Abstract
We examined respiratory sinus arrhythmia (RSA), emotion regulation (ER), and prospective depressive symptoms in children at risk for depression and controls. Of the 65 children (35 boys; 5 – 13 years) in the sample, 39 had a parent with childhood-onset mood disorder and 26 had a parent with no history of major psychiatric disorder. RSA during pre- and post-film baselines and RSA reactivity to sad film clip were measured. Later, children’s ER responses (focusing on sad/distressing affect) were assessed using a parent-reported questionnaire, and depressive symptoms were measured via clinical ratings. Results indicated that, compared to the initial baseline, a greater decrease in RSA (i.e. more vagal withdrawal) in response to the sad film clip predicted more adaptive ER responses and lower levels of clinician-rated depressive symptoms. However, tests for ER as a mediator of the association between RSA reactivity and depressive symptoms were precluded because maladaptive, but not adaptive, ER was associated with depressive symptoms. Overall, results suggest that cardiac vagal withdrawal (a greater decrease in RSA) in response to an emotional stimulus reflects more adaptive parasympathetic activity, which could facilitate children’s ability to effectively manage their sadness and distress and predict lower risk of depressive symptoms over time.
Keywords: RSA, Vagal tone, Emotion Regulation, Depression, Children
Gaining a better understanding of factors that contribute to depression in youth is critical given the disorder’s debilitating nature and possible consequences (e.g., suicide attempts; Tamas et al., 2007). The primary symptoms of childhood depression involve protracted mood such as dysphoria and anhedonia, which implicates an inability to effectively regulate such negative emotions. Children’s inability to regulate emotion, as well as their risk for depression, might stem in part from physiological vulnerabilities. In this paper, we examine one aspect of children’s psychophysiology, respiratory sinus arrhythmia (RSA) or cardiac vagal tone, and test whether it is predictive of their emotion regulation (ER) responses and level of depressive symptoms. Our sample includes a group of children who are at elevated risk for developing depression, by virtue of having a parent with a childhood-onset mood disorder (COMD). These children were contrasted with a control group of children whose parents were free of major psychiatric disorders. We expected that children’s resting RSA and RSA reactivity to an emotional task will predict their ER as well as level of depressive symptoms, and further, that ER will mediate the presumed relationship between RSA and depression. As reviewed below, although RSA and ER links are prevalent in the literature, little work has examined how RSA is associated with depressive symptoms in youth, particularly at-risk children, and no studies have examined ER as an explanatory mechanism.
ER and Depression
Researchers have theorized that depression results from problems with regulating one’s emotions (e.g., Cole, Michel, & Teti, 1994; Keenan & Hipwell, 2005), though limited data have addressed the association between emotion regulation and depression in childhood or adolescence. However, some findings indicate that offspring of a parent with depression, particularly girls, exhibit less adaptive ER than control offspring (Garber, Braafladt, & Zeman, 1991; Silk, Shaw, Skuban, Oland, & Kovacs, 2006). Other work has found that children relying on certain strategies (e.g., less cognitive restructuring, more rumination) to cope with having a depressed parent are more likely to have higher anxiety/depression symptoms (Langrock, Compas, Keller, Merchant, & Copeland, 2002). Similarly, studies with normative samples of youth have shown that maladaptive ER strategies, such as rumination, predict higher levels of depressive symptoms (e.g., Garber, Braafladt, & Weiss, 1995; Schwartz & Koenig, 1996; Silk, Steinberg, & Morris, 2003). Overall, this literature suggests that certain ER strategies (e.g., those reflecting an inability to shift attention from sad feelings) could increase children’s risk for depression, especially for certain subgroups (i.e., those with a depressed parent, girls).
RSA
One physiological measure of particular relevance to ER is RSA. RSA reflects the variation in interbeat intervals of the heart at the frequency of breathing, which is influenced by the parasympathetic branch of the autonomic nervous system, operating through one branch of the vagus nerve. The amplitude of RSA is one way to quantify vagal tone (Porges, Doussard-Roosevelt, & Maiti, 1994). Researchers typically study RSA while individuals are in a resting state or baseline. Also important however is reactivity in response to stressors or other emotional stimuli. A decrease in RSA from an initial baseline is indicative of vagal withdrawal in that the parasympathetic system is withdrawn once a stressor or other stimulus requires attention. RSA reactivity is often computed as task mean minus baseline RSA or residualized scores computed by regressing task mean onto baseline mean (e.g., Alkon, et al., 2003). The term, recovery, is used to describe an individual’s response after the stressor, and has been operationalized by measuring baseline RSA following the task to determine whether individuals return to their initial baseline levels (e.g., Santucci, Silk, Shaw, Gentzler, Fox, & Kovacs, 2008).
According to Porges’ polyvagal theory (e.g., Porges, 1995; Porges, Doussard-Roosevelt, Portales, & Greenspan, 1996), the vagal system plays a key role in preserving physiological homeostasis, where the organism can preserve resources at rest, but still respond to external demands when needed. At rest, parasympathetic activity results in a relatively high vagal tone or RSA, which is associated with slower heart rate (Porges, et al.). Higher RSA would be adaptive in that it reflects a greater capacity for self-regulation or social engagement. When individuals must respond to a challenge, parasympathetic activity or the “vagal brake” is withdrawn, thereby allowing heart rate to increase and the individual to meet environmental demands (Porges, et al., p. 697). Once a stressor has subsided, individuals should show vagal recovery in that they return to baseline levels. Thus, greater withdrawal during stressors and subsequent recovery should be indicative of a more flexible physiological response system, which could facilitate individuals’ ability to respond more adaptively to emotional challenges.
Links to ER
A growing body of research suggests that individual differences in children’s vagal tone or RSA are associated with their regulatory behavior. Higher baseline RSA and a greater decrease in RSA have been found to relate to more positive and less negative affect, less emotion dysregulation, and more effective ER strategies (e.g., Blandon, Calkins, Keane, & O’Brien, 2008; Calkins, 1997; Calkins & Keane, 2004; Fabes, Eisenberg, Karbon, Troyer, & Switzer, 1994; Gottman & Katz, 2002; Hessler & Katz, 2007). Our own research showed that during a waiting task (delay of gratification), children exhibiting less RSA recovery engaged in more maladaptive ER (i.e., negative focus on delay) during a different delay task (Santucci et al., 2008). Associations may be more complex as well, as recent research suggests that 4-year old children with higher baseline RSA whose mother reported clinical levels of depressive symptoms did not show normative increases in emotion regulation by age 7 years that other children did (Blandon, et al., 2008). Thus more work is needed to determine how RSA may predict how ER unfolds over time or may interact with maternal depression. But the overall pattern of findings support the premise that children’s RSA, reactivity, and recovery may be key indicators of their physiological capacity to effectively regulate emotions.
