Abstract
Fifty human immunodeficiency virus (HIV)-infected children participated in an area-under-the plasma concentration–time curve (AUC)-controlled trial of efavirenz and nelfinavir. Pharmacokinetic evaluations were performed at weeks 2, 6, and 56. Efavirenz and nelfinavir doses were adjusted to achieve AUC values of 60–120 and ≥10mgh/l, respectively. Thirty-seven (74%) children met the efavirenz target and 41 (82%) the nelfinavir by week 10. Children with AUC values for both drugs above the first quartile were more likely to reach <400 copies/ml of HIV RNA at week 8. Efavirenz and nelfinavir oral clearance increased 37 and 62% from weeks 2 to 56, respectively, in 34 children who continued on therapy at week 56. AUC values at week 56 were not different between children who did or did not have HIV RNA <400 copies/ml. Dose adjustment to achieve specific AUC values in these children reduced the risk of suboptimal exposure and achieved high rates of virologic suppression.
We designed an open-label evaluation of the pharmacokinetics, safety, and anti-human immunodeficiency virus (HIV) effect of efavirenz and nelfinavir, in combination with nucleoside reverse transcriptase inhibitors (NRTIs), in HIV-infected children. This trial employed a concentration-controlled paradigm to adjust the doses of efavirenz and nelfinavir to meet predetermined area-under-the plasma concentration–time curve (AUC) target ranges. The safety and anti-viral effect of this study have been previously reported.1 This paper reports the pharmacologic characteristics of efavirenz and nelfinavir in the HIV-infected children who participated in this trial.
RESULTS
Pharmacokinetic studies for both efavirenz and nelfinavir were performed on 50 children at weeks 2 and 6. At week 2, the mean and median age of these children were 7.9 and 7 years, respectively, with a range of 3–16 years. These children had a mean and median body weight of 28.1 and 24 kg, respectively, with a range of 13.4–98 kg; the mean and median body surface area were 1 and 0.9m2 and ranged from 0.6 to 2.1m2. Sixteen children discontinued the study before or at week 48: 10 children completed the initial 48-week study and chose not to continue past this primary observation end point; three children met the end point for virologic failure before week 48; one child was withdrawn before week 48 at the request of the family; one child was lost to follow-up before week 48; and one child had to be withdrawn at week 48 because of the lack of availability of the study drug. Thirty-four children remained on study at week 56, and pharmacokinetic evaluations for efavirenz and nelfinavir were repeated. The physical characteristics of these 34 children were (mean, median, and range): age, 8.5, 8, and 5–17 years; weight, 29.9, 26.2, and 16.6–93.7 kg; body surface area, 1, 1, and 0.7–2m2.
In view of the week-2 pharmacokinetic studies, an increase in the efavirenz dose was recommended for 22 children; and a decrease in the dose was recommended for three children. Following the week-6 pharmacokinetic evaluation, an increase in the efavirenz dose was recommended for nine children and a decrease recommended for six children. The dose of nelfinavir was increased in two children following week 2, and in one child after the week-6 pharmacokinetic studies. The number (and percentage) of children who achieved the desired targets for efavirenz was as follows: week 2, 22 of 50 (44%) and week 6, 28 of 48 evaluable AUCs (58%). For nelfinavir, these rates were as follows: week 2, 37 of 45 evaluable AUCs (82%) and week 6, 40 of 50 (80%). At week 10, AUCs were obtained for 13 children. Six achieved the desired target for efavirenz, and three additional children had values less than 10% from the lower threshold and therefore were viewed as having acceptable AUCs. Combined with those who had achieved the target at week 6, this would yield a cumulative rate of 37 (74%) children who had efavirenz AUC values within the target range by week 10. One child had a dose increase of nelfinavir after the week-6 pharmacokinetic study and achieved the target AUC at the week-10 evaluation. This would give a rate of 41 (82%) children who achieved the target nelfinavir AUC at week 10. On the basis of evaluations performed at week 10 in 13 children, the doses of efavirenz were increased in five children and decreased in one, and the dose of nelfinavir was increased in one child. Repeat pharmacokinetic evaluations were not performed based on considerations of the small distance from the target values and the number of evaluations already performed.
