Abstract
Most olfactory bulb (OB) interneurons are derived from neural stem cells in the subventricular zone (SVZ) and migrate to the OB via the rostral migratory stream (RMS). Mature dopaminergic interneurons in the OB glomerular layer are readily identified by their synaptic activity-dependent expression of tyrosine hydroxylase (TH). Paradoxically, TH is not expressed in neural progenitors migrating in the RMS, even though ambient GABA and glutamate depolarize these progenitors. In forebrain slice cultures prepared from transgenic mice containing a GFP reporter gene under the control of the Th 9kb upstream regulatory region, treatment with histone deacetylase (HDAC) inhibitors (either sodium butyrate, Trichostatin A or Scriptaid) induced Th-GFP expression specifically in the RMS independently of depolarizing conditions in the culture media. Th-GFP expression in the glomerular layer was also increased in slices treated with Trichostatin A, but this increased expression was dependent on depolarizing concentrations of KCl in the culture media. Th-GFP expression was also induced in the RMS in vivo by intra-peritoneal injections with either sodium butyrate or valproic acid. Quantitative RT-PCR analysis of neurosphere cultures confirmed that HDAC inhibitors de-repressed Th expression in SVZ-derived neural progenitors. Together, these findings suggest that HDAC function is critical for regulating Th expression levels in both neural progenitors and mature OB dopaminergic neurons. However, the differential responses to the combinatorial exposure of HDAC inhibitors and depolarizing culture conditions indicate that Th expression in mature OB neurons and neural progenitors in the RMS are regulated by distinct HDAC-mediated mechanisms.
Keywords: histone deacetylase inhibition, neurogenesis, differentiation, rostral migratory stream, epigenetics
Introduction
The subventricular zone (SVZ) of the lateral ventricles contains neural stem cells that actively proliferate and generate neural progenitors throughout the life span of vertebrate animals [1; 2]. Although the SVZ continuously generates interneurons throughout the life span of the animal, peak production of occurs in the neonate [3; 4; 5]. SVZ-derived neuronal progenitors tangentially migrate through the rostral migratory stream (RMS) en route to the olfactory bulb (OB) where they become mature interneurons and modulate activity of olfactory sensory neurons and OB mitral/tufted projection neurons [6].
The OB dopaminergic neurons, one of the most extensively studied subsets of OB interneurons, are found predominantly in the glomerular layer and are readily identified by the expression of tyrosine hydroxylase (TH), the rate-limiting enzyme in dopamine biosynthesis [7][8; 9]. OB TH expression in vivo is activity-dependent and requires odor-mediated stimulation of olfactory sensory neurons [10; 11; 12; 13]. In both primary and slice cultures, membrane depolarization and activation of L-type calcium channels are necessary for induction of Th transcription in OB interneurons [14; 15; 16; 17].
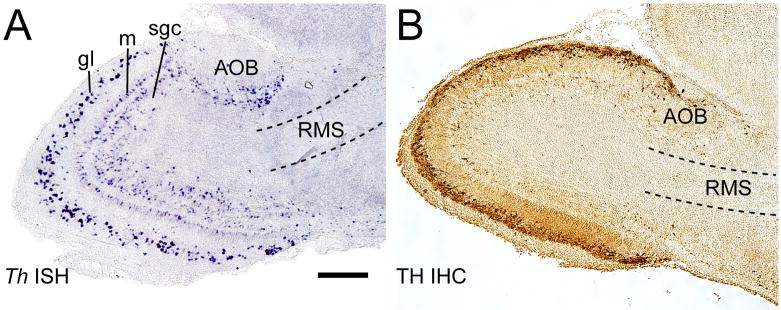
A paradox in the differentiation of OB dopaminergic neurons is the restriction of Th expression to only glomerular and superficial granule cell layers of the OB (Figure 1)[18]. Although Th expression in the OB requires membrane depolarization and L-type calcium channels, migrating progenitors do not express Th in the RMS even though these progenitors receive glutamatergic and GABAergic signals that mediate membrane depolarization and activate L-type channels [19; 20]. The molecular mechanisms responsible for repressing of Th expression in the RMS are unknown.
Figure 1.
