Abstract
Context
Identification of individuals at high risk for cardiovascular events is important for the optimal use of primary and secondary prevention measures.
Objective
To determine whether plasma levels of amino terminal fragment of the prohormone brain-type natriuretic peptide (NT-proBNP) predict cardiovascular events or death independent of other available prognostic tests.
Design, Setting, and Participants
Prospective cohort study (2000–2002) of 987 individuals in California with stable coronary heart disease in the Heart and Soul Study, who were followed up for a mean of 3.7 (range, 0.1–5.3) years.
Main Outcome Measures
The association of baseline NT-proBNP levels with death or cardiovascular events (myocardial infarction, stroke, or heart failure). Traditional clinical risk factors, echocardiographic measures, ischemia, other biomarkers, and New York Heart Association classification were adjusted for to determine whether NT-proBNP levels were independent of other prognostic factors. Receiver operating characteristic (ROC) curves were used to assess the incremental prognostic value of adding NT-proBNP level to these other measures.
Results
A total of 256 participants (26.2%) had a cardiovascular event or died. Each increasing quartile of NT-proBNP level (range of quartile 1, 8.06–73.95 pg/mL; quartile 2, 74–174.5 pg/mL; quartile 3, 175.1–459 pg/mL; quartile 4, ≥460 pg/mL) was associated with a greater risk of cardiovascular events or death, ranging from 23 of 247 (annual event rate, 2.6%) in the lowest quartile to 134 of 246 (annual event rate, 19.6%) in the highest quartile (unadjusted hazard ratio [HR] for quartile 4 vs quartile 1, 7.8; 95% confidence interval [CI], 5.0–12.1; P<.001). Each SD increase in log NT-proBNP level (1.3 pg/mL) was associated with a 2.3-fold increased rate of adverse cardiovascular outcomes (unadjusted HR, 2.3; 95% CI, 2.0–2.6; P<.001), and this association persisted after adjustment for all of the other prognostic measures (adjusted HR, 1.7; 95% CI, 1.3–2.2; P<.001). The addition of NT-proBNP level to standard clinical assessment and complete echocardiographic parameters significantly improved the area under the ROC curves for predicting subsequent adverse cardiovascular outcomes (0.80 for clinical risk factors and echocardiographic parameters plus log NT-proBNP vs 0.76 for clinical risk factors and echocardiographic parameters only; P=.006).
Conclusions
Elevated levels of NT-proBNP predict cardiovascular morbidity and mortality, independent of other prognostic markers, and identify at-risk individuals even in the absence of systolic or diastolic dysfunction by echocardiography. Level of NT-proBNP may help guide risk stratification of high-risk individuals, such as those with coronary heart disease.
Risk stratification for cardiovascular events among the general population and among high-risk individuals is of considerable interest because of the potential for such strategies to help guide use of primary and secondary preventive therapies. Brain-type natriuretic peptide (BNP) and the amino terminal fragment of the prohormone BNP (NT-proBNP) appear to provide prognostic information in individuals following hospital admission for decompensated heart failure1–5 or acute coronary syndrome,6–12 and may also be important markers of long-term prognosis in ambulatory patients.13–18
Despite the potential of a plasma biomarker such as NT-proBNP to aid clinicians in risk stratification, questions remain about the added value of NT-proBNP relative to other available prognostic measures, such as traditional risk factors, left ventricular ejection fraction (LVEF), diastolic dysfunction, left ventricular hypertrophy, and measures of ischemia. Demonstrating the additional usefulness of NT-proBNP level to these other measurements is particularly important for high-risk individuals in which testing with echocardiography, stress tests, and other biomarkers is common and unlikely to be replaced by a single test.
We assessed the association of plasma NT pro-BNP levels with subsequent cardiovascular events and mortality in a cohort of 987 ambulatory patients with coronary heart disease (CHD). To determine the value of NT-proBNP level as an independent predictor of cardiovascular outcomes, we adjusted for other available prognostic measures, including systolic and diastolic dysfunction, left ventricular mass index, inducible ischemia, exercise capacity, C-reactive protein (CRP), cardiac troponin T, and New York Heart Association (NYHA) classification.
METHODS
Study Participants
The Heart and Soul Study is a prospective cohort study investigating how psychosocial factors influence outcomes of patients with CHD. Methods have been described previously.19,20 We recruited outpatients with CHD who were identified through administrative databases from 2 Department of Veterans Affairs Medical Centers in California (San Francisco and Palo Alto), 1 university medical center (University of California, San Francisco), and 9 public health clinics in the Community Health Network of San Francisco. Eligible participants had at least 1 of the following: history of myocardial infarction (MI); angiographic evidence of stenosis of 50% or greater in 1 or more coronary vessels; evidence of exercise-induced ischemia by treadmill electrocardiogram or stress nuclear perfusion imaging; or a history of coronary revascularization.
All eligible patients were invited by mail to make an appointment for a baseline study visit. Patients were excluded if they were unable to walk 1 block or were planning to move out of the local area within 3 years. Between September 2000 and December 2002, a total of 1024 participants were enrolled in the study. For this cross-sectional study, 37 were unable to provide blood samples and were excluded, leaving 987 individuals for this analysis. The institutional review board at each of the sites approved this protocol. All participants provided written informed consent.
Measurement of NT-ProBNP Level
Prior to the study appointment, participants completed an overnight fast except for taking their regularly prescribed medications. Blood samples were drawn into chilled tubes containing EDTA; plasma was aliquoted and stored at −70°C until January 2005. We used the Roche Diagnostics Elecsys NT-proBNP electrochemiluminescence immunoassay (Elecsys proBNP, Roche Diagnostics, Indianapolis, Ind). The assay range is 5 to 35 000 pg/mL. Levels higher than 35 000 were recoded as 35 000 pg/mL. The intraassay and interassay coefficients of variation ranged, respectively, from 1.8% and 2.3% at NT-pro BNP concentrations of 4962 pg/mL to 2.7% and 3.2% at NT-pro BNP concentrations of 175 pg/mL. The technician who ran the assays was blinded to the echocardiographic results.
