Abstract
Serial magnetic resonance imaging (MRI) was performed to investigate the temporal and spatial relationship between the biphasic nature of blood-brain barrier (BBB) opening and in parallel, edema formation following ischemia-reperfusion (I/R) injury in rats. T2-weighted imaging combined with T2-relaxometry mainly for edema assessment was performed at 1 hour post-ischemia, following reperfusion, and at 4, 24, and 48 hours post-reperfusion. T1-weighted imaging was performed pre/post-gadolinium contrast at the last three time points to assess BBB integrity. The biphasic course of BBB opening with significant reduction in BBB permeability at 24 hours post-reperfusion associated with progressive expansion of leaky BBB volume was accompanied by a peak ipsilateral edema formation. In addition, at 4 hours post-reperfusion, edema formation could also be detected at the contralateral striatum as determined by the elevated T2 values that persisted to varying degrees indicative of widespread effects of I/R injury. The observations of this study may indicate a dynamic temporal shift in mechanisms responsible for bi-phasic BBB permeability changes with complex relations to edema formation. Stroke therapy aimed at vasogenic edema and drug delivery for neuroprotection may also be guided according to the functional status of the BBB and these findings should be confirmed in human stroke.
Keywords: Blood-brain barrier, Cerebral edema, Magnetic resonance imaging, Stroke
Introduction
In ischemic stroke therapy, thrombolysis of the occluded cerebral artery is the most beneficial approach to effective reperfusion which aims to salvage the ischemic penumbra (Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group 1995). Rapid reperfusion, although desired, contributes to secondary injury via a cascade of events including hemodynamic disturbances, inflammatory processes, free radical formation and breakdown of the blood-brain barrier (BBB) (del Zoppo 1994; Hacke et al. 1996). When severely impaired, there is an increased risk of deleterious vasogenic edema, brain herniation and death (Hacke et al. 1996). Experimental studies have consistently demonstrated a biphasic pattern in BBB permeability changes following ischemia-reperfusion (I/R) injury, which has not been categorically confirmed in stroke patients (Belayev et al. 1996; Huang et al. 1999; Kuroiwa et al. 1985; Latour et al. 2004). Severe restraints are in place to subject the severely ill patient to time consuming and exhaustive repeated magnetic resonance imaging (MRI) in the acute phase of cerebral edema. In addition, the role of the biphasic BBB opening characteristics for brain edema evolution and resolution is still rather ill defined.
Magnetic resonance imaging (MRI) has the potential to be the standard for assessing BBB permeability characteristics employing small molecule paramagnetic contrast agents (Stoll et al. 2009). Further, quantitative T2 mapping can be performed in parallel to detect dynamic changes in tissue water content which invariably reflects the state of barrier function at the BBB (Verheul et al. 1992). A number of studies have analyzed the temporal evolution of the bi-phasic BBB characteristics and, separately, the temporal course vasogenic edema formation following ischemia-reperfusion (I/R) injury. To the best of our knowledge, no study has been published which simultaneously characterized the complex relationship between BBB opening and vasogenic edema formation in a temporal and spatial manner in identical animals.
In this study, we investigated the dynamics of edema formation in both the dimensions of time and space and in relation to the bi-phasic BBB opening after 90 minutes of transient middle cerebral artery occlusion (tMCAO) in rats.
Materials and Methods
Experimental Design
All experiments were carried out in accordance with the European communities’ council directive (86/609/EEC) and institutional guidelines for animal care after ethics committee approval. Male Wistar rats (Charles River Laboratories, Sulzfeld, Germany) weighing between 250-300 g, were either subjected to 90 minutes of focal cerebral ischemia followed by 48 hours of reperfusion (Experimental group, N=11) or sham operation (Sham group, N=6). Serial MRI was performed at the following time points: Pre-ischemic (Control), 1 hour after ischemia (BR), following reperfusion (AR), 4 hours post-reperfusion (04PR), 24 hours post-reperfusion (24PR) and 48 hours post-reperfusion (48PR). Experimental animals were scanned at six time points (Control, BR, AR, 04PR, 24PR and 48PR), and sham animals at three time points (04PR, 24PR and 48PR).
Surgical procedure
Transient middle cerebral artery occlusion (tMCAO) was performed as previously described (Longa et al. 1989; Spratt et al. 2006). Briefly, anaesthesia was induced with 5% isoflurane in a mixture of 70% nitrous oxide and 30% oxygen. After endotracheal intubation, anaesthesia was maintained using 1.5% isoflurane. The body temperature was maintained at 37-37.5°C throughout surgery. A cannula was inserted into the left femoral vein for contrast agent administration. The left common carotid artery (CCA) bifurcation was exposed through a midline neck incision and the occipital artery branches of the external carotid artery (ECA) were isolated, ligated and dissected. After careful isolation of the internal carotid artery (ICA), a 3 cm long silicone coated, polyamide 4-0 monofilament (Ethicon) was advanced through the ICA to the MCA until mild resistance was felt. After the intra-luminal suture was placed, the neck incision was closed with a silk suture. Sham operation was performed in the same manner, except for MCA occlusion. The animals were then allowed to recover. One hour post occlusion, the animals were re-anesthetized with 1.5% isoflurane and MRI was performed for the time point BR. Following this, animals were subjected to reperfusion by gently pulling back the filament.
MRI
All experiments were conducted on a standard 3T clinical dedicated head MR scanner (Magnetom Allegra, Siemens Healthcare, Erlangen, Germany), where the gradients achieve a 40 mT/m amplitude with a slew rate of 400 T/m/s per axis. The MR scanner was complemented only by a dedicated four-channel phased array rat head coil assembly (RAPID Biomedical GmbH, Rimpar, Germany). All MRI acquisitions throughout this longitudinal study has been conducted at a constant receiver gain, which was initially set during the optimization phase of the standard Siemens product sequences for performing small animal imaging.