Laminar organization of TH expression in the perinatal mouse olfactory bulb. A, Th in situ hybridization (ISH) reveals that Th message is expressed in the glomerular (gl), mitral (m) and superficial granule cell (sgc) layers. However, Th is not expressed in the rostral migratory stream (RMS). B, TH immunohistochemistry (IHC) reveals that TH protein in limited almost exclusively to the glomerular layer, and is also absent from the RMS. The absence of TH protein from the mitral or superficial granule cells layers indicates that TH is post-transcriptionally regulated in these regions. Sagittal sections are from mouse pups approximately 4 days old. Other abbreviations: AOB, accessory olfactory bulb. Bar = 250 μm.
Chromatin remodeling and post-translational modification of histone proteins are established epigenetic molecular mechanisms for regulating neuronal gene transcription in both the developing and mature nervous system [21; 22; 23]. Histone acetylation by histone acetyltransferases (HATs) relaxes chromatin structure and promotes gene expression by increasing transcription-activator protein access to regulatory genomic DNA regions. By contrast, histone deacetylation by histone deacetyltransferases (HDACs) compacts chromatin and represses gene expression by restricting access of transcription-activator proteins. Several studies have reported that chromatin remodeling proteins are critical for OB progenitor proliferation and differentiation [24; 25; 26; 27]. Also, treatment of SVZ-derived neurospheres with pharmacological HDAC inhibitors promotes expression of neuronal differentiation marker genes, such as Tuj1 and NeuroD1 [28]. Although Th expression in certain cultured cell lines can be up-regulated by treatment with HDAC inhibitors [29; 30], the role of histone acetylation in the repression of Th transcription within the RMS is unexplored. In this study, both in vivo and in vitro experimental paradigms test whether HDAC inhibitors are sufficient to induce Th expression in the RMS and OB.
Materials and methods
Animals
Th-GFP transgenic mice expressing an enhanced green fluorescent protein (GFP) reporter driven by 9kb of Th promoter on a C57BL/6xDBA/J background were obtained from Dr. Kazuto Kobayashi [31]. Mice were housed in humidity-controlled cages at 22°C under a 12:12 hour light:dark cycle and provided with food and water ad libitum. All procedures were carried out under protocols approved by the Weill Cornell Medical College Institutional Animal Care and Use Committee and conformed to NIH guidelines.
Slice Culture
The preparation and culture of forebrain organotypic slice cultures from post-natal day 2–3 mouse pups has been previously described [15]. Slices were either cultured in depolarizing conditions containing 25 mM potassium chloride (KCl) or non-depolarizing conditions with media supplemented with 25 mM sodium chloride (NaCl) to match the osmolarity and ionic strength of the depolarizing media. The following HDAC inhibitors were used at the indicated concentrations: sodium butyrate (10 mM, Sigma Corp.), Trichiostatin A (1.2 μM, BioMol Inc.), Scriptaid (6.3 μM, BioMol Inc.) and as a control Nullscript (6.3 μM, BioMol Inc.). Slices were cultured 48 hours at 37°C and 5% CO2 before being analyzed on a Nikon Eclipse 80i fluorescence microscope. Analysis of fluorescence intensity levels in the glomerular layer and RMS were determined as previously described [15].
In vivo HDAC treatment
Th-GFP transgenic mice ranging in age from P3–P4 days were injected with either valproic acid (200 mg/kg) or sodium butyrate (1200 mg/kg) twice a day, for three days. Control mice were injected with saline. After three days, brain sections were prepared for immunofluorescence analysis using previously published methods [32]. GFP expression was visualized using chicken anti-GFP (1:5,000, Chemicon/Millipore Corp.), and secondary antibodies conjugated to either Alexa 488 (1:400, Invitrogen Corp.).
Neurosphere Culture and quantitative PCR
Neurosphere cultures were prepared from wild-type C57BL/6J mouse pups (P2) using previously published methods [33]. RNA from cultures treated with HDAC inhibitors for 48 hours was isolated using the RNeasy kit (Qiagen Inc.). For quantitative analysis, first strand reactions were conducted using SuperScript II first strand synthesis kit (Invitrogen Corp.), and the quantitative PCR reactions were performed on a 7500 Fast Real-time PCR System (Applied Biosystems Inc.). Expression levels for Th, Er81 and Gapdh were measured using TaqMan Gene Expression Assay primer sets (Applied Biosystems Inc.) Mm00447557_m1, Mm00514804_m1 and Mm99999915_g1, respectively, with the TaqMan Universal PCR Master Mix (Applied Biosystems Inc.). Th expression levels were normalized to Gapdh levels, and reported as the mean with error bars representing the standard deviation. Data were analyzed using two-tailed Student T-tests for each gene, and differences were considered significant if p<0.01.