Echocardiographic Measurements
A complete resting 2-dimensional echocardiogram using an Acuson Sequoia ultrasound system (Siemens Medical Solutions, Mountain View, Calif) with a 3.5-MHz transducer and Doppler ultrasound examination, including all standard views and subcostal imaging of the inferior vena cava, was performed just prior to and immediately following exercise treadmill testing. One of us (N.B.S.), who was blinded to the results of the NT-proBNP assay, interpreted all of the echocardiograms. Standard 2-dimensional parasternal short-axis and apical 2- and 4-chamber views during held inspiration were obtained; these were planimetered using a computerized digitization system to determine end-diastolic and end-systolic left ventricular volume.
The LVEF was calculated as (end diastolic volume − end systolic volume)/end diastolic volume. Diastolic dysfunction was defined as the presence of at least 1 of the following: impaired relaxation defined as a ratio of peak mitral early diastolic and atrial contraction velocity(E/A) of 0.75 or less and systolic dominant pulmonary vein flow; pseudonormal defined as an E/A of 0.75 or less than 1.5 and diastolic dominant pulmonary vein flow; or restrictive filling defined as an E/A of 1.5 or greater and diastolic dominant pulmonary vein flow.21 All individuals underwent echocardiography, although the echocardiographic measures necessary for ventricular dysfunction classifications were missing for some (LVEF missing in 2 persons; diastolic dysfunction classification missing in 112 persons because of a rhythm other than sinus, moderate to severe mitral regurgitation, or difficulty determining velocity time integral in the pulmonary vein). We also examined 3 mutually exclusive categories of ventricular dysfunction defined as systolic dysfunction (LVEF <50%), normal systolic function (LVEF ≥50%) with diastolic dysfunction, and normal systolic function without known diastolic dysfunction.
We performed a symptom-limited, graded exercise treadmill test according to a standard Bruce protocol.22 To achieve maximal heart rate, participants who were unable to continue the standard Bruce protocol were switched to lower settings on the treadmill and encouraged to exercise for as long as possible. Continuous, 12-lead electrocardiographic monitoring was performed throughout and exercise capacity was calculated as the total metabolic equivalents achieved at peak exercise (1 metabolic equivalent=3.5 mL of oxygen uptake per kg per minute).23 Participants underwent an echocardiogram immediately after exercise, and inducible ischemia was defined as the presence of wall motion abnormalities at peak exercise that were not present at rest.
Other Measurements
Self-reported age, sex, ethnicity, medical history, and smoking status were determined by questionnaire. The NYHA classification was assessed as a self-reported limitation of physical activity due to cardiovascular symptoms (fatigue, shortness of breath, or chest pain). Responses were dichotomized into NYHA class I or II vs NYHA class III or IV. To assess medication use, participants were instructed to bring their medication bottles to the study appointment and study personnel recorded all current medications.
Creatinine clearance was calculated from 24-hour urine samples. Levels of total cholesterol, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol were measured from sera samples drawn after an overnight fast.
High-sensitivity CRP was measured using the Cobas Integra assay (Roche Diagnostics) in approximately 25% of the participants. The Beckman Extended Range high-sensitivity CRP assay (Beckman Coulter Inc, Fullerton, Calif) was used for the remaining 75%. Because high-sensitivity CRP levels were not normally distributed, this variable was log-transformed.
Cardiac troponin T level was measured using the Elecsys troponin T electrochemiluminescence immunoassay (Roche Diagnostics). The lower limit of detection for this assay is 0.01 ng/mL or greater. These values were dichotomized into detectable vs undetectable cardiac troponin T level.
Cardiovascular Events
The primary outcome was a composite of death from any cause or hospitalization for MI, stroke, or heart failure. The censor date was August 24, 2006. We conducted annual telephone follow-up interviews with participants (or their proxy) and asked about death or hospitalization for “heart trouble.” For any reported event, medical records, electrocardiograms, death certificates, and coroner’s reports were retrieved and reviewed by 2 independent and blinded adjudicators. If the adjudicators agreed on the outcome classification, their classification was binding. In the event of disagreement, the adjudicators conferred, reconsidered their classification, and requested consultation from a third blinded adjudicator.
All-cause mortality was determined by review of death certificates. Nonfatal MI was defined using the American Heart Association diagnostic criteria.24 A CHD death was defined as (1) a death occurring during the same hospitalization in which an acute MI was documented or (2) a death occurring within 1 hour of the onset of terminal symptoms not explained by other etiologies. Stroke was defined as a new neurological deficit not known to be secondary to brain trauma, tumor, infection, or other cause. Heart failure was defined as hospitalization for a clinical syndrome involving at least 2 of the following: paroxysmal nocturnal dyspnea, orthopnea, elevated jugular venous pressure, pulmonary rales, third heart sound, and cardiomegaly or pulmonary edema on chestradiography. These clinical signs and symptoms must have represented a clear change from the normal clinical status and must have been accompanied by either decreased cardiac output as determined by peripheral hypoperfusion (in the absence of other causes such as sepsis or dehydration) or peripheral or pulmonary edema requiring intravenous diuretic, inotropic agents, orvasodilator therapy. Supportive documentation of reduced cardiac output, elevated pulmonary capillary wedge pressure, decreased oxygen saturation, and end-organ hypoperfusion were assessed when available.21 We also examined incident heart failure as an outcome among the 814 individuals who did not report a history of heart failure.
Statistical Analyses
Because baseline levels of NT-proBNP among the study participants were not normally distributed, we analyzed the association between quartiles of NT-proBNP levels and cardiovascular events. Our study had greater than 80% power (2-tailed α=.05) to detect a 5% difference (5% vs 10%) in the annual rate of cardiovascular events among the 246 participants who had NT-proBNP levels in the highest quartile compared with the 247 participants who had NT-proBNP levels in the lowest quartile. Differences in participant characteristics by quartile of NT-proBNP level were compared using χ2 tests for dichotomous variables and analysis of variance for continuous variables. We calculated annual rate of cardiovascular outcomes per quartile of NT-proBNP level.
