Abstract
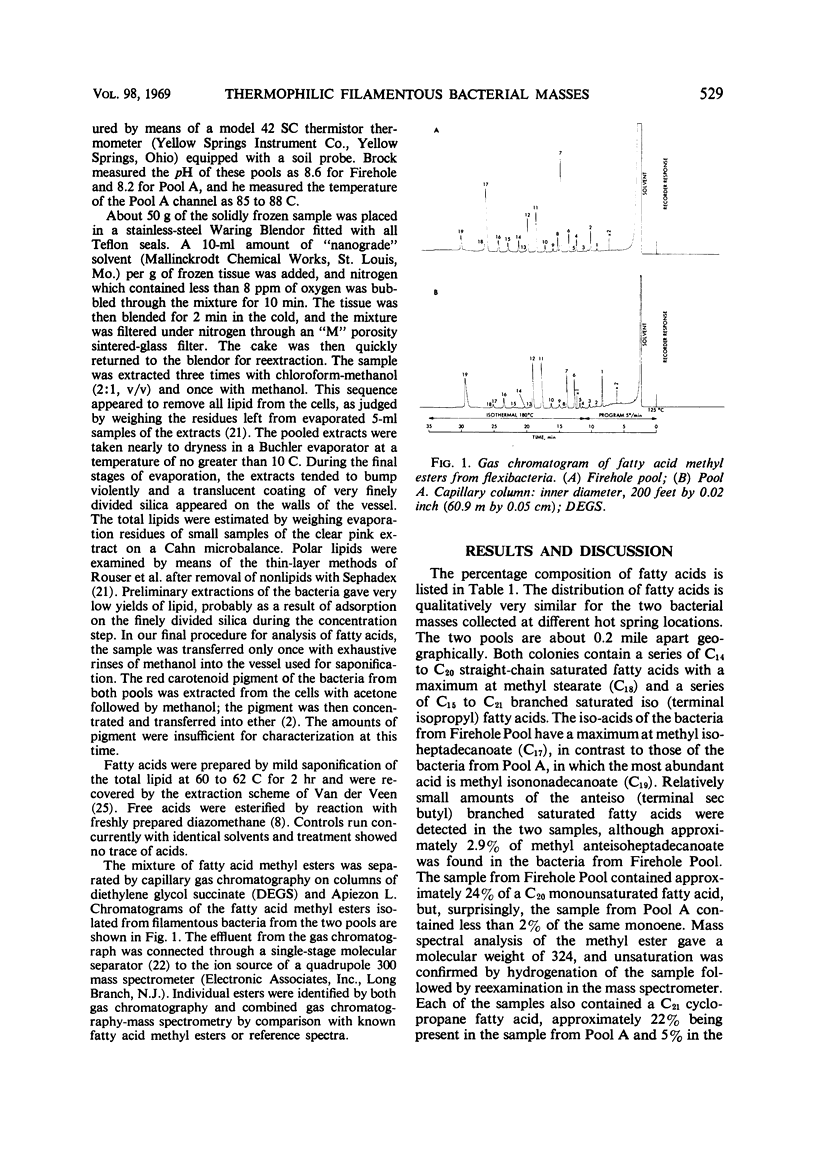
The fatty acid composition of filamentous bacterial masses from two very hot Yellowstone Park springs is not unusual despite the extreme environment. Both populations have a series of C14 to C20 straight-chain acids with a maximum at C18, and a series of saturated iso acids with a maximum at C17 in one case and C19 in the other. The fatty acid pattern of this anomalous group of organisms is like that of bacteria but not of blue-green algae. Both populations have similar polar lipids and identical carotenoids. It is speculated that these organisms may be adapted to their high-temperature environment by means of stable lipoprotein membrane systems.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aasen A. J., Jensen S. L. Carotenoids of flexibacteria. 3. The structures of flexixanthin and deoxy-flexixanthin. Acta Chem Scand. 1966;20(7):1970–1988. doi: 10.3891/acta.chem.scand.20-1970. [DOI] [PubMed] [Google Scholar]
- Aasen A. J., Jensen S. L. The carotenoids of flexibacteria. 2. A new xanthophyll from Saprospira grandis. Acta Chem Scand. 1966;20(3):811–819. doi: 10.3891/acta.chem.scand.20-0811. [DOI] [PubMed] [Google Scholar]
- Bauman A. J., Cameron R. E., Kritchevsky G., Rouser G. Detection of phthalate esters as contaminants of lipid extracts from soil samples stored in standard soil bags. Lipids. 1967 Jan;2(1):85–86. doi: 10.1007/BF02532008. [DOI] [PubMed] [Google Scholar]
- Brian B. L., Gardner E. W. A simple procedure for detecting the presence of cyclopropane fatty acids in bacterial lipids. Appl Microbiol. 1968 Apr;16(4):549–552. doi: 10.1128/am.16.4.549-552.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. D., Brock M. L. Measurement of steady-state growth rates of a thermophilic alga directly in nature. J Bacteriol. 1968 Mar;95(3):811–815. doi: 10.1128/jb.95.3.811-815.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. D. Life at high temperatures. Evolutionary, ecological, and biochemical significance of organisms living in hot springs is discussed. Science. 1967 Nov;158(3804):1012–1019. doi: 10.1126/science.158.3804.1012. [DOI] [PubMed] [Google Scholar]
- Cho K. Y., Salton M. R. Fatty acid composition of bacterial membrane and wall lipids. Biochim Biophys Acta. 1966 Feb 1;116(1):73–79. doi: 10.1016/0005-2760(66)90093-2. [DOI] [PubMed] [Google Scholar]
