Abstract
Context
Most neuroimaging studies of posttraumatic stress disorder (PTSD) have focused on potential abnormalities in the whole hippocampus, but the subfields of this structure, which have distinctive histological characteristics and specialized functions, have not been investigated. Studies of individual subfields may clarify the role of the hippocampus in PTSD.
Objective
To determine if PTSD is associated with structural alterations in specific subfields of the hippocampus.
Design
Case-control study.
Participants
A total of 17 male veterans with combat trauma and PTSD (mean [SD] age, 41[12] years) and 19 age-matched male veterans without PTSD who were recruited from the outpatient mental health clinic of the San Francisco Veterans Affairs Medical Center and by advertising in the community.
Interventions
High-resolution magnetic resonance imaging at 4 T.
Main Outcome Measure
Volumes of hippocampal subfields.
Results
Posttraumatic stress disorder was associated with 11.4%(1.5%) (P = .02) smaller mean (SD) cornu ammonis 3 (CA3)/dentate gyrus subfield volumes, irrespective of age-related alterations, whereas other subfields were spared. Age was associated with reduced volume of the CA1 subfield (P = .03). Total hippocampal volume was also reduced in PTSD by a mean (SD) of 6.5%(0.6%) but, related to both PTSD (P = .05) and age (P = .01), was consistent with the measurements in the subfields.
Conclusions
The findings indicate for the first time in humans that PTSD is associated with selective volume loss of the CA3/dentate gyrus subfields, consistent with animal studies, implying that chronic stress suppresses neurogenesis and dendritic branching in these structures.
Posttraumatic stress disorder (PTSD) is a debilitating condition that can affect individuals who have been exposed to severe emotional or physically life-threatening traumatic events.1 The National Comorbidity Survey estimates that the lifetime prevalence of PTSD is 8% in the general population and 24% in persons exposed to trauma.2 Some symptoms of PTSD may be related to alterations in brain structure that might be detectable with neuroimaging.
Most neuroimaging studies of PTSD have focused on potential abnormalities in the hippocampus, which plays a major role in memory processing and, therefore, is thought to be functionally important in interaction with the amygdala for the pathogenesis of the persistent reexperiencing of symptoms in the context of trauma. The hippocampus is also known to play a crucial role in the biological response to stress.3 Several magnetic resonance imaging (MRI) studies reported smaller hippocampal volumes in patients with PTSD compared with patients without PTSD or controls,4–9 though the findings differed as to whether the effect involved the left or right side or was bilateral. Other studies found no evidence of hippocampal volume deficits in PTSD.10–15 Similarly, longitudinal MRI studies also found no evidence of hippocampal volume loss over time in PTSD.10,16 Other MRI studies have tried to divide the hippocampus into anatomical sections such as the head, body, and tail and reported selective volume deficits of the hippocampal head17 or tail18 in PTSD but others failed to replicate such results.13,15 However, lack of a clear consensus on what actually comprises the anatomical sections of the hippocampus might have compromised the findings. In general, the discrepant findings using MRI have made it difficult to identify the precise role of the hippocampus in PTSD.
The hippocampus is composed of several subfields with distinctive histological characteristics and specialized functions such as the subiculum, the cornu ammonis sectors (CA1-CA3), and the dentate gyrus (DG).19 Compared with division of the hippocampus into head, body, and tail, there is greater consensus on the boundary definitions of the hippocampal subfields. Moreover, animal studies found that stress-related damage to the hippocampus mainly happens in certain subfields20–26 including, specifically, the DG, which contains multipotent adult neural stem cells and is a key site of neurogenesis,27 and the CA3 region, which is a major target of glucocorticoids, a class of steroid hormones that are elevated under conditions of stress.28 Studies of individual subfields may therefore clarify the role of the hippocampus in PTSD.
Recently, we developed a protocol for acquiring and tracing the major hippocampal subfields on high-resolution MRI for studies of neurodegenerative diseases such as Alzheimer disease,29,30 exploiting the increased sensitivity and contrast of MRI at high magnetic fields (4 T). In this study, we used an MRI protocol to study the volumes of hippocampal subfields in PTSD. Specifically, we hypothesized that chronic PTSD selectively affects the DG and CA3 while sparing other subfields, consistent with observations in animals suggesting that chronic stress suppresses neurogenesis in the DG and dendritic branching in the CA3 region.21,24,25,31,32
METHODS
PARTICIPANTS
Subjects were recruited from the outpatient mental health clinic of the San Francisco Veterans Affairs Medical Center and by advertising in the community. After a complete description of the study was given to the participants, written informed consent was obtained. All procedures were approved by the Committees of Human Research at the University of California and the Veterans Affairs Medical center.
The study design required recruitment of 2 groups: veterans with current PTSD and healthy veterans without PTSD. Veterans participating in the study were evaluated by a clinical interviewer using the Structured Clinical Interview for DSM-IV Diagnosis33 and the Clinician Administered PTSD Scale (CAPS),34 and classified into groups. We used an interview version of the Life Stressor Checklist-Revised35 to determine exposure to traumatic events. The Life Stressor Checklist-Revised assesses 21 stressful life events (eg, experiencing or witnessing serious accidents, illnesses, sudden death, and physical and sexual assault). The Structured Clinical Interview for DSM-IV Diagnosis was used to rule out individuals with a lifetime history of psychotic or bipolar disorders and alcohol abuse or dependence within the previous 12 months and drug abuse or dependence within the previous 6 months. Veterans with past but not current PTSD or current sub-syndromal PTSD were also excluded. Other exclusion criteria were neurological illness, head trauma with loss of consciousness greater than 10 minutes, medical disorders affecting brain function, and conditions ineligible for MRI.