To detect ischemic changes especially at the early time points (BR and AR), DW-EPI images were acquired with repetition time (TR) = 3300 ms, echo time (TE) = 105 ms, field of view (FoV) = 6.7 cm, image matrix (IM) = 104 × 104 and at five different ‘b’ values (0, 500, 1000, 1500 & 2000 s/mm2) applied in three orthogonal directions. Trace weighted apparent diffusion co-efficient (ADC) maps were also generated. T2-turbo spin echo (TSE) images were acquired with TR = 3330 ms, TE = 70 ms and Turbo factor = 7.
To assess BBB permeability changes, T1-spin echo (SE) images were acquired before (pre-contrast) and after contrast agent (post-contrast) administration. The images were acquired with TR = 900 ms and TE = 10 ms. After acquiring the pre-contrast images, Gd-DTPA (MW: 590 Da. Magnevist, Shering, Germany) was administered (0.2 mmol/kg) and post-contrast T1-SE images were acquired after 25 minutes. This dose was chosen from preliminary experiments showing that following subtraction and during the calculation of enhanced volumes there was a significant variability in the estimated volumes by two authors of this paper (DRP and DB) when using lower doses of MRI contrast agent (0.1 mmol/kg)
For assessment of brain water content/ edema formation, T2 relaxometry was performed at all time points (Control, BR, AR, 04PR, 24PR & 48PR) using a SE sequence with a TR of 3330 ms and employing 7 TE values (29, 58, 88, 117, 146, 175 & 204 ms).
For TSE and SE acquisitions, a FoV of 2.5 cm, with an IM of 128 × 128 was used. The spin-echo and EPI images had an in-plane resolution of 200 μm and 600 μm respectively with 1 mm slice thickness. Sequences were applied in the following order: DW-EPI, T2-TSE, T2 relaxometry, T1-SE and post-contrast T1-SE.
Data Analysis
All the required parameters were acquired from a single coronal slice (1 mm thick) located at 7 mm posterior to the anterior tip of the frontal cortex. A preliminary analysis had demonstrated that this slice revealed the largest volume of infarct at all three relevant time points with good assessment of the midline shift and was best suited for standardized T2 relaxometry in the striatum and cortex. The variation in infarct volume was also found to be lower for this slice region at 24 and 48 hours, compared to adjacent regions with similar infarct volumes (data not shown).
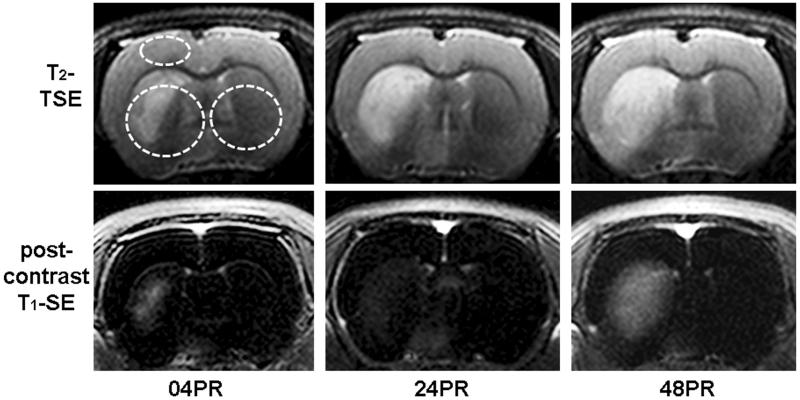
Quantitative T2 relaxometry was performed at three regions of interest (RoIs); the ipsi- and contralateral striatum and on the ipsilateral cortex. In the striatal region, circular RoIs have been defined over the entire striatal region excluding the ventricles (Figure 1).
Figure 1.
Representative images of T2-TSE for edema analysis (upper) and post-contrast T1-SE (lower) for blood-brain barrier permeability at 04, 24 and 48 hours post reperfusion. Highlighted areas (as upper left image) have been considered for T2 relaxometric estimations.
Mono-exponential non-linear curve fitting was performed using Graphpad Prism Version 5.00 for Windows (Graphpad Software, San Diego, California, USA) to determine T2 relaxation parameters. Volumetric estimations of the cerebral hemispheres were again performed on T2-weighted images using the available built-in tools of Siemens Syngo 2004A software (Siemens Healthcare, Erlangen, Germany).
Analysis of BBB permeability changes was conducted on subtracted maps from the pre- and post-contrast T1-SE images to highlight regions of Gd-DTPA extravasation. Gd-DTPA permeable BBB volume (PBV) in cubic centimeters (cm3) representing brain tissue with leaky BBB and the average pixel intensity (T1SIdiff) of the hyper-intense enhanced regions derived from the subtraction maps were calculated using the built-in tools. The obtained values of the mean pixel intensity for the subtracted images (T1SIdiff) are software generated (Siemens syngo 2004A) and standard across all the current generation of Siemens scanners. A product of T1SIdiff and PBV (T1SIdiff x PBV) has been considered to account for the observed temporal and spatial changes in the average pixel intensity of the enhanced regions (T1SIdiff) and the brain volume with leaky BBB (PBV). The T1SIdiff x PBV product serves as an indicator to quantify the overall entry of contrast agent into the brain over time. For sham analysis, the average PBV from the experimental group was projected onto the subtracted images of the sham animals, and T1SIdiff was determined within this region.
Statistical analysis
Throughout the study, values are treated as mean ± standard error (SEM). Repeated measures ANOVA followed by Tukey-Kramer post-hoc tests have been considered for within-group comparisons. For between-group comparisons (experimental v/s sham group) at different time points, unpaired ‘t’ tests with Welch correction were applied. A P value < 0.05 was considered significant.