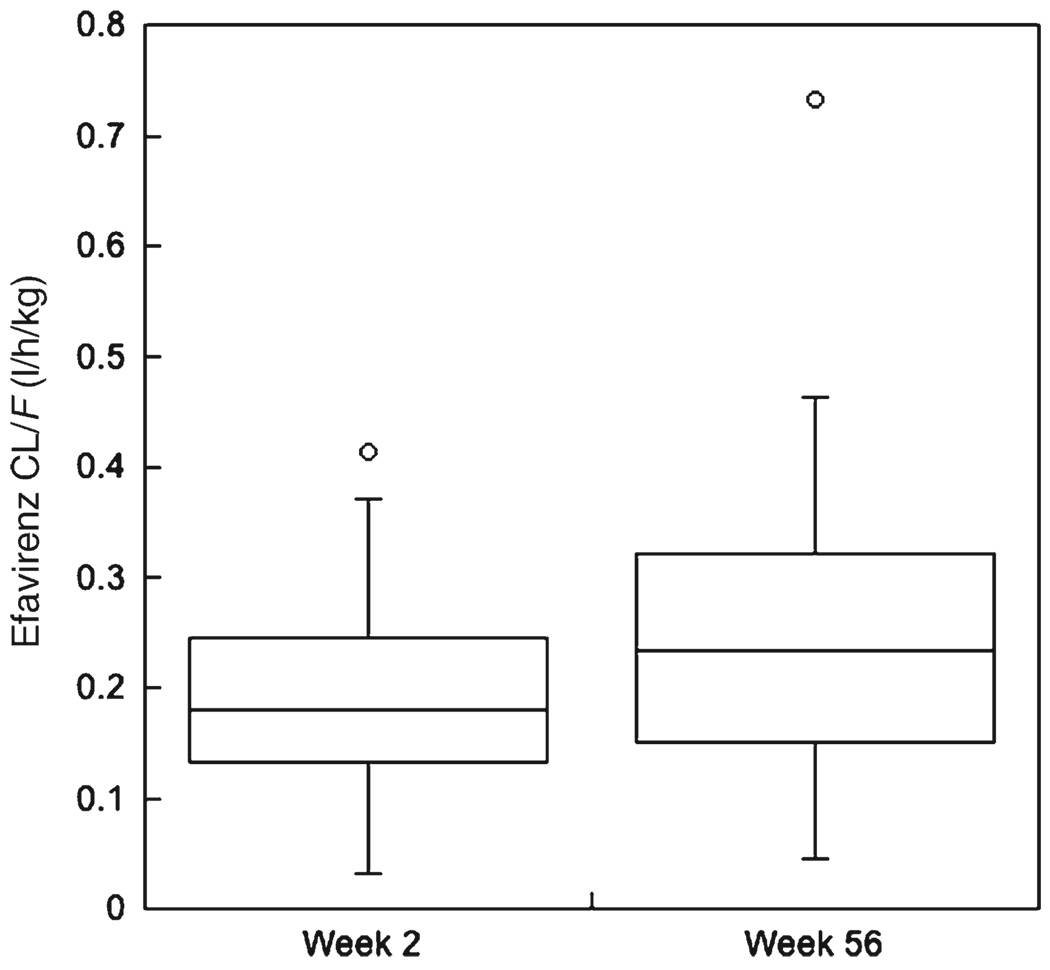
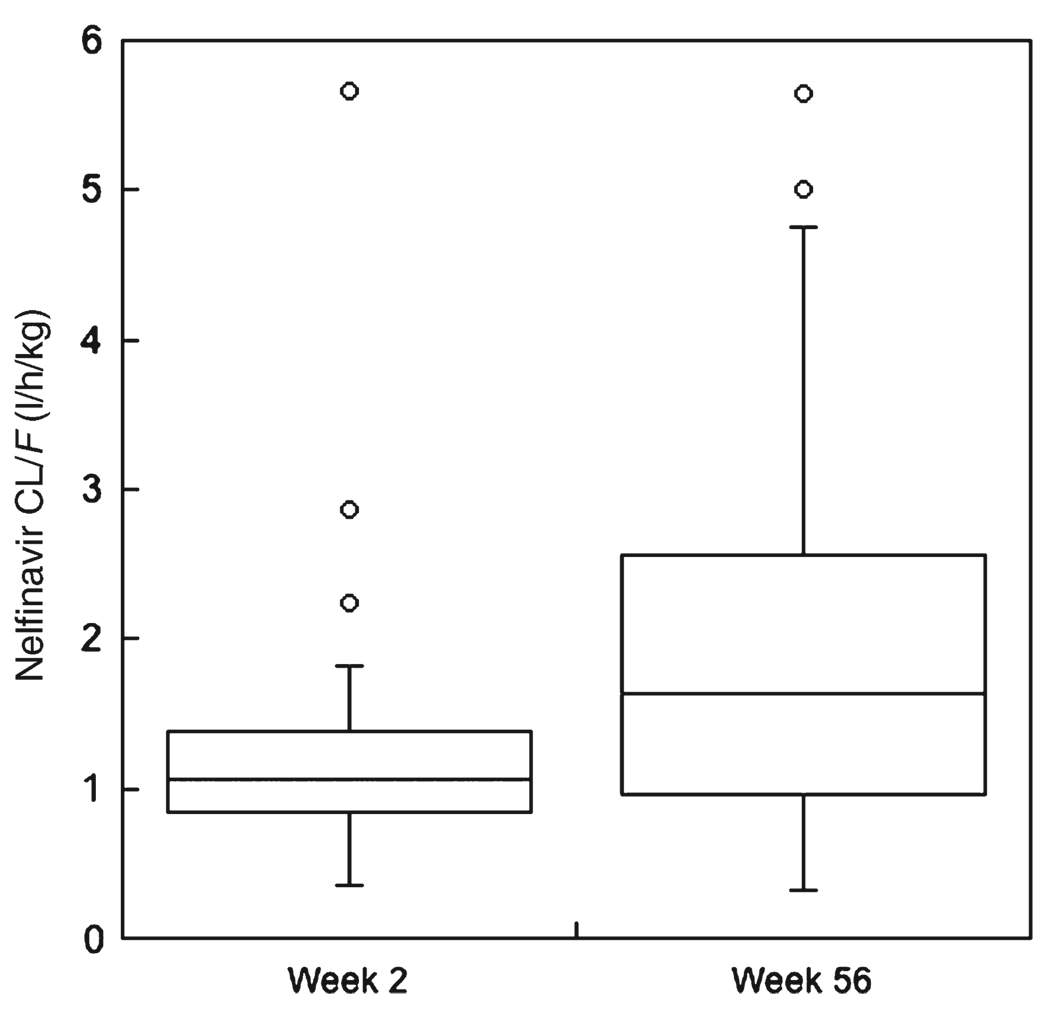
Table 1 and Table 2 present the pharmacokinetic characteristic s of efavirenz and nelfinavir obtained at weeks 2 and 56. Figure 1 and Figure 2 show the apparent oral clearance (CL/F) of efavirenz and nelfinavir only in the 34 children who had pharmacokinetic evaluations at weeks 2, 6, and 56. These figures illustrate statistically significant (P<0.001) increases in CL/F for both drugs between weeks 2 and 56. For efavirenz, CL/F increased an average 37% from a week 2 mean of 0.19 l/h/kg to a week-56 mean of 0.26 l/h/kg. Nelfinavir CL/F increased 62% between weeks 2 and 56, from a mean of 1.3 l/h/kg to a mean of 2.1 l/h/kg.