Links to depression
With adults, evidence on how RSA relates to depression is mixed. In a recent meta analysis, Rottenberg (2007) found that depression had a small to medium effect on resting RSA and also identified several factors that are likely to lead to distorted estimates of depression effects (e.g., diminished physical activity, comorbid disorders, medication status, particular symptom presentations). The relation to RSA reactivity is equivocal as well (Rottenberg, 2007). In a study of adults with major depressive disorder (MDD), Rottenberg, Salomon, Gross, and Gotlib (2005) found that that individuals who fully recovered from an episode exhibited a greater decrease in RSA to a sad film clip 6 months earlier than those whose depression did not remit. But other work has found that a higher level of depressive symptoms relates to increased or decreased RSA reactivity depending on the task (Hughes & Stoney, 2000). Also, depressed adults do not show recovery in RSA following a stressor that nondepressed adults exhibit (Rottenberg, Wilhelm, Gross, & Gotlib, 2003).
The only studies addressing links between RSA and depression in children are those examining symptoms using parent- or self-report measures, and the results are somewhat contradictory. With children from the current project, Forbes, Fox, Cohn, Galles, and Kovacs (2006) found that lower baseline RSA was linked to internalizing symptoms for children at-risk for depression but not for controls. Similarly, Shannon, Beauchaine, Brenner, Neuhaus, and Gatzke-Kopp (2007) found that children with resting RSA below the median reported high levels of depressive symptoms regardless of maternal melancholic depressive symptoms, but for those with resting RSA above the median, children had higher symptoms only when mothers had more melancholic symptoms. Studies also have shown that greater decreases in RSA in response to a stressor are a protective factor against children developing internalizing or externalizing symptoms (El-Sheikh, Harger, & Whitson, 2001; El-Sheikh & Whitson, 2006; Katz & Gottman, 1997). Yet others have found that higher levels of internalizing symptoms (Boyce, Quas, Alkon, Smider, Essex, & Kupfer, 2001) or a combination of both internalizing and externalizing symptoms (Calkins, Graziano, & Keane, 2006) are associated with greater vagal withdrawal to emotional tasks. Also, Ashman, Dawson and Panagiotides (2008) found that offspring of chronically depressed mothers had greater decreases in RSA to a sad film clip than offspring of mothers who had either no depression, stable but mild, or decreasing levels of depression. Thus, as with adults, a better understanding of the associations between children’s RSA, especially RSA reactivity, and depressive symptoms is needed. Our study can contribute to the extant literature by testing whether RSA or change in RSA predicts ER and clinician-rated depressive symptoms, and if these associations vary for at-risk and control children.
The Present Study
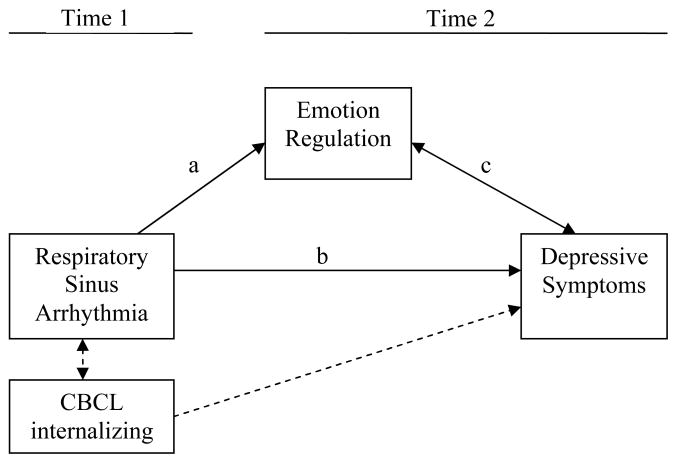
The goal of the present study is to investigate whether children’s RSA predicts their subsequent ER and level of clinical depressive symptoms (see Figure 1). We expect that higher baseline RSA, a larger decrease in RSA (greater vagal withdrawal) during a film clip, or greater recovery (i.e., higher baseline after the task) will predict a greater reliance on adaptive ER strategies and less on maladaptive ER strategies (path a), and prospectively, having fewer depressive symptoms (path b). Furthermore, if we also find that lower scores on adaptive ER or higher scores on maladaptive ER relate to more depressive symptoms (path c), we can test whether ER responses mediate the relationship between RSA and symptom level. We also examine concurrent associations between children’s RSA and internalizing symptom level. This way, when testing RSA’s association to later depressive symptoms (path b), we can control for concurrent symptoms so that we are predicting change in depressive symptoms across time.
Figure 1.
Conceptual model to be examined in sequential steps.
In examining associations among RSA, ER and depressive symptoms, we included group status (at-risk vs. control) and child sex as potential moderating variables. The rationale for these analyses is that different patterns in RSA may be more predictive of depressive symptoms for offspring of a depressed parent (Ashman et al., 2008; Forbes et al., 2006), and some findings that suggest RSA relates differently to emotions or regulation for boys and girls (e.g., Eisenberg, Fabes, Murphy, Maszk, Smith, & Karbon, 1995).
Method
Sample
Sample characteristics
The sample includes 65 children (35 boys, 30 girls) who participated in two studies (psychophysiology study and psychiatric core) within a larger, multidisciplinary program project focusing on risk factors for early onset mood disorders. For the present paper, we selected participants who completed the psychophysiology study between the ages of 6 and 13 years of age (because these children were all given the same protocol using film clips), and had a psychiatric assessment after their psychophysiology visit with a maximum of 2 years between the appointments. The actual interval between children’s 2 appointments ranged from 35 to 658 days (M = 437.5 days). Children’s age ranged from 5.80 to 12.90 (M = 7.93, SD = 1.80) at their psychophysiology visit, and from 6.75 to 14.29 (M = 9.13; SD = 1.91) at their psychiatric visit. The racial breakdown of the sample’s children was 56.9% Caucasian, 21.5% Black, 16.9% Biracial, 1.5% Asian, and 3.1% other. The sample included 26 children of control parents and 39 of an affected parent with a childhood-onset mood disorder (COMD; 27 with unipolar and 12 bipolar). A portion of the participants were siblings (i.e., 5 sibling pairs, and 4 sibling triads). All sample characteristics described above did not vary by whether the child was from the control group or at-risk.
Of the 71 participants who completed one of these particular psychophysiology study appointments (described below) and had a follow-up psychiatric visit, 2 were excluded because of lack of usable ECG data. We also excluded 4 children who were taking a stimulant medication at the time of the psychophysiology appointment.