Twenty participants with PTSD and 20 without PTSD received MRI. All subjects with PTSD had traumatic exposure related to combat. The trauma histories of those without PTSD included 14 individuals who served in combat, 5 without a traumatic life event, and 1 who experienced a traumatic military accident. Four subjects (3 with PTSD and 1 control) had to be excluded because their MRI data showed substantial movement artifacts that led to difficulty visualizing the subfields. Thus, the final analysis included 17 participants with PTSD and 19 control subjects. The groups included a mix of Vietnam (23%), Gulf War (16%), Iraq (54%), and Afghanistan (7%) theater exposure—too few to make much inference about subgroups. Five patients with PTSD were taking antidepressant medications, whereas the other patients and all control subjects were medication free. In addition, 6 patients with PTSD and 3 controls reported a history of drug or alcohol abuse or dependence. Those episodes had occurred an average of 13.6 years (range, 1–40 years) before the study, and none had occurred within a year. The demographics and clinical characteristics of the participants are listed in Table 1.
Table 1.
Demographic Data
| Characteristic | PTSD | Control | P Value |
|---|---|---|---|
| Sample, No. | 17 | 19 | |
| Trauma exposed, No. | 17 | 14 | 0.8a |
| Age, mean (SD), y | 41 (12) | 38 (15) | 0.5 |
| CAPS score, mean (SD)b | 61 (14) | 8 (7) | <.001 |
| ICV, mean (SD), 103 × cm3 | 1.61 (0.12) | 1.71 (0.15) | .04 |
Abbreviations: CAPS, clinician-administered PTSD scale; ICV, intracranial volume; PTSD, posttraumatic stress disorder.
Using a χ2 test, all other comparisons used t tests.
Scale ranged from zero to 136, with higher scores indicating greater PTSD severity.34
MRI ACQUISITION AND DATA PROCESSING
Magnetic resonance imaging scans were performed at the Center for Imaging of Neurodegenerative Diseases at the San Francisco Veterans Affairs Medical Center using a Bruker/Siemens Med-Spec 4T MRI system (Bruker BioSpin, Ettlingen, Germany) equipped with an 8-channel array receiver coil. The MRI scan protocol consisted of a high-resolution T2-weighted fast spin echo sequence (repetition time, 3500 milliseconds; echo time, 19 milliseconds) with a train of 15 spin-echoes per k-space segment, 160° refocusing pulses, and 100% oversampling in the phase-encoding direction to avoid aliasing, yielding a nominal inplane resolution of 0.4 × 0.5 mm. Twenty-four contiguous slices, each 2-mm thick, were acquired in interleaved fashion. The coronal oblique slices were angulated perpendicular to the long axis of the hippocampal formation to achieve consistent images of hippocampal subfields from subject to subject.30 The total acquisition time of the sequence was about 5 ½ minutes. In addition, a volumetric T1-weighted magnetization prepared gradient echo sequence (repetition time, 2300 milliseconds; time following inversion pulse, 950 milliseconds; echo time, 4 milliseconds; 7° excitation pulses; 1 × 1 × 1 mm3 resolution) was acquired to determine total hippocampal volume and a volumetric T2-weighted turbospin echo sequence (repetition time, 3000 milliseconds; echo time, 356 milliseconds; 109 echoes per k-space segment with variable flip angles; 1 × 1 × 2–mm nominal resolution; 120 slices; acquisition time, 3.40 minutes) was collected to measure intracranial volume.
The method used for manual marking of subfields has been described in detail by Mueller et al.30 In brief, after carefully aligning the high-resolution images perpendicular to the long axis of the left or right hippocampus, separately, the tracing of the subfields starts on the first slice on which the head of the hippocampus is no longer visible. On this slice, the hippocampal subfields, subiculum, and entorhinal cortex (ERC) are marked (Figure 1 shows the starting slice). In addition, the ERC is marked on the 2 slices anterior to this starting slice, and the subiculum and the other hippocampal subfields are marked on the 2 slices posterior to the starting slice. Altogether, the hippocampal subfields are marked on 5 consecutive slices, ie, over a length of 10 mm in the rostral part of the hippocampus. The most medial point of the temporal cortex is chosen as the medial border of the ERC, and the medial end of the collateral sulcus is chosen as its lateral border. The CA1/subiculum border is determined by drawing a line perpendicular to the edge of the subiculum, touching the medial border of the hippocampus. The CA1/CA2 border is determined by dividing the line along the longest diameter of the hippocampus by 2 and drawing a line perpendicular to this line. The small region representing CA2 is marked in a squarelike manner, ie, its height at the CA1/2 boundary also determines its length, while its overall shape is determined by the course of the outer boundary of the hippocampus and a hypointense line representing myelinated tissue in the strata moleculare/lacunosum. Because the CA2 is likely to have some overlap with the dorsomedial part of CA1, we considered this region to be a CA1 to CA2 transition zone rather than CA2 exclusively. This represents the only deviation from the original marking protocol.30 The remainder of the hippocampal formation consisting of CA3 and the DG is marked as 1 region (CA3/DG) because there are no reliable landmarks to distinguish between the two structures. In summary, the traced subfields included the left and right ERC, the subiculum, CA1, CA1/2 transition, and CA3/DG. In addition, the volumes of the left and right total hippocampus were determined automatically using the brain parcellation features provided by the FreeSurfer software (Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, Massachusetts),36 which uses a probabilistic brain atlas as input for labeling brain structures. Automatic labeling of the hippocampus with FreeSurfer has been validated36 and tested on different brain pathologies.37 The hippocampal masks derived from FreeSurfer were further visually checked for accuracy and manually refined if necessary. The total intracranial volume (ICV) was determined from the T2-weighted image using the BET (brain extraction tool) program (www.fmrib.ox.ac.uk/fsl; FMRIB Image Analysis Group, Oxford University, Oxford, England). The subfield tracings were performed by an experienced reader (Z.W.) and inspected by a second reader (S.M.). The within-reader reliability of subfield tracings in terms of an intraclass correlation coefficient was better than 0.95 for the CA3/DG region, subiculum, CA1, and ERC, and 0.79 for the CA1/2 transition, which is by far the smallest subfield region. Both readers were blinded to any clinical information.
