Abstract
Background
Protease-activated receptor-1 (PAR-1) is the high-affinity receptor for the coagulation protease thrombin. It is expressed by a variety of cell types in the heart, including cardiomyocytes and cardiac fibroblasts. We have shown that tissue factor (TF) and thrombin contribute to infarct size after cardiac ischemia-reperfusion (I/R) injury. Moreover, in vitro studies have shown that PAR-1 signaling induces hypertrophy of cardiomyocytes and proliferation of cardiac fibroblasts. The purpose of the present study was to investigate the role of PAR-1 in infarction, cardiac remodeling, and hypertrophy after I/R injury. In addition, we analyzed the effect of overexpression of PAR-1 on cardiomyocytes.
Methods and Results
We found that PAR-1 deficiency reduced dilation of the left ventricle and reduced impairment of left ventricular function 2 weeks after I/R injury. Activation of ERK1/2 was increased in injured PAR-1−/− mice compared with wild-type mice; however, PAR-1 deficiency did not affect infarct size. Cardiomyocyte-specific overexpression of PAR-1 in mice induced eccentric hypertrophy (increased left ventricular dimension and normal left ventricular wall thickness) and dilated cardiomyopathy. Deletion of the TF gene in cardiomyocytes reduced the eccentric hypertrophy in mice overexpressing PAR-1.
Conclusions
Our results demonstrate that PAR-1 contributes to cardiac remodeling and hypertrophy. Moreover, overexpression of PAR-1 on cardiomyocytes induced eccentric hypertrophy. Inhibition of PAR-1 after myocardial infarction may represent a novel therapy to reduce hypertrophy and heart failure in humans.
Keywords: proteases; receptor, PAR-1; hypertrophy; ventricular remodeling; myocardial infarction
Myocardial infarction induces structural remodeling of the heart, in which areas of initial infarct are replaced with collagen-rich tissue.1,2 These changes induce cardiac remodeling and hypertrophy, which are associated with reprogramming of cardiac gene expression and induction of the “fetal gene program.” For instance, the fetal genes atrial natriuretic factor (ANF) and B-type natriuretic peptide (BNP) are upregulated as a compensatory mechanism to promote natriuresis and to suppress myocyte hypertrophy.3 Importantly, the MAPK (mitogen-activated protein kinase)/ERK (extracellular signal-regulated kinase) kinase (MEK1)–ERK1/2 pathway has been shown to protect the myocardium from ischemia/reperfusion (I/R) injury.4,5
We have shown that myocardial infarction induces damage to the endothelial barrier that allows leakage of coagulation factors into the myocardium and their activation by tissue factor (TF) expressed by cardiomyocytes.6 Importantly, inhibition of either TF or thrombin significantly reduced the infarct size in rabbit models of cardiac I/R injury.6,7 A recent study showed that the fibrin degradation fragment E1 contributes to inflammation after myocardial infarction by binding to vascular endothelial cadherin at cell-cell junctions in the endothelium and facilitating the recruitment of neutrophils into the myocardium.8
Members of the protease-activated receptor (PAR) family of G-protein–coupled receptors are activated by proteolytic cleavage.9 PAR-1 is expressed by a variety of cell types and is activated by the coagulation protease thrombin, as well as other proteases.9,10 Cleavage of PAR-1 results in activation of Gαq, Gα12/13, and Gαi, as well as downstream signaling pathways, including the MAPK pathways, ERK 1/2 and ERK5.9,11 PAR-1 plays a critical role in the activation of human platelets but is not expressed on mouse platelets.9 Despite the death of ≈50% of PAR-1−/− embryos at mid gestation, adult PAR-1−/− mice have no phenotypic abnormalities in any tissue.12,13
In the heart, PAR-1 is expressed by cardiomyocytes and cardiac fibroblasts.14,15 A recent study showed that PAR-1 expression was increased in the hearts of patients with ischemic and idiopathic dilated cardiomyopathy.16 In addition, PAR-1 expression is increased in the left ventricle (LV) in a mouse model of chronic heart failure.17 In vitro studies using rat neonatal cardiomyocytes demonstrated that activation of PAR-1 induced hypertrophy.14,18 PAR-1–dependent changes included increases in intracellular calcium, protein content and cell size, sarcomeric organization, and ANF expression. Furthermore, activation of PAR-1 on cardiac fibroblasts induces cell proliferation.15 These results strongly suggest that PAR-1 may contribute to cardiac remodeling after injury.19
Methods
Mice
PAR-1+/− mice were backcrossed 11 generations onto a C57Bl/6J background and bred to generate PAR-1+/+ and PAR-1−/− littermate mice.12 Mice overexpressing PAR-1 in cardiomyocytes were generated by construction of a transgene that contained the cardiomyocyte-specific α-myosin heavy chain (α-MHC) promoter and the murine PAR-1 cDNA. Briefly, a 1.3-kbp DNA fragment containing the coding sequence of mouse PAR-1 was cloned into a vector containing the α-MHC promoter (kindly provided by Dr F. Naya). Next, an 8.5-kbp NotI fragment, which contained the α-MHC promoter, the coding sequence for mouse PAR-1, and the human growth hormone polyA sequence, was purified and injected into the pronucleus of fertilized mouse embryos (C57Bl/6J genetic background) by The Scripps Transgenic Core Facility. Transgenic mice were identified by polymerase chain reaction with primers for the human growth hormone polyA sequence. Mice with a cardiomyocyte-specific deletion of the TF gene were generated by crossing TFflox/flox mice with mice expressing Cre recombinase under the control of the α-MHC promoter.20 The present study was performed in accordance with the guidelines of the animal care and use committees of the different institutions and complies with National Institutes of Health guidelines. All experiments used male mice. For the cardiac I/R experiment, mice were 2 to 3 months of age. We used 5 to 14 mice per group for cardiac I/R experiments, 5 to 12 mice per group for echocardiographic analysis, 5 to 14 mice per group for determination of heart weight/body weight ratio, 4 to 6 mice per group for determination of LV lumen area, and 4 to 6 mice per group for measurement of gene expression.