Sample recruitment and case ascertainment
The children in the sample are offspring of adult program project participants who either were diagnosed with a COMD or were control subjects. COMD was operationally defined as depression (MDD and/or dysthymic disorder) which onset by age 14.99, or bipolar spectrum (bipolar I or II or cyclothymic disorder) by age 17.99. Also, to be included in the study, participants had to be free of preexisting major systemic medical disorders, and without evidence of mental retardation. Trained professional-level clinical evaluators from the Psychiatric Evaluation Core of the program project conducted the interviews. Clinicians’ assessments were then evaluated by independent psychiatrists to verify case status. Diagnoses were based on rules specified in the DSM (DSM-III, DSM-IV; American Psychiatric Association, 1980, 1994).
Adult COMD participants (probands) were recruited for the program project in multiple ways: accessing individuals who had participated in clinical research when they were children, through adult mental health clinics, or by advertising in the general community. A portion of the participants had been enrolled in a longitudinal, naturalistic follow-up study of childhood-onset depression where children were followed up to 20 years (Kovacs, Feinberg, Crouse-Novak, Paulauskas, & Finkelstein, 1984; Kovacs, Obrosky, Gatsonis, & Richards, 1997). During childhood and adolescence, their diagnoses were based on the Interview Schedule for Children and Adolescents or its Young Adult version administered separately to the child and one parent (Sherrill & Kovacs, 2000). During adulthood, participants were later given the Structured Clinical Interview for DSM-IV Axis I disorders (SCID, First, Spitzer, Gibbon, & Williams, 1995), which was modified to include childhood-onset and Axis II disorders. A second informant (e.g., parent or partner) also was interviewed. Participants recruited as adults from other research studies, clinics or from the community also were given the SCID, with second informants and pediatric medical records used to document emotional or mood problems in childhood.
At intake, control participants had to be free of any lifetime major psychiatric disorder. Controls were recruited in similar ways: from other studies during childhood or adolescence serving as ‘normal controls’, by using the Cole directory to target neighborhoods matching probands’ socioeconomic status, or through community advertisements (e.g., at Women, Infants, and Children centers).
Procedure
As part of their program project participation, children were involved in a psychophysiology study and also were separately evaluated in a psychiatric assessment core. Children’s electrocardiogram (ECG) data represent a portion of the psychophysiology protocol, which varied slightly by age so that tasks were developmentally appropriate. In the current sample, children either completed the protocol for 6 – 9 year olds (n = 54), or the protocol for 10 –13 year olds (n = 11). All protocols began with a 3-minute resting baseline (BL1), followed by a series of five film clips, and then a 1-minute post-task baseline (BL2). All baselines included 30-second segments where children were asked to keep their eyes closed or alternatively their eyes open and focused on a toy spaceship being held by an experimenter in the room; thus the pre-task baseline included 6 segments whereas the post-task baseline included 2 segments.
The film clips were chosen because of their affective content and were administered in a standardized order: happy, sad, anger-1, fear, anger-2. Due to the nature of our sample with a portion at high-risk for depression and the implications of being unable to regulate sad affect specifically, we chose to focus exclusively on the sad film. The sad clip was an excerpt from the Lion King, in which a young lion wanders off and almost gets run over in a stampede. Children who completed the 10–13 year-old protocol also rated how they felt while watching each film. Results from the larger sample of 10–13 year olds (n = 31) showed that the sad film clip was successful in eliciting the desired emotion in that 88% of children reported that they felt sad, and more children reported feeling sad than any other emotion rated (33% also reported feeling happy, 33% reported anger, 15% reported fear, and 18% reported disgust during the sad film).
Also during the physiology study, children 7 years and older and parents completed questionnaires. As an index of children’s current level of symptoms at the time of the ECG data ascertainment, we examine the Child Behavior Checklist (CBCL) internalizing scores.
Information on children’s ER and depressive symptoms was obtained during a subsequent psychiatric evaluation that involved a semi-structured diagnostic interview with the parent and the child about the child, and the completion of self-rated questionnaires. Relevant ER and symptom measures are described below.
Measures
Emotion regulation
Feelings and My Child is the parent-rated version of the Feelings and Me questionnaire (Kovacs, 2000; Kovacs & George, 2008). Parents rate different emotion regulation strategies that follow the stem, “when my child feels sad or upset, he/she…” using a 3-point scale (ranging from 0 = “not true of my child” to 2 = “many times true of my child”). Two scores were computed: adaptive ER (30 items) and maladaptive ER (24 items), with possible ranges being from 0–60, and 0–48, respectively. For this sample, parent-rated scores for adaptive ER range from 5 – 48 (M = 20.52, SD = 8.60), and for maladaptive ER range from 0 – 27 (M = 6.98, SD = 6.08). The adaptive scale includes ER responses that are likely to downregulate dysphoric feelings (e.g., thinks about feeling better; listens to fun music; talks to family), whereas the maladaptive scale comprises responses that are likely to upregulate dysphoric feelings (e.g., thinks about things being his/her fault; listens to sad music; yells and screams at family). In the larger sample of children (N = 95 at-risk, and N = 66 control), Cronbach’s alphas for adaptive ER were α = .91 for both at-risk and control children, and for maladaptive ER, were α = .86 for at-risk and α = .87 for control children. In this sample, COMD parents rated children lower on adaptive ER and higher on maladaptive ER than control parents rated their children (see Table 1).
Table 1.
Tests of Group Differences (Control or At-risk) for ECG, Emotion Regulation (ER), and Symptom Variables
| Control (N = 25–26) | At-risk (N = 38–39) | ||
|---|---|---|---|
| M (SD) | M (SD) | t-statistic | |
| Child RSA | |||
| Pre-task baseline (BL1) | 7.07 (1.04) | 6.88 (1.16) | .65 |
| RSA reactivity (Sad film – BL1) | −.30 (.61) | −.09 (.71) | −1.02 |
| Post-task baseline (BL2) | 7.14 (.93) | 7.15 (1.28) | −.03 |
| Child ER | |||
| Adaptive ER | 24.00 (8.31) | 18.21 (8.08) | 2.80** |
| Maladaptive ER | 4.69 (5.14) | 8.51 (6.24) | −2.59* |
| Child symptom levels | |||
| Internalizing T-score (CBCL) | 46.27 (7.86) | 55.38 (10.30) | −3.83*** |
| Depressive symptom screen (K-SADS) | 3.52 (.77) | 3.90 (1.19) | −1.41 |
p < .05.
p < .01.
p < .001.