Table 1.
Week-2 and -56 pharmacokinetic characteristics of efavirenz
| Dose | CL/F | |||||||
|---|---|---|---|---|---|---|---|---|
| AUC24 | Cmin | Cmax | ||||||
| Statistic | (mg) | (mg/kg) | (mg/m2) | (mg h/l) | (mg/l) | (mg/l) | (l/h/kg) | (l/h/m2) |
| Efavirenz, n=50 | ||||||||
| Mean | 310 | 12 | 318 | 76 | 2.09 | 4.73 | 0.21 | 5.51 |
| Median | 275 | 12 | 317 | 60 | 1.45 | 4.09 | 0.19 | 5.37 |
| SD | 102 | 1.4 | 14.4 | 58 | 2.16 | 2.76 | 0.09 | 2.42 |
| %CV | 33 | 12 | 5 | 77 | 103.1 | 58.3 | 45.7 | 43.8 |
| Minimum | 200 | 8 | 288 | 26 | 0.11 | 1.76 | 0.03 | 0.74 |
| Maximum | 750 | 15 | 359 | 414 | 13.88 | 19.25 | 0.42 | 11.53 |
| Efavirenz, n=34 | ||||||||
| Mean | 379 | 14 | 376 | 67 | 1.64 | 4.39 | 0.26 | 7.34 |
| Median | 400 | 13 | 367 | 60 | 1.30 | 4.55 | 0.23 | 5.95 |
| SD | 124 | 5 | 116 | 33 | 1.27 | 1.73 | 0.20 | 6.18 |
| %CV | 33 | 36 | 31 | 59 | 77.3 | 39.5 | 74.3 | 84.1 |
| Minimum | 50 | 3 | 69 | 9 | BLQ | 0.90 | 0.05 | 1.71 |
| Maximum | 750 | 23 | 559 | 176 | 6.10 | 9.00 | 1.07 | 36.24 |
AUC24, area under the plasma concentration–time curve from time 0 to 24 h post-dose; BLQ, below the limit of quantitation; Cmin, minimum measured concentration; CL/F, apparent oral clearance; Cmax, observed maximum concentration; %CV, % coefficient of variation.
Table 2.
Week-2 and -56 pharmacokinetic characteristics of nelfinavir
| Dose | CL/F | |||||||
|---|---|---|---|---|---|---|---|---|
| AUC8 | Cmin | Cmax | ||||||
| Statistic | (mg) | (mg/kg) | (mg/m2) | (mg h/l) | (mg/l) | (mg/l) | (l/h/kg) | (l/h/m2) |
| Nelfinvair, n=50 | ||||||||
| Mean | 606 | 24 | 637 | 22 | 1.44 | 4.15 | 2.32 | 61.8 |
| Median | 500 | 24 | 636 | 20 | 1.07 | 3.90 | 1.11 | 30.8 |
| SD | 147 | 5 | 102 | 13 | 1.39 | 2.15 | 4.49 | 110.4 |
| %CV | 24.3 | 21.1 | 16.0 | 59.5 | 96.4 | 51.7 | 193.8 | 178.8 |
| Minimum | 250 | 8 | 274 | 1 | BLQ | 0.28 | 0.36 | 13.6 |
| Maximum | 750 | 30 | 786 | 50 | 6.12 | 8.85 | 29.16 | 705.8 |
| Efavirenz, n=33a | ||||||||
| Mean | 648 | 24 | 656 | 16 | 0.87 | 3.05 | 2.07 | 55.0 |
| Median | 750 | 24 | 652 | 15 | 0.35 | 2.80 | 1.64 | 45.6 |
| SD | 128 | 6 | 120 | 8 | 0.95 | 1.20 | 1.38 | 33.9 |
| %CV | 19.7 | 24.4 | 18.4 | 50.3 | 108.7 | 39.3 | 66.8 | 61.6 |
| Minimum | 400 | 8 | 368 | 4 | BLQ | 1.2 | 0.32 | 14.6 |
| Maximum | 750 | 41 | 987 | 34 | 3.70 | 6.00 | 5.64 | 143.8 |
AUC, area under the plasma concentration–time curve from time 0 to 8 h post-dose; BLQ, below the limit of quantitation; Cmin, minimum measured concentration; CL/F, apparent oral clearance; Cmax, observed maximum concentration; %CV, % coefficient of variation.