Cardiac I/R Injury Models
For the short-term I/R model (30 minutes of ischemia and 2 hours of reperfusion), the surgical protocol and infarct-size determination were performed as described previously21 with some modifications. After occlusion, the suture was released. Briefly, intraperitoneal injection of pentobarbital (100 mg/kg; Abbott Laboratories, Abbott Park, Ill) was used for anesthesia. Mice were intubated orally to provide artificial ventilation (0.3 mL tidal volume, 120 breaths/min). The left anterior descending coronary artery was occluded with a 7-0 silk suture (US Surgical Corp, Norwalk, Conn) passed through PE tubing (US Surgical Corp) to make a Rumel snare. After 30 minutes of ischemia, the snare was released, and the heart was reperfused for 2 hours. Finally, the artery was reoccluded, and 4% Evans Blue dye was injected into the aortic root to delineate the area at risk from not-at-risk myocardium (blue). Hearts were then explanted, rinsed in 0.9% normal saline, and placed in 1% agarose gel (UltraPure agarose, Life Technologies, Gaithersburg, Md) in PBS (pH 7.4). Hearts were sectioned parallel to the AV groove in ≈1-mm sections. Viable and necrotic sections of the area at risk were identified by incubating the hearts in 1% 2,3,5-triphenyltetrazolium chloride (Sigma-Aldrich, St Louis, Mo) for 10 minutes at 37°C followed by 10% neutral buffered formaldehyde for 24 hours. Each section was weighed and photographed. The LV, area at risk, and infarct areas were traced and calculated by computer planimetry (Image J, version 1.21). Infarct volumes were calculated as [(A1×W1)+(A2×W2)+(A3×W3)+(A4×W4)+(A5×W5)], where A is the area of infarct for the slice denoted by the subscript and W is the weight of the respective section. Recombinant hirudin (lepirudin; Hoechst Marion Roussel, Kansas City, Mo) was administered via a single intraperitoneal injection (0.1 mg/kg of body weight) after thoracotomy and 30 minutes before ischemia.
The long-term model (45 minutes of ischemia and 2 weeks of reperfusion) was performed in a similar manner. Mice were anesthetized with 2% halothane and 40% oxygen and maintained with 0.5% halothane and 40% oxygen for the duration of the surgery. Mice were intubated orally to provide artificial ventilation (0.3 mL tidal volume, 120 breaths/min). After left thoracotomy at the fourth intercostal space, the left anterior descending coronary artery was ligated with an 8-0 nylon surgical suture 2.0 mm distal from the tip of the left atria and occluded for 45 minutes. Ischemia was validated via ECG recordings. For determination of infarct and scar formation in the long-term model, heart sections were stained with hematoxylin and eosin, photographed, and analyzed with the National Institutes of Health Scion image system. Viable myocardium was stained pink, whereas infarct was white. The area of infarct was calculated as a percentage of LV area. Four sections from different parts of the heart were measured and averaged for each mouse. Surgery on wild-type (WT) and PAR-1−/− mice was performed in a blinded fashion.
Echocardiography
Echocardiography was performed on conscious mice before and 2 weeks after cardiac I/R injury with an Acuson Sequoia C236 echocardiography machine equipped with a 15-MHz linear probe (Siemens Medical Solutions, Malvern, Pa). LV function was measured by M-mode echocardiography in the short-axis view at the midventricular level. The percentage of fractional shortening was assessed by measuring the end-diastolic and end-systolic diameter [(end-diastolic diameter–end-systolic diameter)/end-diastolic diameter×100 (%)]. Echocardiography on WT, PAR-1−/−, and αMHCPAR-1 mice was performed in a blinded fashion.
Northern and Western Blot Analysis
Hearts were collected and frozen in liquid nitrogen and then stored at −80°C. Levels of ANF, BNP, PAR-1, and GAPDH were determined by Northern blotting. Total RNA was extracted with Trizol reagent (Invitrogen Corp, Carlsbad, Calif), separated by electrophoresis, transferred to a nylon membrane, and hybridized with radiolabeled cDNA probes. Bands were visualized by autoradiography. Protein extracts were generated from mouse hearts as described previously.22 Protein (20 to 25 μg) was subjected to SDS-PAGE and transferred to either nitrocellulose or polyvinylidene difluoride membranes. Murine PAR-1 was detected with a goat anti-murine PAR-1 antibody S-19 (Santa Cruz Biotechnology, Santa Cruz, Calif). Levels of phosphorylated and nonphosphorylated ERK1/2 were detected with antibodies from Cell Signaling Technology (Danvers, Mass). Bands on the Western blots were quantified with a GS-800 scanner (BioRad, Hercules, Calif), and the intensity of the bands was determined with Quantity One software (BioRad).
Immunohistochemistry
Murine PAR-1 expression in fixed heart sections was detected with an anti-mouse PAR-1 antibody S-19 (Santa Cruz Biotechnology) and the Vectastain ABC system (Vector Laboratories, Burlingame, Calif).