Depressive symptoms
From the psychiatric core, we derived the index of depressive symptoms from the “Kiddie” Schedule for Affective Disorders and Schizophrenia (K-SADS, Kaufman et al., 1997). We used symptom levels rather than presence of a depressive disorder because diagnosed disorders were rare events in this young sample (only one at-risk child had dysthymic disorder, and none had MDD or a bipolar disorder). The K-SADS is a widely used, semi-structured, diagnostic interview that assesses lifetime and current rates of psychopathology. The clinician separately interviewed the parent (about the child) and the child, and then assigned overall ratings to each symptom (where 1 = not at all; 2 = subthreshold; and 3 = threshold). We summed clinicians’ ratings of 3 affective symptoms from the depression screening section, namely: a) depressed mood, b) irritability or anger, and c) anhedonia. The possible range of scores is 3 – 9; the actual range was 3 – 8 (M = 3.75, SD = 1.05). Children from the at-risk and control groups did not differ on K-SADS depressive symptoms (see Table 1).
Internalizing Symptoms
To obtain a measure of symptoms that are concurrent with the RSA data, we used the Child Behavior Checklist (CBCL, Achenbach, 1991). The CBCL is a widely-used questionnaire where parents rate children on items assessing emotional or behavioral problems. We used the standardized t-score for internalizing symptoms (M = 51.74, SD = 10.36). As shown in Table 1, COMD parents rated their children higher on CBCL internalizing than control parents rated theirs.
ECG
Children’s ECG was recorded using electrodes placed on children’s rib cage at heart level. Data were recorded and processed using software/equipment from James Long Company (Caroga Lake, NY). A bandpass filter of .01 – 1000 Hz. was used and the data were amplified by a gain of 500. The signal was digitized at a sampling rate of 512 Hz (Bernston et al., 1997), and was re-sampled offline at 1000 Hz.
The processing of ECG data off-line involved a multi-pass algorithm to detect R-waves. Data were later manually checked for missed R-waves or peaks misidentified as R-waves. The R-wave times were converted to interbeat intervals (IBIs) and resampled into equal time intervals of 125 ms. Prorated IBI values were saved for an analysis of the mean and variance of heart period as well as further processing of RSA. RSA was computed using fast fourier transform analysis within the .15 – .50 Hz frequency band, which corresponds to a respiration rate of 9 to 30 breaths per minute. This range reflects high frequency variability due to respiration that is appropriate for children of this age (e.g., Boyce et al., 2001). Power scores were log-transformed to normalize the distribution. Thus, the unit for RSA is logarithmic [ms2], and reflects parasympathetic influence on high-frequency heart rate variability. ECG was processed for the initial 3-minute baseline (BL), the sad film, and the 1-minute post film clip baseline. The particular RSA scores used in analyses are described below.
We examined three RSA indices: 1) pre-task BL RSA (BL1), which is the initial 3-minute resting baseline; 2) post-task BL RSA (BL2), the 1-minute baseline following the films, which indexes children’s vagal recovery after the task; and 3) RSA reactivity, which indexes vagal response to the sad film clip. We computed RSA reactivity by subtracting the pre-task BL RSA from the RSA response during the sad film clip (M = 6.78, SD = 1.26, range of 3.84 – 9.45). Thus, RSA reactivity scores greater than zero indicate an increase as compared to BL1, whereas scores less than zero indicate a decrease (vagal or RSA withdrawal) compared to BL1.
BL1 RSA scores ranged from 4.17 – 9.32 (M = 6.96, SD = 1.11), and BL2 scores ranged from 4.27 – 9.41 (M = 7.14, SD = 1.15). A paired t-test indicated that BL2 RSA was significantly greater than BL1, t (61) = 2.92, p < .01. RSA reactivity scores ranged from –1.83 to 1.43 (M = −.17, SD = .67), and were not significantly correlated with BL1 RSA, r (64) = −.07, p = .56. Children from the control and at-risk groups did not differ significantly on RSA indices (see Table 1).
Results
Preliminary Analyses
Effects of age and sex
To examine effects of child age, correlations were run using age at the psychiatric assessment with ER scores and depression indices, and using age at the psychophysiology assessment with ECG-derived variables and CBCL internalizing. However, no variables were significantly related to child age so it is not considered in further analyses. Also, no child sex differences were found for ER, RSA, or symptom levels.
Bivariate associations among ECG, ER, and symptom variables
As shown in Table 2, adaptive and maladaptive ER scales were not significantly correlated. Adaptive ER was unrelated to symptom levels, but maladaptive ER was positively correlated with internalizing and depressive symptoms. Also, internalizing and depressive symptoms were correlated.
Table 2.
Correlations among RSA Indices, Emotion Regulation (ER), and Depressive Symptoms
| Child ER | Child Symptoms | |||
|---|---|---|---|---|
| Adaptive | Maladaptive | CBCL- Internalizing | K-SADS Depression | |
| Child ER (T2) | ||||
| Adaptive ER | −.14 | −.03 | −.23 | |
| Maladaptive ER | .56*** | .32* | ||
| Child symptom levels | ||||
| CBCL – Internalizing (T1) | .34** | |||
| K-SADS – Depression (T2) | ||||
| Child RSA (T1) | ||||
| Pre-task baseline (BL1) | .11 | .14 | .12 | .07 |
| Reactivity (Sad film – BL1) | −.40*** | .20 | .21 | .38** |
| Post-task baseline (BL2) | .03 | .21 | .23 | .28* |
Note. T1 = Time 1 (psychophysiology study). T2 = Time 2 (psychiatric study).
p < .05.
p < .01.
p < .001.
To examine whether our paths in Figure 1 should be fully tested in multivariate models, we computed bivariate associations between ECG, ER, and depressive symptoms, expecting that RSA would correlate with Time 2 ER and depressive symptoms. Pre-task BL RSA, however, was unrelated to concurrent internalizing and to Time 2 ER and depressive symptoms. But consistent with hypotheses, RSA reactivity was related negatively to adaptive ER, and positively to Time 2 depressive symptoms. In other words, a greater decrease in RSA during the film compared to BL1was linked to better ER and adjustment at time 2. Post-task BL RSA was positively correlated with Time 2 clinical depressive symptoms.
To test whether the time interval between the psychophysiology visit and psychiatric visit affected the associations between RSA and the ER and K-SADS symptoms, we ran partial correlations while controlling for the length of interval between visits. Comparing correlations in Table 2 to the partial correlations, we found all significant associations remained significant, except the post-task BL and K-SADS correlation dropped to a non-significant level, r (67) = .22, p = .08. Based on these analyses, we only examine RSA reactivity in multivariate models.
