This issue of Arteriosclerosis, Thrombosis, and Vascular Biology contains 4 reviews on tissue factor (TF) and 1 on tissue factor pathway inhibitor (TFPI). One review on TF will be published in a later issue. In this editorial, we will briefly revisit the major advances in the field, highlight some of the current controversies, and discuss some of the future challenges. TF (also known as tissue thromboplastin or coagulation factor III) was first identified as a constituent of tissue that when added to plasma activated the clotting cascade— hence the name tissue factor. TF was first purified in 1985,1 and this subsequently led to the cloning of the TF cDNA and gene.2-5 In 1989 Drake and colleagues6 proposed that TF around blood vessels forms a “hemostatic envelope” that initiates clotting after vessel injury. The crystal structure of the extracellular domain of TF bound to Factor VIIa (FVIIa) was reported in 1996.7 In the same year it was discovered that inactivation of the mouse TF gene resulted in embryonic lethality.8-10 Taken together, these studies indicated that TF was essential for hemostasis. Activation of the clotting cascade leads to the generation of thrombin that cleaves fibrinogen to fibrin as well as activates platelets (Figure).
Figure.
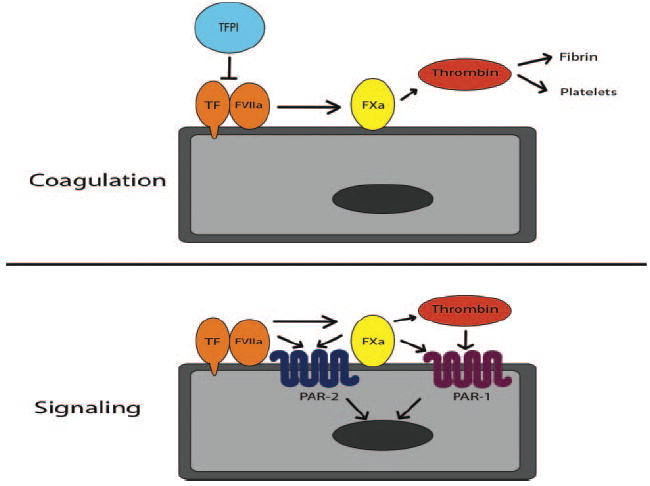
TF coagulant and signaling activities. The TF coagulant activity involves the generation of thrombin, fibrin deposition, and platelet activation. The TF signaling activity involves activation of PAR-2 by FVIIa and FXa, as well activation of PAR-1 by FXa and thrombin.
In 1999, the late Yale Nemerson and colleagues11 reported that there was TF in blood of healthy individuals. They showed that this so-called “blood-borne” or “circulating” TF enhanced thrombosis in an ex vivo model. It was argued that, unlike vessel wall TF, circulating TF would be continuously delivered to the clot and participate in its growth.12,13 Butenas and colleagues14 believe that levels of circulating TF in healthy individuals are extremely low and unlikely to contribute to clotting. However, Monroe and colleagues noted that TF was present throughout thrombotic clots whereas it was only present at the edges of hemostatic clots, suggesting that circulating TF is incorporated into thrombotic clots.15 Clearly, further studies are needed to determine the roles of circulating TF in hemostasis and thrombosis in health and disease.
Other reports indicated that platelets contain very low levels of TF (possibly by binding TF-positive microvesicles, also referred to as microparticles), contain a premRNA (that can be spliced into a mature TF mRNA on activation of the cells), and can synthesize TF (albeit very low levels).16-18 In contrast, other investigators could not detect TF activity in platelets.14 Similar to circulating TF, platelet TF has been proposed to provide an additional source of TF that may enhance the clotting reaction. However, at present there is no experimental evidence to support this hypothesis.
Another controversy arose after the description of a new form of TF called alternatively spliced TF (altTF; also called soluble TF).19 Initial reports suggested that altTF was thrombotic.20 To date this protein has not been isolated from human plasma. Importantly, this truncated protein lacks exon 5 (which encodes the substrate binding site) and exon 6 (which encodes the transmembrane domain that localizes TF in the membrane), both of which are required for TF cofactor activity.21 Indeed, subsequent studies indicate that altTF has no procoagulant activity.22 Therefore, it seems that altTF is unlikely to contribute to either hemostasis or thrombosis. One study suggested a role for altTF in tumor growth and angiogenesis.14
Butenas and colleagues14 discuss the controversial issue of the “encryption-decryption process.” It has been known for many years that lysis of TF-positive cells is associated with a significant increase TF activity. This led to the proposal that TF can exist in two states: a low-activity state (also called “encrypted”) and a high-activity state (also called “decrypted”). Importantly, both states bind FVII and FVIIa. Possible mechanisms of decryption have been reviewed previously.23 One popular mechanism is that TF on the cell surface converted from the low activity to active activity state by interaction with phosphatidlyserine. This anionic phospholipid normally resides in the inner leaflet of the membrane but appears on the cell surface after disruption of the membrane asymmetry. However, at present there are no experimental data to support this hypothesis.