Determination of Lumenal Area of LV and Thickness of LV Wall
Hearts were arrested at diastole by perfusion with PBS/20 mmol/L KCl, fixed in 4% formalin, and embedded in paraffin. Cross sections of hearts at the level of papillary muscles were obtained and stained with hematoxylin and eosin. The lumenal area of the LV was delineated manually and measured with the National Institutes of Health Scion image program. To determine the thickness of the LV wall in the hearts, 5 independent measurements were obtained from different regions of the LV and averaged.
Measurement of Cross-Sectional Myocyte Area
Hearts were cut in cross section just below the level of the papillary muscle. Sections were cut and stained with hematoxylin and eosin. For determination of myocyte cross-sectional areas, 30 to 40 individual cells per section were determined by digitization of the images and computerized pixel counting with the National Institutes of Health Scion image program. Only nucleated cardiomyocytes from areas of transversely cut muscle fibers that contained circular capillary profiles were included in the analysis. One arbitrary square unit equals 20 μm2.
Statistical Analysis
Comparisons between the different groups were performed with a Student t test for unpaired data. Statistical differences were considered significant for probability values <0.05. Data are shown as mean±SEM, unless otherwise indicated in the figure legends.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
PAR-1 Deficiency Does Not Affect Infarct Size After I/R Injury
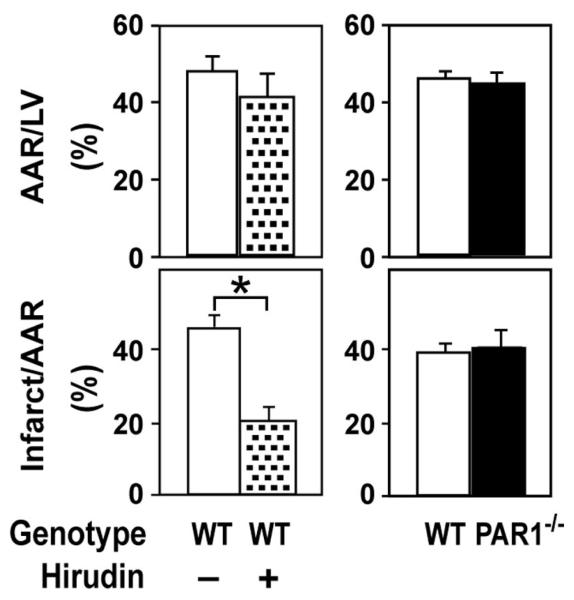
We used a mouse model of short-term cardiac I/R injury to determine the effect of PAR-1 deficiency on infarct size and inflammation. Surprisingly, we found no significant difference in infarct size between PAR-1−/− mice and WT littermates (Figure 1). In contrast, inhibition of thrombin with hirudin decreased infarct size (Figure 1). Neither hirudin treatment nor PAR-1 deficiency affected the size of the area at risk (Figure 1). Furthermore, PAR-1 deficiency did not affect the induction of various inflammatory mediators (interleukin-1β, interleukin-6, macrophage inflammatory protein-2, and monocyte chemotactic protein-1) in the injured hearts (data not shown).
Figure 1.
Role of PAR-1 in infarct size after cardiac I/R injury. Determination of area at risk (AAR) and infarct size in mice subjected to 30 minutes of ischemia and 2 hours of reperfusion. I/R-injured WT mice were treated with either saline (n=8; open bars) or hirudin (n=5; stippled bars). Infarct size was measured in PAR-1−/− mice (n=14; solid bars) and WT littermates (n=10; open bars). *P<0.01.
PAR-1 Deficiency Reduces Cardiac Remodeling After I/R Injury
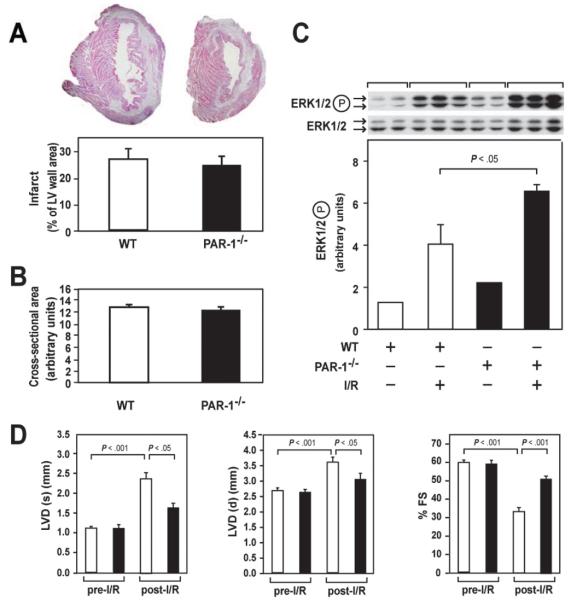
Despite the fact that PAR-1 did not contribute to infarct size, we hypothesized that PAR-1 signaling may play a role in cardiac remodeling after myocardial infarction by inducing hypertrophy of cardiomyocytes and cell proliferation of cardiac fibroblasts. We used a long-term mouse model of cardiac I/R injury that consisted of 45 minutes of ischemia and 2 weeks of reperfusion to induce cardiac remodeling. Consistent with our short-term I/R model, we did not observe a significant difference in infarct size in WT and PAR-1−/− mice 2 weeks after I/R injury (Figure 2A). In addition, the cross-sectional myocyte area was not affected by PAR-1 deficiency (Figure 2B). I/R injury activated ERK1/2 in both WT and PAR-1−/− mice (Figure 2C). Interestingly, the levels of activated ERK1/2 were higher in PAR-1−/− mice than in injured WT mice (Figure 2C). Importantly, echocardiography showed that the hearts of PAR-1−/− mice exhibited reduced dilation of the LV and reduced impairment of LV function compared with WT littermates after cardiac I/R injury (Figure 2D).