Figure 1.

High-resolution magnetic resonance images of the hippocampus. A, A high-resolution (0.4 × 0.5–mm inplane), T2-weighted magnetic resonance image of the brain shows a coronal section through the hippocampus. The zoomed-in image shows a view of the subfields (B) with the tracings in color superimposed (C). CA indicates cornu ammonis; DG, dentate gyrus; ERC, entorhinal cortex; and S, subiculum.
STATISTICAL ANALYSIS
Hippocampal subfields volumes and whole-hippocampal volume were analyzed using a linear model, accounting for the effects of PTSD diagnosis, age, and an interaction between PTSD and age. The volumes were corrected for ICV by cross-correlation, in which the regression coefficient between the volumes and ICV was used to correct individual volumes as a function of each subject’s ICV relative to the mean ICV of all subjects. The brain hemisphere was initially added into the model as covariate to account for left-right differences but, when no significant lateralization effects were found, left and right volumes were averaged. F tests were used to determine if factors added explanatory power and were therefore appropriate for inclusion in the model. Three nested models were fitted by maximum likelihood: the first (base) model included age, and the subsequent models added a PTSD effect and then a further PTSD × age interaction, respectively. The resulting fits were compared sequentially via F tests to determine whether PTSD, age, or an interaction between PTSD and age added significant (P < .05) explanatory power to the base model. No corrections for multiple comparisons were applied for tests involving the subfields because we had an a priori hypothesis for a PTSD effect on a specific subfield (CA3/DG). To further demonstrate that the results are not biased by the inclusion of 5 control subjects without trauma exposure, we performed the analysis with and without the unexposed group included. Finally, we used Spearman rank correlations to determine if subfield volumes in PTSD are associated with PTSD severity, as measured by CAPS scores, using Bonferroni corrections to account for multiple tests of correlations.
RESULTS
The 2 groups were comparable in age (P = .5, t test). Differences in the number of trauma-exposed subjects in the 2 groups were not significant (P = .8, χ2 test). As expected, subjects with PTSD had significantly higher CAPS scores than those without PTSD (P = .001, t test). Somewhat unexpectedly, subjects with PTSD had smaller ICV values than subjects without PTSD (P = .04, t test).
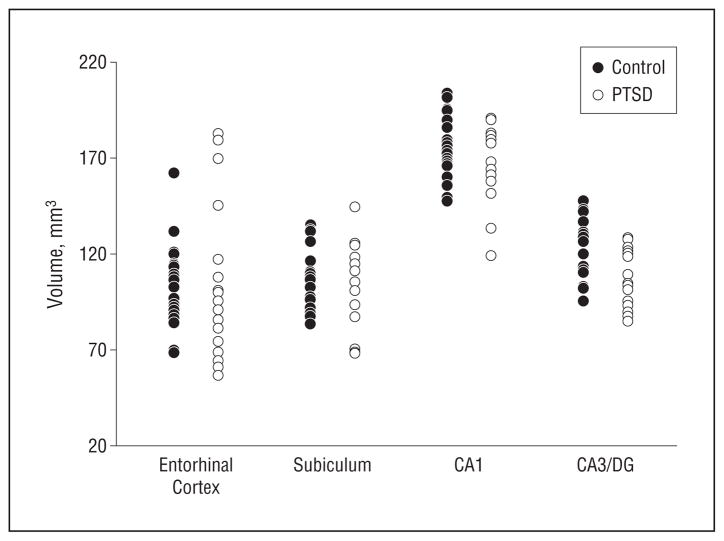
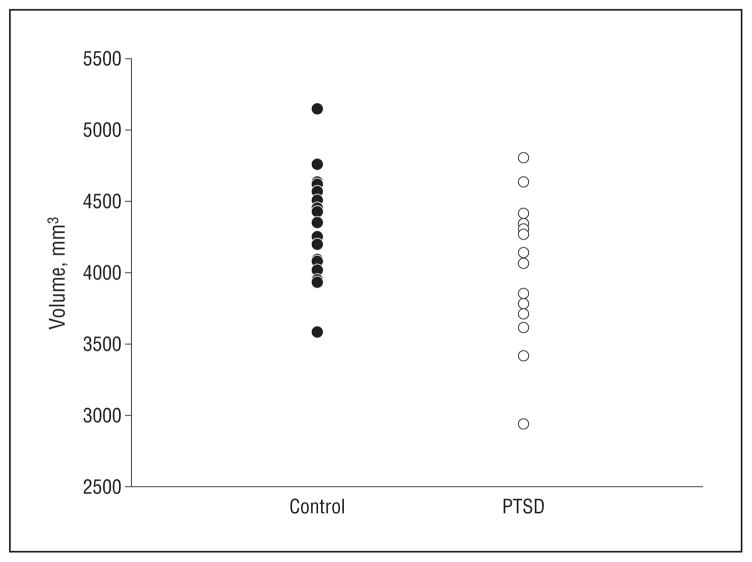
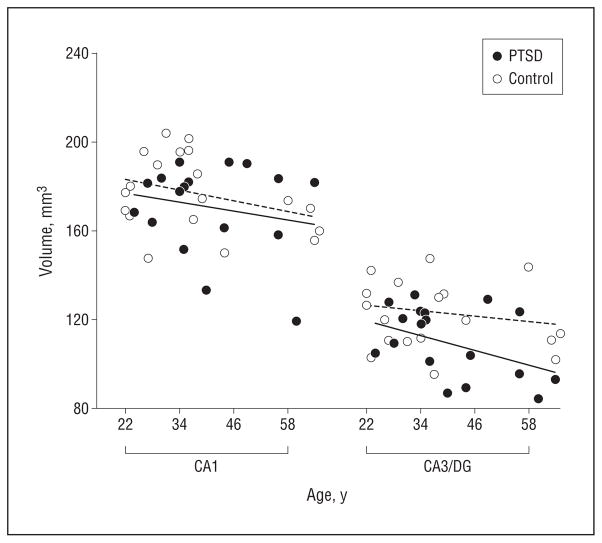
The scatter plots in Figure 2, A and B, depict volumes (left and right side are averaged and adjusted for ICV) of hippocampal subfields by group, and the scatter plot in Figure 3 shows the corresponding total hippocampal volumes. The effects of PTSD and age on subfields and whole hippocampal volumes adjusted for ICV are summarized in Table 2. The subfield CA3/DG was about a mean (SD) of 11.4%(1.5%) smaller in subjects with PTSD than in controls (F1,34 = 5.7; P = .02), yielding an effect size of 0.98. Age was associated with a mean (SD) 2%(1%) volume loss per decade but this did not significantly contribute to the volume reduction of CA3/DG (F1,34 = 2.1; P = .2). In contrast to