Abstract
Introduction
Saliva is a potentially important barrier against respiratory viral infection but its mechanism of action is not well studied.
Methods
We tested the antiviral activities of whole saliva, specific salivary gland secretions, and purified salivary proteins against strains of influenza A virus (IAV) in vitro.
Results
Whole saliva or parotid or submandibular/sublingual secretions from healthy donors inhibited IAV based on hemagglutination inhibition and neutralization assays. This differs from human immunodeficiency virus (HIV), for which only submandibular/sublingual secretions are reported to be inhibitory. Among purified salivary proteins, MUC5B, scavenger receptor cysteine-rich glycoprotein 340 (salivary gp-340), histatins, and human neutrophil defensins (HNPs) inhibited IAV at the concentrations present in whole saliva. In contrast, some abundant salivary proteins (acidic proline-rich proteins and amylase) had no activity, nor did several other less abundant salivary proteins with known activity against HIV (e.g. thrombospondin or serum leukocyte protease inhibitor). Whole saliva and MUC5B did not inhibit neuraminidase activity of IAV and viral neutralizing and aggregating activity of MUC5B was potentiated by the neuraminidase inhibitor oseltamivir. Hence, MUC5B inhibits IAV by presenting a sialic acid ligand for the viral hemagglutinin. The mechanism of action of histatins requires further study.
Conclusions
These findings indicate that saliva represents an important initial barrier to IAV infection and underline the complexity of host defense activity of oral secretions. Of interest, antiviral activity of saliva against IAV and HIV differs in terms of specific glandular secretions and proteins that are inhibitory.
Keywords: histatins, innate immunity, MUC5B
Influenza A virus (IAV) is a major cause of morbidity and mortality in yearly epidemics and there is substantial concern about the development of a new pandemic because of frequent instances of transmission of avian strains to humans (33, 35). The ability of IAV strains to modify their antigenic properties through small incremental mutations and major changes resulting from the exchange of genome segments with those of animal strains (reassortment), results in continued emergence of strains for which prior adaptive immune responses are ineffective. Although essentially everyone can be infected with IAV, certain individuals and groups suffer much more severe illness after infection. It is likely that innate defense mechanisms play an important role in the first crucial days after infection, given the normal delay in development of protective antibodies or T cells. Innate mediators that are important in IAV infection include soluble components [e.g. the collectins, surfactant proteins A and D (SP-A and -D), tumor necrosis factor-α, type I interferons, natural immunoglobulin M (IgM), and complement] and cellular elements (e.g. natural killer cells, dendritic cells, macrophages, and neutrophils) (11-13, 16, 20, 32, 37)]. Although the respiratory tract is the main site of replication for IAV, the oral cavity is a potentially important route for viral transmission. There is a fairly extensive literature regarding the ability of saliva to inhibit HIV but relatively less is known about how it inhibits other viruses, including IAV.
We reported that the saliva of a healthy volunteer donor inhibited IAV hemagglutination (HA) activity and infectivity (12). Innate immune factors contributed strongly to this neutralizing activity because removal of IgA only slightly reduced it. The antiviral activity of saliva differed qualitatively from that of bronchoalveolar lavage (BAL) fluid. In the case of BAL fluid, calcium-dependent lectin activity, contributed largely by SP-D, was predominant (10). We found, however, that SP-D is not present in saliva at sufficient concentrations to account for its anti-influenza activity and that calcium-dependent lectin activity makes a much smaller contribution to the antiviral activity of saliva than to BAL fluid. Salivary glycoprotein 340 (gp-340) did account for some of the antiviral activity of saliva, but activity remained after removal of gp-340 (12).
The goal of this study was to characterize further the mechanisms of antiviral activity of saliva through the use of a panel of saliva donors providing samples obtained specifically from parotid and submandibular/sublingual glands, and purified salivary components.
Materials and methods
Reagents
Thrombospondin (human platelet) and lactoferrin were obtained from Sigma Chemical Co (St Louis, MO). Recombinant human serum leukocyte protein inhibitor (SLPI) was obtained from R&D Systems (Minneapolis, MN). Human neutrophil defensins (HNPs) 1 and 2 were obtained from Bachem (Torrance, CA). Acidic proline rich protein 1 and amylase were isolated from parotid salivary secretions by chromatographic methods involving gel filtration and reverse-phase high-performance liquid chromatography as described elsewhere (17, 25). Histatins 1, 3, and 5 were synthesized by Quality Controlled Biochemicals (http://www.QCB.com) and were >95% pure. Salivary gp-340 preparations were prepared as described from parotid saliva (11). MUC5B was purified by isolation of mucins from human whole saliva using ultracentrifugation of ~500 ml whole saliva under dissociating conditions (8 m urea) as described previously (34), followed by gel filtration over Sephacryl HR 400 in 50 mmol/l Tris–HCl, 6 mol/l urea, 0.5 mol/l NaCl (pH 7.4); column dimensions: 100 × 2.6 cm. Samples of 10 ml mucins (2 mg dry wt/ml) were applied. Salivary gp-340 was purified from human parotid secretions using a Uno Q-6 column (BioRad, Hercules, CA) (23). Recombinant human SP-D dodecamers were prepared in Chinese hamster ovary cells and purified using maltose affinity and gel filtration as described previously (8).
Virus preparation
IAV was grown in the chorioallantoic fluid of 10-day-old chicken eggs and purified on a discontinuous sucrose gradient as previously described (9).