Multivariate Analyses
Because the inclusion of siblings results in non-independent data, we used linear mixed models for all multivariate analyses. Mixed models allow us to examine the fixed factors while including the random factor of family (e.g., Raudenbush & Bryk, 2002). In our reports below, we only report on the fixed effects of interest, but we have accounted for the dependence in the data structure (within family covariance) with the random effects term.
To examine our hypotheses (see Figure 1), we first tested whether RSA reactivity predicts ER (path a) and depressive symptoms (path b) at Time 2. We then tested whether ER predicted clinical depressive symptoms, by entering adaptive and maladaptive ER strategies as predictors while accounting for vagal reactivity (path c). If RSA reactivity is less strongly predictive of K-SADS depressive symptoms in the last model where ER is included compared to the first model, evidence of mediation is suggested (Baron & Kenny, 1986). All continuous predictors were centered (Aiken & West, 1991).
In all models, we also included as covariates: BL1 RSA, child group (at-risk or control), and child sex. When predicting K-SADS depressive symptoms at Time 2 from Time1 RSA reactivity, we also control for Time 1 symptom levels by including CBCL internalizing in the model. Although we tested whether associations among RSA reactivity, ER, and depressive symptoms were moderated by group status or child sex, no significant interaction effects were found. Thus, models reported below do not include interaction terms.
Path a: Vagal reactivity predicting ER
As shown in Table 3, RSA reactivity predicted parent-reported child adaptive ER responses in that a greater decrease in RSA during the film (i.e., greater vagal withdrawal) was related to higher levels of adaptive ER. However, RSA reactivity was unrelated to child maladaptive ER. Child group was a significant predictor of ER, with at-risk children scoring lower on adaptive and higher on maladaptive ER than control children.
Table 3.
Linear Mixed Models Predicting Child Emotion Regulation (ER) and Clinical Depressive Symptoms from RSA Reactivity
| Adaptive ER | Maladaptive ER | K-SADS Depressive Symptoms | |||||||
|---|---|---|---|---|---|---|---|---|---|
| F (1, 59) | Estimate (s.e.) | t | F (1, 59) | Estimate (s.e.) | t | F (1, 57) | Estimate (s.e.) | t | |
| Child sex | 1.25 | −2.21 (1.98) | −1.12 | .01 | −.11 (1.46) | −.08 | .24 | −.12 (.25) | −.49 |
| Child group | 6.77* | 5.25 (2.01) | 2.60* | 7.34** | −4.04 (1.49) | −2.71** | .01 | −.03 (.29) | −.10 |
| Pre-task baseline RSA (BL1) | .27 | .45 (.87) | .52 | 2.20 | .96 (.65) | 1.48 | .15 | .04 (.11) | .39 |
| RSA reactivity (Sad film – BL1) | 7.98** | −4.20 (1.49) | 2.83** | 1.87 | 1.50 (1.10) | 1.37 | 8.50** | .56 (.19) | 2.92** |
| CBCL internalizing | -- | -- | -- | -- | -- | -- | 4.09* | .03 (.01) | 2.02* |
p < .05.
p < .01.
Child sex: 0 = boys; 1 = girls. Group: 0 = control; 1 = at-risk.
BL = baseline. s.e. = standard error.
Path b: RSA reactivity predicting future depressive symptoms
As expected, RSA reactivity to the film was positively related to a higher level of clinician-rated depressive symptoms at Time 2 (see Table 3), and even when controlling for concurrent internalizing symptoms.
Path c: ER relating to depressive symptoms
Next, we examined whether ER is associated with K-SADS depressive symptoms (Table 4). We included adaptive and maladaptive ER along with RSA reactivity as predictors (Baron & Kenny, 1986). However, with RSA reactivity in the equation, adaptive and maladaptive ER responses were no longer related to depressive symptoms. Thus, a test of mediation is precluded.
Table 4.
Linear Mixed Model Predicting Children’s Depressive Symptoms (K-SADS scores) from Emotion Regulation (ER) and RSA Reactivity
| K-SADS Depressive Symptoms | |||
|---|---|---|---|
| F (1, 60) | Estimate (s.e.) | t | |
| Child sex | .44 | −.17 (.26) | −.66 |
| Child group | .14 | .12 (.31) | .37 |
| Adaptive ER | 1.02 | −.02 (.02) | −1.01 |
| Maladaptive ER | .77 | .02 (.02) | .87 |
| Pre-task baseline RSA (BL1) | .08 | .03 (.11) | .30 |
| CBCL internalizing | 2.49 | .03 (.02) | 1.58 |
| RSA reactivity (Sad film – BL1) | 5.10* | .46 (.21) | 2.56* |
p < .05.
Child sex: 0 = boys; 1 = girls. Group: 0 = control; 1 = at-risk. s.e. = standard error.
Discussion
Overall, the present study adds to the extant literature supporting vagal reactivity as a potential contributor to children’s emotional resilience and adjustment. Consistent with hypotheses, we found that RSA reactivity in response to a sad film clip predicted both children’s emotion regulation responses to dysphoria and their level of depressive symptoms over time. However, because RSA reactivity only related to adaptive ER, but only maladaptive ER was linked to depressive symptoms in bivariate analyses, ER thus did not serve as a mediating mechanism to explain vagal reactivity’s association with depressive symptoms. Also unexpected, baseline levels of RSA, either pre- or post-task, were unrelated to emotion regulation and depressive symptoms. Nevertheless, our study offers new evidence that RSA reactivity may be a particularly important predictor of the development of emotion regulation and depressive symptoms across time.
RSA or vagal reactivity is central to understanding how children react when sad or upset. As the parasympathetic system is involved in modulating emotional arousal and returning to homeostasis, it may aid one’s ability to avoid being overwhelmed with negative emotions and consequently rely on more constructive coping strategies. Thus, as it has been proposed, the withdrawal of vagal brake in response to emotional stimuli may be indicative of a more flexible neurological system (e.g., Porges, 1995). In our study, RSA reactivity related to the use of adaptive, but not maladaptive ER responses. It is unclear why the amount of RSA reactivity is more predictive of positive ER responses. RSA reactivity has been linked to ER operationalized in a variety of ways (e.g., general levels of emotion dysregulation as reported in a self-report interview, a rating dial reflecting valence of emotions during a peer provocation task; Hessler & Katz, 2007). Although some specificity in its associations have been reported (e.g., Hessler & Katz), more research is needed to determine if particular aspects of ER are more relevant to RSA reactivity than others.