CA3/DG, the subfield CA1 was not significantly associated with PTSD (F1,34 = 1.4; P = .3; mean [SD], 0.4%[2%] volume loss compared with controls) but showed a significant relationship with age (F1,34 = 5.1; P = .03; mean [SD], 3.0%[1.5%] volume loss per decade). Similarly, the CA1/2 transition was not significantly associated with PTSD but also showed a dependence on age (F1,34 = 8.9; P = .005; mean [SD], 3%[2%] volume loss per decade). Relations between the volumes of subfields and ICV varied and reached significance only for CA1/2 transition. Whole-hippocampal volume showed an effect of PTSD (F1,34 = 4.2; P = .05) as well as age (F1,34 = 6.7; P = .01), with PTSD explaining 31% and age 56% of the total variance. No region showed a significant PTSD × age interaction, which is not surprising given that the study was designed for tests of additive effects, whereas interactions of more than 35% change only were detectable with 90% confidence. The different effects of PTSD and age on CA3/DG and CA1 are depicted in the regression plots of subfield volumes vs age in Figure 4. A further analysis in which controls without exposure to trauma were excluded did not substantially alter the results. We also tested if subfield volumes or total hippocampal volume correlated with severity of PTSD, as measured with CAPS scores, but found no significant correlation for any of the volumes (all P = .7). Lastly, taking drug or alcohol abuse or dependence into consideration did not significantly alter the effect of PTSD on CA3/DG.
Figure 2.
Scatterplots of volumes of hippocampal subfields and entorhinal cortex by group. All volumes are corrected for variations in intracranial volume between subjects. The CA1/2 transition is not listed because the volumes are very small and difficult to trace compared with the other subfields. CA, cornu ammonis; DG, dentate gyrus; and PTSD, participants with posttraumatic stress disorder.
Figure 3.
Scatterplot of total hippocampal volume by group. The volumes are corrected for variations in intracranial volume between subjects. PTSD indicates participants with posttraumatic stress disorder.
Table 2.
Volumes of Subfields and Total Hippocampus in Subjects With PTSD and Controls and Associations With PTSD and Age
| Mean (SD) Volume, mm3 |
|||||
|---|---|---|---|---|---|
| Subfield | PTSD | Control | Contrast | F Valuea | P Value |
| CA3/DG | 109 (15) | 123 (16) | PTSD | 5.72 | .02 |
| Age | 2.13 | .17 | |||
| CA1 | 170 (20) | 177 (17) | PTSD | 1.41 | .24 |
| Age | 5.16 | .03 | |||
| CA1/2 transition | 7 (0.7) | 7 (0.8) | PTSD | 0.17 | .68 |
| Age | 8.92 | .005 | |||
| Entorhinal cortex | 105 (41) | 103 (22) | PTSD | 0.02 | .89 |
| Age | <.01 | .98 | |||
| Subiculum | 105 (21) | 106 (16) | PTSD | 0.22 | .62 |
| Age | 1.24 | .27 | |||
| Hippocampus | 2035 (234) | 2178 (181) | PTSD | 4.21 | .05 |
| Age | 6.73 | .01 | |||
Abbreviations: CA, cornu ammonis; PTSD, posttraumatic stress disorder.
F tests by maximum likelihood with 34 df for each test.
Figure 4.
Depiction of the dissociation between the effects of posttraumatic stress disorder (PTSD) and aging on subfields, separately for cornu ammonis 1 (CA1) and CA3 and the dentate gyrus (CA3/DG). The solid and dashed lines represent regressions of subfield volumes against age by group. This shows a PTSD effect on CA3/DG but not on CA1 after accounting for age.