In 2006, a radically different hypothesis was proposed to explain the mechanism of TF decryption. Hogg and colleagues24 suggested that TF activity was regulated by the formation of a disulfide bond between cys186 and cys209. The idea that TF activity was modulated by a redox reaction became very popular. However, many investigators in the field have questioned the validity of this model.14 In this issue of ATVB, Bach25 raises several concerns about the model. Most notably, the crystal structure shows that cys186 and cys209 are buried in the interface between TF and FVIIa. Therefore, it is unclear how proteins, such as protein disulfide isomerase, gain access to these residues to form the disulfide bond. Despite enthusiasm for this new model, it seems that the most likely mechanism that increases TF activity is via interaction with phosphatidylserine. Interestingly, this would increase TF activity on damaged cells and help to preserve vascular integrity.
Kretz and colleagues discuss the sources of TF that drive thrombosis in different animal models. This review will be published in a later issue. Experimental studies have shown that vessel wall TF is the major source of TF in models involving loss of the endothelium, such as the carotid artery injury model. In contrast, hematopoietic cell–derived microvesicles contribute to thrombosis in cremaster arterioles subjected to heat injury. It should be noted that healthy mice are used in these experiments. Further studies are needed using mice with elevated levels of TF in the vessel wall, such as those with atherosclerosis, or within the circulation, such as endotoxemic mice. In addition, mice with tumors have elevated levels of circulating tumor-derived TF-positive microvesicles, which activates the clotting system and may enhance local thrombosis. In cancer patients, these microvesicles may increase the risk of venous thrombosis.
Another review in this issue of ATVB is by Schaffner and Ruf.26 It summarizes our current knowledge on TF signaling (Figure). The authors propose that TF signaling activity increases tumor growth by induction of the angiogenic switch. This is mediated by binding FVIIa, activation of PAR-2, and expression of proangiogenic genes. Indeed, in one model system tumor growth was reduced in mice lacking PAR-2 but not in mice lacking PAR-1. However, many human tumor cells express both PAR-1 and PAR-2, and activation of these two receptors induces a similar array of proangiogenic genes. Therefore, human cancers may use both PAR-1 and PAR-2 signaling to enhance their growth. In contrast, the TF coagulant activity may increase metastasis via the generation of thrombin, PAR-1 signaling, and the formation of fibrin. As noted by Schaffner and Ruf,26 inhibition of either TF:FVIIa signaling or PAR-2 signaling will not affect thrombin generation and downstream pathways and may be less effective at reducing tumor growth compared with inhibition of both TF signaling and coagulant activities.
Milsom and colleagues27 discuss the possible link between TF, tumorigenesis, and cancer stem cells. They propose that expression of TF and PARs by cancer stem cells allow them to respond to coagulation proteases (FVIIa, FXa, and thrombin). This would result in fibrin deposition, activation of platelets, expression of proangiogenic and proinflammatory mediators, all of which would modulate the local microenvironment and enhance tumorigenesis. This may explain how the presence of TF on tumor cells (and possibly cancer stem cells) contributes to tumor growth and metastasis.
In the last review, Hackeng and Rosing28 discuss the recent finding that protein S acts as a cofactor for TFPI. Protein S has long been known to be a cofactor for the anticoagulant activated protein C. Now we find that protein S stimulates the inhibition of Factor Xa by TFPI about 10-fold. What is less clear is whether or not protein S enhances TFPI inhibition of the TF:Factor VIIa:Factor Xa ternary complex.
We have learned a tremendous amount about TF since its purification and cloning. It is the major cellular trigger of the clotting cascade and clearly plays an essential role in hemostasis. Pathological expression of TF within atherosclerotic plaques likely drives arterial thrombosis, and elevated levels of circulating TF may enhance venous thrombosis. Further studies are needed to define the mechanism by which TF contributes to tumor growth and other disease processes.