Results
Treatment of forebrain slice cultures with HDAC inhibitors
Organotypic forebrain slice cultures were used to test the role of HDAC activity on Th expression in the RMS and OB because they preserve the complex in vivo cellular architecture and neural connections. Previous studies have demonstrated that OB interneuron progenitor proliferation and migration, as well as activity-dependent Th expression, can be effectively studied with the slice culture paradigm [15; 34]. In this study, slices were prepared from transgenic mice expressing GFP under the control of the 9kb Th upstream region. GFP expression in this strain is a sensitive and readily-detectable reporter of Th promoter activity.
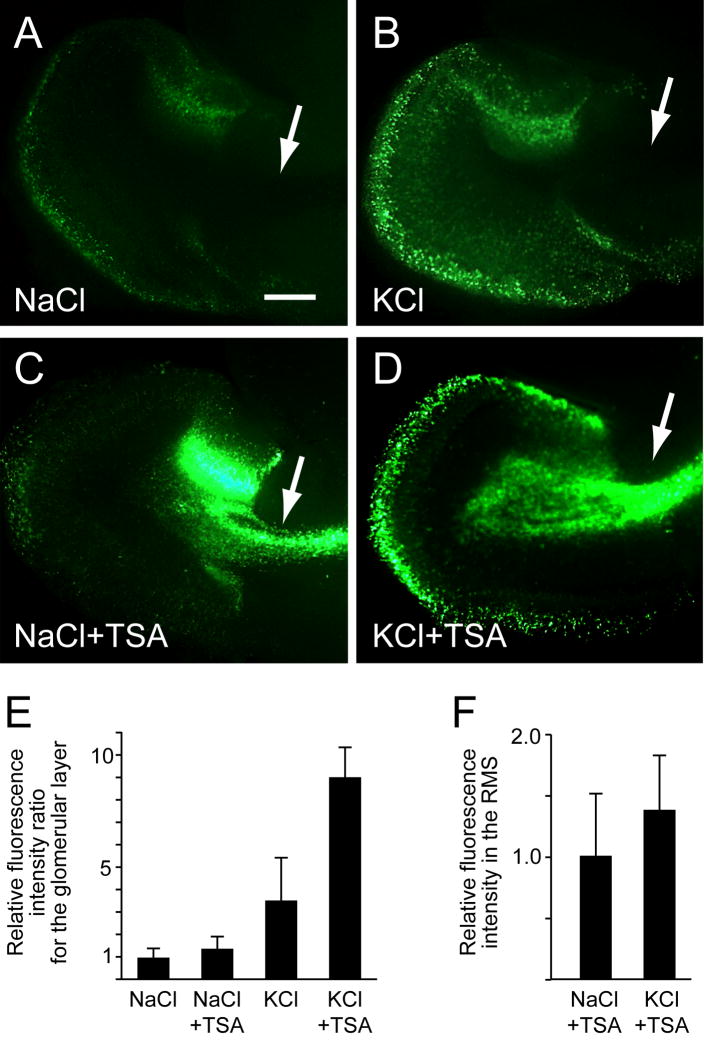
Th-GFP expression can be induced in slices cultured with depolarizing concentrations of KCl [15; 35]. Similar to the OB in vivo, depolarization-induced Th expression in slice cultures induced in the OB glomerular and superficial granule cell layers, but not in the RMS (Figure 2A and 2B). However, Th promoter activity was induced in the RMS when slices were treated with the HDAC inhibitor Trichostatin A (TSA; Supplemental Figure 1) in both depolarizing (supplemented with KCl) and non-depolarizing (supplemented with NaCl) culture media (Figure 2C and 2D).
Figure 2.