We used multivariable Cox proportional hazards models to compare the rate of cardiovascular outcomes across quartiles of NT-proBNP level. We further examined risk associated with the continuous value of NT-proBNP level after log-transformation to normalize the distribution, and expressed risk per SD increase of log NT-proBNP level. We adjusted for known clinical prognostic factors (age, sex, smoking status, systolic blood pressure, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, history of diabetes, and creatinine clearance), echocardiographic measures (LVEF, diastolic dysfunction, left ventricular mass index), measures of ischemia (presence of inducible ischemia and exercise capacity), serum biomarkers (log CRP and cardiac troponin T), and NYHA classification. All models were run with and without adjustment for baseline use of β-blockers, renin-angiotensin system inhibitors, aspirin, and statins, and we verified proportionality assumptions and absence of colinearity for all models.
In a supplementary analysis, we used smoothed linear splines to examine the association between NT-proBNP level and cardiovascular outcomes across NT-proBNP values from 0 to 2000 pg/mL. We also explored possible cut points to distinguish low-risk and high-risk populations. We used receiver operating characteristic (ROC) curves to determine the incremental prognostic value of NT-proBNP measures added to other measures. We tested for interactions between log NT-proBNP level and age, sex, creatinine clearance of less than 60 mL/min, ventricular dysfunction, inducible ischemia, log CRP, and β-blocker and renin-angiotensin inhibitor use, and conducted stratified analyses by variables found to have significant interactions (P<.10). All analyses were performed using SAS software version 9.1 (SAS Institute Inc, Cary, NC).
RESULTS
The median NT-proBNP level among the 987 participants was 174.8 pg/mL (interquartile range, 73.95–459 pg/mL). Compared with those in the lowest NT-proBNP quartile (8.06–73.95 pg/mL), participants in the highest quartile (≥460 pg/mL) were older and more likely to be white. Participants in the highest NT-proBNP quartile were more likely to have clinical risk factors for adverse cardiovascular events, including a history of hypertension, MI, or revascularization, higher systolic blood pressure, and lower creatinine clearance (Table 1). Participants in the highest NT-proBNP quartile were also more likely to be taking β-blockers, renin-angiotensin inhibitors, and statins. Each of the other prognostic markers was also significantly associated with increasing levels of NT-proBNP (Table 2).
Table 1.
Baseline Characteristics of 987 Individuals With Stable Coronary Heart Disease by Amino Terminal Fragment of the Prohormone Brain-Type Natriuretic Peptide (NT-proBNP) Quartile*
| NT-proBNP Quartiles, pg/mL |
|||||
|---|---|---|---|---|---|
| 1 (8.06–73.95) (n = 247) | 2 (74–174.5) (n = 247) | 3 (175.1–459) (n = 247) | 4 (≥460) (n = 246) | P Value† | |
| Age, mean (SD), y | 61 (10) | 67 (10) | 68 (10) | 72 (11) | <.001 |
| Men | 197 (80) | 210 (85) | 188 (76) | 209 (85) | .03 |
| Race/ethnicity | |||||
| White | 133 (54) | 143 (58) | 154 (62) | 165 (67) | .02 |
| Black | 52 (21) | 39 (16) | 30 (12) | 39 (16) | .06 |
| Other | 62 (25) | 64 (26) | 63 (26) | 42 (17) | .06 |
| Medical history | |||||
| Myocardial infarction | 114 (47) | 118 (48) | 135 (55) | 160 (66) | <.001 |
| Stroke | 24 (10) | 30 (12) | 43 (17) | 43 (17) | .03 |
| Heart failure | 19 (8) | 26 (11) | 52 (21) | 76 (31) | <.001 |
| Diabetes | 57 (23) | 56 (23) | 66 (27) | 80 (33) | .05 |
| Revascularization | 122 (50) | 146 (59) | 146 (59) | 164 (67) | .002 |
| Current smoking | 64 (26) | 44 (18) | 49 (20) | 39 (16) | .03 |
| Measurement | |||||
| Systolic BP, mean (SD), mm Hg | 129 (17) | 131 (20) | 134 (21) | 137 (25) | <.001 |
| Cholesterol, mean (SD), mg/dL | |||||
| Total | 184 (42) | 178 (40) | 179 (43) | 171 (45) | .01 |
| Low-density lipoprotein | 109 (32) | 104 (34) | 104 (34) | 101 (35) | .03 |
| High-density lipoprotein | 45 (13) | 46 (13) | 46 (15) | 45 (15) | .90 |
| Creatinine clearance, mean (SD), mL/min | 99 (27) | 86 (26) | 77 (24) | 61 (24) | <.001 |
| <60 mL/min | 14 (6) | 35 (15) | 58 (25) | 122 (54) | <.001 |
| Current medication use | |||||
| β-Blocker | 100 (40) | 142 (57) | 160 (65) | 165 (67) | <.001 |
| ACE inhibitor | 91 (37) | 113 (46) | 138 (56) | 163 (66) | <.001 |
| Aspirin | 192 (78) | 200 (81) | 199 (81) | 171 (70) | .008 |
| Statin | 143 (58) | 169 (68) | 167 (68) | 154 (63) | .05 |
Abbreviations: ACE, angiotensin-converting enzyme; BP, blood pressure.
SI conversion factor: To convert total, low-density lipoprotein, and high-density lipoprotein cholesterol to mmol/L, multiply by 0.0259.
Values are expressed as number (percentage) unless otherwise indicated.
Analysis of variance was used for continuous variables and the χ2 test was used for categorical variables.
Table 2.
Baseline Measures of Other Known Prognostic Markers by Amino Terminal Fragment of the Prohormone Brain-Type Natriuretic Peptide (NT-proBNP) Quartile Among 987 Individuals With Stable Coronary Heart Disease*
| NT-proBNP Quartiles, pg/mL | |||||
|---|---|---|---|---|---|
| 1 (8.06–73.95) (n = 247) | 2 (74–174.5) (n = 247) | 3 (175.1–459) (n = 247) | 4 (≥460) (n = 246) | P Value† | |
| LVEF, mean (SD), % | 65 (6) | 64 (7) | 61 (10) | 56 (12) | <.001 |
| Diastolic dysfunction | 51 (22) | 77 (33) | 97 (44) | 114 (59) | <.001 |
| Left ventricular mass index, mean (SD) | 87 (18) | 92 (21) | 98 (23) | 115 (32) | <.001 |
| Inducible ischemia | 23 (10) | 39 (17) | 71 (31) | 84 (41) | <.001 |
| Exercise capacity, mean (SD), METs | 8.9 (3.6) | 8.1 (3.2) | 6.7 (2.8) | 5.5 (2.6) | <.001 |
| C-reactive protein, mean (SD), mg/dL | 3.02 (3.81) | 3.34 (5.40) | 5.86 (11.36) | 6.14 (9.66) | <.001 |
| Detectable troponin T | 3 (1) | 3 (1) | 8 (3) | 44 (18) | <.001 |
| NYHA class III or IV | 43 (17) | 45 (18) | 59 (24) | 72 (29) | .004 |
Abbreviations: LVEF, left ventricular ejection fraction; MET, metabolic equivalent; NYHA, New York Heart Association.