Acknowledgments
We acknowledge Claire Mackman for preparing the figure.
Footnotes
Disclosures N.M. is on the Scientific Advisory Board of Thrombotargets.
Contributor Information
Nigel Mackman, Division of Hematology and Oncology, Department of Medicine, University of North Carolina at Chapel Hill.
Mark Taubman, Aab Cardiovascular Research Institute and Department of Medicine, University of Rochester, NY.
References
- 1.Broze GJ, Leykam JE, Schwartz BD, Miletich JP. Purification of human brain tissue factor. J Biol Chem. 1985;260:10917–10920. [PubMed] [Google Scholar]
- 2.Spicer EK, Horton R, Bloem L, Bach R, Williams KR, Guha A, Kraus J, Lin TC, Nemerson Y, Konigsberg WH. Isolation of cDNA clones coding for human tissue factor: primary structure of the protein and cDNA. Proc Natl Acad Sci U S A. 1987;84:5148–5152. doi: 10.1073/pnas.84.15.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morrissey JH, Fakhrai H, Edgington TS. Molecular cloning of the cDNA for tissue factor, the cellular receptor for the initiation of the coagulation protease cascade. Cell. 1987;50:129–135. doi: 10.1016/0092-8674(87)90669-6. [DOI] [PubMed] [Google Scholar]
- 4.Scarpati EM, Wen D, Broze JGJ, Miletich JP, Flandermeyer RR, Siegel NR, Sadler JE. Human tissue factor: cDNA sequence and chromosome localization of the gene. Biochemistry. 1987;26:5234–5238. doi: 10.1021/bi00391a004. [DOI] [PubMed] [Google Scholar]
- 5.Mackman N, Morrissey JH, Fowler B, Edgington TS. Complete sequence of the human tissue factor gene, a highly regulated cellular receptor that initiates the coagulation protease cascade. Biochemistry. 1989;28:1755–1762. doi: 10.1021/bi00430a050. [DOI] [PubMed] [Google Scholar]
- 6.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Am J Pathol. 1989;134:1087–1097. [PMC free article] [PubMed] [Google Scholar]
- 7.Banner DW, D’Arcy A, Chene C, Winkler FK, Guha A, Konigsberg WH, Nemerson Y, Kirchhofer D. The crystal structure of the complex of blood coagulation factor VIIa with soluble tissue factor. Nature. 1996;380:41–46. doi: 10.1038/380041a0. [DOI] [PubMed] [Google Scholar]
- 8.Carmeliet P, Mackman N, Moons L, Luther T, Gressens P, Van Vlaenderen I, Demunck H, Kasper M, Breier G, Evrard P, Muller M, Risau W, Edgington T, Collen D. Role of tissue factor in embryonic blood vessel development. Nature. 1996;383:73–75. doi: 10.1038/383073a0. [DOI] [PubMed] [Google Scholar]
- 9.Bugge TH, Xiao Q, Kombrinck KW, Flick MJ, Holmback K, Danton MJS, Colbert MC, Witte DP, Fujikawa K, Davie EW, Degen JL. Fatal embryonic bleeding events in mice lacking tissue factor, the cell-associated initiator of blood coagulation. Proc Natl Acad Sci U S A. 1996;93:6258–6263. doi: 10.1073/pnas.93.13.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toomey JR, Kratzer KE, Lasky NM, Stanton JJ, Broze GJ., Jr Targeted disruption of the murine tissue factor gene results in embryonic lethality. Blood. 1996;88:1583–1587. [PubMed] [Google Scholar]
- 11.Giesen P, Rauch U, Bohrmann B, Kling D, Roque M, Fallon J, Badimon JJ, Riederer M, Nemerson Y. Blood-borne tissue factor: another view of thrombosis. Proc Natl Acad Sci U S A. 1999;96:2311–2315. doi: 10.1073/pnas.96.5.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hathcock JJ, Nemerson Y. Platelet deposition inhibits tissue factor activity: in vitro clots are impermeable to factor Xa. Blood. 2004;104:123–127. doi: 10.1182/blood-2003-12-4352. [DOI] [PubMed] [Google Scholar]