Induction of Th-GFP expression in the RMS and OB of forebrain slice cultures by TSA. A, weak basal Th-GFP expression in the glomerular and superficial granule cells layers, but not in the RMS (arrow), was detected in slices cultured in non-depolarizing media (supplemented with NaCl). B, Th-GFP expression was induced in the glomerular and superficial granule cell layers, but not in the RMS (arrow), in slices cultured with depolarizing media (supplemented with KCl). C, Th-GFP was induced in the RMS (arrow) of slices cultured in non-depolarizing media with TSA. D, Th-GFP expression was induced in the glomerular and superficial granule cell layers, as well as the RMS (arrow), in slices cultured with depolarizing media and TSA. E, relative fluorescence intensity ratios of GFP expression in the glomerular layer revealed TSA increased Th-GFP expression in the glomerular layer only when the slices were cultured in depolarizing media. F, depolarizing concentrations of KCl in the culture media did not affect the Th-GFP fluorescence intensity in the RMS. Bar = 250 μm.
Although TSA did not induce Th-GFP expression in glomerular layer under non-depolarizing culture conditions, analysis of the fluorescence intensity of KCl-depolarized slices revealed that TSA did significantly increased Th-GFP expression in the glomerular layer (Figure 2E). By contrast, TSA did not increase the fluorescence intensity of the RMS in depolarized slices relative to non-depolarized slices (Figure 2F). These findings indicate that HDAC activity modulates Th expression in both progenitors and mature OB neurons, but these findings also suggest that Th expression is regulated by distinct HDAC-mediated mechanisms in progenitors and mature OB neurons.
The ability to induce Th promoter activity in the RMS was not limited to TSA. Scriptaid, a hydroxamic acid compound similar to TSA, also induced Th-GFP expression was also induced in the RMS (Supplemental Figures 1 and 2). By contrast, Nullscript, an inactive analog of Scriptaid, did not induce Th-GFP expression under identical culture conditions (Supplemental Figures 1 and 2). These findings with Scriptaid and Nullscript confirmed that the induction of GFP expression by Scriptaid resulted from inhibition of HDAC enzymes and not from the ability of the hydroxamic acid functional group to chelate zinc or iron from other enzymes in the cell. Nullscript is structurally similar to Scriptaid, except that the aliphatic linker region of Nullscript is two carbons shorter and this difference prevents Nullscript from functioning as an effective HDAC inhibitor [36]. The short-chain fatty acid HDAC inhibitors sodium butyrate (NaB) also induced Th promoter activity in the RMS of cultured slices (Supplemental Figures 1 and 2).
Intraperitoneal injections with HDAC inhibitors
To test whether HDAC inhibitors could also induce Th promoter activity in the RMS in vivo, pups (aged P3–P4) were injected twice-a-day for three days with NaB. As shown in Figure 3A, this treatment induced GFP expression in the RMS. Examination of GFP-expressing cells in the RMS of mice treated with HDAC inhibitors revealed that these cells had leading and trailing processes that were consistent with migrating progenitor cells (Figure 3B). Similar results were also observed with mice treated with another short-chain fatty acid, valproic acid (VPA; Supplemental Figure 3). By contrast, Th-GFP expression was not observed in saline injected control littermates (Figure 3C).
Figure 3.
In vivo induction of Th-GFP expression in the RMS by sodium butyrate (NaB). A, the RMS of a mouse treated with NaB contained Th-GFP expression in the RMS (outlined with dashed line). B, high magnification image of boxed region in A revealed leading and trailing processes of GFP expressing cells in the RMS. C, in contrast, Th-GFP was not observed in the RMS of saline injected control littermates. Bar = 125 μm for A and C, and 25 μm for B.
Unlike the NaB and VPA short-chain fatty acid compounds, intraperitoneal administration of either TSA or Scriptaid did not induce detectable GFP expression in the RMS (data not shown). However, this discrepancy may result from the low efficiency of the hydroxamic acid compounds crossing the blood-brain-barrier. Unlike short-chain fatty acids which readily penetrate the blood-brain-barrier [37; 38], hydroxamic acid compounds, such as TSA and Scriptaid, may require co-administration of carrier molecules, such as cyclodextrin, to improve in vivo brain delivery [39].