Figure 2.
Role of PAR-1 in cardiac remodeling after cardiac I/R injury. A, Representative cross sections of hearts from WT and PAR-1−/− mice subjected to 45 minutes of ischemia and 2 weeks of reperfusion. Sections were stained with hematoxylin and eosin. The normal myocardium is pink, whereas the infarct is white. Infarct size was quantified in WT (n=10) and PAR-1−/− (n=10) hearts. B, Cross-sectional area of cardiomyocytes in the hearts of WT and PAR-1−/− mice after I/R injury. Six mice were analyzed for each group, and 30 to 40 cardiomyocytes were measured per mouse. C, Levels of phosphorylated ERK1/2 in hearts of WT and PAR-1−/− mice before and after I/R injury. Two or 3 mice are shown per group. Data are mean±SD. D, Echocardiographic analysis of LV diameter (LVD) at diastole (d) and systole (s) and LV fractional shortening (FS) before and after myocardial infarction induced by 45 minutes of ischemia and 2 weeks of reperfusion. Results from WT (n=12; open bars) and PAR-1−/− (n=12; solid bars) mice are shown.
Generation of Mice Overexpressing PAR-1 on Cardiomyocytes
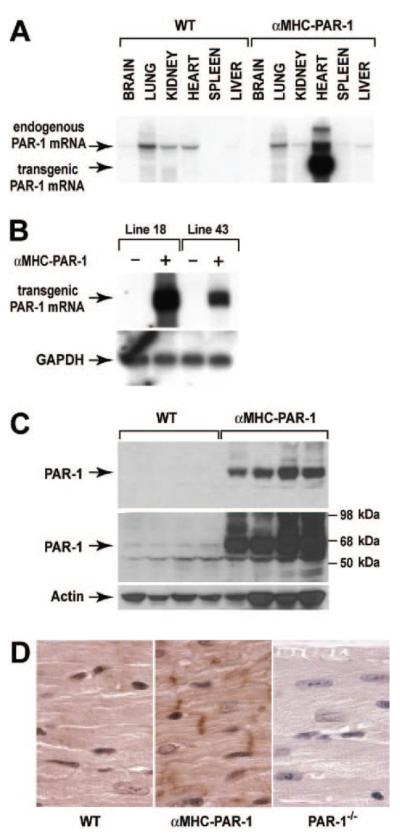
Next, we generated transgenic mice that overexpressed PAR-1 on cardiomyocytes. Two transgenic mouse lines (αMHC-PAR-1 line 18 and αMHC-PAR-1 line 43) were made that exhibited different levels of cardiac-specific PAR-1 mRNA expression (Figure 3A and 3B). PAR-1 protein expression was detected in hearts of αMHC-PAR-1 mice line 18 by Western blotting at much higher levels than in hearts of WT littermates (Figure 3C). Immunohistochemical studies showed that PAR-1 was localized to the plasma membrane, intercalated discs, and the cytoplasm of cardiomyocytes in the hearts of αMHC-PAR-1 mice (Figure 3D). Low levels of PAR-1 were detected in the hearts of WT mice, and no PAR-1 was present in the hearts of PAR-1−/− mice (Figure 3D).
Figure 3.
Characterization of mice overexpressing PAR-1 in cardiomyocytes. A, PAR-1 mRNA expression was analyzed by Northern blotting in various tissues from transgenic (αMHC-PAR-1; line 18) and nontransgenic (WT) mice. Ten micrograms of total RNA was analyzed in each lane. PAR-1 mRNA expressed by the transgene is smaller than PAR-1 mRNA from the endogenous PAR-1 gene because it lacks most of the 3′ untranslated region. The larger bands may represent partially spliced transcripts. B, Comparison of PAR-1 mRNA expression in hearts from transgenic lines 18 and 43. GAPDH was used as a loading control. C, PAR-1 protein expression in αMHC-PAR-1 (line 18) mice and WT littermates was analyzed by Western blotting. Two exposures (shorter on top and longer below) are included to show the relative levels of PAR-1 in the 2 groups of mice. Four mice from each group are shown. D, PAR-1 expression in the hearts of WT, αMHC-PAR-1, and PAR-1−/− mice was analyzed by immunohistochemistry. Original magnification ×1000.
PAR-1 Overexpression on Cardiomyocytes Induces Eccentric Hypertrophy and Dilated Cardiomyopathy
PAR-1 signaling has been shown to induce activation of ERK1/2 and hypertrophy of cultured cardiomyocytes.14,18 Therefore, we determined whether overexpression of PAR-1 induced activation of ERK1/2 and heart hypertrophy in mice. αMHC-PAR-1 mice exhibited a significant increase in activation of ERK1/2 and expression of the fetal genes ANF and BNP compared with WT littermates at 6 months (Figure 4A and 4B). We also observed an age-dependent increase in the heart weight:body weight ratio (Figure 4C). The weight of other organs (lung, kidney, and liver) compared with body weight was unchanged in αMHC-PAR-1 mice (data not shown). Older αMHC-PAR-1 line 43 mice (12 months of age) also exhibited an increase in heart weight:body weight ratio (4.97±0.36 versus 4.54±0.26 in αMHC-PAR-1 mice and WT littermate controls, respectively; P<0.05; n=15 for each group).
Figure 4.