Results
Three out of 11 animals did not survive the duration of this study, making for an effective N = 8 for the experimental group. Representative images for T2-TSE and T1SIdiff are shown in Figure 01 for 04PR, 24PR, and 48PR time points.
Bi-phasic BBB opening and progression of brain volume with leaky BBB following ischemia-reperfusion injury as investigated by post-contrast T1-sequences
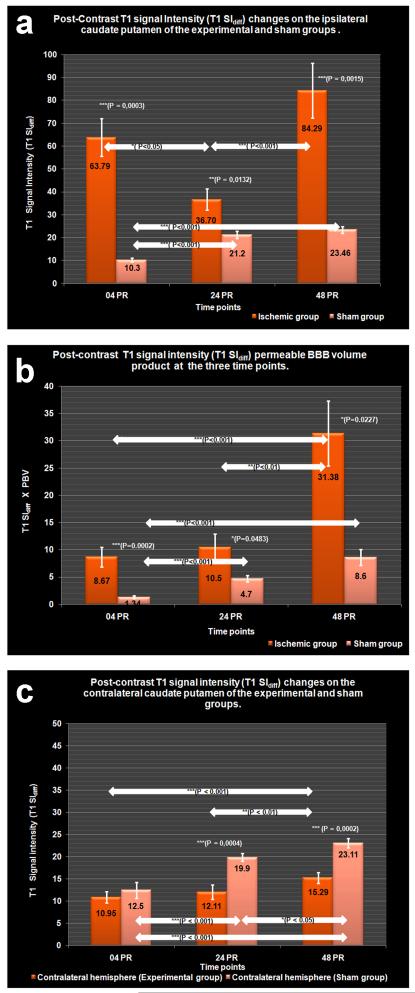
The temporal profiles of BBB leakage (post-contrast T1SIdiff) and overall extravasated contrast agent (T1SIdiff x PBV product) for the ipsilateral hemisphere are compared to sham (Figure 2a and b). Post-contrast T1SIdiff profiles of the contralateral striatum are again compared to that of sham (Figure 2c).
Figure 2.
a.) BBB permeability changes as represented by post-contrast signal intensity (T1 SIdiff), changes on the ipsilateral striatum of experimental group in comparison to sham.
b.) Brain tissue with leaky BBB as measured by signal intensity (T1 SIdiff) permeable BBB volume (PBV) product at the ipsilateral striatum of experimental and sham groups.
c.) BBB permeability changes as represented by post-contrast signal intensity changes at the contralateral striatum of experimental and sham groups.
At 04PR, the post-contrast T1SIdiff value at the ipsilateral striatum of the experimental group was found to be significantly elevated (63.79 ± 8.2) compared to sham group (10.3 ± 0.79, P = 0.0003). The brain volume with leaky BBB (PBV) at the ipsilateral hemisphere of the experimental group was 0.13 ± 0.02 cm3 and the T1SIdiff x PBV value was also significantly higher (8.67 ± 1.77) to that of sham (1.34 ± 0.23, P = 0.0002). In contrast, there was a non-significant drop in the post-contrast T1SIdiff intensity at the contralateral hemisphere (10.95 ± 1.28) when compared to sham (12.5 ± 1.8).
By 24PR, post-contrast T1SIdiff (36.70 ± 4.61) declined significantly to that at 04 PR (P < 0.05). However, the observed value was still significantly higher than sham control (21.2 ± 1.623, P = 0.0132). The PBV doubled in volume (0.265 ± 0.032 cm3) and was significantly more widespread compared to 04PR (P < 0.05). The T1SIdiff x PBV demonstrated a non significant increase (10.50 ± 2.43) compared to 04PR, but was significantly higher than sham (4.7 ± 0.60, P = 0.0483). On the contralateral side, the post-contrast T1SIdiff intensity (12.11 ± 1.61) was not significantly increased in comparison to 04PR, while being significantly lower than sham values (19.9 ± 0.85, P = 0.0004).
The 48PR post-contrast T1SIdiff value (84.29 ± 11.99) was significantly higher than 24PR (P < 0.001) and sham (23.46 ± 1.52; P = 0.0015). The increase in the ipsilateral PBV (0.36 ± 0.055 cm3) was non-significant, but the T1SIdiff x PBV product (31.38 ± 5.98) was significantly higher compared to 24PR (P < 0.01) and also to that of sham (8.6 ± 1.44, P = 0.0227). On the contralateral side, the post-contrast intensity (15.29 ± 1.19) was significantly higher compared to 24PR (P < 0.01), while being significantly lower than sham (23.11 ± 1.088, P = 0.0002).
Ipsilateral and remote edema formation as investigated by the temporal profile of transverse relaxation times
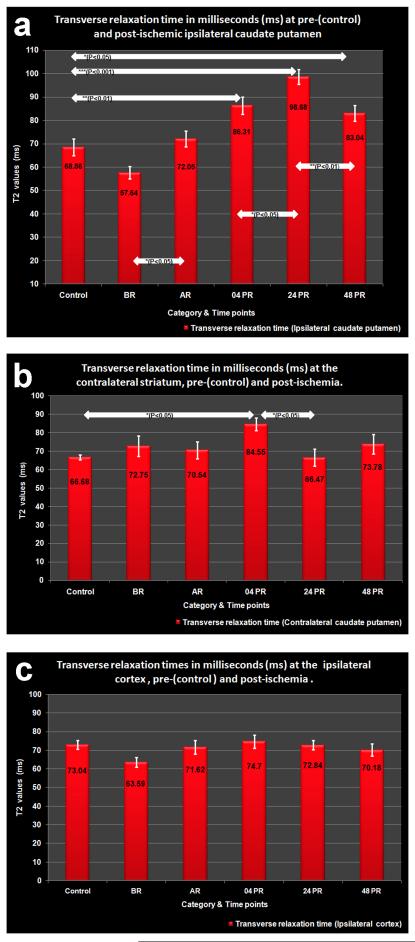
Transverse relaxation times from healthy, ischemic, and reperfused tissues are shown for the ipsi- and contralateral striatum along with the cortical region on the ipsilateral side (Figure 3a-c).