Source of normal donor saliva
Normal donor saliva was obtained by simple expectoration into 50-ml tubes. Whole native saliva was not processed further. Whole saliva supernatant was treated by centrifugation at 10,000 g to remove mucinous precipitate and addition of 1% penicillin and streptomycin to inhibit bacterial growth. Parotid saliva and submandibular/sublingual saliva were obtained using specially designed cups as described elsewhere (2). One set of parotid and submandibular/sublingual secretions was not processed further before the assays. An additional set of parotid, submandibular, and sublingual secretions [each obtained separately as described elsewhere (2)] were lyophilized and then reconstituted in distilled water and frozen in aliquots for testing. Saliva samples were obtained after informed consent as approved by the Boston University School of Medicine Institutional Review Board for Human Research.
Viral inhibition assays
Inhibition of HA was measured by serially diluting potential host defense proteins in round-bottom 96-well plates (Serocluster U-Vinyl plates; Costar, Cambridge, MA) using phosphate-buffered saline as a diluent as described previously (12). The minimum concentration of protein required to fully inhibit the hemagglutinating activity of the viral suspension was determined by noting the highest dilution of protein that still inhibited hemagglutination. If no inhibition of HA activity was observed at the highest protein concentration used then the value is expressed as > the maximal protein concentration. Viral neutralization was measured using a fluorescent focus assay as described previously (14). In brief, Madin–Darby caning kidney cell monolayers were prepared in 96-well plates and infected for 7 h with IAV that had been treated with either control buffer, saliva, or various salivary proteins. Viral infectivity was quantified by counting cells positive for the expression of IAV nucleoprotein. Neuraminidase inhibition of IAV was measured as described using an enzyme-linked microplate assay in which Arachis hypogaea peanut lectin was used to detect β-d-galactose-N-acetylglucosamine sequences exposed after the removal of sialic acid from fetuin (31).
Measurement of HNP levels in saliva
HNPs 1–3 in saliva were measured using a commercially available sandwich enzyme-linked immunosorbent assay (ELISA; Cell Sciences, Canton, MA) as described (37).
Results
Comparison of IAV inhibitory activity of saliva from various donors
As shown in Fig. 1A, whole saliva supernatant samples from six different healthy donors had dose-related neutralizing activity. All of the whole saliva supernatant samples also had significant inhibitory activity on viral HA assays (Table 1). We also tested neutralizing and HA-inhibiting whole native saliva from three healthy donors (not centrifuged after isolation) and this had activity similar to the whole saliva supernatant (Figs 1B and 2).
Fig. 1.
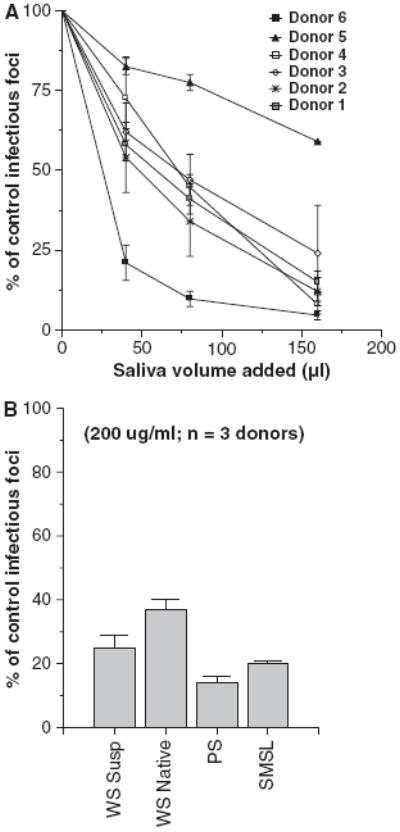
Saliva of healthy volunteers inhibits infectivity of influenza A virus (IAV). Whole saliva obtained from a panel of healthy volunteer donors was tested for the ability to neutralize the Phil82 strain of IAV using the infectious focus assay (A). Each saliva preparation caused significant dose-related reduction in viral infectivity. Results are expressed as mean % of control infectivity (n = 3 or more for each saliva sample). The neutralizing activity of whole saliva (supernatant or native), or parotid or SMSL secretions from three donors was compared (B). Neutralizing activity was present in all of these preparations (all P < 0.05 compared to control). Results were normalized to 200 μg/ml saliva protein.
Table 1.
Comparative hemagglutination (HA) inhibitory activity of lyophilized salivary gland secretions (μg/ml)
| Phil82 | PR-8 | Sendai virus | |
|---|---|---|---|
| Whole saliva supernatant | 20 ± 6 | 12.5 ± 2.6 | 50 ± 16 |
| Parotid secretions | 21 ± 2 | 27 ± 9.5 | 84 ± 0 |
| Submandibular secretions | 6.1 ± 0.8 | 4.3 ± 0.35 | 25 ± 5 |
Results are mean ± SEM of three or more experiments and are expressed as μg/ml of the indicated salivary preparations or purified proteins required to inhibit HA activity of 40 HA units of the Phil82 or PR-8 strain of influenza A virus or Sendai virus. Whole saliva, parotid and submandibular results are mean values for six, two, and four donors, respectively.
Fig. 2.