Values are expressed as number (percentage) unless otherwise indicated.
Analysis of variance was used for continuous variables and the χ2 test was used for categorical variables.
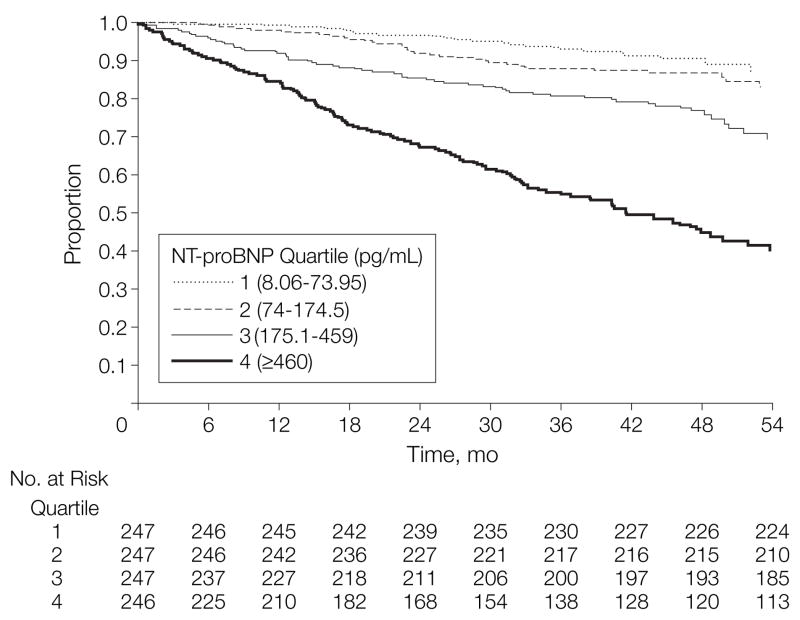
Participants were followed up for a mean of 3.7 (range, 0.1–5.3) years. During this period, 256 (26.2%) participants developed a cardiovascular event or died; only 9 individuals (1%) were lost to follow-up. The event rate increased with each successive quartile of NT-proBNP level (Figure). The same graded increase in risk associated with increasing NT-proBNP concentration was observed for each of the events included in the composite outcome (Table 3). Individuals with NT-proBNP concentrations in the highest quartile had a nearly 8-fold increased rate of cardiovascular events or death compared with those in the lowest quartile (unadjusted hazard ratio [HR], 7.8; 95% confidence interval [CI], 5.0–12.1; P<.001).
Figure.
Survival Free of Cardiovascular Events or Death by NT-proBNP Quartile
NT-proBNP indicates amino terminal fragment of the prohormone brain-type natriuretic peptide.
Table 3.
Adverse Cardiovascular Outcomes by Quartile of Amino Terminal Fragment of the Prohormone Brain-Type Natriuretic Peptide (NT-proBNP)*
| NT-proBNP Quartiles, pg/mL† | |||||
|---|---|---|---|---|---|
| Total | 1 (8.06–73.95) | 2 (74–174.5) | 3 (175.1–459) | 4 (≥460) | |
| All-cause mortality | 158 (4.3) | 12 (1.3) | 21 (2.2) | 35 (3.8) | 90 (10.7) |
| CHD death | 34 (0.9) | 2 (0.2) | 4 (0.4) | 6 (0.7) | 22 (2.6) |
| Myocardial infarction | 88 (2.5) | 10 (1.1) | 14 (1.5) | 23 (2.6) | 41 (5.3) |
| Stroke | 27 (0.7) | 4 (0.4) | 3 (0.3) | 4 (0.4) | 16 (2.0) |
| Heart failure | 112 (3.2) | 3 (0.3) | 8 (0.8) | 21 (2.4) | 80 (11.2) |
| Incident heart failure with no history of heart failure (n = 814) | 67 (2.3) | 2 (0.2) | 7 (0.8) | 11 (1.5) | 47 (9.0) |
| Any event or death | 256 (7.6) | 23 (2.6) | 37 (4.0) | 62 (7.4) | 134 (19.6) |
Abbreviation: CHD, coronary heart disease.
Values expressed as number (annual rate).
P<.001 for all comparisons except for CHD death in which P = .001; calculated using χ2 test for trend.
We evaluated the continuous NT-proBNP values and found each SD increase in log NT-proBNP (1.3 pg/mL) was associated with an increased rate of adverse cardiovascular outcomes, even after adjusting for other prognostic markers, including clinical factors, echocardiographic parameters, ischemia, serum biomarkers, and functional limitations (Table 4). The addition of baseline medication use to the full model did not alter this association (adjusted HR for quartile 4 [≥460 pg/mL] vs quartile 1 [8.06–73.95 pg/mL], 3.9; 95% CI, 1.9–8.0; adjusted HR for each SD increase in log NT-proBNP, 1.8; 95% CI, 1.4–2.3).
Table 4.
Association of NT-proBNP With Any Cardiovascular Event or Death After Adjusting for Available Prognostic Markers
| HR (95% CI)* |
||
|---|---|---|
| Quartile 4 vs Quartile 1 | Log NT-proBNP per 1-SD Increase | |
| Unadjusted | 7.8 (5.0–12.1) | 2.3 (2.0–2.6) |
| Adjusted model | ||
| 1† | 5.2 (2.9–9.3) | 2.1 (1.8–2.5) |
| 2‡ | 4.3 (2.1–7.8) | 2.0 (1.6–2.5) |
| 3§ | 3.1 (1.5–6.4) | 1.7 (1.4–2.2) |
| 4|| | 3.4 (1.7–6.9) | 1.7 (1.3–2.2) |
| 5¶ | 3.4 (1.7–6.9) | 1.7 (1.3–2.2) |
Abbreviations: CI, confidence interval; HR, hazard ratio; NT-proBNP, amino terminal fragment of the prohormone brain-type natriuretic peptide.