In our study, RSA reactivity also was related to depressive symptoms as predicted in that children who had higher levels of clinician-rated depressive symptoms had earlier shown less of a decrease in RSA than those with fewer symptoms. Most studies on RSA and depression in adults have focused on baseline RSA rather than reactivity. Yet, some available data do similarly show that depression is related to a lack of RSA withdrawal when presented with emotional stimuli or stressors (Rottenberg, Clift, Bolden, & Salomon, 2007; Rottenberg et al., 2005). Our study is the first to suggest that children who show a greater decrease in RSA to a sad film clip may be at decreased risk to develop depressive symptoms.
These results were not moderated by group. Thus, given comparable levels of RSA reactivity, at-risk children were likely to have similar levels of symptoms as control children. Although all of our proband parents had a diagnosed COMD, they are hetereogenous in the type and course of their affective illnesses. In the future, it may be fruitful to examine parental mood disorder at a finer level (e.g., considering the number or the course of episodes, specific disorder or symptom presentation) given Shannon et al. (2007)’s finding for maternal melancholic (but not non-melancholic) depression interacting with children baseline vagal tone and Ashman et al. (2008)’s differentiation between offspring of mothers with chronic depression and those with mild but stable or decreasing depression when examining children’s vagal response to film clips.
Although we predicted and found support that a greater decrease in RSA in response to a sad film is related to fewer depressive symptoms, this finding is inconsistent with some earlier work showing that children higher in internalizing symptoms exhibit excessive vagal reactivity (Boyce et al., 2001; Calkins et al., 2006). One consideration is the particular outcome of interest. We predicted clinical levels of the affective depressive symptoms in our study, whereas Calkins et al. found high vagal reactivity for those high in both internalizing and externalizing and Boyce et al included over-anxious and separation anxiety symptoms in their internalizing group. In another study, parasuicidal female adolescents showed lower RSA at baseline and during a sad film clip compared to a control group (Crowell, Beauchaine, McCauley, Smith, Stevens, & Sylvers, 2005). Thus, possible comorbid symptoms or disorders may account for some discrepancies across studies. There also may be particular depressive symptoms that show distinct (and conflicting) relations to RSA (see Rottenberg, 2007). Other reasons underlying cross-study differences might be due to the nature of the sample, the baseline procedure, or the particular task. The sample as a whole, even with a portion of our sample being at elevated risk for depression, is still quite normative given the sample’s distribution on the CBCL and K-SADS. Regarding task effects, adults with higher levels of depressive symptoms were found to have greater withdrawal in RSA during a speech task but less withdrawal during a cold pressor task than those with fewer symptoms (Hughes & Stoney, 2000). More research is needed to get a clearer picture of under what circumstances and why RSA reactivity is associated with depression.
Our mediation hypothesis was not supported. This may be due to how we operationalized ER. Perhaps a more multifaceted index of ER (e.g., including also child-report and observational data) would explain more shared variance between RSA reactivity and depression. However, future work could examine other potential mechanisms that may underlie RSA reactivity’s relation with depressive symptoms in children. According to Polyvagal Theory (Porges, 1995, 2007; Porges et al., 1996) as well as supporting research, this vagal system is responsible for behaviors that affect one’s ability for social communication and for remaining calm or preventing excessive emotionality. Findings with children have shown that higher baseline RSA is correlated with a variety of factors that reflect good child adjustment, such as social skills or social engagement (Eisenberg et al., 1995; Fox & Field, 1989; Graziano, Keane, & Calkins, 2007). Studying other factors that may relate to both RSA reactivity and depression may help to elucidate our findings.
We did not find evidence that mean levels of RSA indices differed for at-risk and control children. Our analyses thus far with adults also show no differences comparing COMD versus control groups for women’s baseline RSA (Santucci, Galles, Cohn, & Fox, 2007) or for adults’ RSA reactivity in response to an anticipated speech (Bodas, Edgar-Perez, Cohn, Fox, & Kovacs, 2008). However, given that RSA shows moderate heritability (e.g., Dubreuil, et al., 2003; Sinnreich, Friedlander, Sapoznikkov, & Kark, 1998; Snieder, Boomsma, Van Doornen, & De Geus, 1997) and that depression arises from multiple causes, it is more than likely that only a subset of affected adults evidence maladaptive RSA and only a subset of their at-risk children inherits this type of physiological vulnerability.
We did find however, that children’s ER included higher rates of maladaptive strategies and lower rates of adaptive strategies than those of control children. This pattern is consistent with other findings on offspring of depressed parents (Garber et al., 1991; Silk et al., 2006). Also, as measured by parent ratings (but not by clinician ratings), at-risk children had higher levels of symptoms than did control children. The lack of group differences on the clinician-rated depression index may reflect the greater stringency of clinical ratings and the restricted number of K-SADS items available to form this index. It should be noted, however, that our sample was quite young (89% were less than 12 years old at their psychiatric appointment) and half had no clinical affective symptoms of depression. Both elevated depressive symptoms and clinically diagnosable depressive disorders are far more likely in to mid- and late-adolescence (e.g., Kessler, Avenevoli, Merikangas, 2001), particularly among girls (e.g., Angold & Rutter, 1992; Hankin, Abramson, Moffitt, Silva, McGee, & Angell, 1998). Although offspring of a depressed parent are at 3 times greater risk for developing depression or other disorders compared to controls (e.g., Weissman, Wickramaratne, Nomura, Warner, Pilowsky, & Verdeli, 2006), that risk is not yet evident in this young sample.
Our findings should be evaluated in light of several study limitations. First, the sample size was modest and may have limited our ability to detect small effects. Second, as mentioned our sample was positively skewed for child age, which likely contributed to the low levels of symptoms and perhaps to the lack of sex differences in RSA or reactivity given that sex differences have been found for adolescents but not children (Allen & Matthews, 1997). Third, RSA has been shown to relate to many disorders and problem behaviors (see Beauchaine, 2001, for a review); although we have a particular interest in mood disorders, RSA indices could be examined within a wider net of psychopathology or disorders that may be comorbid with depression. Fourth, if we had psychiatric assessments at later follow-up periods on these youth, we may have been able to predict the development of depressive disorders rather than symptom levels. Despite these limitations, our study contributes to existing knowledge on RSA reactivity and ER and depression in children by testing a comprehensive model predicting emotion regulation and depressive symptoms across time, and by examining both offspring of depressed parents and controls.
Acknowledgments
This study was supported by the NIMH Program Project Grant #MH56193, HHSA, Washington, DC, USA. Authors would like to thank the families who participated in this project and Jon Rottenberg for his helpful comments on this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Amy L. Gentzler, West Virginia University
Aimee K. Santucci, University of Pittsburgh
Maria Kovacs, University of Pittsburgh.