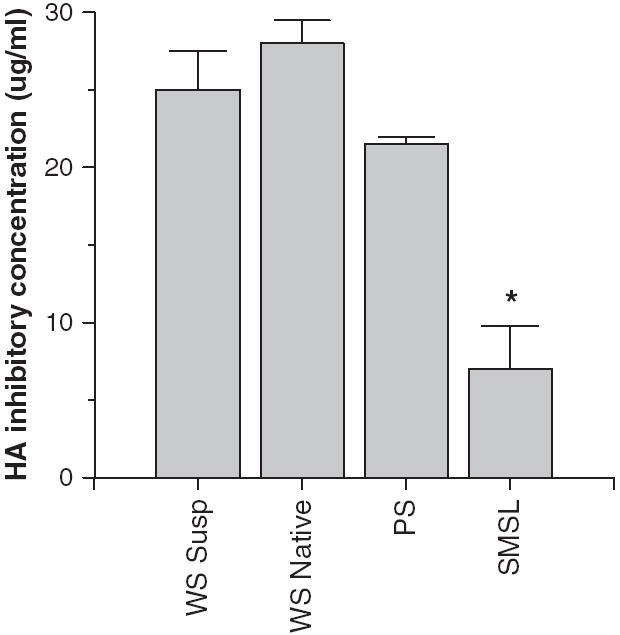
Hemagglutination (HA) inhibitory activity of whole saliva or parotid or submandibular/sublingual (SMSL) secretions. Mean HA inhibitory activity of whole saliva (supernatant or native) was compared to that of parotid or SMSL secretions from three donors. SMSL secretions had significantly greater HA inhibitory activity than either whole saliva preparation (*P < 0.02) when for the amount of protein per saliva sample. Greater activity in this assay is reflected by a lower concentration of the saliva preparation needed to inhibit a fixed concentration (40 HAU) of IAV.
Antiviral activity of specific salivary gland secretions
To determine which components of whole saliva contain antiviral activity we obtained samples of whole saliva supernatant, native whole saliva, and parotid or submandibular+sublingual (SMSL) secretions from three separate donors and tested their activity without further processing or freezing of the samples. All of the freshly isolated saliva preparations had neutralizing activity against Phil82 IAV (Fig. 1B). Interestingly, in this assay the parotid secretions had a similar activity to the SMSL secretions and both had greater activity than whole native saliva. These saliva preparations all inhibited HA activity of Phil82 IAV (Fig. 2). SMSL secretions were significantly more effective than whole saliva or parotid secretions at inhibiting HA activity when samples were compared based on protein concentration. The concentrations presented for the HA inhibition assay were the minimum amount of salivary protein required to fully inhibit 40 HA units of virus, so that a lower concentration indicates greater activity.
To carry out more extensive experimentation, we lyophilized samples obtained from parotid and submandibular glands (the submandibular secretions were obtained separately from sublingual secretions in this case using a specialized device). These samples were then reconstituted with distilled water and frozen in aliquots. Secretions from each of these glands had HA inhibitory activity against the Phil82 viral strain (Table 1). The results obtained with the lyophilized salivary secretion samples were very similar to those obtained with the freshly isolated samples (see Fig. 2). To determine if HA inhibitory activity of SM secretions was heat labile we boiled an aliquot of the sample from one donor. Boiling reduced the HA inhibitory activity of this sample from 7 ± 2.8 to 18.5 ± 5 μg/ml for the Phil82 strain of virus (P < 0.01; n = 3 determinations). Sublingual secretions were also isolated from a single donor and these had strong activity (i.e. 1.2 μg/ml for Phil82 viral strain). The lyophilized samples also neutralized the Phil82 viral strain to 39 ± 12 and 57 ± 16% of control for SM and parotid secretions, respectively (all P < 0.05 compared to control; 100 μg/ml secretions used; data not shown).
The various secretions also inhibited HA activity of the PR-8 strain of IAV (a mouse-adapted laboratory strain) and, to a lesser extent, that of Sendai virus (a murine paramyxovirus) (Table 1). The PR-8 strain and Sendai virus preferentially bind to sialic acids in an α(2, 3) linkage whereas Phil82 (like other common human strains) is selective for the α(2,6) linkage. The finding that saliva or specific saliva components inhibited these strains indicates activity against strains favoring both types of sialic acid linkage.
Comparison of HA inhibiting and neutralizing activity of specific salivary proteins
We tested the HA inhibitory activity of various purified salivary proteins against IAV. Histatins, proline-rich proteins (PRP), amylase, HNPs 1 and 2, and SLPI had no detectable activity against the Phil82 strain of IAV at concentrations up to 100 μg/ml (n = 3 or more experiments; data not shown). Lactoferrin had no activity up to 12.5 μg/ml (data not shown). As shown in Table 2, MUC5B and salivary gp-340 had significant activity against the Phil82 strain and also against the PR-8 and Sendai virus strains. Thrombospondin had slight HA inhibiting activity on some assays for the PR-8 strain of IAV and inhibited HA activity of the Brazil78 strain at a concentration of 5.8 ± 0.8 μg/ml (n = 3; data not shown). We tested these proteins for viral neutralization using concentrations in the range found in saliva (Fig. 3). Amylase, acidic PRPs, thrombospondin, lactoferrin, and SLPI did not cause significant neutralization of the Phil82 strain of IAV either; however, MUC5B and histatins 1 and 3 (but not 5) did. HNP1 and HNP2 inhibited infectivity (as previously reported) (13).
Table 2.
Hemagglutination (HA) inhibition caused by purified salivary proteins (μg/ml)
| Phil82 | PR-8 | Sendai virus | |
|---|---|---|---|
| MUC5B | 0.65 ± 0.05 | 2.1 ± 0.9 | 4.6 ± 0.9 |
| Lactoferrin | >12.5 | >12.5 | |
| Salivary gp-340 | 0.22 ± 7 | 0.05 ± 0.01 | 0.13 ± 0.02 |
| Thrombospondin | >7.5 | ≥4.4 |
Results are mean ± SEM of three or more experiments and are expressed as μg/ml of the indicated purified proteins required to inhibit HA activity of 40 HA units of the Phil82 or PR-8 strain of influenza A virus or Sendai virus. Thrombospondin had detectable HA inhibitory activity against the PR-8 strain of IAV in three of five experiments and not in the other two (hence, ≥4.4).