One AUC was not evaluable.
Figure 1.
Box plot of efavirenz apparent oral clearance (CL/F, in l/h/kg) at weeks 2 and 56. CL/F at week 56 was significantly faster (P < 0.001, signed-rank test) than at week 2.
Figure 2.
Box plot of nelfinavir apparent oral clearance (CL/F, in l/h/kg) at weeks 2 and 56. CL/F at week 56 was significantly faster (P < 0.001, signed-rank test) than at week 2.
Table 3 presents a comparison of efavirenz and nelfinavir pharmacokinetic characteristics in those children who had ≤ 400 versus > 400 copies/ml of HIV RNA at week 8. This comparison shows that the week-2 and -6 AUC of efavirenz was related to virologic response, whereas only the week-2 AUC for nelfinavir was related to response. Quantitative exposure–response thresholds for these relationships were best discriminated by AUC values at week 2 above or below the first quartile. Only 50% of children who had efavirenz AUC values less than the first quartile (49 mg h/l) reached less than 400 copies/ml of HIV RNA by week 8 compared with 86% of children who had AUC values greater than 49 mg h/l (P = 0.01). Similarly, 42% of children with nelfinavir AUC values less than the first quartile (10 mg h/l) had an undetectable level of HIV RNA at week 8 compared with 89% of those who had AUC values above the first quartile (P < 0.001). Finally, logistic regression found that the week-2 AUC values for efavirenz and nelfinavir, with each drug adjusted for the effect of the other, had independent contributions to predicting an undetectable HIV RNA at week 8. The adjusted odds ratios (and 95% confidence intervals) were efavirenz AUC (per 16 mg h/l), 2.29 (1.10–4.77), and nelfinavir AUC (per 5 mg h/l), 1.54 (1.04–2.28 ). Table 4 gives the pharmacokinetic characteristics of efavirenz and nelfinavir in the 34 subjects who continued on the study at week 56 and comparisons with those who had plasma HIV RNA levels ≤ 400 versus > 400 copies/ml and by change in CD4 cell count from baseline to week 48.
Table 3.
Comparison of efavirenz and nelfinavir pharmacokinetic characteristics with the proportion of children ≤ 400 vs > 400 copies/ml of HIV RNA at week 8
| Efavirenz | Nelfinavir | |||
|---|---|---|---|---|
| Plasma HIV RNA at week 8 | AUC24 (mg h/l) | C24 (mg/l) | AUC8 (mg h/l) | C8 (mg/l) |
| Pharmacokinetic parameters at week 2 | ||||
| ≤ 400 copies/ml (n=37) | 69.4 | 1.9 | 21.4 | 2.8 |
| > 400 copies/ml (n=12) | 43.5 | 1.3 | 6.9 | 1.5 |
| P-valuea | 0.002 | 0.01 | 0.01 | 0.038 |
| Pharmacokinetic parameters at week 6 | ||||
| ≤ 400 copies/ml (n=37) | 72.2 | 1.8 | 20.8 | 2.8 |
| > 400 copies/ml (n=12) | 46.0 | 1.9 | 18.4 | 1.6 |
| P-valuea | 0.07 | 0.36 | 0.36 | 0.11 |
AUC24, area under the plasma concentration–time curve from time 0 to 24 h post-dose; AUC8, area under the plasma concentration–time curve from time 0 to 8 h post-dose; C24, measured concentration 24 h post-dose; C8, measured concentration 8 h post-dose; HIV, human immunodeficiency virus.
Wilcoxon rank-sum test.
Table 4.