- 13.Nemerson Y. A different view of thrombosis. Blood Coagul Fibrinol. 2000;11:S1–S2. doi: 10.1097/00001721-200004001-00001. [DOI] [PubMed] [Google Scholar]
- 14.Butenas S, Orfeo T, Mann KG. Tissue factor in coagulation: which? where? when? Arterioscler Thromb Vasc Biol. 2009;29:1989–1996. doi: 10.1161/ATVBAHA.108.177402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman M, Whinna HC, Monroe DM. Circulating tissue factor accumulates in thrombi, but not in hemostatic plugs. J Thromb Haemost. 2006;4:2092–2093. doi: 10.1111/j.1538-7836.2006.02085.x. [DOI] [PubMed] [Google Scholar]
- 16.Zillmann A, Luther T, Muller I, Kotzsch M, Spannagl M, Kauke T, Oelschlagel U, Zahler S, Engelmann B. Platelet-associated tissue factor contributes to the collagen-triggered activation of blood coagulation. Biochem Biophys Res Com. 2001;281:603–609. doi: 10.1006/bbrc.2001.4399. [DOI] [PubMed] [Google Scholar]
- 17.Panes O, Matus V, Saez CG, Quiroga T, Pereira J, Mezzano D. Human platelets synthesize and express functional tissue factor. Blood. 2007;109:5242–5250. doi: 10.1182/blood-2006-06-030619. [DOI] [PubMed] [Google Scholar]
- 18.Schwertz H, Tolley ND, Foulks JM, Denis MM, Risenmay BW, Buerke M, Tilley RE, Rondina MT, Harris EM, Kraiss LW, Mackman N, Zimmerman GA, Weyrich AS. Signal-dependent splicing of tissue factor pre-mRNA modulates the thrombogenicity of human platelets. J Exp Med. 2006;203:2433–2440. doi: 10.1084/jem.20061302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bogdanov VY, Balasubramanian V, Hathcock J, Vele O, Lieb M, Nemerson Y. Alternatively spliced human tissue factor: a circulating, soluble, thrombogenic protein. Nature Med. 2003;9:458–462. doi: 10.1038/nm841. [DOI] [PubMed] [Google Scholar]
- 20.Szotowski B, Antoniak S, Poller W, Schultheiss HP, Rauch U. Procoagulant soluble tissue factor is released from endothelial cells in response to inflammatory cytokines. Circ Res. 2005;96:1233–1239. doi: 10.1161/01.RES.0000171805.24799.fa. [DOI] [PubMed] [Google Scholar]
- 21.Mackman N. Alternatively spliced tissue factor - One cut too many? Thromb Haemost. 2007;97:5–8. doi: 10.1160/th06-11-0670. [DOI] [PubMed] [Google Scholar]
- 22.Censarek P, Bobbe A, Schror K, Weber AA. Alternatively spliced human tissue factor (asHTF) is not procoagulant. Thromb Haemost. 2007;97:11–14. [PubMed] [Google Scholar]
- 23.Bach RR. Tissue Factor encryption. Arterioscler Thromb Vasc Biol. 2006;26:456–461. doi: 10.1161/01.ATV.0000202656.53964.04. [DOI] [PubMed] [Google Scholar]
- 24.Chen VM, Ahamed J, Versteeg HH, Berndt MC, Ruf W, Hogg PJ. Evidence for activation of tissue factor by an allosteric disulfide bond. Biochemistry. 2006;45:12020–12028. doi: 10.1021/bi061271a. [DOI] [PubMed] [Google Scholar]
- 25.Bach RR. What’s wrong with the allosteric disulfide bond hypothesis? Arterioscler Thromb Vasc Biol. 2009;29:1997–1998. doi: 10.1161/ATVBAHA.109.194985. [DOI] [PubMed] [Google Scholar]
- 26.Schaffner F, Ruf W. Tissue factor and PAR-2 signaling in the tumor microenvironment. Arterioscler Thromb Vasc Biol. 2009;29:1999–2004. doi: 10.1161/ATVBAHA.108.177428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milsom C, Magnus N, Meeham B, Al-Nedawi K, Garnier D, Rak J. Tissue factor and cancer stem cells- is there a linkage? Arterioscler Thromb Vasc Biol. 2009;29:2005–2014. doi: 10.1161/ATVBAHA.108.177444. [DOI] [PubMed] [Google Scholar]
- 28.Hackeng TM, Rosing J. Protein S as a cofactor for TFPI. Arterioscler Thromb Vasc Biol. 2009;29:2015–2020. doi: 10.1161/ATVBAHA.108.177436. [DOI] [PubMed] [Google Scholar]