COMMENT
We have 3 major findings. First, PTSD was associated with reduced volumes of the CA3/DG, regardless of age or when limited to trauma-exposed individuals, while other subfields were spared. This finding is in agreement with our a priori hypothesis and is also consistent with animal studies suggesting that chronic stress suppresses neurogenesis and dendritic branching in these regions. Second, age was associated with reduced volumes of the CA1 and CA1/2 transition, consistent with previous subfield MRI studies in aging.30 Third, both PTSD and age contributed to the total hippocampal volume reduction. Our findings suggest that PTSD is associated selectively with volume loss of the CA3/DG subfield.
Our finding of reduced CA3/DG subfield volumes in PTSD is consistent with studies in animals suggesting that stressful conditions can induce structural remodeling of the interconnected CA3/DG fields of the hippocampus. The main processes that are thought to be involved in stress-related remodeling include suppression of neurogenesis, reduced branching of dendrites, and reduced synaptic and neuronal plasticity. First, suppressed neurogenesis could explain the volume reduction of CA3/DG in PTSD because the DG is one of the few regions of the adult brain where neurogenesis occurs.38 Neurogenesis can produce a large pool of new granule cells in the DG,39 which is considered essential for the formation of memory,40 and memory plays an important role in development of PTSD. Repeated restraint stress in rats suppressed neurogenesis and significantly reduced the total number of granule cells and the granule cell layer volume of DG.24 In another study on Swiss albino mice, the expression of brain-derived neurotrophic factor in DG decreased after 5 weeks of a chronic mild stress procedure.41 Reduced brain-derived neurotrophic factor may inhibit neurogenesis because it is associated with the growth and differentiation of stem cells into neurons in hippocampus.42 The physiological effects of stress have also been shown to inhibit the process of neurogenesis in primates.21 Although it is not clear if suppressed neurogenesis leads to measurable effects in humans, our finding of smaller CA3/DG volumes in PTSD is consistent with depressed neurogenesis.
Second, diminished dendritic branching could also be responsible for the reduction of CA3/DG volumes in PTSD. The hippocampus is characterized by a relative abundance of glucocorticoid receptors43,44 and is especially vulnerable to elevated levels of glucocorticoids. Prolonged high levels of glucocorticoids may have direct and indirect neurotoxic effects on the hippocampus.45,46 A major target for glucocorticoids in the hippocampus is the CA3 region. Rats that were subjected to physical restraint daily for a period of 3 to 4 weeks had significant atrophy and reduced branching of apical dendrites in pyramidal neurons of the CA3 region.22,47 These changes can be blocked by glucocorticoid inhibitors. Chronic psychosocial stress also led to a decrease in the number of branch points and total dendritic length in the apical dendritic trees of CA3 pyramidal neurons.20,23 Alternatively, insufficient glucocorticoid signaling can also have damaging effects either via decreased neurotrophic effects on neurons48,49 or promoting inflammatory responses in the brain.50
Third, diminished synaptic or neuronal plasticity could be responsible for reduced CA3/DG volumes in PTSD.51 A number of studies have described changes in synaptic plasticity and neuron density in response to repetitive stress including suppression of long-term potentiation in DG granule cells and CA3 pyramidal neurons52,53 and retraction of thorny excrescences25 in the CA3 region. Stress-induced elevation of glucocorticoid levels cause pronounced loss of synapses in the CA3.32,54 The prolonged stress of emotional pain26 or immobilization55 also leads to a decrease in neurons in the CA3 subfield in rats.
Although our results of reduced CA3/DG volumes in PTSD are, overall, consistent with the results of stress studies in animals, implying that trauma exposure caused the reduction in PTSD, it is also possible that vulnerability to PTSD exists before trauma exposure. An MRI study of homozygous twins discordant for trauma exposure found that hippocampal volumes were smaller in both the exposed and unexposed members of the twin pairs in which one of the twins developed PTSD compared with twin pairs without PTSD.6 This implies that a smaller hippocampus constitutes a risk factor for the development of stress-related psychopathology. Our finding of a decreased ICV in PTSD, consistent with the finding by Woodward et al,56 would add further support that abnormalities in brain structure may increase vulnerability to developing PTSD. In particular, a smaller ICV could potentially indicate adverse early brain development that might predispose to PTSD. However, the volume reduction of CA3/DG in PTSD cannot simply be explained as bias toward a smaller ICV because the other subfields showed no significant effect of PTSD. A prospective study is necessary to elucidate cause or effect of PTSD on volume reductions. Furthermore, the finding that subjects with PTSD had smaller ICV values than those without PTSD warrants further studies on a larger population.
Another finding was that the volume reduction of CA1 was associated with aging but not significantly with PTSD. The finding of a selective age effect on CA1 replicates prior MRI studies of subfields in aging from our laboratory.30 The CA1/2 transition region showed similar results to the CA1 region, but the CA1-2 transition is very small compared with the other subfields and difficult to measure precisely. It is possible that greater PTSD severity leads to more widespread damage, eventually involving also the CA1 region and other subfields. Given the number of subjects in this study, we had the sensitivity to detect about 15% volume difference in subfields between the groups with 90% power and an α = .05. Nonetheless, the findings suggest that CA3/DG is more selectively vulnerable to PTSD than other subfield regions.