Induction of Th expression in SVZ-derived neurosphere cultures
To confirm that HDAC inhibitors could induce Th expression in SVZ-derived progenitors, neurospheres cultured from dissociated SVZ cells were treated with various HDAC inhibitors for 48 hours. Quantitative RT-PCR analysis of the treated neurospheres showed that Th expression was significantly up-regulated by the presence of HDAC inhibitors (Figure 4). Although Th transcription was increased by administration of HDAC inhibitors, immunohistochemical analysis of the treated neurosphere cultures found no evidence for TH protein expression (not shown). This finding was consistent with both the HDAC inhibitor treated slice cultures and in vivo results that also found no immunohistochemical evidence for TH protein expression in the RMS despite the induction of Th-GFP expression (not shown).
Figure 4.
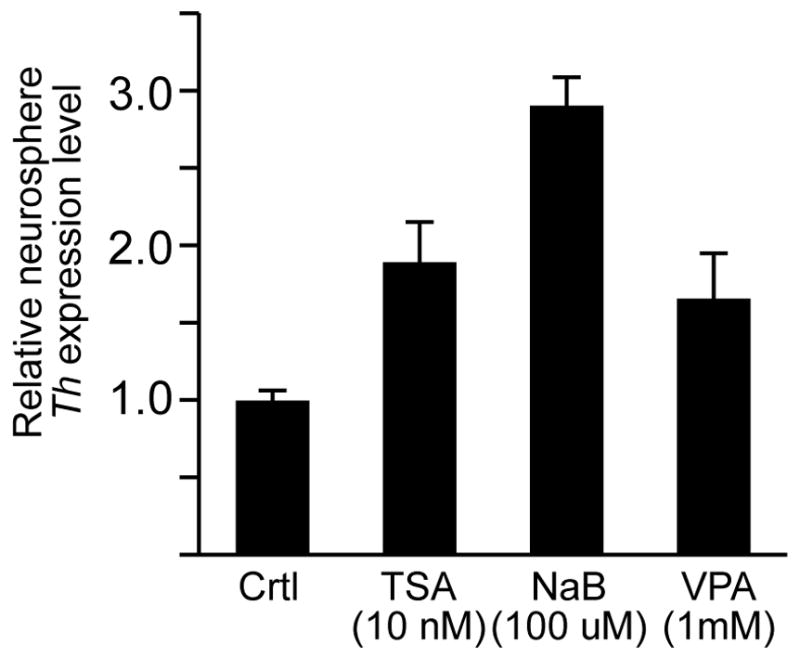
Induction of Th expression in SVZ-derived neurosphere cultures by HDAC inhibitors. Quantitative RT-PCR analysis showed that Th expression levels were increased in neurospheres treated for 48 hours with either TSA, NaB or VPA.
Discussion
Using both in vivo and in vitro experimental paradigms, the current study showed that Th promoter activity in the RMS is induced by HDAC inhibitors. This induction of Th expression suggests that the transcription factors necessary to activate Th transcription are present in at least some of the migrating neural progenitors in the RMS, but HDAC activity prevents these proteins from up-regulating Th expression. HDAC activity in the neural progenitors of the RMS may be necessary to maintain high levels of acetylated histones and compact the genomic DNA at the Th locus in order to reduce transcription factor protein access to the Th promoter. However, an alternative, but not mutually exclusive possibility is that the HDAC enzymes may target transcription factors that require acetylation to mediate activation of Th transcription.
The fluorescence intensity analysis of the slice cultures revealed that HDAC activity also regulates Th expression levels in the glomerular layer. However, the increased Th expression in the glomerular layer mediated by HDAC inhibitors was dependent on KCl-mediated depolarization. These findings suggest that HDAC activity modulates OB Th expression levels of in response to synaptic activity. In contrast to the glomerular layer, HDAC inhibitors induced Th promoter activity in the RMS in both depolarizing and non-depolarizing culture media, but depolarizing concentrations of KCl did not increase the Th-GFP expression levels. This differential response to the combinatorial exposure to HDAC inhibitors and depolarizing concentrations of KCl suggests that Th expression in mature OB neurons and RMS progenitors is regulated by distinct HDAC-mediated mechanisms. Consistent with this possibility, recent studies have reported that HDAC1 and HDAC2 are differentially expressed in neural progenitors and mature olfactory sensory neurons in the olfactory epithelium [40]. The possible differential expression of HDAC1 and HDAC2 in the RMS and OB is currently being investigated.