P<.001 for all comparisons except for model 3 comparison of quartile 4 with quartile 1 in which P = .002.
Adjusted for clinical risk factors (age, sex, history of diabetes, current smoking, systolic blood pressure, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, continuous creatinine clearance).
Adjusted for clinical risk factors in model 1 plus echocardiographic parameters (left ventricular ejection fraction, diastolic dysfunction, left ventricular mass index).
Adjusted for models 1 and 2 plus inducible ischemia and treadmill exercise capacity (metabolic equivalents).
Adjusted for models 1, 2, and 3 plus serum biomarkers (C-reactive protein concentration and detectable troponin T).
Adjusted for models 1 through 4 plus New York Heart Association classification.
Level of NT-proBNP predicted each of the individual events included in the composite outcome, with the exception of MI (Table 5). The magnitude of the association was greatest for NT-proBNP level predicting incident heart failure among the 814 individuals without a history of heart failure. These associations remained unchanged after adjusting for baseline medication use.
Table 5.
Association of Log Amino Terminal Fragment of the Prohormone Brain-Type Natriuretic Peptide (NT-proBNP) per 1 SD Increase With Specific Cardiovascular Outcomes After Adjusting for Other Prognostic Markers
| HR (95% CI) |
||||||
|---|---|---|---|---|---|---|
| All-Cause Mortality | CHD Death | Myocardial Infarction | Stroke | Heart Failure | Incident Heart Failure With No History of Heart Failure | |
| Unadjusted | 2.3 (2.0–2.7) | 2.8 (2.0–3.9) | 2.0 (1.6–2.4) | 1.8 (1.3–2.6) | 3.4 (2.8–4.0) | 3.6 (2.9–4.5) |
| Adjusted model | ||||||
| 1* | 2.0 (1.6–2.4) | 2.6 (1.7–4.0) | 1.7 (1.3–2.3) | 1.8 (1.1–3.0) | 3.6 (2.8–4.7) | 3.8 (2.7–5.3) |
| 2† | 2.0 (1.6–2.6) | 3.2 (1.8–5.6) | 1.6 (1.2–2.3) | 1.7 (0.9–3.4) | 2.8 (2.0–4.1) | 3.1 (1.9–5.1) |
| 3‡ | 1.7 (1.3–2.3) | 2.9 (1.5–5.6) | 1.3 (0.9–1.8) | 1.8 (0.9–3.8) | 2.5 (1.7–3.7) | 2.7 (1.5–4.8) |
| 4§ | 1.6 (1.2–2.1) | 2.6 (1.3–5.0) | 1.2 (0.8–1.8) | 1.8 (0.9–3.7) | 2.4 (1.6–3.7) | 2.6 (1.5–4.8) |
| 5|| | 1.6 (1.2–2.2) | 2.6 (1.3–5.0) | 1.3 (0.9–1.8) | 1.8 (0.9–3.8) | 2.4 (1.6–3.7) | 2.7 (1.5–4.8) |
Abbreviations: CHD, coronary heart disease; CI, confidence interval; HR, hazard ratio.
Adjusted for clinical risk factors (age, sex, history of diabetes, current smoking, systolic blood pressure, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, continuous creatinine clearance).
Adjusted for clinical risk factors in model 1 plus echocardiographic parameters (left ventricular ejection fraction, diastolic dysfunction, left ventricular mass index).
Adjusted for models 1 and 2 plus inducible ischemia and treadmill exercise capacity (metabolic equivalents).
Adjusted for models 1, 2, and 3 plus serum biomarkers (C-reactive protein concentration and detectable troponin T).
Adjusted for models 1 through 4 plus New York Heart Association classification.
We used spline functions to examine possible nonlinear associations between NT-proBNP level and adverse cardiovascular events. Individuals with NT-proBNP levels greater than 500 pg/mL were at highest risk for future events, and the association between NT-proBNP level and adverse outcomes was linear above 500 pg/mL. At NT-proBNP levels between 50 and 500 pg/mL, each increase in NT-proBNP level was more predictive of future adverse events, and based on this observation, we explored possible cut points at lower NT-proBNP concentrations that would distinguish low-risk and high-risk individuals (Table 6). These post-hoc analyses suggested that 100 pg/mL may be optimal for distinguishing high and low risk, particularly among older individuals. Participants aged 70 years or older with NT-proBNP levels greater than 100 pg/mL had more than 3 times the rate of adverse events compared with those with lower NT-proBNP levels.
Table 6.
Association of Various Cut Points of Amino Terminal Fragment of the Prohormone Brain-Type Natriuretic Peptide (NT-proBNP) Cardiovascular Events or Death
| No. of Events/No. at Risk for Cardiovascular Events or Death by Cut Point Position |
||||
|---|---|---|---|---|
| NT-proBNP Level, pg/mL | Above | Below | Adjusted HR (95% CI)* | P Value |
| Entire cohort (N = 987) | ||||
| >50 vs ≤50 | 241/828 | 15/150 | 1.4 (0.7–2.7) | .30 |
| >100 vs ≤100 | 225/659 | 31/319 | 2.2 (1.4–3.6) | .001 |
| >125 vs ≤125 | 218/594 | 38/384 | 2.1 (1.4–3.3) | .007 |
| >150 vs ≤150 | 205/532 | 51/446 | 1.8 (1.2–2.7) | .006 |
| Age <70 y (n = 554) | ||||
| >50 vs ≤50 | 89/427 | 11/127 | 1.7 (0.7–4.2) | .20 |
| >100 vs ≤100 | 79/313 | 21/241 | 1.7 (0.9–3.4) | .10 |
| >125 vs ≤125 | 78/275 | 22/279 | 2.0 (1.0–3.9) | .04 |
| >150 vs ≤150 | 74/238 | 26/316 | 2.0 (1.0–3.9) | .04 |
| Age ≥70 y (n = 424) | ||||
| >50 vs ≤50 | 152/401 | 4/23 | 0.9 (0.3–2.7) | .90 |
| >100 vs ≤100 | 146/346 | 10/78 | 3.2 (1.4–7.0) | .004 |
| >125 vs ≤125 | 140/319 | 16/105 | 2.2 (1.2–4.0) | .01 |
| >150 vs ≤150 | 131/294 | 25/130 | 1.6 (0.9–2.7) | .10 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Adjusted for clinical risk factors (age, sex, history of diabetes, current smoking, systolic blood pressure, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, continuous creatinine clearance), echocardiographic parameters (left ventricular ejection fraction, diastolic dysfunction, left ventricular mass index, inducible ischemia, and treadmill exercise capacity [metabolic equivalents]), serum biomarkers (C-reactive protein concentration and detectable troponin T), and New York Heart Association classification.