Fig. 3.
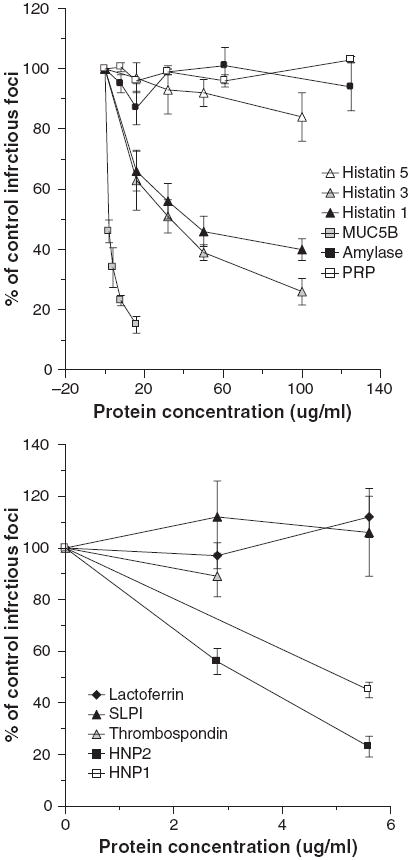
Histatins 1 and 3, MUC5B, and human neutrophil defensins (HNPs) inhibit the infectivity of influenza A virus (IAV). Inhibition of infectivity of the Phil82 strain of IAV was tested using the fluorescent focus assay as in Fig. 1. In the left panel more abundant salivary proteins [histatins, proline-rich proteins (PRPs), amylase, and MUC5B] were preincubated with the virus and then inoculated on Madin–Darby canine kidney cells. Histatins 1 and 3 and MUC5B significantly inhibited infectivity at all the concentrations tested (P < 0.01 compared to control for all). Results are mean ± SEM of three or more experiments. PRP, amylase, and histatin 5 did not inhibit infectivity. In the right panel less abundant salivary proteins were tested. Among these only HNPs 1 and 2 inhibited infectivity.
We have reported that purified salivary or lung gp-340 causes 50% inhibition of IAV infectivity at concentrations under 100 ng/ml. The level of gp-340 in saliva was measured at 492 ± 40 ng/ml and removal of gp-340 reduced antiviral activity (12). The levels of HNPs 1–3 in saliva samples of donors 1–5 were found to be 2.7 ± 0.4 μg/ml (range 2.1–4.2) (measured by ELISA as described in the Materials and methods; data not shown). MUC5B is abundant in SMSL secretions and whole saliva (~10–200 μg/ml) (15, 24). Hence, among the proteins indicated in Fig. 3, histatins 1 and 3, HNPs, and MUC5B all could contribute to neutralizing activity of whole saliva. MUC5B would not, however, account for the activity of parotid secretions.
Effects of whole saliva, MUC5B, and salivary gp-340 on viral neuraminidase (NA) activity
Inhibition of influenza viral NA activity is another mechanism through which salivary proteins might be protective against viral infection. NA inhibitors like oseltamivir and zanamivir are currently the most effective antiviral agents available for treatment of IAV infection (18). NA inhibitors are inactive in the infectivity assay used in this paper because this assay targets only the first cycle of viral replication (unlike plaque assays) (31). Whole saliva from several donors and lyophilized submandibular secretions did not cause any detectable NA inhibition (data not shown). In addition, MUC5B did not cause any inhibition of NA activity of the Phil82 strain of IAV at concentrations up to 200 μg/ml (data not shown). Salivary gp-340 did not inhibit NA activity of the Phil82 strain of IAV (data not shown); however, it did cause inhibition of the PR-8 strain in this assay (Fig. 4). Salivary gp-340 preparations from two donors were tested and one had significantly greater NA inhibitory activity against PR-8. This preparation has a greater density of α(2, 3)-linked sialic acids, as previously reported (11), which probably accounts for its greater NA inhibitory activity against the PR-8 strain.
Fig. 4.
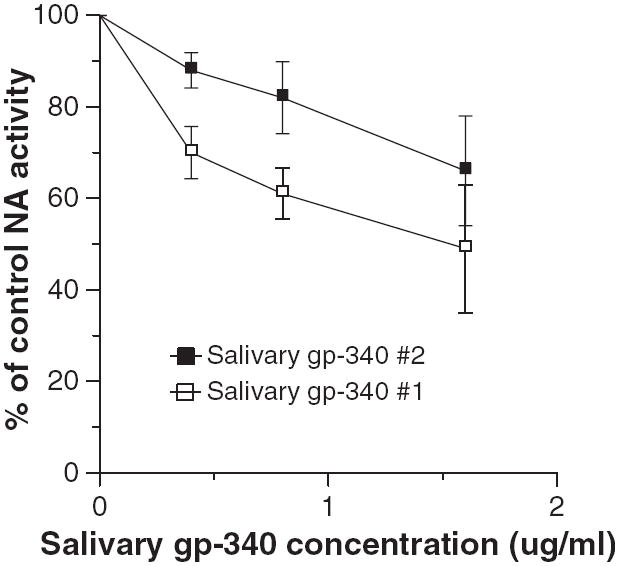
Salivary glycoprotein 340 (gp-340) inhibits neuraminidase (NA) activity of the PR-8 strain of influenza A virus (IAV). The PR-8 strain of IAV was preincubated with the indicated concentrations of salivary gp-340 isolated from two different saliva donors. Salivary gp-340 from donor #1 caused significantly more inhibition at concentrations of 0.4 and 0.8 μg/ml compared to that of donor #2 (results mean ± SEM of four experiments).