Comparison of efavirenz and nelfinavir pharmacokinetic characteristics at week 56 with the proportion of children with HIV RNA ≤ 400 and > 400 copies/ml at week 48
| AUC24 (mg h/l) |
C24(mg/l) |
Cmax (mg/l) |
CL/F (l/h/kg) | % Δ CL/F weeks 2–56 |
No. at target AUC (%) |
|
|---|---|---|---|---|---|---|
| Efavirenz pharmacokinetic characteristics (mean and range) at week 56 | ||||||
| Plasma HIV RNA at week 48 (N) | ||||||
| ≤ 400 copies/ml (n=27) | 68 (25–176) | 1.66 (0.20–6.20) | 4.55 (1.50–9.00) | 0.24 (0.05–0.73) | 34 (−45–181) | 14 (52%) |
| >400 copies/ml (n=7) | 64 (9–148) | 1.58 (BLQ–4.40) | 3.74 (0.90–6.80) | 0.37 (0.08–1.07) | 63 (−75–227) | 2 (29%) |
| P-value | 0.55 | 0.97 | 0.34 | 0.39 | 0.33 | 0.41* |
| CD4 cell difference from weeks 0 to 48 | ||||||
| P-value | 0.12 | 0.73 | 0.07 | 0.32 | 0.24 | |
|
AUC8 (mg h/l) |
C8 (mg/l) |
Cmax (mg/l) |
CL/F (l/h/kg) |
% Δ CL/F week 2–56 |
No. at AUC target (%) |
|
| Nelfinavir pharmacokinetic characteristics (mean and range) at week 56 | ||||||
| Plasma HIV RNA at week 48 (N) | ||||||
| ≤ 400 copies/ml (n=27) | 16 (4–34) | 0.8 (BLQ–3.8) | 3.0 (1.2–6.0) | 1.80 (0.40–5.60) | 80 (−33–475) | 22 (81%) |
| > 400 copies/ml (n=6)a | 14 (5–27) | 0.6 (BLQ–2.0) | 3.2 (1.2–4.8) | 2.96 (0.86–5.00) | 91 (−53–258) | 3 (50%) |
| P-value | 0.44 | 0.42 | 0.85 | 0.20 | 0.78 | 0.14* |
| CD4 cell difference from weeks 0 to 48 | ||||||
| P-value | 0.11 | 0.52 | 0.31 | 0.05 | 0.01 | |
AUC24, area under the plasma concentration–time curve from time 0 to 24 h post-dose; AUC8, area under the plasma concentration–time curve from time 0 to 8 h post-dose; BLQ, below the limit of quantitation; C24, measured concentration 24 h post-dose; C8, measured concentration 8 h post-dose; HIV, human immunodeficiency virus; CL/F, apparent oral clearance; Cmax, observed maximum concentration.
All P-values are Wilcoxon rank-sum test except those designated with an *, which is from the Fisher‘s Exact test.
One AUC for nelfinavir was not evaluable in the > 400copies/ml group.
DISCUSSION
The primary objectives of this study were to determine a dosing regimen for efavirenz, given in combination with nelfinavir and at least one NRTI, in children and to evaluate the safety and virologic responses of this combination antiretroviral regimen. Before the initiation of this study, efavirenz had not been administered to children. Thus, this study had phase I objectives of establishing the pharmacokinetic profile and pediatric dose of efavirenz and phase II objectives of evaluating safety and efficacy. To meet these objectives, this study employed an AUC-controlled strategy for both efavirenz and nelfinavir. The target AUC range for efavirenz was selected to find a dose of efavirenz that achieved concentrations at least equivalent to those of the “ average” adult receiving a dose shown to be safe and associated with a significant virologic response. For nelfinavir, the target AUC choice differed from that of efavirenz because nelfinavir was already approved by the US Food and Drug Administration (FDA) for treatment of children and adults; and thus, theoretically, there was no need to find the right dose for children, but rather the objective was to set a “safety net” to identify children who had unacceptably low concentrations and may be at an increased risk for a suboptimal virologic response. At week 10, 74 and 82% of children in this study had AUC values in the target range for efavirenz and nelfinavir, respectively. These data, in conjunction with previously published virologic response data, indicating that 81% of children on treatment achieved a viral load of less than 400 copies/ml at week 48 (70% had less than 50 copies/ml) and that the regimen was safe and well tolerated, provide support to conclude that this study met the intended objectives.1,2
The pharmacokinetic characteristics found for efavirenz and nelfinavir in these children indicated two important differences compared with adults. First, the mean week-2 CL/F for efavirenz of 0.21 l/h/kg is approximately 1.5-fold faster than in adults, and for nelfinavir, the mean week-2 CL/F of 2.32 l/h/kg is approximately 3.4-fold faster. These data reinforce that children are pharmacokinetically distinct from adults. For efavirenz and nelfinavir, simply scaling adult doses to children on a body weight basis would have substantially underdosed these children, and highlights the need for pediatric-specific dosing recommendations. The pharmacokinetic characteristics of nelfinavir in these children compared well with those reported in other smaller studies.3 Second, in the group of 34 children who had pharmacokinetic evaluations performed at both weeks 2 and 56, there were significant changes in their pharmacokinetic characteristics for both efavirenz and nelfinavir over this 1-year period. The CL/F of efavirenz increased by 37%, whereas that for nelfinavir increased by 62%. The physiologic change in these children that resulted in an increase in CL/F for efavirenz and nelfinavir between weeks 2 and 56 cannot be identified with certainty. However, the pharmacokinetic evidence points to a change in metabolic capacity rather than bioavailability. For example, the 34 children who had pharmacokinetic evaluations at both weeks 2 and 56 had a mean efavirenz elimination half-life at week 2 of 40.9 h and at week 56 of 29.3 h. Had the increase in CL/F been due to a change in bioavailability and not metabolic capacity, the half-life would have remained unchanged. These data indicate a need for an improved understanding of the ontogeny of the drug-metabolizing pathways involved in efavirenz and nelfinavir clearance. A contribution of other factors to the change in drug clearance over time, such as HIV infection itself and associated opportunistic infections, cannot be excluded. These data indicate that age- or weight-based (doses in this study were adjusted if weight changed by 25%) dosing strategies may not reliably capture developmental changes in drug disposition and that ongoing monitoring of plasma concentrations for drugs such as antiretroviral agents where there are profound consequences of therapeutic failure, is necessary.