Areas under receiver operating characteristic curves (AUROC) were compared to determine the additional prognostic value of NT-proBNP level used in conjunction with measures available by clinical assessment, echocardiography, and cardiac stress testing. The addition of NT-proBNP level to clinical risk factors was significantly more predictive of adverse cardiovascular outcomes than clinical risk factors alone (AUROC, 0.80 for clinical risk factors plus log NT-proBNP vs 0.73 for clinical risk factors alone; P<.001), or clinical risk factors plus echocardiographic parameters (AUROC, 0.80 for clinical risk factors plus echocardiographic parameters plus log NT-proBNP level vs 0.76 for clinical risk factors plus echocardiographic parameters only; P=.006). A trend toward more predictive capability was also observed when NT-proBNP level was added to clinical risk factors, echocardiographic parameters, and ischemia and treadmill exercise capacity (AUROC, 0.80 for clinical risk factors, echocardiographic parameters, ischemia, and treadmill exercise capacity plus log NT-proBNP level vs 0.79 for clinical risk factors, echocardiographic parameters, ischemia, and treadmill exercise capacity only; P=.07), as well as these factors plus CRP and cardiac troponin T (AUROC, 0.81 for clinical risk factors, echocardiographic parameters, ischemia, and treadmill exercise capacity, log CRP, and cardiac troponin T plus log NT-proBNP vs 0.79 for clinical risk factors, echocardiographic parameters, ischemia, treadmill exercise capacity, log CRP, and cardiac troponin T only; P=.09).
The association between log NT-proBNP level and the composite outcome did not vary by age, inducible ischemia, creatinine clearance, or log CRP (P for interaction>.10). However, we did find significant interactions between NT-proBNP level and LVEF (P=.05) and use of cardiovascular medications (P=.01). Although we observed the association between log NT-proBNP level and cardiovascular outcomes among all individuals, the association was somewhat more pronounced among the 865 individuals with LVEF greater than 50% (adjusted HR, 2.0; 95% CI, 1.7–2.4) than among the 111 individuals with LVEF of 50% or less (adjusted HR, 1.8; 95% CI, 1.2–2.7). We further examined whether the association varied by the presence of diastolic dysfunction among those with LVEF greater than 50% (P for interaction=.06) and found again that NT-proBNP level was associated with adverse cardiovascular events among all individuals, with a somewhat stronger association among the 278 individuals with LVEF greater than 50% and diastolic dysfunction(adjustedHR,2.4;95% CI, 1.8–3.3) than among the 496 with LVEF greater than 50% without known diastolic dysfunction (adjusted HR, 1.7; 95% CI, 1.2–2.3). Log NT-proBNP level appeared more strongly associated with adverse cardiovascular outcomes among the 232 participants not taking a β-blocker or renin-angiotensin inhibitor (adjusted HR, 3.5; 95% CI, 2.3–5.4) than among the 184 participants taking a renin-angiotensin inhibitor only (adjustedHR,2.7;95%CI,1.9–3.9), the 242 participant staking a β-blocker only(adjusted HR, 1.9; 95% CI, 1.4–2.7), or the 320 participant staking both medications (adjusted HR, 1.8; 95% CI, 1.4–2.4).
COMMENT
In an ambulatory population of 987 individuals with stable CHD, we found that NT-proBNP level predicts subsequent cardiovascular events and death, independent of traditional cardiac risk factors and other prognostic markers commonly used to assess risk, even when these measurements are used in combination. These findings suggest that a simple blood test for NT-proBNP level may aid in the risk stratification of high-risk patients, such as those with CHD, and may guide further testing and treatment strategies aimed at reducing future cardiovascular morbidity and mortality.
By examining the independent association of NT-proBNP level with multiple cardiovascular outcomes in a high-risk ambulatory population with known CHD, this study extends the existing literature on NT-proBNP level and prognosis in several important ways. Previous studies have found that NT-proBNP level is predictive of subsequent morbidity and mortality independent of systolic dysfunction14,15,25 and other biomarkers including high-sensitivity CRP.13,14 However, these findings leave open the possibility that elevations in NT-proBNP level may simply reflect elevated filling pressures due to diastolic dysfunction or inducible ischemia, which were not accounted for in these studies. We describe an independent association between elevated NT-proBNP concentration and cardiovascular morbidity and mortality after accounting for multiple prognostic factors including systolic and diastolic dysfunction, left ventricular mass, inducible ischemia, exercise capacity, CRP, cardiac troponin T, and functional status, suggesting that these parameters are not the sole explanation for the prognostic power of NT-proBNP level.
Why elevations in NT-proBNP level predict adverse cardiovascular outcomes is a subject of substantial investigation. Elevations of NT-proBNP level may reflect subclinical levels of ventricular dysfunction or inducible ischemia not detectable by standard echocardiographic or stress test measures.19 Because natriuretic peptides are secreted from the ventricle in response to wall stress from volume or pressure overload, elevations in NT-proBNP level may also signal important adverse hemodynamic alterations not captured in these other measures. Elevated NT-proBNP level may also signal vascular dysfunction, in which the natriuretic peptides produce changes in vascular smooth muscle proliferation or contractility, in part via cyclic GMP signaling cascades or nitric oxide synthesis.26,27 Although elucidating the exact biological mechanism whereby elevations in NT-proBNP level are associated with future cardiovascular morbidity and mortality is outside the scope of this study, it is notable that the postulated mechanisms are not easily assessed by current standard cardiovascular tests. Importantly, our results suggest that elevated NT-proBNP level predicts adverse events strongly in patients without echocardiographic evidence of systolic or diastolic dysfunction.