Phenotype of mice overexpressing PAR-1 in cardiomyocytes. A, Western blot analysis of the levels of phosphorylated ERK1/2 in the hearts of WT and αMHC-PAR-1 mice at 6 months of age. Three mice are shown per group. Data are shown as mean±SD. B, Northern blot analysis of ANF and BNP mRNA expression in the LV of 6-month-old WT and αMHC-PAR-1 mice (5 mice per group). Levels of ANF and BNP mRNA normalized to GAPDH are shown (right). C, The heart weight:body weight (HW:BW) ratio was determined in 4-, 6-, and 10-month-old WT mice and αMHC-PAR-1 mice (5 to 10 mice per group). D, Representative hearts from 10-month-old WT and αMHC-PAR-1 mice. E, Representative cross sections of hearts from 6-month-old WT and αMHC-PAR-1 mice cut at the level of the papillary muscle (left) and quantitation of the lumenal area of the LV and thickness of the LV free wall in WT (n=6) and αMHC-PAR-1 (n=6) mice (right). F, Cross-sectional area of cardiomyocytes in hearts of WT and αMHC-PAR-1 (line 18) mice. Six mice were analyzed for each group, and 30 to 40 cardiomyocytes were measured per mouse. In panels with bars, results from WT mice (open bars) and αMHC-PAR-1 mice (solid bars) are shown.
At 10 months of age, the hearts of αMHC-PAR-1 line 18 mice were grossly larger than those of WT littermates (Figure 4D). Histological analysis of the hearts showed that the lumenal area of the LV was increased significantly without a change in the thickness of the free LV wall (Figure 4E). In addition, no difference was present in the cross-sectional area of cardiomyocytes in the hearts of αMHC-PAR-1 mice and WT littermates (Figure 4F). We used transthoracic echocardiography to independently measure the diameter of the LV and the thickness of the ventricular walls, as well as LV function. Figure 5A shows representative M-mode echocardiographic images of the hearts of αMHC-PAR-1 and WT mice. Consistent with our histological analysis, we found that the diameter of the LV was increased significantly in αMHC-PAR-1 mice compared with WT littermates at 10 months of age (Figure 5B). In contrast, no difference in the thickness of the anterior and posterior LV wall was present between the 2 groups of mice (Figure 5B). As expected, LV function was significantly reduced in αMHC-PAR-1 mice compared with WT littermates (Figure 5B).
Figure 5.
Echocardiographic analysis of heart size and function in αMHC-PAR-1 mice. A, Representative M-mode echocardiographic images of an αMHC-PAR-1 mouse and a WT littermate control at 10 months of age. B, LV diameter (LVD), anterior and posterior LV wall thickness, and LV function (percentage of fractional shortening, %FS) were measured at diastole (d) and systole (s) by echocardiography in 10-month-old mice. WT (open bars) and αMHC-PAR-1 (solid bars) mice are shown (5 mice per group).
Cardiomyocyte-Specific Deletion of the TF Gene Reduces Hypertrophy in αMHC-PAR-1 Mice
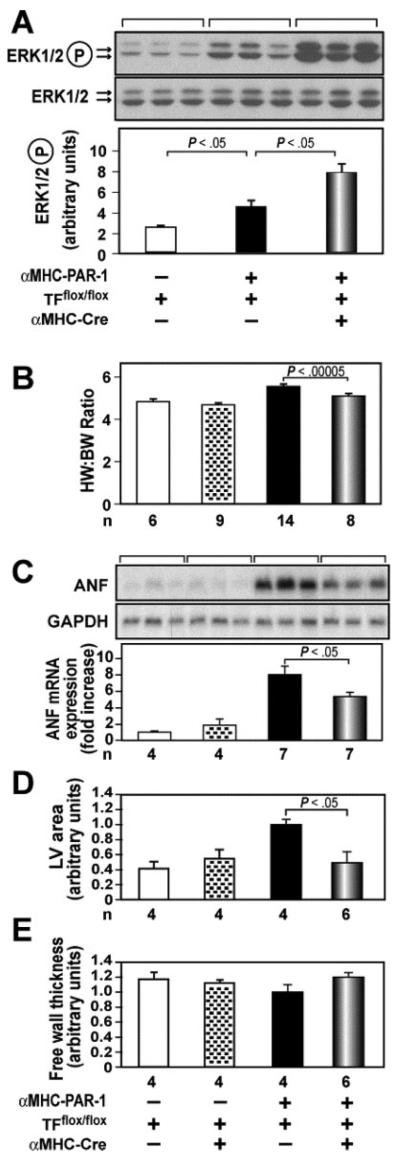
To test the hypothesis that coagulation proteases activate PAR-1 signaling in αMHC-PAR1 mice, we determined the effect of deleting the TF gene in cardiomyocytes. αMHC-PAR-1 mice with cardiomyocyte-specific deletion of the TF gene (αMHC-PAR-1/TFflox/flox/αMHC-Cre) were generated in 2 steps from crosses between αMHC-PAR-1 and TFflox/flox/αMHC-Cre mice. These mice were then crossed with TFflox/flox mice to generate 4 different groups of mice. Deletion of the TF gene led to a further increase in activation of ERK1/2 in αMHC-PAR-1 mice but did not change the level of activated ERK1/2 in WT mice (Figure 6A and data not shown). In addition, deletion of the TF gene significantly reduced the heart weight:body weight ratio and ANF mRNA expression in the hearts of αMHC-PAR-1/TFflox/flox/αMHC-Cre mice compared with αMHC-PAR-1/TFflox/flox littermate controls (Figure 6B and 6C). Moreover, the lumenal area of the LV was significantly reduced in αMHC-PAR-1/TFflox/flox/αMHC-Cre mice without any effect on the thickness of the LV free wall (Figure 6D and 6E). To exclude the possibility that the presence of the αMHC-Cre transgene reduced PAR-1 expression from the αMHC-PAR-1 transgene because of competition for transcription factors, we analyzed PAR-1 mRNA expression in αMHC-PAR-1/TFflox/flox/αMHC-Cre mice and αMHC-PAR-1/TFflox/flox littermate controls. Similar levels of PAR-1 mRNA expression were observed in these 2 groups of mice (data not shown).