Those children who reached undetectable HIV RNA at week 8 had higher efavirenz and nelfinavir AUCs and trough concentrations at week 2 than those who had HIV RNA levels > 400 copies/ml. These exposure–response relationships were supported by analyses showing that significantly more children who had AUC values for efavirenz and nelfinavir greater than the first quartile had HIV RNA ≤ 400 copies/ml, and additionally by logistic regression analyses showing that the week-2 AUC values had independent contributions to predicting an undetectable HIV RNA at week 8. These findings are consistent with other literature demonstrating exposure–response relationships and treatment guidelines for minimum trough concentrations of efavirenz and nelfinavir for antiretroviral naive adults.4 For example, efavirenz trough concentrations < 1 mg/l were associated with a 50% rate of virologic failure versus a 22% rate when concentrations were between 1 and 4 mg/l in a population of 130 adult patients receiving antiretroviral therapy for more than 3 months.5 Morning trough concentrations of nelfinavir less than 1.4mg/l (also expressed as a concentration ratio of less than 0.9) were found to increase significantly the risk of virologic failure.6 A prospective study of nelfinavir therapeutic drug monitoring in 92 adult patients demonstrated an improved virologic response after 1 year of follow-up with 81% of subjects who received therapeutic drug monitoring having HIV RNA < 500 copies/ml compared with 59% of those who received standard dose therapy.7
The number (and percent) of children who achieved the desired targets for efavirenz and nelfinavir at the week-56 pharmacokinetic evaluation were 16 (47%) and 25 (74%), respectively. There was no difference in the proportion of children who achieved the target and had or did not have HIV RNA < 400 copies/ml, although these comparisons were limited by the small sample size, and any child with an HIV RNA > 10,000 copies/ml at week 48 was excluded from further participation. The change in CD4 cell count from baseline to week 48 was weakly associated with efavirenz Cmax and nelfinavir CL/F at week 56. The proportion of children who achieved the target AUC values at week 56 was less than that at week 10, and can be attributed to significant, and unexpected, increases in efavirenz and nelfinavir oral clearance. There was also no difference in pharmacokinetic characteristics at week 56 between those children with HIV RNA above and below 400 copies/ml. These data suggest that the cause of viremia in these children likely cannot be attributed to differences in pharmacokinetics. One logical consideration is poor adherence. We have previously shown in children in this study that those who had high variability in their monthly random efavirenz concentrations over time, as a putative metric of low adherence, were significantly more likely to experience virologic rebound.8
The antiretroviral regimen and innovative dosing strategies used in this study demonstrated a high rate of virologic success in these HIV-infected children. A comparison of pediatric antiretroviral therapy studies found that those in which doses were adjusted based on measured concentrations of antiretroviral drugs resulted in superior virologic responses compared with those that used fixed-dose regimens. 9 This study accomplished its objectives without prior pharmacokinetic knowledge of efavirenz in children, by using an AUC-controlled paradigm and targeting adult levels of systemic exposure (but not adult doses). This approach was also extended to nelfinavir; although there was an FDA-approved dose for children, there was quite limited pediatric experience. Collectively, the pediatric HIV therapeutic experience advances an argument for therapeutic drug monitoring of antiretroviral agents in this population. In fact, such a recommendation is included in the British HIV Association treatment guidelines and discussed in those of the USA Working Group on Medical Management of HIV-Infected Children.10,11 Knowledge of genetic differences in drug-metabolizing enzymes will improve our understanding of interpatient variability in pharmacokinetics and pharmacodynamics. For example, recent data for efavirenz in children indicate that the CYP2B6-G516T polymorphism accounts for a 2.3-fold difference in clearance on a l/h/m2 basis.12 The integration of pharmacogenomics with therapeutic drug monitoring might improve the application and usefulness of this strategy.
The challenges of antiretroviral therapy, indeed all pharmacologic interventions in children, are not trivial and require, for example, the availability of suitable pediatric formulations, knowledge of age-specific pharmacokinetic characteristics, and the ability to account for the effects of developmental changes in drug pharmacokinetics over time. We believe that the strategy described here can be extended to other antiretroviral agents and other drugs to increase the effectiveness and efficiency of pediatric drug development.
METHODS
Human subjects and study design
Eligibility criteria included age of 3–16 years; plasma HIV-1 RNA > 400 copies/ml using the reverse transcription-polymerase chain reaction (Amplicor™) HIV Monitor assay; non-nucleoside reverse transcriptase inhibitor- and protease inhibitor-naive; receipt of at least one NRTI; and the ability to swallow capsules. History and physical examination were obtained at baseline and weeks 2, 4, 6, 8, and every 4 weeks thereafter. Specimens for plasma HIV-1 RNA, lymphocyte subsets, complete blood count, and differential, chemistries, and urinalysis were obtained at baseline and weeks 2, 4, 8, and every 4 weeks thereafter. The primary virologic end point was the proportion of children who had plasma HIV RNA ≤ 400 copies/ml at week 48. Children who had not met the definition of virologic failure were offered the opportunity to participate in an extension of the study for a total of 208 weeks. Virologic failure was defined as less than a 1 log10 drop in plasma HIV RNA copy number by week 12 after study treatment was initiated or HIV RNA in plasma > 10,000 copies/ml in a patient who previously achieved plasma HIV RNA < 400 copies/ml, confirmed by a second measurement within 30 days. The Division of AIDS Toxicity Table for Grading Severity of Pediatric Adverse Experiences (0, none; 1, mild; 2, moderate; 3, severe; and 4, life-threatening) was used. Criteria for treatment discontinuation included a single grade 4 or repeated grade 3 toxicity attributable to study treatment or poor compliance (< 80% of efavirenz or nelfinavir consumed over a 1-month period). This trial was conducted at 18 Pediatric AIDS Clinical Trials Group (PACTG) sites. Institutional Review Boards at each participating site approved the study. Written informed consent was obtained from parents/guardians of participating children.