Only a few studies have examined the ability of NT-proBNP level to predict multiple types of cardiovascular outcomes in an ambulatory population, and most of these have focused on community-dwelling adults without known cardiovascular disease.14,18 We have examined multiple outcomes among individuals with known CHD, a population at highest risk for future cardiovascular morbidity and mortality and therefore a population with the potential to benefit from risk stratification. However, individuals with CHD are also more likely to undergo various different types of cardiovascular testing to assess risk, and the true value of any new prognostic marker must be measured against existing tests. Demonstrating the added value of NT-proBNP measurements to the various types of testing that a patient with CHD is likely to undergo may make it more likely that such a test could be of benefit in clinical practice. We found that NT-proBNP level significantly increased predictive capability when added to standard measures used to assess risk. Furthermore, there was a linear association between NT-proBNP levels and future events among those at greatest risk (NT-proBNP level >500 pg/mL); and NT-proBNP levels less than 100 pg/mL signaled a very low risk of future adverse events.
Similar to studies of community-dwelling populations, we found that elevated levels of NT-proBNP predict a variety of cardiovascular outcomes. The mechanism for the association between NT-proBNP level and the risk of stroke that we and others14,18 have observed has yet to be elucidated, although a link between a genetic polymorphism of atrial natriuretic peptide and the risk of stroke has been described.28 We did observe an association between elevated NT-proBNP level and the risk of MI, although the magnitude was less than the other cardiovascular events and was significantly attenuated after adjusting for the other prognostic markers. Although this weaker association may simply reflect the relatively high baseline risk for this outcome in our cohort (in which more than half of participants had a history of prior MI), similar results have been noted in other studies of community-dwelling populations14,18 as well and merit further investigation.
We observed the strongest association between NT-proBNP level and risk of incident heart failure. The ability to identify individuals at risk for developing heart failure has considerable appeal because the early initiation of preventive therapies may alter the course of this disease with very high rates of morbidity and mortality. Consistent with preventive therapies lowering risk is our observation that elevations in NT-proBNP level were most strongly predictive of future adverse events in individuals not taking renin-angiotensin converting enzyme inhibitors or β-blockers at baseline. However, the utility of an algorithm for risk stratification that includes NT-proBNP level ultimately rests in the ability to alter disease trajectory, and whether individuals identified as high risk on the basis of an elevated NT-proBNP level benefit from additional therapies beyond those already indicated on the basis of their comorbid conditions is not known.
Several limitations should be noted when interpreting our results. Because the sample was predominantly male, our findings may not be as generalizable to women. We defined incident heart failure by hospitalization for this disease; individuals who were diagnosed and treated for new-onset heart failure in the outpatient setting were therefore not captured in this analysis, and thus our results may have been biased to the detection of more acute presentations of heart failure.
In summary, we found that NT-proBNP level is a predictor of adverse cardiovascular events among ambulatory individuals with stable CHD, independent of other prognostic markers available by echocardiography, ischemic stress testing, or serum measurement. Use of a simple blood test for NT-proBNP level may help guide risk stratification and may focus efforts toward preventing adverse cardiovascular events in high-risk patients with stable CHD.
Acknowledgments
Funding/Support: This study was supported by grants to Dr Bibbins-Domingo from the Robert Wood Johnson Foundation (Amos Medical Faculty Development award), the National Heart, Lung, and Blood Institute (contract N01-HC-95095), and the University of California, San Francisco, Research Evaluation and Allocation Committee. The Heart and Soul Study was funded by the Department of Veterans Affairs (Washington, DC), the National Heart, Lung, and Blood Institute (grant R01 HL079235), the American Federation for Aging Research (Paul Beeson Scholars Program), the Robert Wood Johnson Foundation (Faculty Scholars Program), the Nancy Kirwan Heart Research Fund, San Francisco, Calif, and the Ischemia Research and Education Foundation. The NT-proBNP assays were funded by Roche Diagnostics Corporation, Indianapolis, Ind.
Role of the Sponsor: Neither the funding organizations nor Roche Diagnostics had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation or approval of the manuscript.
Footnotes
Financial Disclosures: None reported.
Author Contributions: Drs Bibbins-Domingo and Whooley had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Bibbins-Domingo, Whooley.
Acquisition of data: Bibbins-Domingo, Whooley.
Analysis and interpretation of data: Bibbins-Domingo, Gupta, Na, Wu, Schiller, Whooley.
Drafting of the manuscript: Bibbins-Domingo, Gupta, Na, Schiller, Whooley.
Critical revision of the manuscript for important intellectual content: Bibbins-Domingo, Wu, Schiller, Whooley.
Statistical analysis: Bibbins-Domingo, Gupta, Na, Whooley.
Obtained funding: Bibbins-Domingo, Whooley.
Administrative, technical, or material support: Bibbins-Domingo, Wu, Whooley.
Study supervision: Bibbins-Domingo, Whooley.