Figure 6.
Deletion of the TF gene in cardiomyocytes reduces cardiac hypertrophy in αMHC-PAR1 mice. A, Levels of phosphorylated ERK1/2 were analyzed by Western blotting. Three mice were analyzed per group. Data are shown as mean±SD. B, Heart weight:body weight (HW:BW) ratio. C, Levels of ANF mRNA expression in the different groups of mice were normalized to levels of GAPDH mRNA. The Northern blot above shows 3 representative mice from each group. D and E, Measurement of lumenal area of the LV and thickness of LV free wall. Representative cross sections of hearts cut at the level of the papillary muscle from αMHC-PAR1/TFflox/flox mice with or without the αMHC-Cre transgene are shown. Quantitation is shown below. n indicates number of mice in each group. Data are shown for 4-month-old male mice on a mixed genetic background (75% C57Bl/6 and 25% Sv129). αMHC-PAR1 mice on this genetic background developed hypertrophy faster than αMHC-PAR1 mice on a C57Bl/6 genetic background.
Discussion
In this study, we showed that PAR-1 signaling plays a role in pathological remodeling after cardiac I/R injury. Importantly, we found that PAR-1 deficiency significantly reduced LV dilation and impairment of LV function after myocardial infarction. PAR-1−/− mice exhibited an increase in the activation of ERK1/2 in their hearts compared with WT mice. Previous studies have shown that the MEK1-ERK1/2 pathway protects the myocardium after I/R injury,4,5 which suggests that the increase in ERK1/2 signaling in PAR-1−/− mice may explain, in part, the reduced remodeling. We propose that thrombin activation of PAR-1 after I/R injury leads to activation of ERK1/2 and pathways that contribute to hypertrophy; however, other proteases may also activate PAR-1 in the injured heart. We speculated that PAR-1 signaling may limit the activation of ERK1/2 from other receptors. This may explain why deletion of PAR-1 results in loss of pathological signaling, increased ERK/12 phosphorylation, and reduced remodeling.
We found that PAR-1 deficiency did not affect infarct size in either short-term or long-term I/R models. In contrast, a recent study found that inhibition of PAR-1 with a selective PAR-1 antagonist reduced infarct size in a rat model of cardiac I/R injury.23 This difference could be due to the use of different models of I/R injury or off-target effects of the antagonist.
PAR-1 overexpression on cardiomyocytes induced eccentric hypertrophy in the hearts of αMHC-PAR-1 mice. This form of hypertrophy results from an increase in serial but not parallel assembly of sarcomeres in cardiomyocytes.24,25 Thrombin cleavage of PAR-1 leads to the Gαq-dependent activation of ERK1/2 and ERK5.11 We observed an increase in ERK1/2 phosphorylation in the hearts of αMHC-PAR-1 mice, which indicated a change in intracellular signaling associated with overexpression of PAR-1. Interestingly, over-expression of Gαq in cardiomyocytes also leads to eccentric hypertrophy and dilated cardiomyopathy.26 In addition, a recent study showed that activation of the MEK5-ERK5 pathway induces serial assembly of sarcomeres in vitro and eccentric hypertrophy in mice.27 Therefore, overexpression of signaling intermediates downstream of PAR-1 produces phenotypes that are remarkably similar to that observed in mice overexpressing PAR-1 on cardiomyocytes. Further studies are required to determine the role of the Gαq, MEK5-ERK5, and MEK1-ERK1/2 signaling pathways in PAR-1–dependent eccentric hypertrophy.
Deletion of the TF gene in cardiomyocytes resulted in a decrease in eccentric hypertrophy in αMHC-PAR-1 mice. We speculate that low levels of thrombin are generated continually in the normal myocardium in a TF-dependent manner. In αMHC-PAR-1 mice, this basal thrombin is “sensed” by PAR-1 overexpressed on cardiomyocytes and is translated into pathological signaling within the cardiomyocyte. In addition, assembly of the TF:factor VIIa:factor Xa ternary complex on the surface of the cardiomyocyte may activate PAR-1. Deletion of TF on cardiomyocytes would reduce the generation of thrombin and PAR-1 activation, which would result in a reduction in pathological signaling. The increase in ERK1/2 activation in αMHC-PAR-1 mice lacking the TF gene in cardiomyocytes may be due to the loss of TF-dependent inhibitory pathways. Indeed, a recent study showed that deletion of the TF cytoplasmic domain resulted in an increase in lipopolysaccharide activation of ERK1/2 in macrophages.28 Residual activation of PAR-1 overexpressed on cardiomyocytes may occur from thrombin generated from TF on cardiac fibroblasts or other proteases within the myocardium, such as matrix metalloproteinase-1.29
The results of the present study indicate that PAR-1 plays an important role in cardiac remodeling after myocardial infarction. Importantly, a recent study showed that PAR-1 expression was increased in human end-stage heart failure.16 Despite extensive research, heart hypertrophy and failure are a leading cause of death worldwide.1 Thus, a need exists for the development of new therapies for heart disease. Inhibition of pathological PAR-1 signaling with PAR-1 antagonists such as mimetic peptides,30 pepducins,31 or antibodies after myocardial infarction may be an effective therapy for the treatment of hypertrophy and heart failure.
CLINICAL PERSPECTIVE.
