Abstract
Hyperpolarization can increase the sensitivity of NMR/MRI experiments but the primary limitation is the T1 decay of magnetization. Due to its long T1, hyperpolarized 89Y nucleus makes an excellent candidate as an in vivo spectroscopy/imaging probe. Here we report the 89Y chemical shift dependence upon pH for two hyperpolarized 89Y(III) complexes and demonstrate how such complexes can be used as sensitive spectroscopy/imaging agents to measure pH.
Dynamic nuclear polarization (DNP) of a NMR sample can significantly increase sensitivity by creating nuclear spin polarization levels that are much higher than ambient temperature Boltzman levels. DNP is based on the transfer of electron spin polarization from a stable free radical to coupled nuclear spins by microwave irradiation in a frozen glass matrix at low temperatures (around 1K). The method gained practical importance when it was demonstrated that compounds hyperpolarized in the solid state could be dissolved and transferred into an NMR magnet for spectrum acquisition with negligible loss of polarization.1 Liquid state DNP NMR offers dramatic signal enhancements, 10,000-fold or more, for some 13C and 15N enriched compounds. Such increases in sensitivity have made it possible to perform molecular/functional imaging of nuclei other than 1H. Hyperpolarized 13C labeled substrates, particularly [1-13C]-pyruvate, have successfully been used to study metabolism in various normal and diseased tissues.2 While the advantages of 13C labeled metabolites as imaging agents are obvious, the typical longitudinal relaxation time (T1) of 13C nuclei (few seconds to ∼1 minute) limits the metabolic processes that can be studied by hyperpolarized 13C compounds. The time constraint emerging from the inevitable decay of polarization motivates the search for long T1 agents. Among the NMR active nuclei, 89Y in its diamagnetic 3+ oxidation state has one of the longest T1 relaxation times known (600 s or longer).3 This very long T1 combined with a favorable spin quantum number (1/2), sharp NMR line width (3-5 Hz) and 100% natural abundance makes hyperpolarized 89Y attractive as a potential in vivo imaging and spectroscopy probe.3
In addition, Y(III) is a pseudo-lanthanide so ligand systems developed for Gd(III)-based MRI contrast agents can be used to safely chelate Y(III) for biomedical applications. Most importantly, the sensitivity of the chemical shift of 89Y(III) to its chemical environment could be exploited in the design of probes to image physiological parameters such as pH, temperature, and other indices of metabolism in vivo. Among the physiological parameters that describe a particular state of an organism, pH is considered to be one of the most important. Extracellular pH is tightly regulated and even small deviations from normal are indicative of a metabolic abnormality. For example, the acidic microenvironment of various tumors due to the Warburg effect is well documented.4 Imaging of extracellular pH around a tumor could provide relevant information for tumorogenesis and therapy; however, current methods based on microelectrodes, 31P or 1H NMR are either invasive, have poor spatial resolution, and/or offer a poor signal-to-noise (SNR).5 The successful application of hyperpolarized 89Y(III) chelates as pH responsive agents requires complexes that exhibit a significant change in the coordination environment around the Y(III) ion as a function of pH in the physiologically relevant pH range (pH 5 to 8). We have selected two ligands, DOTP (1) and DO3A-NTs (2) (Figure 1) because Ln(III) complexes of these ligands have been reported to exhibit pH dependent properties in the desired pH range.6,7 The 31P chemical shift of several LnDOTP complexes show a marked pH dependence as a result of the protonation of the noncoordinating phosphonate oxygens, while GdDO3A-NTs exhibits pH-dependent relaxivity due to the intramolecular coordination of the tosyl nitrogen side-arm.
Figure 1.
Ligands studied in this work.
Because of the combined effects of a low γ, low sensitivity and long T1, acquiring 89Y NMR data on thermally polarized samples is impractical, often requiring days even for concentrated solutions. Our preliminary experiments have shown that various chelated forms of Y(III) including YDOTP can be polarized with currently available commercial hardware using the trityl radical (OX63) as a polarizing agent.3 While only a modest signal enhancement was observed previously for YDOTP (298-fold over thermal equilibrium at 310 K), we are now able to routinely achieve much higher enhancements (∼3000-fold) by optimizing the sample preparation prior to DNP (see Supporting Information).