In both the in vivo and in vitro model systems studied, TH protein expression was never detected in the RMS despite the induction of Th promoter activity by HDAC inhibitors. These findings were consistent with previous observations that TH protein expression in the OB is limited to the glomerular layer, even though there are substantial levels of Th message in other regions, such as the superficial granule cell layer [18]. Thus, HDAC inhibitors are sufficient to induce Th transcription in the RMS, but not sufficient to overcome the apparent post-transcriptional repression of Th message in progenitor cells.
The findings in this study have implications for neuroprotection and repair strategies. The systemic administration of HDAC inhibitors did not broadly induce Th-GFP expression in forebrain, rather the effects were region-specific. The HDAC inhibitors induced expression in only a subset of cells in the RMS and up-regulated expression in the depolarized glomerular region, which already express Th. The underlying mechanism for this region-specific response is likely that the cells in which Th-GFP was either induced or up-regulated already expressed the appropriate complement of proteins that are necessary to mediate Th transcription. Thus, the ability for HDAC inhibitors, or other classes of drugs, to either induce or up-regulate expression of genes necessary for neural protection and repair may be dependent on the pre-existing transcriptome of the cell. Together, the findings in this study reveal a novel epigenetic regulatory mechanism for Th in the OB, and highlight challenges for development of drug-based neuroprotection and repair strategies.
Supplementary Material
Acknowledgments
Funding provided by NIH R01 DC008955 and BMRI
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chapouton P, Jagasia R, Bally-Cuif L. Adult neurogenesis in non-mammalian vertebrates. Bioessays. 2007;29:745–57. doi: 10.1002/bies.20615. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Buylla A, Lois C. Neuronal stem cells in the brain of adult vertebrates. Stem Cells. 1995;13:263–72. doi: 10.1002/stem.5530130307. [DOI] [PubMed] [Google Scholar]
- 3.Altman J, Das GD. Autoradiographic and histological studies of postnatal neurogenesis. I. A longitudinal investigation of the kinetics, migration and transformation of cells incorporating tritiated thymidine in neonate rats, with special reference to postnatal neurogenesis in some brain regions. J Comp Neurol. 1966;126:337–89. doi: 10.1002/cne.901260302. [DOI] [PubMed] [Google Scholar]
- 4.Bayer SA. 3H-thymidine-radiographic studies of neurogenesis in the rat olfactory bulb. Exp Brain Res. 1983;50:329–40. doi: 10.1007/BF00239197. [DOI] [PubMed] [Google Scholar]
- 5.Hinds JW. Autoradiographic study of histogenesis in the mouse olfactory bulb. I. Time of origin of neurons and neuroglia. J Comp Neurol. 1968;134:287–304. doi: 10.1002/cne.901340304. [DOI] [PubMed] [Google Scholar]
- 6.Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–93. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- 7.Cave JW, Baker H. Dopamine systems in the forebrain. In: Pasterkamp RJ, Smidt MP, Burbach JPH, editors. Development and Engineering of Dopamine Neurons. Landes BioScience; Austin: 2008. [Google Scholar]
- 8.Hokfelt T, Johansson O, Fuxe K, Goldstein M, Park D. Immunohistochemical studies on the localization and distribution of monoamine neuron systems in the rat brain II. Tyrosine hydroxylase in the telencephalon. Med Biol. 1977;55:21–40. [PubMed] [Google Scholar]
- 9.Halasz N, Ljungdahl A, Hokfelt T, Johansson O, Goldstein M, Park D, Biberfeld P. Transmitter histochemistry of the rat olfactory bulb. I. Immunohistochemical localization of monoamine synthesizing enzymes. Support for intrabulbar, periglomerular dopamine neurons. Brain Res. 1977;126:455–74. doi: 10.1016/0006-8993(77)90597-2. [DOI] [PubMed] [Google Scholar]