Oseltamivir and SP-D potentiate the antiviral activities of MUC5B
MUC5B alone caused slight reversible viral aggregation as assessed by light transmission through a stirred viral suspension (Fig. 5; left panel). Increased concentrations of MUC5B caused a greater initial aggregation response that was also reversible over a similar time–course (data not shown). We presumed that this reflected cleavage of sialic acids on the mucin by the viral neuraminidase. This concept was supported by the finding that addition of the neuraminidase inhibitor oseltamivir to the assay caused a dramatic sustained viral aggregation response to MUC5B. Note that oseltamivir alone causes no viral aggregation. SP-D also inhibits viral neuraminidase activity (31); used alone it strongly aggregates IAV particles. We tested a low concentration of SP-D and found that this had additive viral aggregating effects when combined with MUC5B (Fig. 5; right panel).
Fig. 5.
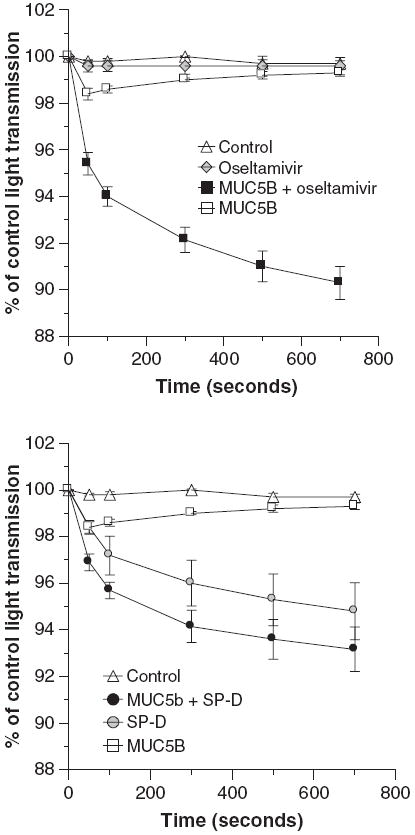
Oseltamivir and surfactant protein D (SP-D) have cooperative viral aggregating activity when combined with MUC5B. Viral aggregation was assessed by decreases in light transmission through a stirred suspension of Phil82 influenza A virus. The left panel shows the effect of adding oseltamivir (1 μg/ml) to MUC5B and the right panel the effect of adding SP-D (0.2 μg/ml) to MUC5B in this assay. MUC5B alone (8 μg/ml) caused a significant decrease in light transmission after 50 and 100 s but it was no longer different from control after that (n = 5; P < 0.05 compared to control at 50 and 100 s). Oseltamivir alone did not cause any aggregation but markedly potentiated the effect of MUC5B (significant at all time-points by analysis of variance). SP-D caused aggregation on its own and also increased activity of MUC5B. The combination of MUC5B and SP-D was significantly greater than MUC5B alone but not than SP-D alone by analysis of variance.
We also tested the combined effects of MUC5B and either oseltamivir or SP-D on viral HA activity or infectivity (Fig. 6). For these assays we used concentrations of MUC5B or SP-D that caused low or partial inhibition on their own. Addition of oseltamivir again caused a dramatic increase in viral neutralizing or HA inhibiting activity of MUC5B. An additive interaction of SP-D and MUC5B was again seen in these assays.
Fig. 6.
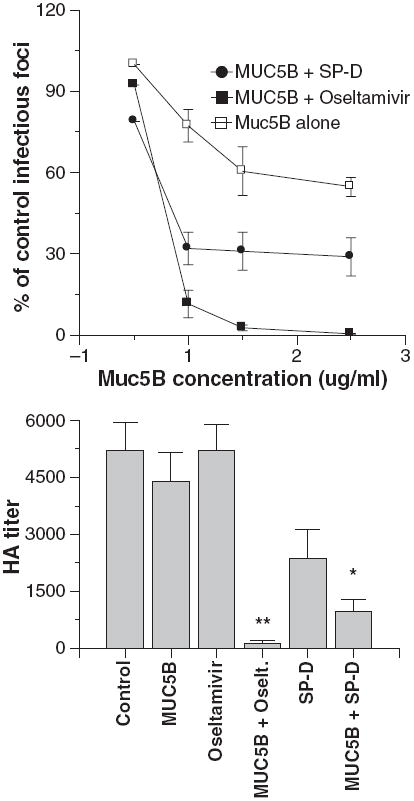
Oseltamivir and surfactant protein D (SP-D) potentiate viral neutralization and hemagglutination (HA) inhibition caused by MUC5B. In the left panel viral neutralization was measured as in Fig. 3. The Phil82 strain of virus was preincubated with the indicated concentrations of MUC5B alone or with MUC5B combined with either 250 ng/ml oseltamivir or 6 ng/ml human SP-D dodecamers. Inhibition caused by the combinations of either SP-D or oseltamivir with MUC5B caused significantly greater neutralization than either treatment alone (n = 5; significance assessed by analysis of variance). HA titers were measured on viral samples used in the aggregation assays shown in Fig. 5 and are shown in the right panel. The combination of oseltamivir and mucin caused markedly greater inhibition than either agent alone (n = 4; significant by analysis of variance as indicated by **). The combination of SP-D and MUC5B caused significantly greater inhibition than MUC5B alone but not than SP-D alone (indicated by *).