References
- 1.Berger R, Huelsman M, Strecker K, et al. B-type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation. 2002;105:2392–2397. doi: 10.1161/01.cir.0000016642.15031.34. [DOI] [PubMed] [Google Scholar]
- 2.Gardner RS, Ozalp F, Murday AJ, et al. N-terminal pro-brain natriuretic peptide: a new gold standard in predicting mortality in patients with advanced heart failure. Eur Heart J. 2003;24:1735–1743. doi: 10.1016/j.ehj.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Groenning BA, Raymond I, Hildebrandt PR, Nilsson JC, Baumann M, Pedersen F. Diagnostic and prognostic evaluation of left ventricular systolic heart failure by plasma N-terminal pro-brain natriuretic peptide concentrations in a large sample of the general population. Heart. 2004;90:297–303. doi: 10.1136/hrt.2003.026021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonagh TA, Cunningham AD, Morrison CE, et al. Left ventricular dysfunction, natriuretic peptides, and mortality in an urban population. Heart. 2001;86:21–26. doi: 10.1136/heart.86.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsutamoto T, Wada A, Maeda K, et al. Attenuation of compensation of endogenous cardiac natriuretic peptide system in chronic heart failure. Circulation. 1997;96:509–516. doi: 10.1161/01.cir.96.2.509. [DOI] [PubMed] [Google Scholar]
- 6.de Lemos JA, Morrow DA, Bentley JH, et al. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med. 2001;345:1014–1021. doi: 10.1056/NEJMoa011053. [DOI] [PubMed] [Google Scholar]
- 7.James SK, Lindahl B, Siegbahn A, et al. N-terminal pro-brain natriuretic peptide and other risk markers for the separate prediction of mortality and subsequent myocardial infarction in patients with unstable coronary artery disease. Circulation. 2003;108:275–281. doi: 10.1161/01.CIR.0000079170.10579.DC. [DOI] [PubMed] [Google Scholar]
- 8.Mega JL, Morrow DA, de Lemos JA, et al. B-type natriuretic peptide at presentation and prognosis in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2004;44:335–339. doi: 10.1016/j.jacc.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 9.Morrow DA, de Lemos JA, Sabatine MS, et al. Evaluation of B-type natriuretic peptide for risk assessment in unstable angina/non-ST-elevation myocardial infarction. J Am Coll Cardiol. 2003;41:1264–1272. doi: 10.1016/s0735-1097(03)00168-2. [DOI] [PubMed] [Google Scholar]
- 10.Omland T, Persson A, Ng L, et al. N-terminal pro-B-type natriuretic peptide and long-term mortality in acute coronary syndromes. Circulation. 2002;106:2913–2918. doi: 10.1161/01.cir.0000041661.63285.ae. [DOI] [PubMed] [Google Scholar]
- 11.Richards AM, Nicholls MG, Yandle TG, et al. Plasma N-terminal pro-brain natriuretic peptide and adrenomedullin. Circulation. 1998;97:1921–1929. doi: 10.1161/01.cir.97.19.1921. [DOI] [PubMed] [Google Scholar]
- 12.Morrow DA, de Lemos JA, Blazing MA, et al. Prognostic value of serial B-type natriuretic peptide testing during follow-up of patients with unstable coronary artery disease. JAMA. 2005;294:2866–2871. doi: 10.1001/jama.294.22.2866. [DOI] [PubMed] [Google Scholar]
- 13.Blankenberg S, McQueen MJ, Smieja M, et al. Comparative impact of multiple biomarkers and N-terminal pro-brain natriuretic peptide in the context of conventional risk factors for the prediction of recurrent cardiovascular events in the Heart Outcomes Prevention Evaluation (HOPE) Study. Circulation. 2006;114:201–208. doi: 10.1161/CIRCULATIONAHA.105.590927. [DOI] [PubMed] [Google Scholar]
- 14.Kistorp C, Raymond I, Pedersen F, Gustafsson F, Faber J, Hildebrandt P. N-terminal pro-brain natriuretic peptide, C-reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. JAMA. 2005;293:1609–1616. doi: 10.1001/jama.293.13.1609. [DOI] [PubMed] [Google Scholar]
- 15.Kragelund C, Gronning B, Kober L, Hildebrandt P, Steffensen R. N-terminal pro-B-type natriuretic peptide and long-term mortality in stable coronary heart disease. N Engl J Med. 2005;352:666–675. doi: 10.1056/NEJMoa042330. [DOI] [PubMed] [Google Scholar]
- 16.Ndrepepa G, Braun S, Niemoller K, et al. Prognostic value of N-terminal pro-brain natriuretic peptide in patients with chronic stable angina. Circulation. 2005;112:2102–2107. doi: 10.1161/CIRCULATIONAHA.105.550715. [DOI] [PubMed] [Google Scholar]
- 17.Omland T, Richards AM, Wergeland R, Vik-Mo H. B-type natriuretic peptide and long-term survival in patients with stable coronary artery disease. Am J Cardiol. 2005;95:24–28. doi: 10.1016/j.amjcard.2004.08.058. [DOI] [PubMed] [Google Scholar]
- 18.Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 19.Bibbins-Domingo K, Ansari M, Schiller NB, Massie B, Whooley MA. B-type natriuretic peptide and ischemia in patients with stable coronary disease. Circulation. 2003;108:2987–2992. doi: 10.1161/01.CIR.0000103681.04726.9C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA. 2003;290:215–221. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redfield MM, Jacobsen SJ, Burnett JC, Jr, et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 22.Gibbons RJ, Balady GJ, Bricker JT, et al. Committee to Update the 1997 Exercise Testing Guidelines. ACC/AHA 2002 guideline update for exercise testing: summary article. Circulation. 2002;106:1883–1892. doi: 10.1161/01.cir.0000034670.06526.15. [DOI] [PubMed] [Google Scholar]
- 23.American College of Sports Medicine. Guidelines for Exercise Testing and Prescription. 6. Baltimore, Md: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 24.Luepker RV, Apple FS, Christenson RH, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108:2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 25.Schnabel R, Rupprecht HJ, Lackner KJ, et al. Analysis of N-terminal-pro-brain natriuretic peptide and C-reactive protein for risk stratification in stable and unstable coronary artery disease: results from the AtheroGene study. Eur Heart J. 2005;26:241–249. doi: 10.1093/eurheartj/ehi036. [DOI] [PubMed] [Google Scholar]
- 26.Ahluwalia A, MacAllister RJ, Hobbs AJ. Vascular actions of natriuretic peptides. Basic Res Cardiol. 2004;99:83–89. doi: 10.1007/s00395-004-0459-6. [DOI] [PubMed] [Google Scholar]
- 27.D’Souza SP, Davis M, Baxter GF. Autocrine and paracrine actions of natriuretic peptides in the heart. Pharmacol Ther. 2004;101:113–129. doi: 10.1016/j.pharmthera.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Rubattu S, Stanzione R, Di Angelantonio E, et al. Atrial natriuretic peptide gene polymorphisms and risk of ischemic stroke in humans. Stroke. 2004;35:814–818. doi: 10.1161/01.STR.0000119381.52589.AB. [DOI] [PubMed] [Google Scholar]