- 10.Baker H, Kawano T, Margolis FL, Joh TH. Transneuronal regulation of tyrosine hydroxylase expression in olfactory bulb of mouse and rat. J Neurosci. 1983;3:69–78. doi: 10.1523/JNEUROSCI.03-01-00069.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker H. Unilateral, neonatal olfactory deprivation alters tyrosine hydroxylase expression but not aromatic amino acid decarboxylase or GABA immunoreactivity. Neurosci. 1990;36:761–771. doi: 10.1016/0306-4522(90)90018-y. [DOI] [PubMed] [Google Scholar]
- 12.Baker H, Cummings DM, Munger SD, Margolis JW, Franzen L, Reed RR, Margolis FL. Targeted deletion of a cyclic nucleotide-gated channel subunit (OCNC1): Biochemical and morphological consequences in adult mice. J Neurosci. 1999;19:9313–9321. doi: 10.1523/JNEUROSCI.19-21-09313.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker H, Farbman AI. Olfactory afferent regulation of the dopamine phenotype in the fetal rat olfactory system. Neurosci. 1993;52:115–134. doi: 10.1016/0306-4522(93)90187-k. [DOI] [PubMed] [Google Scholar]
- 14.McMillian MK, Mullis SB, Wu GC, Hudson PM, Pennypacker KR, Hong JS. Regulation of tyrosine hydroxylase in olfactory bulb cultures: selective inhibition of depolarization-induced increase by endogenous opioids. Brain Res. 1994;658:105–11. doi: 10.1016/s0006-8993(09)90015-4. [DOI] [PubMed] [Google Scholar]
- 15.Akiba Y, Sasaki H, Huerta PT, Estevez AG, Baker H, Cave JW. gamma-Aminobutyric acid-mediated regulation of the activity-dependent olfactory bulb dopaminergic phenotype. J Neurosci Res. 2009 doi: 10.1002/jnr.22055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cigola E, Volpe BT, Lee JW, Franzen L, Baker H. Tyrosine hydroxylase expression in primary cultures of olfactory bulb: role of L-type calcium channels. J Neurosci. 1998;18:7638–7649. doi: 10.1523/JNEUROSCI.18-19-07638.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puche AC, Shipley MT. Odor-induced, activity-dependent transneuronal gene induction in vitro: mediation by NMDA receptors. J Neurosci. 1999;19:1359–70. doi: 10.1523/JNEUROSCI.19-04-01359.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saino-Saito S, Sasaki H, Volpe BT, Kobayashi K, Berlin R, Baker H. Differentiation of the dopaminergic phenotype in the olfactory system of neonatal and adult mice. J Comp Neurol. 2004;479:389–98. doi: 10.1002/cne.20320. [DOI] [PubMed] [Google Scholar]
- 19.Darcy DP, Isaacson JS. L-type calcium channels govern calcium signaling in migrating newborn neurons in the postnatal olfactory bulb. J Neurosci. 2009;29:2510–8. doi: 10.1523/JNEUROSCI.5333-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Platel JC, Dave KA, Bordey A. Control of neuroblast production and migration by converging GABA and glutamate signals in the postnatal forebrain. J Physiol. 2008;586:3739–43. doi: 10.1113/jphysiol.2008.155325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsieh J, Gage FH. Chromatin remodeling in neural development and plasticity. Curr Opin Cell Biol. 2005;17:664–71. doi: 10.1016/j.ceb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Feng J, Fouse S, Fan G. Epigenetic regulation of neural gene expression and neuronal function. Pediatr Res. 2007;61:58R–63R. doi: 10.1203/pdr.0b013e3180457635. [DOI] [PubMed] [Google Scholar]
- 23.Langley B, Gensert JM, Beal MF, Ratan RR. Remodeling chromatin and stress resistance in the central nervous system: histone deacetylase inhibitors as novel and broadly effective neuroprotective agents. Curr Drug Targets CNS Neurol Disord. 2005;4:41–50. doi: 10.2174/1568007053005091. [DOI] [PubMed] [Google Scholar]