Discussion
We show that whole saliva supernatant and whole native saliva from a panel of volunteer donors has substantial IAV neutralizing activity. It is of interest that the activity of whole saliva supernatant varied among the donors, although all donors tested had considerable antiviral activity. The viral neutralizing activity of whole saliva was present in SM, SL and parotid secretions and was evident to varying degrees in all donors tested and in samples that were freshly isolated and unprocessed or lyophilized and stored frozen. It is of interest that SM, SL, and parotid secretions all caused viral inhibition. The HA inhibitory activity of SMSL secretions was significantly greater than that of parotid secretions or whole saliva on a protein basis, although the neutralizing activity was not greater for SMSL secretions. These results suggest partial differences in mechanisms of antiviral activity of SMSL vs. parotid secretions (e.g. with mucins playing a greater role in the activity of SMSL secretions). The discovery of IAV neutralizing activity in parotid saliva is useful because these secretions are largely free of mucins and contain a number of specific protein components that remain fairly intact during isolation.
The most abundant proteins in parotid saliva are amylase (~650–800 μg/ml), acidic proline-rich proteins (PRPs) (~200–800 μg/ml), and histatins and statherin (~30–55 μg/ml each) (24). Other components include salivary gp-340 (or salivary agglutinin) and a variety of other potential host defense proteins present at low concentrations. We have identified at least two components of parotid secretions that could contribute to its neutralizing activity (i.e. gp-340 and histatins 1 and 3). Mucins are present in SMSL but not parotid saliva. It is of interest that parotid saliva inhibited IAV because this has not been found to inhibit HIV (1, 19). Several components of saliva have been reported to inhibit HIV infectivity, including mucins, salivary gp-340, defensins, SLPI, basic PRPs, and thrombospondin (3, 6, 7, 25, 26, 28). Another innate immune protein in saliva that has reported antiviral activity is lactoferrin (27).
Overall our results, taken together with those of earlier studies, suggest that mechanisms of inhibition by saliva of HIV and IAV differ. Some salivary proteins known to inhibit HIV do not inhibit IAV (i.e. SLPI) or inhibited it minimally and inconsistently (i.e. thrombospondin). Also note that thrombospondin is mainly present in SMSL saliva so that it could not account for the inhibitory activity of parotid saliva (4). Physiological concentrations of MUC5B, HNPs, and salivary gp-340 inhibit both IAV and HIV. Of interest, a peptide derived from histatin 5 has been shown to inhibit HIV, although histatin 5 did not inhibit IAV in our study (6). Mucins probably contribute significantly to the antiviral activity of SM and SL secretions. It should be noted that we only tested MUC5B. MUC7 is another important component of saliva that has anti-HIV activity (7) and could have activity against IAV as well. The finding of antiviral activity of histatins 1 and 3 is novel and of interest and further studies will be needed to elucidate the mechanism. It is possible that these histatins have a mechanism of activity similar to other cationic peptides like defensins and their activity against other viruses should also be explored. One possible mechanism of action could be viral membrane disruption (5).
Despite strong viral neutralizing activity for saliva and several specific salivary proteins, we were not able to detect significant NA inhibition by whole or SM saliva. Consistent with this, MUC5B did not cause any NA inhibition. Salivary gp-340 did cause measurable inhibition of NA activity of the PR-8 strain of IAV but the active concentrations were higher than those that could be achieved even using saliva at a one to one dilution in the NA assay (the mean concentration of gp-340 in undiluted saliva is 492 ± 40 ng/ml) (12). The NA inhibitory activity of salivary gp-340 for the PR-8 virus parallels the density of α(2,3)-linked sialic acids on gp-340. It is possible therefore that some people have greater ability to inhibit infectivity or NA activity of avian-like strains, which prefer this linkage. In any case, it does not appear from our results that NA inhibition plays a major role in the antiviral activity of saliva.
It is likely that both salivary gp-340 and MUC5B inhibit infectivity and HA activity of IAV by presenting sialic acid ligands that bind to the viral HA preventing attachment to target cells. In the case of MUC5B this effect appears to be strongly attenuated by the ability of the viral NA to cleave the sialic acids of the mucin. This was demonstrated by showing marked enhancement of viral aggregating, neutralizing, and HA-inhibiting activity by oseltamivir. This confirms earlier findings obtained with bovine submaxillary mucin (36) and implies that NA inhibitors may in part act by potentiating the activity of innate inhibitors like MUC5B. We have previously shown that oseltamivir does not increase antiviral activities of salivary gp-340 and this suggests that sialic acids of salivary gp-340 are not as readily cleaved as those of MUC5B. This may therefore account for the increased intrinsic antiviral activity of salivary gp-340 as compared to MUC5B and for its ability to inhibit NA activity.
Our findings significantly increase understanding of the distinctive antiviral properties of saliva with respect to influenza viruses and support the notion that saliva may provide an important initial barrier to influenza infection. It remains possible that other components of saliva also contribute to the innate inhibition of infectivity of IAV. We did not have access to basic PRPs and MUC7, which are reported to inhibit HIV, and it will be useful to test their activity against IAV in future studies. Furthermore, we have not taken into account the complex interactions among various salivary components, including protein–protein interactions and micelle formation (21, 29, 30), that could impact importantly on antiviral activity of specific components (22). We have found significant interactions between specific components of BAL fluid (36). We also reported that salivary gp-340 and HNPs bind strongly to SP-D and inhibit its activity (11, 13). We now show that MUC5B has additive effects when combined with SP-D. This interaction could occur in the lung environment where both proteins are expressed. Future studies could explore further such cooperative or competitive interactions among salivary and respiratory tract proteins.