Target AUC strategies
The target 24-h AUC (AUC24) for efavirenz was 60–120 mg h/l (190–380 µmh; 1 µm = 0.315 mg/l). This range represents the 50th to twice the 50th percentile of AUC24 values in adults receiving the recommended dose of 600 mg/day.13 This range was chosen to find a dose of efavirenz for administration in children, which would achieve systemic concentrations that were not less than the typical (average) adult. For nelfinavir, the target 8-h AUC (AUC8) was ≥ 10 mg h/l, which is approximately the 10th percentile for adults receiving 750 mg thrice daily and children receiving the recommended dose of 20–30 mg/kg thrice daily.14 The 10th percentile was selected for nelfinavir as a threshold that represented unacceptably low concentrations, which could place the children at risk for therapeutic failure and emergence of drug-resistant viral strains.
The starting dose of efavirenz for all children was allometrically scaled to body size by the formula: child dose (mg/day)=(child weight in kg/70 kg)0.7 × 600 mg/day. Doses were rounded to the nearest 25 mg increment. Efavirenz was given once daily in the morning. Efavirenz capsules (50, 75, or 100 mg) were initially provided by DuPont Pharmaceutical Company (Wilmington, Delaware) and subsequently by Bristol Myers Squibb Company (Princeton, NJ). The recommended pediatric dose for nelfinavir, 20–30 mg/kg thrice daily, was used. Nelfinavir tablets (250 mg) and powder (50 mg/g) were initially provided by Agouron Pharmaceuticals (La Jolla, CA) and subsequently Pfizer (New York, NY). At entry, subjects were maintained on current NRTIs or switched to new NRTIs, at the discretion of site investigators.
Pharmacokinetic studies
Efavirenz and nelfinavir doses were adjusted if target AUC values were not achieved. AUCs for efavirenz and nelfinavir were determined at weeks 2 and 6 in all children; those children who had not achieved efavirenz or nelfinavir concentrations within the target range had additional pharmacokinetic evaluations at week 10. Blood samples were obtained pre-dose and 2, 5, 6, 8, 12, and 24 h post-dose. Plasma concentrations were determined within 2 weeks at a PACTG Pharmacology Laboratory by high-performance liquid chromatography. Total assay variability (composite of within- and between-day variability) of the efavirenz and nelfinavir assays was 1–4.5 and 1.7–10.3%, respectively. The limits of quantitation were 0.020 mg/l for both efavirenz and nelfinavir. The AUC within the dosing interval (24 h for efavirenz and 8 h for nelfinavir) was calculated with non-compartmental methods and the linear trapezoidal rule.15 If target AUCs were not achieved, efavirenz and/or nelfinavir doses were adjusted proportionally under the assumption of linear pharmacokinetics. Dose adjustments were implemented at the study visit 2 weeks after the AUC was obtained (i.e., week 4 for the AUC determined at week 2). The maximum dose increase allowed following each pharmacokinetic evaluation for efavirenz was 200 mg, whereas up to a 50% increase in the nelfinavir dose was allowed. Doses of efavirenz and nelfinavir were increased in children who had a 25% increase in body weight since their last in-range AUC. Those children who continued on this study after week 48 all had a repeat pharmacokinetic study (as described above) at week 56 with dose adjustment if necessary, as described.
Relationships between pharmacokinetic characteristics at weeks 2 and 6 and virologic response at week 8 were evaluated. Week 8 was selected as the time point for virologic response in this comparison because it reflected changes in drug dosing made at week 2 and at least in part made at week 6, but would not be overly confounded by adherence to the antiretroviral regimen that may change over time. The Wilcoxon rank-sum test was used to evaluate differences in the pharmacokinetic characteristics of efavirenz and nelfinavir between subjects who achieved undetectable levels of HIV RNA ( < 400 copies/ml) at week 8 versus those who did not. To test whether children in various quartiles of AUC values had significantly different rates of reaching an undetectable level of HIV RNA at week 8, χ2 tests were used. Finally, logistic regression was used to test whether the AUCs of efavirenz and nelfinavir each contributed to predicting whether a subject will achieve undetectable HIV RNA at week 8. Relationships between efavirenz and nelfinavir pharmacokinetic characteristics determined at the week 56 visit for those children who continued in the study and virologic response at week 48 were also evaluated. These time points were selected because the week 48 plasma HIV RNA level determined whether a subject could continue the study (i.e., a plasma HIV RNA < 10,000 copies/ml), and the week-56 pharmacokinetic study was required in all children who did continue past week 48. TheWilcoxon rank-sum test was used to compare pharmacokinetic characteristics and Fisher’s exact test was used to compare proportions of subjects who achieved target AUC values and undetectable HIV RNA. Regression analyses were used to evaluate the difference in CD4 cells from baseline to week 48 and efavirenz and nelfinavir pharmacokinetic characteristics.