- 24.Lim DA, Huang YC, Swigut T, Mirick AL, Garcia-Verdugo JM, Wysocka J, Ernst P, Alvarez-Buylla A. Chromatin remodelling factor Mll1 is essential for neurogenesis from postnatal neural stem cells. Nature. 2009;458:529–33. doi: 10.1038/nature07726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–7. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fasano CA, Dimos JT, Ivanova NB, Lowry N, Lemischka IR, Temple S. shRNA knockdown of Bmi-1 reveals a critical role for p21-Rb pathway in NSC self-renewal during development. Cell Stem Cell. 2007;1:87–99. doi: 10.1016/j.stem.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Merson TD, Dixon MP, Collin C, Rietze RL, Bartlett PF, Thomas T, Voss AK. The transcriptional coactivator Querkopf controls adult neurogenesis. J Neurosci. 2006;26:11359–70. doi: 10.1523/JNEUROSCI.2247-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siebzehnrubl FA, Buslei R, Eyupoglu IY, Seufert S, Hahnen E, Blumcke I. Histone deacetylase inhibitors increase neuronal differentiation in adult forebrain precursor cells. Exp Brain Res. 2007;176:672–8. doi: 10.1007/s00221-006-0831-x. [DOI] [PubMed] [Google Scholar]
- 29.DeCastro M, Nankova BB, Shah P, Patel P, Mally PV, Mishra R, La Gamma EF. Short chain fatty acids regulate tyrosine hydroxylase gene expression through a cAMP-dependent signaling pathway. Brain Res Mol Brain Res. 2005;142:28–38. doi: 10.1016/j.molbrainres.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Kim HS, Park JS, Hong SJ, Woo MS, Kim SY, Kim KS. Regulation of the tyrosine hydroxylase gene promoter by histone deacetylase inhibitors. Biochem Biophys Res Commun. 2003;312:950–7. doi: 10.1016/j.bbrc.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 31.Matsushita N, Okada H, Yasoshima Y, Takahashi K, Kiuchi K, Kobayashi K. Dynamics of tyrosine hydroxylase promoter activity during midbrain dopaminergic neuron development. J Neurochem. 2002;82:295–304. doi: 10.1046/j.1471-4159.2002.00972.x. [DOI] [PubMed] [Google Scholar]
- 32.Saino-Saito S, Cave JW, Akiba Y, Sasaki H, Goto K, Kobayashi K, Berlin R, Baker H. Er81 and CaMKIV identify anatomically and phenotypically defined subsets of mouse olfactory bulb interneurons. J Comp Neurol. 2007;502 doi: 10.1002/cne.21293. [DOI] [PubMed] [Google Scholar]
- 33.Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–4. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- 34.Wang DD, Krueger DD, Bordey A. GABA depolarizes neuronal progenitors of the postnatal subventricular zone via GABAA receptor activation. J Physiol. 2003;550:785–800. doi: 10.1113/jphysiol.2003.042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akiba Y, Sasaki H, Saino-Saito S, Baker H. Temporal and spatial disparity in cFOS expression and dopamine phenotypic differentiation in the neonatal mouse olfactory bulb. Neurochem Res. 2007;32:625–34. doi: 10.1007/s11064-006-9134-7. [DOI] [PubMed] [Google Scholar]
- 36.Su GH, Sohn TA, Ryu B, Kern SE. A novel histone deacetylase inhibitor identified by high-throughput transcriptional screening of a compound library. Cancer Res. 2000;60:3137–42. [PubMed] [Google Scholar]
- 37.Cornford EM, Diep CP, Pardridge WM. Blood-brain barrier transport of valproic acid. J Neurochem. 1985;44:1541–50. doi: 10.1111/j.1471-4159.1985.tb08793.x. [DOI] [PubMed] [Google Scholar]
- 38.Dhopeshwarkar GA, Mead JF. Uptake and transport of fatty acids into the brain and the role of the blood-brain barrier system. Adv Lipid Res. 1973;11:109–42. doi: 10.1016/b978-0-12-024911-4.50010-6. [DOI] [PubMed] [Google Scholar]
- 39.Hockly E, Richon VM, Woodman B, Smith DL, Zhou X, Rosa E, Sathasivam K, Ghazi-Noori S, Mahal A, Lowden PA, Steffan JS, Marsh JL, Thompson LM, Lewis CM, Marks PA, Bates GP. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, ameliorates motor deficits in a mouse model of Huntington’s disease. Proc Natl Acad Sci U S A. 2003;100:2041–6. doi: 10.1073/pnas.0437870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacDonald JL, Roskams AJ. Histone deacetylases 1 and 2 are expressed at distinct stages of neuro-glial development. Dev Dyn. 2008;237:2256–67. doi: 10.1002/dvdy.21626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.