Acknowledgments
The research was supported by National Institutes of Health grant HL69031 (K.L.H.) and NIH/NIDCR grants DE05672 (F.O.), DE07652(F.O.), DE1495(F.O.), and DE16699 (E.H.). We would like to thank Dr Erika Crouch for providing SP-D for these studies.
Footnotes
The authors have no conflict of interest related to research presented in this manuscript.
References
- 1.Archibald DW, Cole GA. In vitro inhibition of HIV-1 infectivity by human salivas. AIDS Res Hum Retroviruses. 1990;6:1425–1432. doi: 10.1089/aid.1990.6.1425. [DOI] [PubMed] [Google Scholar]
- 2.Bolscher JG, Nazmi K, Ran LJ, et al. Inhibition of HIV-1 IIIB and clinical isolates by human parotid, submandibular, sublingual and palatine saliva. Eur J Oral Sci. 2002;110:149–156. doi: 10.1034/j.1600-0722.2002.11175.x. [DOI] [PubMed] [Google Scholar]
- 3.Crombie R, Kawasaki K, Hojo K, Laurence J. Peptides derived from salivary thrombospondin-1 replicate its anti-HIV effect: potential role in microbicide development. J Acquir Immune Defic Syndr. 2001;27:91–93. doi: 10.1097/00126334-200105010-00016. [DOI] [PubMed] [Google Scholar]
- 4.Crombie R, Silverstein RL, MacLow C, Pearce SF, Nachman RL, Laurence J. Identification of a CD36-related thrombospondin 1-binding domain in HIV-1 envelope glycoprotein gp120: relationship to HIV-1-specific inhibitory factors in human saliva. J Exp Med. 1998;187:25–35. doi: 10.1084/jem.187.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.den Hertog AL, van Marle J, van Veen HA, et al. Candidacidal effects of two antimicrobial peptides: histatin 5 causes small membrane defects, but LL-37 causes massive disruption of the cell membrane. Biochem J. 2005;388:689–695. doi: 10.1042/BJ20042099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groot F, Sanders RW, ter Brake O, et al. Histatin 5-derived peptide with improved fungicidal properties enhances human immunodeficiency virus type 1 replication by promoting viral entry. J Virol. 2006;80:9236–9243. doi: 10.1128/JVI.00796-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Habte HH, Mall AS, de Beer C, Lotz ZE, Kahn D. The role of crude human saliva and purified salivary MUC5B and MUC7 mucins in the inhibition of human immunodeficiency virus type 1 in an inhibition assay. Virol J. 2006;3:99. doi: 10.1186/1743-422X-3-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartshorn K, Chang D, Rust K, White M, Heuser J, Crouch E. Interactions of recombinant human pulmonary surfactant protein D and SPD multimers with influenza A. Am J Physiol. 1996;271:L753–762. doi: 10.1152/ajplung.1996.271.5.L753. [DOI] [PubMed] [Google Scholar]
- 9.Hartshorn KL, Collamer M, Auerbach M, Myers JB, Pavlotsky N, Tauber AI. Effects of influenza A virus on human neutrophil calcium metabolism. J Immunol. 1988;141:1295–1301. [PubMed] [Google Scholar]
- 10.Hartshorn KL, Crouch EC, White MR, et al. Evidence for a protective role of pulmonary surfactant protein D (SP-D) against influenza A viruses. J Clin Invest. 1994;94:311–319. doi: 10.1172/JCI117323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartshorn KL, Ligtenberg A, White MR, et al. Salivary agglutinin and lung scavenger receptor cysteine-rich glycoprotein 340 have broad anti-influenza activities and interactions with surfactant protein D that vary according to donor source and sialylation. Biochem J. 2006;393:545–553. doi: 10.1042/BJ20050695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartshorn KL, White MR, Mogues T, Ligtenberg T, Crouch E, Holmskov U. Lung and salivary scavenger receptor glycoprotein-340 contribute to the host defense against influenza A viruses. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1066–1076. doi: 10.1152/ajplung.00057.2003. [DOI] [PubMed] [Google Scholar]
- 13.Hartshorn KL, White MR, Tecle T, Holmskov U, Crouch EC. Innate defense against influenza A virus: activity of human neutrophil defensins and interactions of defensins with surfactant protein D. J Immunol. 2006;176:6962–6972. doi: 10.4049/jimmunol.176.11.6962. [DOI] [PubMed] [Google Scholar]
- 14.Hartshorn KL, White MR, Voelker DR, Coburn J, Zaner K, Crouch EC. Mechanism of binding of surfactant protein D to influenza A viruses: importance of binding to haemagglutinin to antiviral activity. Biochem J. 2000;351(Pt 2):449–458. [PMC free article] [PubMed] [Google Scholar]
- 15.Helmerhorst EJ, Oppenheim FG. Saliva: a dynamic proteome. J Dent Res. 2007;86:680–693. doi: 10.1177/154405910708600802. [DOI] [PubMed] [Google Scholar]
- 16.Jayasekera JP, Moseman EA, Carroll MC. Natural antibody and complement mediate neutralization of influenza virus in the absence of prior immunity. J Virol. 2007;81:3487–3494. doi: 10.1128/JVI.02128-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen JL, Lamkin MS, Oppenheim FG. Adsorption of human salivary proteins to hydroxyapatite: a comparison between whole saliva and glandular salivary secretions. J Dent Res. 1992;71:1569–1576. doi: 10.1177/00220345920710090501. [DOI] [PubMed] [Google Scholar]