Our results of independent effects of PTSD and age on whole-hippocampal volume reduction in PTSD are in agreement with our findings of the subfields that the CA3/DG region is affected by PTSD and the CA1 by aging. A meta-analysis also suggested that smaller hippocampal volume may be related to PTSD duration or aging,57 and age-related hippocampal atrophy is well documented.58,59 Simultaneous effects of PTSD and aging on whole-hippocampal volume may, therefore, explain some inconsistencies in previous results of whole-hippocampal volume loss in PTSD, especially when aging was not fully taken into account. Similarly, age-related volume changes of the hippocampus may have obscured PTSD-related changes in studies that did not find significant differences between PTSD and control subjects. However, CA3/DG cannot account for all volume effects of the whole hippocampus related to PTSD. First, the whole hippocampus included the fimbria, a prominent band of white matter that anatomically does not belong to any subfield, and second, our tracing of subfields did not extend all the way to the hippocampal tail, where the delineation of structures becomes increasingly difficult. Moreover, CA1 and subiculum are also numerically smaller—though not significantly—in PTSD, adding to the overall difference between the volume effects on the CA3/DG and whole hippocampus related to PTSD. Nevertheless, our data imply that the CA3/DG is more affected in PTSD than the other subfields and, therefore, their assessment is likely more specific for PTSD than assessments of whole hippocampus.
While our subfield findings are promising, several limitations should be noted. First, the gene type of apolipoprotein E (APOE), which plays a fundamental role in the maintenance and repair of neurons,60 has been associated with smaller CA3/DG volumes in normal aging and in patients with Alzheimer disease.29 We do not have information about the participants’ APOE genotypes and therefore cannot account for variations in APOE profiles. It is possible, then, that variations in the APOE profile or an interaction between APOE and PTSD, and not PTSD per se, are responsible for the volume variations of CA3/DG. Second, we have no reliable information about the duration of PTSD. It is possible that the extent to which PTSD is associated with reduced CA3/DG volumes may be modulated by the duration of the disease. Furthermore, a few patients with PTSD were taking antidepressant medication but we do not believe that the difference in medication exposure between the patients with PTSD and controls explains the decreased hippocampal volume in PTSD. If anything, more use of antidepressants might introduce a negative bias to find less volume in PTSD given that there is evidence that antidepressant treatment increases the size of the hippocampus.61 Third, we could not separate the effect of trauma exposure because most of our subjects were trauma exposed. To separate the effects of PTSD from those of trauma exposure, 3 groups are needed, including trauma-exposed controls and subjects with PTSD and non–trauma-exposed controls. Finally, the number of subjects in this study is relatively small, limiting generalization. The findings need to be replicated in a new cohort of subjects.
In conclusion, the results of this study indicate for the first time that PTSD is associated with a selective reduction of the CA3/DG subfield in the human hippocampus, irrespective of age-related effects. Future studies are required to clarify the potential role of APOE status and PTSD duration on the CA3/DG volume reduction in PTSD, and longitudinal studies should help determining causality.
Acknowledgments
Funding/Support: This study was supported in part by grants from the Mental Illness Research and Education Clinical Center of the US Veterans Health Administration, Office of Research and Development, and the Department of Defense and the National Center for Research Resource of National Institutes of Health grant RR23953.
Role of the Sponsor: Resources and the use of facilities were provided by the Veterans Administration Medical Center, San Francisco, California.
Footnotes
Financial Disclosure: None reported.
References
- 1.Pine DS. Anxiety disorders: clinical features. In: Sadock BJ, Sadock VA, editors. Kaplan and Sadock’s Comprehensive Textbook of Psychiatry. Philadelphia, PA: Lippincott Williams & Wilkins; 2000. pp. 1476–1490. [Google Scholar]
- 2.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52 (12):1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 3.Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57(10):925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- 4.Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, Delaney RC, McCarthy G, Charney DS, Innis RB. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152(7):973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gurvits TV, Shenton ME, Hokama H, Ohta H, Lasko NB, Gilbertson MW, Orr SP, Kikinis R, Jolesz FA, McCarley RW, Pitman RK. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol Psychiatry. 1996;40(11):1091–1099. doi: 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5(11):1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hedges DW, Allen S, Tate DF, Thatcher GW, Miller MJ, Rice SA, Cleavinger HB, Sood S, Bigler ED. Reduced hippocampal volume in alcohol and substance naive Vietnam combat veterans with posttraumatic stress disorder. Cogn Behav Neurol. 2003;16(4):219–224. doi: 10.1097/00146965-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, Capelli S, McCarthy G, Innis RB, Charney DS. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse: a preliminary report. Biol Psychiatry. 1997;41(1):23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woodward SH, Kaloupek DG, Streeter CC, Kimble MO, Reiss AL, Eliez S, Wald LL, Renshaw PF, Frederick BB, Lane B, Sheikh JI, Stegman WK, Kutter CJ, Stewart LP, Prestel RS, Arsenault NJ. Hippocampal volume, PTSD, and alcoholism in combat veterans. Am J Psychiatry. 2006;163(4):674–681. doi: 10.1176/ajp.2006.163.4.674. [DOI] [PubMed] [Google Scholar]