Heart failure is a debilitating disease with poor prognosis. Numerous G-protein–coupled receptors have been implicated in the progression of cardiac dysfunction, and several G-protein–coupled receptors are the target of current heart failure therapeutics. Prior data have established an important vascular role for protease-activated receptor-1 (PAR-1) G-protein–coupled receptor signaling in thrombosis and hemostasis. Previous in vitro data had suggested a role for PAR-1 signaling in neonatal cardiomyocyte hypertrophy; however, little was known regarding a role for PAR-1 signaling in the heart in vivo. Importantly, PAR-1 expression is significantly elevated in human end-stage heart disease. In the present report, we found that cardiac overexpression of PAR-1 in transgenic mice resulted in dilated cardiomyopathy, which suggests that elevated PAR-1 expression in human heart failure contributes to pathological cardiac remodeling. The pathological role of excess cardiac PAR-1 signaling was confirmed using mice with genetic ablation of PAR-1, which led to improved cardiac function after myocardial infarction. Surprisingly, the cardioprotection observed in PAR-1–null animals after myocardial infarction occurred despite a lack of significant reduction in infarct size. Collectively, the present data demonstrate an important role for cardiac PAR-1 signaling in pathological cardiac remodeling and suggest therapeutic potential for PAR-1 inhibition in the treatment of cardiac hypertrophy and heart failure.
Acknowledgments
We acknowledge the technical assistance of B. Pedersen, C. Rothnie, T. Thinnes, and T. Ludovic.
Funding Sources
This work was supported by a grant from the National Institutes of Health (HL71053 to Dr Mackman) and an American Heart Association scientific development grant (043543T to Dr Blaxall).
Footnotes
Disclosures
None.
References
- 1.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 2.Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol. 2005;45:657–687. doi: 10.1146/annurev.pharmtox.45.120403.095802. [DOI] [PubMed] [Google Scholar]
- 3.Blaxall BC, Spang R, Rockman HA, Koch WJ. Differential myocardial gene expression in the development and rescue of murine heart failure. Physiol Genomics. 2003;15:105–114. doi: 10.1152/physiolgenomics.00087.2003. [DOI] [PubMed] [Google Scholar]
- 4.Li DY, Tao L, Liu H, Christopher TA, Lopez BL, Ma XL. Role of ERK1/2 in the anti-apoptotic and cardioprotective effects of nitric oxide after myocardial ischemia and reperfusion. Apoptosis. 2007;11:923–930. doi: 10.1007/s10495-006-6305-6. [DOI] [PubMed] [Google Scholar]
- 5.Lips DJ, Bueno OF, Wilkins BJ, Purcell NH, Kaiser RA, Lorenz JN, Voisin L, Saba-El-Leil MK, Meloche S, Pouyssegur J, Pages G, De Windt LJ, Doevendans PA, Molkentin JD. MEK1-ERK2 signaling pathway protects myocardium from ischemic injury in vivo. Circulation. 2007;109:1938–1941. doi: 10.1161/01.CIR.0000127126.73759.23. [DOI] [PubMed] [Google Scholar]
- 6.Erlich JH, Boyle EM, Jr, Labriola J, Kovacich JC, Santucci RA, Fearns C, Morgan EN, Yun W, Luther T, Kojikawa O, Martin TR, Pohlman TH, Verrier ED, Mackman N. Inhibition of the tissue factor-thrombin pathway limits infarct size after myocardial ischemia-reperfusion injury by reducing inflammation. Am J Pathol. 2000;157:1849–1862. doi: 10.1016/S0002-9440(10)64824-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golino P, Ragni M, Cirillo P, D'Andrea D, Ravera A, Buono C, Corcione N, Vigorito F. Recombinant human, active site-blocked factor VII reduces infarct size and the NO-reflow phenomenon in a rabbit model of myocardial ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2000;278:H1507–H1516. doi: 10.1152/ajpheart.2000.278.5.H1507. [DOI] [PubMed] [Google Scholar]
- 8.Petzelbauer P, Zacharowski PA, Miyazaki Y, Friedl P, Wickenhauser G, Castellino FJ, Groger M, Wolff K, Zacharowski K. The fibrin-derived peptide Bbeta15-42 protects the myocardium against ischemiareperfusion injury. Nat Med. 2005;11:298–304. doi: 10.1038/nm1198. [DOI] [PubMed] [Google Scholar]
- 9.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 10.Riewald M, Ruf W. Orchestration of coagulation protease signaling by tissue factor. Trends Cardiovasc Med. 2002;12:149–154. doi: 10.1016/s1050-1738(02)00153-6. [DOI] [PubMed] [Google Scholar]
- 11.Marinissen MJ, Servitja JM, Offermanns S, Simon MI, Gutkind JS. Thrombin protease-activated receptor-1 signals through Gq- and G13-initiated MAPK cascades regulating c-Jun expression to induce cell transformation. J Biol Chem. 2003;278:46814–46825. doi: 10.1074/jbc.M305709200. [DOI] [PubMed] [Google Scholar]
- 12.Darrow AL, Fung-Leung W-P, Ye RD, Santulli RJ, Cheung W-M, Derian CK, Burns CL, Damiano BP, Zhou L, Keenan CM, Peterson PA, Andrade-Gordon P. Biological consequences of thrombin receptor deficiency in mice. Thromb Haemost. 1996;76:860–866. [PubMed] [Google Scholar]