Nathan A. Fox, University of Maryland, College Park
References
- Achenbach TM. Manual for the child behavior checklist/4–18 and 1991 profile. Burlington: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. Thousand Oaks, CA: Sage Publications; 1991. [Google Scholar]
- Alkon A, Goldstein LH, Smider N, Essex MJ, Kupfer DJ, Boyce WT. Developmental and contextual influences on autonomic reactivity in young children. Developmental Psychobiology. 2003;42:64–78. doi: 10.1002/dev.10082. [DOI] [PubMed] [Google Scholar]
- Allen MT, Matthews KA. Hemodynamic responses to laboratory stressors in children and adolescents: The influences of age, race, and gender. Psychophysiology. 1997;34:329–339. doi: 10.1111/j.1469-8986.1997.tb02403.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. Washington, DC: Author; 1980. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Ashman SB, Dawson G, Panagiotides H. Trajectories of maternal depression over 7 years: Relations with child psychophysiology and behavior and role of contextual risks. Development and Psychopathology. 2008;20:55–77. doi: 10.1017/S0954579408000035. [DOI] [PubMed] [Google Scholar]
- Angold A, Rutter M. Effects of age and pubertal status on depression status in a large clinical sample. Development and Psychopathology. 1992;4:5–28. [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Beauchaine T. Vagal tone, development, and Gray’s motivational theory: toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Bernston GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, et al. Heart rate variability: Origins, methods, and interpretative caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Blandon AY, Calkins SD, Keane SP, O’Brien M. Individual differences in trajectories of emotion regulation processes: The effects of maternal depressive symptomatology and children’s physiological regulation. Developmental Psychology. 2008;44:1110–1123. doi: 10.1037/0012-1649.44.4.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodas J, Edgar-Perez K, Cohn J, Fox NA, Kovacs M. Physiological response to anticipated public speaking in adults with a history of childhood-onset depression. 2008 Manuscript submitted for publication. [Google Scholar]
- Boyce WT, Quas J, Alkon A, Smider NA, Essex MJ, Kupfer DJ, et al. Autonomic reactivity and psychopathology in middle childhood. British Journal of Psychiatry. 2001;179:144–150. doi: 10.1192/bjp.179.2.144. [DOI] [PubMed] [Google Scholar]
- Calkins SD. Cardiac vagal tone indices of temperamental reactivity and behavioral regulation in young children. Developmental Psychobiology. 1997;31:125–135. doi: 10.1002/(sici)1098-2302(199709)31:2<125::aid-dev5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Graziano PA, Keane SP. Cardiac vagal regulation differentiates among children at risk for behavior problems. Biological Psychology. 2006;74:144–153. doi: 10.1016/j.biopsycho.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD, Keane SP. Cardiac vagal regulation across the preschool period: Stability, continuity, and implications for childhood adjustment. Developmental Psychobiology. 2004;45:101–112. doi: 10.1002/dev.20020. [DOI] [PubMed] [Google Scholar]
- Cole PM, Michel MK, Teti LO. The development of emotion regulation and dysregulation: A clinical perspective. Monographs Society of Research in Child Development. 1994;59(2–3):73–100. Serial No. 240. [PubMed] [Google Scholar]
- Crowell SE, Beauchaine TP, McCauley E, Smith CJ, Stevens AL, Sylvers P. Psychological, autonomic, and serotonergic correlates of parasuicide among adolescent girls. Development and Psychopathology. 2005;17:1105–1127. doi: 10.1017/s0954579405050522. [DOI] [PubMed] [Google Scholar]
- Dubreuil E, Ditto B, Dionne G, Pihl RO, Tremblay RE, Boivin M, Perusse D. Familiality of heart rate and cardiac-related autonomic activity in five-month-old twins: The Quebec newborn twins study. Psychophysiology. 2003;40:849–862. doi: 10.1111/1469-8986.00103. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Murphy B, Maszk P, Smith M, Karbon M. The role of emotionality and regulation in children’s social functioning: a longitudinal study. Child Development. 1995;66:1360–1384. [PubMed] [Google Scholar]
- El-Sheikh M, Harger J, Whitson SM. Exposure to interparental conflict and children’s adjustment and physical health: The moderating role of vagal tone. Child Development. 2001;72:1617–1636. doi: 10.1111/1467-8624.00369. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Whitson SA. Longitudinal relations between marital conflict and child adjustment: Vagal regulation as a protective factor. Journal of Family Psychology. 2006;20:30–39. doi: 10.1037/0893-3200.20.1.30. [DOI] [PubMed] [Google Scholar]
- Fabes RA, Eisenberg N, Karbon N, Troyer N, Switzer G. The relations of children’s emotion regulation to their vicarious emotional responses and comforting behaviors. Child Development. 1994;65:1678–1693. doi: 10.1111/j.1467-8624.1994.tb00842.x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P, Version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- Forbes EE, Fox NA, Cohn JF, Galles SF, Kovacs M. Children’s affect regulation during a disappointment: Psychophysiological responses and relation to parent history of depression. Biological Psychology. 2006;71:264–277. doi: 10.1016/j.biopsycho.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Fox NA, Field TM. Individual differences in preschool entry behavior. Journal of applied developmental psychology. 1989;10:527–540. [Google Scholar]
- Garber J, Braafladt N, Weiss B. Affect regulation in depressed and nondepressed children and young adolescents. Development and Psychopathology. 1995;7:93–115. [Google Scholar]
- Garber J, Braafladt N, Zeman J. The regulation of sad affect: An information-processing perspective. In: Garber J, Dodge KA, editors. The Development of Emotion Regulation and Dysregulation. New York: Cambridge University Press; 1991. pp. 208–240. [Google Scholar]
- Gottman JM, Katz LK. Children’s emotion reactions to stressful parent-child interactions: The link between emotion regulation and vagal tone. Marriage and Family Review. 2002;34:265–283. [Google Scholar]
- Graziano PA, Keane SP, Calkins SD. Cardiac vagal regulation and early peer status. Child Development. 2007;78:264–278. doi: 10.1111/j.1467-8624.2007.00996.x. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Siliva PA, McGee R, Angell KE. Development of depression from preadolescence to young adulthood: Emerging gender differences in a 10-year longitudinal study. Journal of Abnormal Psychology. 1998;107:128–140. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- Hessler DM, Katz LF. Children’s emotion regulation: Self-report and physiological response to peer provocation. Developmental Psychology. 2007;43:27–38. doi: 10.1037/0012-1649.43.1.27. [DOI] [PubMed] [Google Scholar]
