Scholar]
- 125.Guyton JR, Bays HE. Safety considerations with niacin therapy. Am J Cardiol. 2007;99(6A):22C-31C [DOI] [PubMed] [Google Scholar]
- 126.Brown WV, Goldberg AC, Guyton JR, Knopp RH. Clinical lipidology roundtable: the use of niacin. J Clin Lipidol. 2009;3:65-69 [DOI] [PubMed] [Google Scholar]
- 127.American Society of Health-System Pharmacists ASHP Therapeutic Position Statement on the safe use of niacin in the management of dyslipidemias. Am J Health Syst Pharm. 1997;54(24):2815-2819 [DOI] [PubMed] [Google Scholar]
- 128.Benner JS, Tierce JC, Ballantyne CM, et al. Follow-up lipid tests and physician visits are associated with improved adherence to statin therapy. Pharmacoeconomics 2004;22(Suppl 3):13-23 [DOI] [PubMed] [Google Scholar]
- 129.Cefali EA, Simmons PD, Stanek EJ, McGovern ME, Kissling CJ. Aspirin reduces cutaneous flushing after administration of an optimized extended-release niacin formulation. Int J Clin Pharmacol Ther. 2007;45(2):78-88 [DOI] [PubMed] [Google Scholar]
- 130.Thakkar RB, Kashyap ML, Lewin AJ, Krause SL, Jiang P, Padley RJ. Acetylsalicylic acid reduces niacin extended-release-induced flushing in patients with dyslipidemia. Am J Cardiovasc Drugs 2009;9(2):69-79 [DOI] [PubMed] [Google Scholar]
- 131.Alves JD, Steinhagen-Thiessen E, Darioli R, Hostalek U, Vogt A. Influence of the timing of low-dose aspirin on tolerability of prolonged-release nicotinic acid in patients at elevated cardiovascular risk. Curr Med Res Opin. 2008;24(10):2815-2820 [DOI] [PubMed] [Google Scholar]
- 132.Jungnickel PW, Maloley PA, Vander Tuin EL, Peddicord TE, Campbell JR. Effect of two aspirin pretreatment regimens on niacin-induced cutaneous reactions. J Gen Intern Med. 1997;12(10):591-596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dunn RT, Ford MA, Rindone JP, Kwiecinski FA. Low-dose aspirin and ibuprofen reduce the cutaneous reactions following niacin administration. Am J Ther. 1995;2(7):478-480 [DOI] [PubMed] [Google Scholar]
- 134.Whelan AM, Price SO, Fowler SF, Hainer BL. The effect of aspirin on niacin-induced cutaneous reactions. J Fam Pract. 1992;34(2):165-168 [PubMed] [Google Scholar]
- 135.Oberwittler H, Baccara-Dinet M. Clinical evidence for use of acetyl salicylic acid in control of flushing related to nicotinic acid treatment. Int J Clin Pract. 2006;60(6):707-715 [DOI] [PubMed] [Google Scholar]
- 136.Lai E, Wenning LA, Crumley TM, et al. Pharmacokinetics, pharmacodynamics, and safety of a prostaglandin D2 receptor antagonist. Clin Pharmacol Ther. 2008;83(6):840-847 [DOI] [PubMed] [Google Scholar]
- 137.Lai E, DeLepeleire I, Crumley TM, et al. Suppression of niacin-induced vasodilation with an antagonist to prostaglandin D2 receptor subtype 1. Clin Pharmacol Ther. 2007;81(6):849-857 [DOI] [PubMed] [Google Scholar]
- 138.Dishy V, Liu F, Ebel DL, et al. Effects of aspirin when added to the prostaglandin D2 receptor antagonist laropiprant on niacin-induced flushing symptoms. J Clin Pharmacol. 2009;49(4):416-422 [DOI] [PubMed] [Google Scholar]
- 139.Paolini JF, Mitchel YB, Reyes R, et al. Effects of laropiprant on nicotinic acid-induced flushing in patients with dyslipidemia. Am J Cardiol. 2008;101(5):625-630 [DOI] [PubMed] [Google Scholar]
- 140.Maccubbin D, Bays HE, Olsson AG, et al. Lipid-modifying efficacy and tolerability of extended-release niacin/laropiprant in patients with primary hypercholesterolaemia or mixed dyslipidaemia. Int J Clin Pract. 2008;62(12):1959-1970 [DOI] [PubMed] [Google Scholar]
- 141.Paolini JF, Bays HE, Ballantyne CM, et al. Extended-release niacin/laropiprant: reducing niacin-induced flushing to better realize the benefit of niacin in improving cardiovascular risk factors. Cardiol Clin. 2008;26(4):547-560 [DOI] [PubMed] [Google Scholar]
- 142.Maccubbin D, Koren MJ, Davidson M, et al. Flushing profile of extended-release niacin/laropiprant versus gradually titrated niacin extended-release in patients with dyslipidemia with and without ischemic cardiovascular disease. Am J Cardiol. 2009;104(1):74-81 [DOI] [PubMed] [Google Scholar]
- 143.Ding RW, Kolbe K, Merz B, de Vries J, Weber E, Benet LZ. Pharmacokinetics of nicotinic acid-salicylic acid interaction. Clin Pharmacol Ther. 1989;46(6):642-647 [DOI] [PubMed] [Google Scholar]
- 144.EMEA CHMP Assessment Report for Tredaptive: European Medicines Agency, Evaluation of Medicines for Human Use Doc. Ref. #: EMEA/348364/2008 ed. London, UK: European Medicines Agency; 2008. [Google Scholar]
- 145.Kalogeromitros D, Makris M, Chliva C, Aggelides X, Kempuraj D, Theoharides TC. A quercetin containing supplement reduces niacin-induced flush in humans. Int J Immunopathol Pharmacol. 2008;21(3):509-514 [DOI] [PubMed] [Google Scholar]
- 146.Papaliodis D, Boucher W, Kempuraj D, Theoharides TC. The flavonoid luteolin inhibits niacin-induced flush. Br J Pharmacol. 2008;153(7):1382-1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shen HC, Ding FX, Deng Q, et al. Discovery of novel tricyclic full agonists for the G-protein-coupled niacin receptor 109A with minimized flushing in rats. J Med Chem. 2009;52(8):2587-2602 [DOI] [PubMed] [Google Scholar]
- 148.Meier AS, Richardson BA, Hughes JP. Discrete proportional hazards models for mismeasured outcomes. Biometrics 2003;59(4):947-954 [DOI] [PubMed] [Google Scholar]
- 149.Turnbull BW. The empirical distribution function with arbitrarily grouped, censored, and truncated data. J R Stat Soc B. 1976;38:290-295 [Google Scholar]
- 150.Sagar KA, Smyth MR. A comparative bioavailability study of different aspirin formulations using on-line multidimensional chromatography. J Pharm Biomed Anal. 1999;21:383-392 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.