Figure 3.
a.) Tissue water content/edema as determined from T2 relaxation values at pre- (=control) and post- ischemic ipsilateral striatum.
b.) Tissue water content/edema as determined from T2 relaxation values at pre-(=control) and post- ischemic contralateral striatum.
c.) Tissue water content/edema as determined from T2 relaxation values at pre- (=control) and post- ischemic ipsilateral cortex.
The control T2 values indicative of increased water content obtained at the cortex was 73.04 ± 2.313 ms with little inter-hemispherical difference while the striatal values were 68.56 ± 3.58 ms and 66.68 ± 1.38 ms at the ipsi- and contralateral hemispheres, respectively.
During tMCAO (BR) the T2 values dropped at the ipsilateral cortex (63.59 ± 2.52 ms) and striatum (57.64 ± 2.625 ms), while the contralateral striatal T2 value was slightly elevated (72.75 ± 5.617 ms). However, none of these changes were statistically significant. Following reperfusion (AR), the T2 value at the ipsilateral striatum showed a significant increase to 72.05 ± 3.4 ms (P < 0.05), whereas the cortical value incremented insignificantly (71.62 ± 3.7 ms) compared to BR. Again, the contralateral striatal T2 values declined (70.54 ± 4.6 ms) insignificantly to that of BR.
By 04PR, the T2 values at the ipsilateral (86.31 ± 3.71 ms; P < 0.01) and contralateral striatum (84.55 ± 3.36 ms, P < 0.05) were significantly elevated compared to the corresponding control values. However, the cortical T2 values showed only a marginal increase (74.7 ± 3.59 ms) to that of the control. The corresponding ipsilateral hemispherical volume was 0.60 ± 0.01 cm3.
At 24PR, the ipsilateral striatal T2 value (98.68 ± 3.12 ms) increased significantly compared to both 04PR (P < 0.05) and control (P < 0.001) associated with significant increase in the ipsilateral hemispheric volume (0.62 ± 0.02 cm3, P < 0.05). The corresponding cortical T2 value (72.84 ± 2.41 ms) was non-significantly reduced to that at 04PR. The contralateral striatal T2 value (66.47 ± 4.56 ms) was also significantly (P < 0.05) lower to that at 04PR.
By 48PR, T2 value at the ipsilateral striatum (83.04 ± 3.4 ms) showed significant (P < 0.01) decline compared to 24PR, but was still significantly (P < 0.05) higher when compared to that of control and is accompanied by a significant fall in hemispheric volume (0.59 ± 0.02 cm3, P < 0.01). The corresponding cortical values (70.18 ± 3.3 ms) declined insignificantly from that at 24PR and the T2 value at the contralateral striatum (73.78 ± 5.17 ms) showed a marginal rise compared to that of 24PR.
Discussion
Employing serial MRI at 3T we detected the phasic nature of BBB opening following I/R injury. The injured BBB demonstrated dynamic temporal changes in permeability characteristics and might mediate local and remote vasogenic edema. Further, the first phase of differential permeability characteristics at the BBB lasting up to 24 hours led to progressive edema accumulation whereas the second phase did not contribute to edema formation and may mediate edema resorption.
Upon reperfusion, three distinct phases of increased BBB permeability changes attributed to endothelial paracellular/tight junction (TJ) opening have been documented (Belayev et al. 1996; Huang et al. 1999; Kuroiwa et al. 1985). Increased BBB permeability (also termed BBB opening, BBB disruption, BBB leakage) is commonly characterized by the extravasation of blood-pool agents that under normal physiological conditions do not cross the BBB to enter the brain parenchyma. Such blood-pool agents include the Evans Blue (EB) dye/albumin complex (MW 65961 Da), 3H-sucrose (MW 342 Da) for histological studies and Gd-DTPA (MW 590 Dalton) for in vivo for MRI, amongst others (Belayev et al. 1996; Huang et al. 1999; Kuroiwa et al. 1985; Stoll et al. 2009). It is conceivable, given the differences in molecular weight, charge and protein binding characteristics of the tracer and detection methods, sensitivity of these methods for altered BBB permeability may differ considerably. Following reperfusion, the initial change in BBB permeability is attributed to acute elevations in regional cerebral blood flow (rCBF), which is then followed by the characteristic “biphasic” permeability response. Since, this is short lasting and hemodynamic in origin, we focused our attention on the subsequent pathologic ‘biphasic’ opening. In principle, at least three components of I/R injury are likely to contribute and act in sequential, but, overlapping time courses: 1. Direct injury to the neurovascular unit. 2. Resultant inflammation and repair. 3. Regenerative strategies such as angiogenesis.