ACKNOWLEDGMENTS
Stuart E Starr, MD, the original chair of this protocol; Bobbie Graham and Suzanne Siminski, MS, Frontier Science and Technology Research Foundation, Buffalo, NY; Lynette Purdue, PharmD, and James McNamara, MD, National Institute of Allergy and Infectious Diseases, Rockville, MD; Douglas Manion, MD, and David Kornhauser, MD, from Dupont Pharmaceuticals Company; Pamela Clax, DPM, Merril Gersten, MD, and Mark Becker, PharmD, with Agouron Pharmaceuticals are acknowledged. This study was supported by the Pediatric AIDS Clinical Trial Group (PACTG protocol 382), funded by the National Institute of Allergy and Infectious Diseases (NIAID) and the National Institute of Child Health and Human Development (NICHD) Domestic and International Pediatric and Maternal HIV Clinical Trials Network supported by the NICHD; General Clinical Research Centers, National Center for Research Resources (at several participating sites); Dupont Pharmaceuticals Company, Bristol Myers Squibb Company, Agouron Pharmaceuticals, and Pfizer.
Footnotes
CONFLICT OF INTEREST
The authors declared no conflict of interest.
References
- 1.Starr SE, et al. Combination therapy with efavirenz, nelfinavir, and nucleoside reverse-transcriptase inhibitors in children infected with human immunodeficiency virus type 1. Pediatric AIDS Clinical Trials Group 382 Team. N. Engl. J. Med. 1999;341:1874–1881. doi: 10.1056/NEJM199912163412502. [DOI] [PubMed] [Google Scholar]
- 2.Spector SA, et al. Patterns of plasma human immunodeficiency virus type 1 RNA response to highly active antiretroviral therapy in infected children. J. Infect. Dis. 2000;182:1769–1773. doi: 10.1086/317621. [DOI] [PubMed] [Google Scholar]
- 3.Krogstad P, et al. Treatment of human immunodeficiency virus 1-infected infants and children with the protease inhibitor nelfinavir mesylate. Clin. Infect. Dis. 1999;28:1109–1118. doi: 10.1086/514759. [DOI] [PubMed] [Google Scholar]
- 4.DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents—a Working Group of the Office of AIDS Research Advisory Council. [October, 10, 2006];Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. www.aidsinfo.nih.gov.
- 5.Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, Buclin T. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS. 2001;15:71–75. doi: 10.1097/00002030-200101050-00011. [DOI] [PubMed] [Google Scholar]
- 6.Burger DM, et al. Treatment failure of nelfinavir-containing triple therapy can largely be explained by low nelfinavir plasma concentrations. Ther. Drug Monit. 2003;25:73–80. doi: 10.1097/00007691-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Burger D, et al. Therapeutic drug monitoring of nelfinavir and indinavir in treatment-naive HIV-1-infected individuals. AIDS. 2003;17:1157–1165. doi: 10.1097/00002030-200305230-00007. [DOI] [PubMed] [Google Scholar]
- 8.Brundage RC, Yong FH, Fenton T, Spector SA, Starr SE, Fletcher CV. Intrapatient variability of efavirenz concentrations as a predictor of virologic response to antiretroviral therapy. Antimicrob. Agents Chemother. 2004;48:979–984. doi: 10.1128/AAC.48.3.979-984.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Rossum AM, Fraaij PL, de Groot R. Efficacy of highly active antiretroviral therapy in HIV-1 infected children. Lancet Infect. Dis. 2002;2:93–102. doi: 10.1016/s1473-3099(02)00183-4. [DOI] [PubMed] [Google Scholar]
- 10.Gazzard B, et al. British HIV Association (BHIVA) guidelines for the treatment of HIV-infected adults with antiretroviral therapy (2006) HIV Med. 2006;7:487–503. doi: 10.1111/j.1468-1293.2006.00424.x. [DOI] [PubMed] [Google Scholar]
- 11.Working Group on Antiretroviral Therapy and Medical Management of HIV-Infected Children. [October 26, 2006];Guidelines for the use of antiretroviral agents in pediatric HIV infection. www.aidsinfo.nih.gov.
- 12.Saitoh A, et al. Efavirenz pharmacokinetics in HIV-1-infected children are associated with CYP2B6-G516T polymorphism. J. Acquir. Immune Defic. Syndr. 2007 doi: 10.1097/QAI.0b013e318040b29e. [DOI] [PubMed] [Google Scholar]
- 13.Bristol Myers Squibb Company. Package Insert. Princeton, NJ: Bristol Myers Squibb Company; 2006. Sustiva (efavirenz) [Google Scholar]
- 14.Agouron Pharmaceuticals. Package Insert. La Jolla, CA: Agouron Pharmaceuticals; 2006. Viracept (nelfinavir mesylate) [Google Scholar]
- 15.Gibaldi M, Perrier D. Pharmacokinetics. New York, NY: Marcel Dekker; 1982. [Google Scholar]