- Hughes JW, Stoney CM. Depressed mood is related to high-frequency heart rate variability during stressors. Psychosomatic Medicine. 2000;62:796–803. doi: 10.1097/00006842-200011000-00009. [DOI] [PubMed] [Google Scholar]
- Katz LF, Gottman JM. Buffering children from marital conflict and dissolution. Journal of Clinical Child Psychology. 1997;26:157–171. doi: 10.1207/s15374424jccp2602_4. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Keenan K, Hipwell AE. Preadolescent clues to understanding depression in girls. Clinical Child and Family Psychology Review. 2005;8:89–105. doi: 10.1007/s10567-005-4750-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, Merikangas KR. Mood disorders in children and adolescents: An epidemiologic perspective. Biological Psychiatry. 2001;49:1002–1014. doi: 10.1016/s0006-3223(01)01129-5. [DOI] [PubMed] [Google Scholar]
- Kovacs M. The “Feelings and Me’ emotion regulatory strategy utilization questionnaires. Pittsburgh, PA: University of Pittsburgh School of Medicine; 2000. [Google Scholar]
- Kovacs M, Feinberg TL, Crouse-Novak MA, Paulauskas SL, Finkelstein R. Depressive disorders in childhood. I. A longitudinal prospective study of characteristics and recovery. Archives of General Psychiatry. 1984;41:229–237. doi: 10.1001/archpsyc.1984.01790140019002. [DOI] [PubMed] [Google Scholar]
- Kovacs M, George C. Self-regulatory responses to sadness among young adults with histories of juvenile-onset depression. 2007 Manuscript submitted for publication. [Google Scholar]
- Kovacs M, Obrosky DS, Gatsonis C, Richards C. First episode major depressive and dysthymic disorder in childhood: Clinical and sociodemographic factors in recovery. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:777–784. doi: 10.1097/00004583-199706000-00014. [DOI] [PubMed] [Google Scholar]
- Langrock AM, Compas BE, Keller G, Merchant MJ, Copeland ME. Coping with the stress of parental depression: Parents’ reports of children’s coping, emotional, and behavioral problems. Journal of Clinical Child and Adolescent Psychology. 2002;31:312–324. doi: 10.1207/S15374424JCCP3103_03. [DOI] [PubMed] [Google Scholar]
- Porges SW. Orienting in a defensive world: mammalian modifications of our evolutionary heritage: A polyvagal theory. Psychophysiology. 1995;32:301–328. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. The Polyvagal Perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales AL, Greenspan SI. Infant regulation of the vagal “brake” predicts child behavior problems: A psychobiological model of social behavior. Developmental Psychobiology. 1996;29:697–712. doi: 10.1002/(SICI)1098-2302(199612)29:8<697::AID-DEV5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Maiti AK. Vagal tone and the physiological regulation of emotion. Monographs of the Society for Research in Child Development. 1994;59(2–3) Serial No. 240. [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. 2. Thousand Oaks, CA: Sage Publications; 2002. [Google Scholar]
- Rottenberg J. Cardiac vagal tone in depression: A critical analysis. Biological Psychology. 2007;74:200–211. doi: 10.1016/j.biopsycho.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Clift A, Bolden S, Salomon K. RSA fluctuation in major depressive disorder. Psychophysiology. 2007;44:450–458. doi: 10.1111/j.1469-8986.2007.00509.x. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Salomon K, Gross JJ, Gotlib IH. Vagal withdrawal to a sad film predicts subsequent recovery from depression. Psychophysiology. 2005;42:277–281. doi: 10.1111/j.1469-8986.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Wilhelm FH, Gross JJ, Gotlib IH. Vagal rebound during resolution of tearful crying among depressed and nondepressed individuals. Psychophysiology. 2003;40:1–6. doi: 10.1111/1469-8986.00001. [DOI] [PubMed] [Google Scholar]
- Santucci AK, Galles SJ, Cohn JF, Fox NA. High frequency heart rate variability in women with a history of depression. 2007 Manuscript submitted for publication. [Google Scholar]
- Santucci AK, Silk JS, Shaw DS, Gentzler AL, Fox NA, Kovacs M. Vagal tone and temperament as predictors of emotion regulation strategies in young children. Developmental Psychobiology. 2008;50:205–216. doi: 10.1002/dev.20283. [DOI] [PubMed] [Google Scholar]
- Schwartz JAJ, Koening LJ. Response styles and negative affect among adolescents. Cognitive Therapy Research. 1996;20:13–36. [Google Scholar]
- Shannon KE, Beauchaine TP, Brenner SL, Neuhaus E, Gatzke-Kopp L. Familial and temperamental predictors of resilience in children at risk for conduct disorder and depression. Development and Psychopathology. 2007;19:701–727. doi: 10.1017/S0954579407000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrill JT, Kovacs M. Interview schedule for children and adolescents (ISCA) Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:67–75. doi: 10.1097/00004583-200001000-00018. [DOI] [PubMed] [Google Scholar]
- Silk JS, Shaw DS, Skuban EM, Oland AA, Kovacs M. Emotion regulation strategies in offspring of childhood-onset depressed mothers. Journal of Child Psychology and Psychiatry. 2006;47:69–78. doi: 10.1111/j.1469-7610.2005.01440.x. [DOI] [PubMed] [Google Scholar]
- Silk JS, Steinberg L, Morris AS. Adolescents’ emotion regulation in daily life: Links to depressive symptoms and problem behavior. Child Development. 2003;74:1869–1880. doi: 10.1046/j.1467-8624.2003.00643.x. [DOI] [PubMed] [Google Scholar]
- Sinnreich R, Friedlander Y, Sapoznikov D, Kark JD. Familial aggregation of heart rate variability based on short recordings – the kibbutzim family study. Human Genetics. 1998;103:34–40. doi: 10.1007/s004390050779. [DOI] [PubMed] [Google Scholar]
- Snieder H, Boomsma DI, Van Doornen LJP, De Geus EJC. Heritability of respiratory sinus arrhythmia: Dependency on task and respiration rate. Psychophysiology. 1997;34:317–328. doi: 10.1111/j.1469-8986.1997.tb02402.x. [DOI] [PubMed] [Google Scholar]
- Tamas Z, Kovacs M, Gentzler AL, Tepper P, Kiss E, Kapornai K, Vetró Á. The relations of temperament and emotion self-regulation with suicidal behaviors in a clinical sample of depressed children in Hungary. Journal of Abnormal Child Psychology. 2007;35:640–652. doi: 10.1007/s10802-007-9119-2. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Wickramaratne P, Nomura Y, Warner V, Pilowsky D, Verdeli H. Offspring of depressed parents: 20 years later. American Journal of Psychiatry. 2006;163:1001–1008. doi: 10.1176/ajp.2006.163.6.1001. [DOI] [PubMed] [Google Scholar]