MR imaging of BBB permeability characteristics
Following 4 hours of reperfusion and consistent with studies using EB, we observed the first significant increase of BBB permeability as indicated by an increase of T1SIdiff (Fig. 2a). This observation denotes the first phase of the biphasic permeability changes and may be attributed to increased inflammatory and oxidative stress on the BBB, in conjunction with enzymatic degradation of the extracellular matrix (Heo et al. 2005; Wang and Shuaib 2007). Inflammatory mediators, such as thrombin, histamine and bradykinin cause BBB permeability changes by actin polymerization-dependent endothelial cell (EC) rounding and formation of inter-endothelial gaps (Ahmmed and Malik 2005). Proteolytic enzymes like matrix metalloproteinases (MMPs) mediate the degradation of TJ proteins (TJPs) at such an early time point (Yang et al. 2007). The deleterious role of oxidative stress has also been demonstrated by the reversible disruptive effect of hydrogen peroxide on the physiological, biochemical, and immunocytochemical parameters of TJP integrity (Meyer et al. 2001). RNA levels of vascular endothelial growth factor (VEGF) and Angiopoetin-2 (Ang-2) has been described to increase as early as 1–3 hours after ischemia, while proteins peak later during severe vasogenic edema. Since, the angiogenic and endothelial cell destabilizing effects has been shown to lag behind their peak levels, their contributory roles in this initial opening seems to be minimal (Croll and Wiegand 2001). A correlation has been demonstrated between neutrophil infiltration into the brain parenchyma and the overlying time-frame of the biphasic permeability responses (Sandoval and Witt 2008). Peak neutrophil infiltration may however not be the cause but may be facilitated by the disassembly of TJPs as a result of MMP activation and/or due to oxidative stress following I/R injury. Since, the observed enhancement being a characteristic feature of tMCAO and is not demonstrated by permanent MCAO models, it is reasonable to speculate that the increased oxidative stress, resulting from reperfusion may be the principal factor contributing to the reversible TJP disruption and thereby bringing about the observed Gd-DTPA extravasation.
The first phase of the biphasic BBB permeability was followed by a significant drop in BBB permeability measured by T1SIdiff at 24 hours, however, complete recovery of BBB integrity did not occur as T1SIdiff were still higher than that of sham (Fig.2a). In fact, brain tissue with leaky BBB (as measured by PBV) doubled in volume compared to that at 4 hours post-reperfusion and the net amount of extravasated Gd-DTPA as measured as T1SIdiff x PBV product remained almost similar to that of the 4 hour post-reperfusion.
Even though the precise mechanisms of this partial recovery in barrier function found at this time are not well understood, two independent mechanisms might contribute. Reactive oxygen species (ROS) mediated disassembly of TJPs has been shown to reverse within a period of 6 hours, while ongoing effects of inflammatory mediators maintain the EC rounding resulting in an endothelial gap so large that even fully functional TJPs cannot form functional tight junctions limiting paracellular diffusion (Meyer et al. 2001). Such a situation at the BBB may still allow the extravasation of water while the free flow of Gd-DTPA molecules is partially hindered by the re-assembled TJPs causing the observed drop in post-contrast T1SIdiff.
Following these observations at 24PR, we observed an increase in BBB permeability and brain tissue with leaky BBB as indicated by both, T1SIdiff and PBV, at 48PR. The angiogenic and endothelial cell destabilizing effect of VEGF and Ang2 levels are known to peak at 48PR and may contribute to the observed BBB permeability changes (Croll and Wiegand 2001). Monocyte chemoattractant protein-1 (MCP-1) secretion by both astrocytes and brain endothelial cells also coincide well with the time course of events described and directly alters the BBB permeability characteristics associated with redistribution of occludin, ZO-1, ZO-2, and claudin-5. This also facilitates the entry of neutrophils to the site of the lesion (Stamatovic et al. 2005). Infiltrating neutrophils, being a rich source of MMP-9, have also been demonstrated to mediate vasogenic edema and hemorrhagic transformation in a transient ischemic model of stroke (Gidday et al. 2005).
We observed a consistent increase of brain tissue with leaky BBB (PBV) up to 48 PR, with the most dramatic increase observed at the 24PR. Interestingly, the entire PBV presented rather homogenously at the time points of 24 and 48 PR in- and outside the initial ischemic core (Figure 1).
Throughout the experiment, the BBB permeability as measured by T1SIdiff at the contralateral side of the experimental group has been consistently lower than that of sham controls probably due to compression of cerebral vessels due to raised intracranial pressure by cerebral edema. Also, we observed increase in post-contrast T1SIdiff values in the sham group as well as in the contralateral hemisphere of the experimental group most likely attributed to isoflurane anesthesia. Although not thoroughly investigated, isoflurane has shown to induce cerebrovascular dilation mediated through endothelial nitric oxide synthase (eNOS) and the formed nitric oxide (NO) in humans and animals in a dose-dependent manner (Werner et al. 2005). The confounding effects of isoflurane on the observed ipsilateral T1SIdiff may not be as direct as this region also possesses the infarct core along with profound edema. Low basal cerebrovascular tone results in a decreased capacity to vasodilate whereas high cerebrovascular tone expands the auto-regulatory range (Werner et al. 2005). Persistent edema observed at the ipsilateral hemisphere with increased ICP results in low cerebrovascular tone in order to maintain adequate CBF. Any additional effect of isoflurane to cause further vasodilatation is rather doubtful.
Edema development in relation to BBB permeability
Quantification of edema formation in both hemispheres using MRI was performed by T2-relaxometry over time and correlated to BBB permeability in parallel. At the early time point of 4PR, we could detect increased brain water content/edema as indicated by an elevated T2 value not only at the ipsi- but also at the contralateral striatum. Such remote effects at the non-ischemic contralateral hemisphere have thus far been demonstrated in permanent MCAO and cold brain injury models only (Izumi et al. 2002; Shinohara et al. 1990). In permanent MCAO peak edema at the contralateral regions was only apparent at 24-48 hours post occlusion. However, in the cold brain injury model, edema mediated by leaked protein was observed at the lesion site and periphery after one hour and by 24–48 hours the leaked protein was strongly stained at both the hemispheres (Shinohara et al. 1990). The authors attribute this to ‘routed protein migration’ and consider such ‘remote’ effects within the realm of ‘Diaschisis’. A similar assumption may also be valid in the case tMCAO for two reasons. First, the initial BBB permeability change would have resulted in the extravasation of serum proteins, which, along with the cellular debris following necrosis could proliferate through the white matter tracts. Second, with an intact BBB at the contralateral striatum, net transport of water need to be driven by osmotic forces due to solute movements as there are no primary active, ATP-driven, water pumps (Kimelberg 2004).