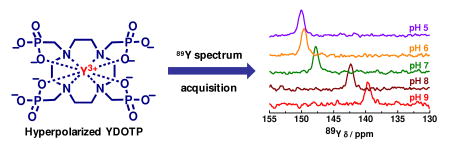
The 89Y chemical shift of hyperpolarized 89YDOTP as a function of pH is shown in Figure 2. Given the long T1 of YDOTP, the entire 89Y chemical shift versus pH dataset was collected using a single batch of hyperpolarized YDOTP in about 3 min. In comparison, collecting this entire titration curve on thermally polarized samples would have required 24 hr for each pH value. A single 89Y(III) resonance was seen at all pH values showing that the protonated and deprotonated species are in fast exchange. The 89Y chemical shift gradually decreases from 150 ppm to 140 ppm between pH 5 and 8 following a reverse sigmoid curve as the electronic shielding of the 89Y nucleus decreases with increasing protonation of the complex at the noncoorodinating phosphonate oxygens.6 The apparent macroscopic protonation constant of the complex could be determined by fitting this curve (pKa = 7.6). Thermally polarized 31P NMR spectra of YDOTP were also run as a function of pH to confirm the hyperpolarized Y(III) results. The 31P chemical shift dispersion followed a similar trend (Figure S1) and fitting the data gave a protonation constant (pKa) of 5.7. While this value is in agreement with the average pKa's of the four protonation steps reported previously for other LnDOTP complexes,6 the pKa value obtained from the 89Y chemical shift dispersion is significantly higher and reflects the fact that the coordination environment of the central Y-ion is strongly affected by protonation of the first noncoordinating oxygen while further protonations have much less effect. This is likely the consequence of the relaxation of the coordination cage around the Y-ion occurring on protonation, as has been suggested for protonated LnDOTP comlexes.6
Figure 2.
89Y chemical shift dispersion of hyperpolarized YDOTP ( ) and YDO3A-NTs (
) and YDO3A-NTs ( ) as a function of pH (9.4 T and 25°C). The YDOTP data were collected from a single sample adjusted to different pH values after hyperpolarization while the YDO3A-NTs data required collection on different hyperpolarized samples due to rapid relaxation.
) as a function of pH (9.4 T and 25°C). The YDOTP data were collected from a single sample adjusted to different pH values after hyperpolarization while the YDO3A-NTs data required collection on different hyperpolarized samples due to rapid relaxation.
The 89Y chemical shift dispersion of YDO3A-NTs in the pH range of 4 to 9 followed an opposite trend and could be approximated by a sigmoid curve as it increased from 132 ppm at pH 4 to about 157 ppm at pH 9. This trend can be explained by the pH dependent intramolecular coordination of the tosyl N-atom whereby two metal bound water molecules are replaced by the sulfonamide pendant arm resulting in a decreased shielding of the 89Y nucleus.7 The apparent pKa obtained from this curve (5.8) is in reasonable agreement with the pKa value reported for EuDO3A-NTs (6.4) by luminescence measurements.7
Surprisingly, no hyperpolarized 89Y signal for YDO3A-NTs could be observed at various pH values between pH 5 and 7. Likewise, we were unable to generate a signal from thermally polarized samples in this pH range. One possible explanation for this loss of signal could be that the two different chemical species present in solution, presumably the Y(III)-coordinated versus uncoordinated tosyl N-atom, are exchanging at a critical rate that results in extreme line-broadening at these intermediate pH values. This hypothesis is supported by variable pH 1H NMR studies in which exchange broadened spectra were recorded at pH 5, 6 and 7 indicating the presence of several species (Figure S2). These results are in agreement with the 1H NMR studies performed on the Eu(III) complex DO3A-NTs in which cooperative arm rotation was also observed in addition to the intramolecular ligation of the tosyl N-atom.7
Long longitudinal relaxation times are essential for in vivo imaging of hyperpolarized materials so the T1 of YDOTP was measured at three different pH values to evaluate the sensitivity of T1 to pH. Magnetization decay curves such as that shown in Figure 3 were fitted to give T1 values of 202, 123, and 57 s at pH 4, 7, and 9, respectively. In human blood serum, the T1 was found to be about 20% shorter (see Supporting Information). These data indicate that YDOTP has an adequately long T1 at pH 7 for in vivo imaging. In comparison, the T1 of hyperpolarized 13C-bicarbonate (H13CO3-), which has been used for imaging pH in vivo, is ∼10 s.8 At present we do not have a satisfactory explanation for the unexpectedly large changes in T1 with pH although a plausible explanation might involve the interaction of quadrupolar 23Na+ ions with the highly charged [YDOTP]5- anion above pH 8 which may allow an additional relaxation pathway for the 89Y nucleus. Ln-complexes of DOTP have been reported to bind Na+ relatively strongly with a binding constant (log K) of approximately 2.6.6
Figure 3.