- 10.Bonne O, Brandes D, Gilboa A, Gomori JM, Shenton ME, Pitman RK, Shalev AY. Longitudinal MRI study of hippocampal volume in trauma survivors with PTSD. Am J Psychiatry. 2001;158(8):1248–1251. doi: 10.1176/appi.ajp.158.8.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, Frustaci K, Ryan ND. AE Bennett research award: developmental traumatology part II: brain development. Biol Psychiatry. 1999;45(10):1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- 12.Fennema-Notestine C, Stein MB, Kennedy CM, Archibald SL, Jernigan TL. Brain morphometry in female victims of intimate partner violence with and without posttraumatic stress disorder. Biol Psychiatry. 2002;52(11):1089–1101. doi: 10.1016/s0006-3223(02)01413-0. [DOI] [PubMed] [Google Scholar]
- 13.Golier JA, Yehuda R, De Santi S, Segal S, Dolan S, de Leon MJ. Absence of hippocampal volume differences in survivors of the Nazi Holocaust with and without posttraumatic stress disorder. Psychiatry Res. 2005;139(1):53–64. doi: 10.1016/j.pscychresns.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Schuff N, Neylan TC, Lenoci MA, Du AT, Weiss DS, Marmar CR, Weiner MW. Decreased hippocampal N-acetylaspartate in the absence of atrophy in posttraumatic stress disorder. Biol Psychiatry. 2001;50(12):952–959. doi: 10.1016/s0006-3223(01)01245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yehuda R, Golier JA, Tischler L, Harvey PD, Newmark R, Yang RK, Buchsbaum MS. Hippocampal volume in aging combat veterans with and without posttraumatic stress disorder: relation to risk and resilience factors. J Psychiatr Res. 2007;41(5):435–445. doi: 10.1016/j.jpsychires.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 16.De Bellis MD, Hall J, Boring AM, Frustaci K, Moritz G. A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol Psychiatry. 2001;50(4):305–309. doi: 10.1016/s0006-3223(01)01105-2. [DOI] [PubMed] [Google Scholar]
- 17.Vythilingam M, Luckenbaugh DA, Lam T, Morgan CA, III, Lipschitz D, Charney DS, Bremner JD, Southwick SM. Smaller head of the hippocampus in Gulf War-related posttraumatic stress disorder. Psychiatry Res. 2005;139(2):89–99. doi: 10.1016/j.pscychresns.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Bonne O, Vythilingam M, Inagaki M, Wood S, Neumeister A, Nugent AC, Snow J, Luckenbaugh DA, Bain EE, Drevets WC, Charney DS. Reduced posterior hippocampal volume in posttraumatic stress disorder. J Clin Psychiatry. 2008;69(7):1087–1091. doi: 10.4088/jcp.v69n0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duvernoy HM, Cattin F, Naidich TP, et al. The Human Hippocampus: Functional Anatomy, Vascularization and Serial Sections with MRI. 3. New York, NY: Springer-Verlag; 2005. [Google Scholar]
- 20.Magariños AM, McEwen BS, Flugge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996;16(10):3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci U S A. 1998;95(6):3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conrad CD, LeDoux JE, Magarinos AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113(5):902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- 23.McKittrick CR, Magarinos AM, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Chronic social stress reduces dendritic arbors in CA3 of hippocampus and decreases binding to serotonin transporter sites. Synapse. 2000;36(2):85–94. doi: 10.1002/(SICI)1098-2396(200005)36:2<85::AID-SYN1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 24.Pham K, Nacher J, Hof PR, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci. 2003;17(4):879–886. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- 25.Stewart MG, Davies HA, Sandi C, Kraev IV, Rogachevsky VV, Peddie CJ, Rodriguez JJ, Cordero MI, Donohue HS, Gabbott PL, Popov VI. Stress suppresses and learning induces plasticity in CA3 of rat hippocampus: a three-dimensional ultrastructural study of thorny excrescences and their postsynaptic densities. Neuroscience. 2005;131(1):43–54. doi: 10.1016/j.neuroscience.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 26.Shiryaeva NV, Vshivtseva VV, Mal’tsev NA, Sukhorukov VN, Vaido AI. Neuron density in the hippocampus in rat strains with contrasting nervous system excitability after prolonged emotional-pain stress. Neurosci Behav Physiol. 2008;38(4):355–357. doi: 10.1007/s11055-008-0049-4. [DOI] [PubMed] [Google Scholar]
- 27.Duan X, Kang E, Liu CY, Ming GL, Song H. Development of neural stem cell in the adult brain. Curr Opin Neurobiol. 2008;18(1):108–115. doi: 10.1016/j.conb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McEwen BS. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583(2–3):174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mueller SG, Schuff N, Raptentsetsang S, Elman J, Weiner MW. Selective effect of Apo e4 on CA3 and dentate in normal aging and Alzheimer’s disease using high resolution MRI at 4 T. Neuroimage. 2008;42(1):42–48. doi: 10.1016/j.neuroimage.2008.04.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mueller SG, Stables L, Du AT, Schuff N, Truran D, Cashdollar N, Weiner MW. Measurement of hippocampal subfields and age-related changes with high resolution MRI at 4T. Neurobiol Aging. 2007;28(5):719–726. doi: 10.1016/j.neurobiolaging.2006.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sunanda, Rao MS, Raju TR. Effect of chronic restraint stress on dendritic spines and excrescences of hippocampal CA3 pyramidal neurons: a quantitative study. Brain Res. 1995;694(1–2):312–317. doi: 10.1016/0006-8993(95)00822-8. [DOI] [PubMed] [Google Scholar]