By 24 hours, the observation of a constant amount of extravasated contrast agent into the brain as observed by a constant T1SIdiff x PBV product to that at 4PR accompanied by a peak edema observed by T2 values and volumetric swelling of the ipsilateral hemisphere may indicate an ongoing unperturbed water shift from the vascular to the brain compartment. Contrary to the earlier time point, the observed edema is unlikely to be caused by significant BBB leakage for proteins as indicated by the partial recovery of BBB function at this time point. Potential candidates mediating edema accumulation may include members of the non-selective cationic transient receptor potential (TRP) channels, which serve as redox sensors in the vascular endothelium (Groschner et al. 2004). TRPs may form the molecular basis of oxidant-activated cation channels and are increasingly recognized for their role in regulating barrier function (Ahmmed and Malik 2005; Simard et al. 2007). Some members of the TRP family, especially the canonical TRPs (TRPCs) are well recognized for their lack of cation specificity and the ability to generate substantial monovalent conductances that govern membrane potential and cationic gradients. According to the biophysical properties reported so far, inward currents through most TRPC channels are carried by both Na+ and Ca2+ ions in physiological conditions (Eder et al. 2005). However, the entry of Na+ has been implicated in physiological Ca2+ signalling phenomena such as Ca2+ oscillations and also in pathophysiological Ca2+ overload (Craner et al. 2004; Paltauf-Doburzynska et al. 2000). Such a mechanism would explain the observation that BBB permeability to sodium occurs at 12-48 hours following MCA occlusion, which, together with an antecedent intracellular shift to sodium, cause a massive water and sodium influx (Gotoh et al. 1985). Again, continued oxidative stress resulting from reperfusion, entry of neutrophils and the generation of superoxide by microglia activated by extravasated albumin and other serum components may continuously drive the up-regulation and proliferation of such ion channels and therefore would be responsible for the massive edema observed at these times (Abraham et al. 2002).
At 24 hours post reperfusion, we also observe a significant drop in the T2 value at the contralateral striatum compared to the T2 value at 04 PR when remote edema has been consistently demonstrated attributed to extravasated protein migration through the white matter tracts. In the immediate absence of any scavenging mechanisms to remove those protein and/or their degradation products, we expected a further increases of vascular edema at this time point especially in the presence of the up regulation of ion channels as discussed before. The unexpected significant fall in the T2 values at 24 hours, however, may possibly be due to the reduction in the oxy-hemoglobin content resulting from vessel compression by the existing bilateral edema and the increased cerebral oxygen extraction and/or decreased volume of oxygenated blood volume. In this scenario the resultant T2 value being a function of both water content and the blood oxygenation effects happens to be tilted downwards.
Consistent with previous studies we found a decrease in brain water content/edema as indicated by the fall in T2 values and a reduction in ipsilateral hemispherical volume at 48hours despite an increase of BBB permeability and BBB disrupted area as indicated by both, T1SIdiff and PBV (Belayev et al. 1996; Kuroiwa et al. 1985; Wang and Shuaib 2007). This indicates resorption of vasogenic edema possibly brought about by a variety of mechanisms including the uptake of extravasated proteins by glial cells and the resulting reduction in oncotic force, migration of edema into the cerebrospinal fluid spaces and also due to effects from proliferating endothelium. (Hatashita and Hoff 1988; Hatashita and Hoff 1990; Klatzo 1985). Off late, selective water channels at the astrocytic end feet, the aquaporins (AQPs), especially aquaporin-4, have also been proposed to play an important role in the patho-physiology of brain edema both in rodents and humans with a peak at 48PR (Zador et al. 2009). Further, the T2 values at the contralateral striatum demonstrated a non-significant but a re-bound increase in values. Since this value being higher than that of the control, possibly indicate the persistent presence of remote edema at these times which was duly masked at the 24 hour time point due to the increased ICP.
T2-relaxometry is also influenced by flow effects, alteration in the amounts of oxy- and deoxy-hemoglobin, tissue oxygenation and the exchange of nuclear spin magnetization between the “free” and “bound” proton pools (Calamante et al. 1999). We observed this ‘blood oxygenation level dependant’ (BOLD) effect in the very early phase of ischemia by a consistent fall in the T2 values which affected the whole ischemic hemisphere even in regions that did not demonstrate pannecrosis (Longa et al. 1989; Young et al. 2007). As expected, following reperfusion, the increased inflow of oxygenated blood resulting from reactive hyperemia which invariably develops after the release of arterial occlusion elevated the transverse relaxation time at the ipsilateral striatum.
Biphasic BBB permeability – a surrogate marker for the ‘new penumbra’?
In a recent editorial, Eng Lo coined the term ‘new penumbra’ to define the transition of most targets of ischemic stroke therapy (VEGF, MMPs, NO, glutamate release, JNK pathway, amongst others) from mediators of injury to be the effectors of repair following acute stroke (Lo 2008). Indeed, the lack of understanding of this ‘Janus-faced’ nature of many targets of neuroprotection in stroke research might represent the increasing frustration with a number of the proposed neuroprotectants (Endres et al. 2008). The observed phasic permeability characteristics of the BBB and the resultant shifts in edema may possibly be orchestrated by a variety of mediators, from growth factors (i.e. VEGF, epidermal growth factor, brain derived growth factor), MMPs, NO to inflammatory cytokines (i.e. IL-6, IL-1ß, C-reactive protein, TNF-α). Such, intricate and highly complex interactions resulting in dynamic shifts in the BBB permeability characteristics cannot be solely considered to mediate edema alone. The proposed role of the expression of ion channels might also have a larger role in mediating the adaptive mechanisms of the injured brain by exerting its plastic behaviour. Pharmacotherapy for neuroprotection and/or edema prevention in stroke therapy may be carefully characterized as the same drug moiety may cause beneficial and detrimental effects on BBB function, functional improvement and ultimately stroke outcome when given early, late or throughout the phase of ischemic injury. Overall, MRI may provide a translational read-out of BBB function reflecting the transition from injury and repair.