- 13.Connolly AJ, Ishihara H, Kahn ML, Farese RV, Jr, Coughlin SR. Role of the thrombin receptor in development and evidence for a second receptor. Nature. 1996;381:516–519. doi: 10.1038/381516a0. [DOI] [PubMed] [Google Scholar]
- 14.Sabri A, Muske G, Zhang H, Pak E, Darrow A, Andrade-Gordon P, Steinberg SF. Signaling properties and functions of two distinct cardiomyocyte protease-activated receptors. Circ Res. 2000;86:1054–1061. doi: 10.1161/01.res.86.10.1054. [DOI] [PubMed] [Google Scholar]
- 15.Sabri A, Short J, Guo J, Steinberg SF. Protease-activated receptor-1-mediated DNA synthesis in cardiac fibroblast is via epidermal growth factor receptor transactivation: distinct PAR-1 signaling pathways in cardiac fibroblasts and cardiomyocytes. Circ Res. 2002;91:532–539. doi: 10.1161/01.res.0000035242.96310.45. [DOI] [PubMed] [Google Scholar]
- 16.Moshal KS, Tyagi N, Moss V, Henderson B, Steed M, Ovechkin A, Aru GM, Tyagi SC. Early induction of matrix metalloproteinase-9 transduces signaling in human heart end stage failure. J Cell Mol Med. 2005;9:704–713. doi: 10.1111/j.1582-4934.2005.tb00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moshal KS, Tyagi N, Henderson B, Ovechkin AV, Tyagi SC. Protease-activated receptor and endothelial-myocyte uncoupling in chronic heart failure. Am J Physiol Heart Circ Physiol. 2005;288:H2770–H2777. doi: 10.1152/ajpheart.01146.2004. [DOI] [PubMed] [Google Scholar]
- 18.Glembotski CC, Irons CE, Krown KA, Murray SF, Sprenkle AB, Sei CA. Myocardial alpha-thrombin receptor activation induces hypertrophy and increases atrial natriuretic factor gene expression. J Biol Chem. 1993;268:20646–20652. [PubMed] [Google Scholar]
- 19.Steinberg SF. The cardiovascular actions of protease-activated receptors. Mol Pharmacol. 2005;67:2–11. doi: 10.1124/mol.104.003103. [DOI] [PubMed] [Google Scholar]
- 20.Pawlinski R, Tencati M, Holscher T, Pedersen B, Voet T, Tilley RE, Marynen P, Mackman N. Role of cardiac myocyte tissue factor in heart hemostasis. J Thromb Hemost. 2007;5:1693–1700. doi: 10.1111/j.1538-7836.2007.02649.x. [DOI] [PubMed] [Google Scholar]
- 21.Palazzo AJ, Jones SP, Girod WG, Anderson DC, Granger DN, Lefer DJ. Myocardial ischemia-reperfusion injury in CD18- and ICAM-1-deficient mice. Am J Physiol. 1998;275:H2300–H2307. doi: 10.1152/ajpheart.1998.275.6.H2300. [DOI] [PubMed] [Google Scholar]
- 22.Liu L, Tsai J, Aird WC. Egr-1 gene is induced by the systemic administration of the vascular endothelial growth factor and the epidermal growth factor. Blood. 2000;96:1772–1781. [PubMed] [Google Scholar]
- 23.Strande JL, Hsu A, Su J, Fu X, Gross GJ, Baker JE. SCH 79797, a selective PAR1 antagonist, limits myocardial ischemia/reperfusion injury in rat hearts. Basic Res Cardiol. 2007;4:350–358. doi: 10.1007/s00395-007-0653-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chien KR, Olson EN. Converging pathways and principles in heart development and disease: CV@CSH. Cell. 2002;110:153–162. doi: 10.1016/s0092-8674(02)00834-6. [DOI] [PubMed] [Google Scholar]
- 25.Chien KR. Stress pathways and heart failure. Cell. 1999;98:555–558. doi: 10.1016/s0092-8674(00)80043-4. [DOI] [PubMed] [Google Scholar]
- 26.Mende U, Kagen A, Cohen A, Aramburu J, Schoen FJ, Neer EJ. Transient cardiac expression of constitutively active Galphaq leads to hypertrophy and dilated cardiomyopathy by calcineurin-dependent and independent pathways. Proc Natl Acad Sci U S A. 1998;95:13893–13898. doi: 10.1073/pnas.95.23.13893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicol RL, Frey N, Pearson G, Cobb M, Richardson J, Olson EN. Activated MEK5 induces serial assembly of sarcomeres and eccentric cardiac hypertrophy. EMBO J. 2001;20:2757–2767. doi: 10.1093/emboj/20.11.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahamed J, Niessen F, Kurokawa T, Lee YK, Bhattacharjee G, Morrissey JH, Ruf W. Regulation of macrophage procoagulant responses by the tissue factor cytoplasmic domain in endotoxemia. Blood. 2007;109:5251–5259. doi: 10.1182/blood-2006-10-051334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumor-igenesis of breast cancer cells. Cell. 2005;120:303–313. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 30.Andrade-Gordon P, Maryanoff B, Derian CK, Zhang HC, Addo MF, Darrow A, Eckhardt A, Hoekstra W, McComsey D, Oksenberg D, Reynolds E, Santulli RJ, Scarborough RM, Smith CE, White KB. Design, synthesis, and biological characterization of a peptide-mimetic antagonist for a tetheredligand receptor. Proc Natl Acad Sci U S A. 1999;96:12257–12262. doi: 10.1073/pnas.96.22.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Covic L, Misra M, Badar J, Singh C, Kuliopulos A. Pepducin-based intervention of thrombin-receptor signaling and systemic platelet activation. Nat Med. 2002;8:1161–1165. doi: 10.1038/nm760. [DOI] [PubMed] [Google Scholar]