- 18.Kandel R, Hartshorn KL. Novel strategies for prevention and treatment of influenza. Expert Opin Ther Targets. 2005;9:1–22. doi: 10.1517/14728222.9.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Kazmi SH, Naglik JR, Sweet SP, et al. Comparison of human immunodeficiency virus type 1-specific inhibitory activities in saliva and other human mucosal fluids. Clin Vaccine Immunol. 2006;13:1111–1118. doi: 10.1128/CDLI.00426-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LeVine A, Whitsett J, Hartshorn K, Korfhagen T. Surfactant protein D enhances clearance of influenza A virus from the lung in vivo. J Immunol. 2001;167:5868–5873. doi: 10.4049/jimmunol.167.10.5868. [DOI] [PubMed] [Google Scholar]
- 21.Ligtenberg AJ, Bikker FJ, De Blieck-Hogervorst JM, Veerman EC, Nieuw Amerongen AV. Binding of salivary agglutinin to IgA. Biochem J. 2004;383:159–164. doi: 10.1042/BJ20040265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ligtenberg AJ, Veerman EC, Nieuw Amerongen AV, Mollenhauer J. Salivary agglutinin/glycoprotein-340/DMBT1: a single molecule with variable composition and with different functions in infection, inflammation and cancer. Biol Chem. 2007;388:1275–1289. doi: 10.1515/BC.2007.158. [DOI] [PubMed] [Google Scholar]
- 23.Ligtenberg TJ, Bikker FJ, Groenink J, et al. Human salivary agglutinin binds to lung surfactant protein-D and is identical with scavenger receptor protein gp-340. Biochem J. 2001;359:243–248. doi: 10.1042/0264-6021:3590243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oppenheim FG, Salih E, Siqueira WL, Zhang W, Helmerhorst EJ. Salivary proteome and its genetic polymorphisms. Ann N Y Acad Sci. 2007;1098:22–50. doi: 10.1196/annals.1384.030. [DOI] [PubMed] [Google Scholar]
- 25.Robinovitch MR, Ashley RL, Iversen JM, Vigoren EM, Oppenheim FG, Lamkin M. Parotid salivary basic proline-rich proteins inhibit HIV-I infectivity. Oral Dis. 2001;7:86–93. [PubMed] [Google Scholar]
- 26.Salvatore M, Garcia-Sastre A, Ruchala P, Lehrer RI, Chang T, Klotman ME. Alpha-defensin inhibits influenza virus replication by cell-mediated mechanism(s) J Infect Dis. 2007;196:835–843. doi: 10.1086/521027. [DOI] [PubMed] [Google Scholar]
- 27.Sano H, Nagai K, Tsutsumi H, Kuroki Y. Lactoferrin and surfactant protein A exhibit distinct binding specificity to F protein and differently modulate respiratory syncytial virus infection. Eur J Immunol. 2003;33:2894–2902. doi: 10.1002/eji.200324218. [DOI] [PubMed] [Google Scholar]
- 28.Skott P, Lucht E, Ehnlund M, Bjorling E. Inhibitory function of secretory leukocyte proteinase inhibitor (SLPI) in human saliva is HIV-1 specific and varies with virus tropism. Oral Dis. 2002;8:160–167. doi: 10.1034/j.1601-0825.2002.01807.x. [DOI] [PubMed] [Google Scholar]
- 29.Soares RV, Lin T, Siqueira CC, et al. Salivary micelles: identification of complexes containing MG2, sIgA, lactoferrin, amylase, glycosylated proline-rich protein and lysozyme. Arch Oral Biol. 2004;49:337–343. doi: 10.1016/j.archoralbio.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Soares RV, Siqueira CC, Bruno LS, Oppenheim FG, Offner GD, Troxler RF. MG2 and lactoferrin form a heterotypic complex in salivary secretions. J Dent Res. 2003;82:471–475. doi: 10.1177/154405910308200613. [DOI] [PubMed] [Google Scholar]
- 31.Tecle T, White MR, Crouch EC, Hartshorn KL. Inhibition of influenza viral neuraminidase activity by collections. Arch Virol. 2007;152:1731–1742. doi: 10.1007/s00705-007-0983-4. [DOI] [PubMed] [Google Scholar]
- 32.Tecle T, White MR, Gantz D, Crouch EC, Hartshorn KL. Human neutrophil defensins increase neutrophil uptake of influenza A virus and bacteria and modify virus-induced respiratory burst responses. J Immunol. 2007;178:8046–8052. doi: 10.4049/jimmunol.178.12.8046. [DOI] [PubMed] [Google Scholar]
- 33.Trampuz A, Prabhu RM, Smith TF, Baddour LM. Avian influenza: a new pandemic threat? Mayo Clin Proc. 2004;79:523–530. doi: 10.4065/79.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veerman EC, van den Keybus PA, Valentijn-Benz M, Nieuw Amerongen AV. Isolation of different high-Mr mucin species from human whole saliva. Biochem J. 1992;283(Pt 3):807–811. doi: 10.1042/bj2830807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Webby RJ, Webster RG. Are we ready for pandemic influenza? Science. 2003;302:1519–1522. doi: 10.1126/science.1090350. [DOI] [PubMed] [Google Scholar]
- 36.White MR, Crouch E, van Eijk M, et al. Cooperative anti-influenza activities of respiratory innate immune proteins and neuraminidase inhibitor. Am J Physiol Lung Cell Mol Physiol. 2005;288:L831–840. doi: 10.1152/ajplung.00365.2004. [DOI] [PubMed] [Google Scholar]
- 37.White MR, Tecle T, Crouch EC, Hartshorn KL. Impact of neutrophils on antiviral activity of human bronchoalveolar lavage fluid. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1293–1299. doi: 10.1152/ajplung.00266.2007. [DOI] [PubMed] [Google Scholar]