However, confirming similar characterization of BBB changes in humans being a prerequisite for such timely pharmacotherapy, demands great efforts from acute stroke patients and neuroradiologists in the early hours following thrombolysis. In addition, due to the inherent variability in human stroke, the biphasic BBB behaviour may not occur as uniform as in experimental settings but in overlapping time intervals, which may complicate analysis of MRI data. Using a human 3T MR scanner with identical sequences to the application in human stroke allows us to employ identical analysis as used in this study.
Summary
In the present MRI study conducted on a relatively low field clinical scanner we were able to define the timely correlation between the biphasic opening of the BBB and the dynamics of brain water content during the first 48 hours after focal cerebral ischemia. The phasic phenomena of altered BBB permeability might not only contribute to local but also possibly mediate widespread edematous changes including resorption at late time points. MRI equivalents of BBB permeability characteristics and edema formation are exceedingly complex and continuously influenced by raised ICP, altered CBF, BOLD effects and effects of inhalational anesthetics. The biphasic BBB opening and edema formation as demonstrated here may reflect injury and repair following ischemia and reperfusion at the neurovascular unit and needs to be confirmed in acute stroke patients employing similar MRI sequences. Stroke therapy aimed at the vasogenic edema and neuroprotection strategies may be guided according to the functional status of the BBB.
Acknowledgements
D.R. Pillai was supported by a grant of the “Bayerische Forschungsgemeinschaft” and the Bavarian State Ministry of Sciences, Research and the Arts, “ForNeuroCell” grant. We like to thank ‘Achtzehn Medical Service’ (Erlangen, Germany) for donation of the anaesthetic equipment.
References
- Abraham CS, Harada N, Deli MA, Niwa M. Transient forebrain ischemia increases the blood-brain barrier permeability for albumin in stroke-prone spontaneously hypertensive rats. Cell Mol Neurobiol. 2002;22:455–462. doi: 10.1023/A:1021067822435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmmed GU, Malik AB. Functional role of TRPC channels in the regulation of endothelial permeability. Pflugers Arch. 2005;451:131–142. doi: 10.1007/s00424-005-1461-z. [DOI] [PubMed] [Google Scholar]
- Belayev L, Busto R, Zhao W, Ginsberg MD. Quantitative evaluation of blood-brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res. 1996;739:88–96. doi: 10.1016/s0006-8993(96)00815-3. [DOI] [PubMed] [Google Scholar]
- Calamante F, Lythgoe MF, Pell GS, Thomas DL, King MD, Busza AL, Sotak CH, Williams SR, Ordidge RJ, Gadian DG. Early changes in water diffusion, perfusion, T1, and T2 during focal cerebral ischemia in the rat studied at 8.5 T. Magn Reson Med. 1999;41:479–485. doi: 10.1002/(sici)1522-2594(199903)41:3<479::aid-mrm9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Craner MJ, Hains BC, Lo AC, Black JA, Waxman SG. Co-localization of sodium channel Nav1.6 and the sodium-calcium exchanger at sites of axonal injury in the spinal cord in EAE. Brain. 2004;127:294–303. doi: 10.1093/brain/awh032. [DOI] [PubMed] [Google Scholar]
- Croll SD, Wiegand SJ. Vascular growth factors in cerebral ischemia. Mol Neurobiol. 2001;23:121–135. doi: 10.1385/MN:23:2-3:121. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ. Microvascular changes during cerebral ischemia and reperfusion. Cerebrovasc Brain Metab Rev. 1994;6:47–96. [PubMed] [Google Scholar]
- Eder P, Poteser M, Romanin C, Groschner K. Na(+) entry and modulation of Na(+)/Ca(2+) exchange as a key mechanism of TRPC signaling. Pflugers Arch. 2005;451:99–104. doi: 10.1007/s00424-005-1434-2. [DOI] [PubMed] [Google Scholar]
- Endres M, Engelhardt B, Koistinaho J, Lindvall O, Meairs S, Mohr JP, Planas A, Rothwell N, Schwaninger M, Schwab ME, Vivien D, Wieloch T, Dirnagl U. Improving outcome after stroke: overcoming the translational roadblock. Cerebrovasc Dis. 2008;25:268–278. doi: 10.1159/000118039. [DOI] [PubMed] [Google Scholar]
- Gidday JM, Gasche YG, Copin JC, Shah AR, Perez RS, Shapiro SD, Chan PH, Park TS. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol. 2005;289:H558–568. doi: 10.1152/ajpheart.01275.2004. [DOI] [PubMed] [Google Scholar]
- Gotoh O, Asano T, Koide T, Takakura K. Ischemic brain edema following occlusion of the middle cerebral artery in the rat. I: The time courses of the brain water, sodium and potassium contents and blood-brain barrier permeability to 125I-albumin. Stroke. 1985;16:101–109. doi: 10.1161/01.str.16.1.101. [DOI] [PubMed] [Google Scholar]
- Groschner K, Rosker C, Lukas M. Role of TRP channels in oxidative stress. Novartis Found Symp. 2004;258:222–230. discussion 231-225, 263-226. [PubMed] [Google Scholar]
- Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R. ‘Malignant’ middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol. 1996;53:309–315. doi: 10.1001/archneur.1996.00550040037012. [DOI] [PubMed] [Google Scholar]
- Hatashita S, Hoff JT. Biomechanics of brain edema in acute cerebral ischemia in cats. Stroke. 1988;19:91–97. doi: 10.1161/01.str.19.1.91. [DOI] [PubMed] [Google Scholar]