- 32.Sandi C, Davies HA, Cordero MI, Rodriguez JJ, Popov VI, Stewart MG. Rapid reversal of stress induced loss of synapses in CA3 of rat hippocampus following water maze training. Eur J Neurosci. 2003;17(11):2447–2456. doi: 10.1046/j.1460-9568.2003.02675.x. [DOI] [PubMed] [Google Scholar]
- 33.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for Axis I DSM-IV Disorders. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1994. [Google Scholar]
- 34.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a clinician-administered PTSD scale. J Trauma Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 35.Wolfe JW, Kimerling R, Brown PJ, Chrestman KR, Levin K. Psychometric review of The Life Stressor Checklist-Revised. In: Stamm BH, editor. Measurement of Stress, Trauma, and Adaptation. Lutherville, MD: Sidran Press; 1996. pp. 198–201. [Google Scholar]
- 36.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 37.Khan AR, Wang L, Beg MF. FreeSurfer-initiated fully-automated subcortical brain segmentation in MRI using large deformation diffeomorphic metric mapping. Neuroimage. 2008;41(3):735–746. doi: 10.1016/j.neuroimage.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85(2):523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- 39.Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435(4):406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- 40.Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10(3):355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- 41.Li S, Wang C, Wang W, Dong H, Hou P, Tang Y. Chronic mild stress impairs cognition in mice: from brain homeostasis to behavior. Life Sci. 2008;82(17–18):934–942. doi: 10.1016/j.lfs.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 42.Palmer TD, Takahashi J, Gage FH. The adult rat hippocampus contains primordial neural stem cells. Mol Cell Neurosci. 1997;8(6):389–404. doi: 10.1006/mcne.1996.0595. [DOI] [PubMed] [Google Scholar]
- 43.De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19(3):269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 44.McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 45.McEwen BS, Gould EA, Sakai RR. The vulnerability of the hippocampus to protective and destructive effects of glucocorticoids in relation to stress. Br J Psychiatry Suppl. 1992;(15):18–23. [PubMed] [Google Scholar]
- 46.Sapolsky RM. Stress, glucocorticoids, and damage to the nervous system: the current state of confusion. Stress. 1996;1(1):1–19. doi: 10.3109/10253899609001092. [DOI] [PubMed] [Google Scholar]
- 47.Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69(1):89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- 48.Sloviter RS, Valiquette G, Abrams GM, Ronk EC, Sollas AL, Paul LA, Neubort S. Selective loss of hippocampal granule cells in the mature rat brain after adrenalectomy. Science. 1989;243(4890):535–538. doi: 10.1126/science.2911756. [DOI] [PubMed] [Google Scholar]
- 49.Neylan TC, Schuff N, Lenoci M, Yehuda R, Weiner MW, Marmar CR. Cortisol levels are positively correlated with hippocampal N-acetylaspartate. Biol Psychiatry. 2003;54(10):1118–1121. doi: 10.1016/S0006-3223(03)01974-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller AH. Inflammation versus glucocorticoids as purveyors of pathology during stress: have we reached the tipping point? Biol Psychiatry. 2008;64(4):263–265. doi: 10.1016/j.biopsych.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takemura NU, Kato N. Adult neurogenesis and systemic adaptation: animal experiments and clinical perspectives for PTSD. Prog Brain Res. 2008;167:99–109. doi: 10.1016/S0079-6123(07)67007-1. [DOI] [PubMed] [Google Scholar]
- 52.Pavlides C, Watanabe Y, McEwen BS. Effects of glucocorticoids on hippocampal long-term potentiation. Hippocampus. 1993;3(2):183–192. doi: 10.1002/hipo.450030210. [DOI] [PubMed] [Google Scholar]
- 53.Pavlides C, Nivon LG, McEwen BS. Effects of chronic stress on hippocampal long-term potentiation. Hippocampus. 2002;12(2):245–257. doi: 10.1002/hipo.1116. [DOI] [PubMed] [Google Scholar]
- 54.Tata DA, Marciano VA, Anderson BJ. Synapse loss from chronically elevated glucocorticoids: relationship to neuropil volume and cell number in hippocampal area CA3. J Comp Neurol. 2006;498(3):363–374. doi: 10.1002/cne.21071. [DOI] [PubMed] [Google Scholar]
- 55.Mizoguchi K, Kunishita T, Chui DH, Tabira T. Stress induces neuronal death in the hippocampus of castrated rats. Neurosci Lett. 1992;138(1):157–160. doi: 10.1016/0304-3940(92)90495-s. [DOI] [PubMed] [Google Scholar]
- 56.Woodward SH, Kaloupek DG, Streeter CC, Kimble MO, Reiss AL, Eliez S, Wald LL, Renshaw PF, Frederick BB, Lane B, Sheikh JI, Stegman WK, Kutter CJ, Stewart LP, Prestel RS, Arsenault NJ. Brain, skull, and cerebrospinal fluid volumes in adult posttraumatic stress disorder. J Trauma Stress. 2007;20(5):763–774. doi: 10.1002/jts.20241. [DOI] [PubMed] [Google Scholar]
- 57.Kitayama N, Vaccarino V, Kutner M, Weiss P, Bremner JD. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. J Affect Disord. 2005;88(1):79–86. doi: 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 58.Du AT, Schuff N, Chao LL, Kornak J, Jagust WJ, Kramer JH, Reed BR, Miller BL, Norman D, Chui HC, Weiner MW. Age effects on atrophy rates of entorhinal cortex and hippocampus. Neurobiol Aging. 2006;27(5):733–740. doi: 10.1016/j.neurobiolaging.2005.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morra JH, Tu Z, Apostolova LG, et al. Automated mapping of hippocampal atrophy in 1-year repeat MRI data from 490 subjects with Alzheimer’s disease, mild cognitive impairment, and elderly controls. Neuroimage. 2009;45(1 suppl):S3–15. doi: 10.1016/j.neuroimage.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc Natl Acad Sci U S A. 2006;103(15):5644–5651. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry. 2003;54(7):693–702. doi: 10.1016/s0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]