- Friedman S. M. Protein-synthesizing machinery of thermophilic bacteria. Bacteriol Rev. 1968 Mar;32(1):27–38. doi: 10.1128/br.32.1.27-38.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaughran E. R. THE THERMOPHILIC MICROORGANISMS. Bacteriol Rev. 1947 Sep;11(3):189–225. doi: 10.1128/br.11.3.189-225.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holton R. W., Blecker H. H., Stevens T. S. Fatty acids in blue-green algae: possible relation to phylogenetic position. Science. 1968 May 3;160(3827):545–547. doi: 10.1126/science.160.3827.545. [DOI] [PubMed] [Google Scholar]
- Ikawa M. Bacterial phosphatides and natural relationships. Bacteriol Rev. 1967 Mar;31(1):54–64. doi: 10.1128/br.31.1.54-64.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANEDA T. Biosythesis of branched chain fatty acids. I. Isolation and identification of fatty acids from Bacillus subtilis (ATCC 7059). J Biol Chem. 1963 Apr;238:1222–1228. [PubMed] [Google Scholar]
- Leo R. F., Parker P. L. Branched-chain Fatty acids in sediments. Science. 1966 Apr 29;152(3722):649–650. doi: 10.1126/science.152.3722.649. [DOI] [PubMed] [Google Scholar]
- Moss C. W., Cherry W. B. Characterization of the C15 branched-chain fatty acids of Corynebacterium acnes by gas chromatography. J Bacteriol. 1968 Jan;95(1):241–242. doi: 10.1128/jb.95.1.241-242.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols B. W., Harris R. V., James A. T. The lipid metabolism of blue-green algae. Biochem Biophys Res Commun. 1965 Jul 26;20(3):256–262. doi: 10.1016/0006-291x(65)90356-6. [DOI] [PubMed] [Google Scholar]
- O'leary W. M. THE FATTY ACIDS OF BACTERIA. Bacteriol Rev. 1962 Dec;26(4):421–447. doi: 10.1128/br.26.4.421-447.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker P. L., Van Baalen C., Maurer L. Fatty acids in eleven species of blue-green algae: geochemical significance. Science. 1967 Feb 10;155(3763):707–708. doi: 10.1126/science.155.3763.707. [DOI] [PubMed] [Google Scholar]
- Tornabene T. G., Bennett E. O., Oró J. Fatty acid and aliphatic hydrocarbon composition of Sarcina lutea grown in three different media. J Bacteriol. 1967 Aug;94(2):344–348. doi: 10.1002/path.1700940212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornabene T. G., Gelpi E., Oró J. Identification of fatty acids and aliphatic hydrocarbons in Sarcina lutea by gas chromatography and combined gas chromatography-mass spectrometry. J Bacteriol. 1967 Aug;94(2):333–343. doi: 10.1128/jb.94.2.333-343.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Veen J., Medwadowski B. F., Olcott H. S. Losses of fatty acids during the saponification extraction of small samples. Lipids. 1968 Mar;3(2):189–190. doi: 10.1007/BF02531743. [DOI] [PubMed] [Google Scholar]