- Hatashita S, Hoff JT. Brain edema and cerebrovascular permeability during cerebral ischemia in rats. Stroke. 1990;21:582–588. doi: 10.1161/01.str.21.4.582. [DOI] [PubMed] [Google Scholar]
- Heo JH, Han SW, Lee SK. Free radicals as triggers of brain edema formation after stroke. Free Radic Biol Med. 2005;39:51–70. doi: 10.1016/j.freeradbiomed.2005.03.035. [DOI] [PubMed] [Google Scholar]
- Huang ZG, Xue D, Preston E, Karbalai H, Buchan AM. Biphasic opening of the blood-brain barrier following transient focal ischemia: effects of hypothermia. Can J Neurol Sci. 1999;26:298–304. doi: 10.1017/s0317167100000421. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Haida M, Hata T, Isozumi K, Kurita D, Shinohara Y. Distribution of brain oedema in the contralateral hemisphere after cerebral infarction: repeated MRI measurement in the rat. J Clin Neurosci. 2002;9:289–293. doi: 10.1054/jocn.2001.0966. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK. Water homeostasis in the brain: basic concepts. Neuroscience. 2004;129:851–860. doi: 10.1016/j.neuroscience.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Klatzo I. Brain oedema following brain ischaemia and the influence of therapy. Br J Anaesth. 1985;57:18–22. doi: 10.1093/bja/57.1.18. [DOI] [PubMed] [Google Scholar]
- Kuroiwa T, Ting P, Martinez H, Klatzo I. The biphasic opening of the blood-brain barrier to proteins following temporary middle cerebral artery occlusion. Acta Neuropathol. 1985;68:122–129. doi: 10.1007/BF00688633. [DOI] [PubMed] [Google Scholar]
- Latour LL, Kang DW, Ezzeddine MA, Chalela JA, Warach S. Early blood-brain barrier disruption in human focal brain ischemia. Ann Neurol. 2004;56:468–477. doi: 10.1002/ana.20199. [DOI] [PubMed] [Google Scholar]
- Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med. 2008;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Meyer TN, Schwesinger C, Ye J, Denker BM, Nigam SK. Reassembly of the tight junction after oxidative stress depends on tyrosine kinase activity. J Biol Chem. 2001;276:22048–22055. doi: 10.1074/jbc.M011477200. [DOI] [PubMed] [Google Scholar]
- Paltauf-Doburzynska J, Frieden M, Spitaler M, Graier WF. Histamine-induced Ca2+ oscillations in a human endothelial cell line depend on transmembrane ion flux, ryanodine receptors and endoplasmic reticulum Ca2+-ATPase. J Physiol. 2000;524(Pt 3):701–713. doi: 10.1111/j.1469-7793.2000.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008;32:200–219. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Shinohara Y, Ohsuga H, Ohsuga S, Takizawa S, Haida M. Routed protein migration after protein extravasation and water leakage caused by cold injury. Acta Neurochir Suppl (Wien) 1990;51:90–92. doi: 10.1007/978-3-7091-9115-6_31. [DOI] [PubMed] [Google Scholar]
- Simard JM, Kent TA, Chen M, Tarasov KV, Gerzanich V. Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol. 2007;6:258–268. doi: 10.1016/S1474-4422(07)70055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt NJ, Fernandez J, Chen M, Rewell S, Cox S, van Raay L, Hogan L, Howells DW. Modification of the method of thread manufacture improves stroke induction rate and reduces mortality after thread-occlusion of the middle cerebral artery in young or aged rats. J Neurosci Methods. 2006;155:285–290. doi: 10.1016/j.jneumeth.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Stamatovic SM, Shakui P, Keep RF, Moore BB, Kunkel SL, Van Rooijen N, Andjelkovic AV. Monocyte chemoattractant protein-1 regulation of blood-brain barrier permeability. J Cereb Blood Flow Metab. 2005;25:593–606. doi: 10.1038/sj.jcbfm.9600055. [DOI] [PubMed] [Google Scholar]
- Stoll G, Kleinschnitz C, Meuth SG, Braeuninger S, Ip CW, Wessig C, Nolte I, Bendszus M. Transient widespread blood-brain barrier alterations after cerebral photothrombosis as revealed by gadofluorine M-enhanced magnetic resonance imaging. J Cereb Blood Flow Metab. 2009;29:331–341. doi: 10.1038/jcbfm.2008.129. [DOI] [PubMed] [Google Scholar]
- The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- Verheul HB, Berkelbach van der Sprenkel JW, Tulleken CA, Tamminga KS, Nicolay K. Temporal evolution of focal cerebral ischemia in the rat assessed by T2-weighted and diffusion-weighted magnetic resonance imaging. Brain Topogr. 1992;5:171–176. doi: 10.1007/BF01129046. [DOI] [PubMed] [Google Scholar]
- Wang CX, Shuaib A. Critical role of microvasculature basal lamina in ischemic brain injury. Prog Neurobiol. 2007;83:140–148. doi: 10.1016/j.pneurobio.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Werner C, Lu H, Engelhard K, Unbehaun N, Kochs E. Sevoflurane impairs cerebral blood flow autoregulation in rats: reversal by nonselective nitric oxide synthase inhibition. Anesth Analg. 2005;101:509–516. doi: 10.1213/01.ANE.0000160586.71403.A4. table of contents. [DOI] [PubMed] [Google Scholar]
- Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- Young AR, Ali C, Duretete A, Vivien D. Neuroprotection and stroke: time for a compromise. J Neurochem. 2007;103:1302–1309. doi: 10.1111/j.1471-4159.2007.04866.x. [DOI] [PubMed] [Google Scholar]
- Zador Z, Stiver S, Wang V, Manley GT. Role of aquaporin-4 in cerebral edema and stroke. Handb Exp Pharmacol. 2009;190:159–170. doi: 10.1007/978-3-540-79885-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]