T1 decay of hyperpolarized YDOTP magnetization at pH 4 ( ), 7 (
), 7 ( ), and 9 (
), and 9 ( ), with n=2 and an error of approximately ±10 sec.
), with n=2 and an error of approximately ±10 sec.
We were unable to measure the T1 of YDO3A-NTs due to the rapid decay of the hyperpolarized signal. Although this makes YDO3A-NTs less attractive for in vivo imaging, it does provide insight into possible relaxation mechanisms that must be avoided in developing further long T1 89Y agents.
In summary, we have demonstrated the potential of using hyperpolarized 89YDOTP as a pH sensor. The chemical shift of this complex changes about 10 ppm over the pH range 5-9 due to protonation of the non-coordinated phosphonate oxygen atoms in the complex. Although the T1 of 89Y in YDOTP is also pH dependent, ranging from 202 s at pH 4 to 57 s at pH 9, it is sufficiently long for in vivo applications at physiological pH values. The pH dependent 89Y chemical shift of YDO3A-NTs is even larger (20 ppm) over this same pH range but chemical exchange processes in this molecule cause both line broadening and rapid T1 decay of the 89Y signal making it unattractive for imaging applications. Finally, we managed to achieve significant signal enhancements (over 3000-fold) by optimizing the sample preparation, an important consideration for future in vivo spectroscopy and imaging applications.
Supplementary Material
Acknowledgments
The authors acknowledge financial support from the National Institutes of Health (1 R21 EB009147-01 and and P41 RR-02584).
Footnotes
Supporting Information Available: Synthesis of the yttrium complexes, DNP sample preparation and 1H, 31P and 89Y NMR experimental details and data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Proc Natl Acad Sci. 2003;100:10158–10163. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallagher FA, Kettunen MI, Brindle KM. Progress in Nuclear Magnetic Resonance Spectroscopy. 2009;55:285–295. [Google Scholar]
- 3.Merritt ME, Harrison C, Kovacs Z, Kshirsagar P, Malloy CR, Sherry AD. J Am Chem Soc. 2007;129:12942–12943. doi: 10.1021/ja075768z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu PP, Sabatini DM. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 5.van Sluis R, Bhujwalla ZM, Raghunand N, Ballesteros P, Alvarez J, Cerdán S, Galons JP, Gillies RJ. Magn Reson Med. 1999;41:743–50. doi: 10.1002/(sici)1522-2594(199904)41:4<743::aid-mrm13>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 6.Sherry AD, Ren J, Huskens J, Brucher E, Toth E, Geraldes CFCG, Castro MMCA, Cacheris WP. Inorg Chem. 1996;35:4604–4612. [Google Scholar]
- 7.Lowe MP, Parker D, Reany O, Aime S, Botta M, Castellano G, Gianolio E, Pagliarin R. J Am Chem Soc. 2001;123:7601–7609. doi: 10.1021/ja0103647. [DOI] [PubMed] [Google Scholar]
- 8.Gallagher FA, Kettunen MI, Day SE, Hu DE, Ardenkjaer-Larsen JH, in't Zandt R, Jensen PR, Karlsson M, Golman K, Lerche MH, Brindle KM. Nature. 2008;453:940–943. doi: 10.1038/nature07017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





