March is deep vein thrombosis awareness month in the United States. To mark this event, this issue of Arteriosclerosis, Thrombosis, and Vascular Biology contains a series of reviews on new anticoagulants that either are in development or have been approved for clinical use. Particularly exciting are the new oral inhibitors of thrombin (dabigatran etexilate) and factor Xa (rivaroxaban).
Thrombosis is the leading cause of morbidity and mortality in the Western world. Arterial thrombosis leads to myocardial infarction and stroke. Arterial clots are platelet-rich and fibrin-poor (so-called white clots). They are generated at sites of vascular injury under high shear rates and are prevented or treated with antiplatelet drugs.1 However, because of the fact that the thrombin is a potent activator of platelets and that arterial clots contain fibrin, anticoagulant drugs are also used to prevent arterial thrombosis. Venous thromboembolism (VTE), which includes deep vein thrombosis and pulmonary embolism, is the third leading cause of death after myocardial infarction and stroke. It is estimated that 2 million people have deep vein thrombosis in the United States, and of these 600 000 have a pulmonary embolism (one-third of which are fatal).2 Venous clots are fibrin-rich and platelet-poor (socalled red clots) because of the incorporation of red cells and low levels of platelets. They are prevented or treated with anticoagulant drugs.1
There are 3 major groups of patients that are administered anticoagulant drugs to treat or prevent venous thrombosis: (1) patients with VTE, who have had VTE, or are at risk for VTE, such as patients undergoing hip and knee replacement surgery; (2) patients at risk for stroke because of atrial fibrillation; and (3) acute coronary syndrome patients.
Before discussing the anticoagulant drugs that are available to treat thrombosis, we will briefly summarize the coagulation protease cascade. Our knowledge of the cascade is based on many years of biochemical studies, the phenotype of patients with defects in the system, and, more recently, on knockout studies in mice. Rapid formation of a clot is required to limit bleeding after vascular injury. Because this cascade is essential for hemostasis, the major side effect of overdosing with an anticoagulant drug is bleeding. Patients with deficiencies in either factor VIII (hemophilia A) or factor IX (hemophilia B) have mild to severe bleeding, depending on the extent of the deficiency. Studies with mice have shown that an absence of components of the extrinsic pathway (tissue factor and factor VII) or the common pathway (factor V, factor X, and prothrombin) is not compatible with survival.3 In contrast, mice lacking components of the intrinsic pathway (factor VIII, factor IX, factor XI, and factor XII) survive. Factor VIII−/− and factor IX−/− mice have excessive bleeding after hemostatic challenge, whereas factor XI−/− and factor XII−/− mice have no hemostatic defects but are protected in thrombosis models.4
The coagulation cascade can be divided into 2 phases: initiation and propagation (Figure). The extrinsic pathway (tissue factor–factor VIIa) initiates the clotting cascade by activation of factor X and, to a lesser extent, factor IX, which leads to the generation of small amounts of thrombin because of the absence of the cofactors factor V and VIII. However, the tissue factor–factor VIIa is rapidly inactivated in a factor Xa-dependent manner by tissue factor pathway inhibitor. The small amount of thrombin generated in the first phase then initiates the propagation phase by activating the cofactors factor V and factor VIII, as well as factor XI and factor XIII. A recent study showed that polyphosphate released by platelets activates factor XII, thus providing an alternative route for activation of factor XI5 (Figure). The propagation phase is mediated by the intrinsic pathway, particularly the tenase complex (factor VIIIa–factor IXa), and this results in the generation of large amounts of thrombin and fibrin formation. The cell-based model of thrombosis proposes that platelets are the major thrombogenic surface for the propagation phase.6 Thrombin also activates platelets by cleavage of protease activated receptors.7
Figure.
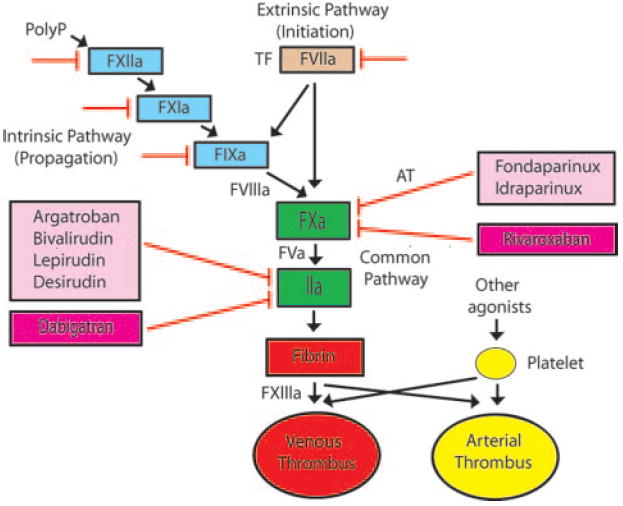
Targets of new anticoagulant drugs. The tissue factor (TF)–factor VIIa complex initiates clotting, the intrinsic pathway mediates propagation, and the common pathway results in the generation of large amounts of thrombin (factor IIa), which is the central protease in the coagulation cascade. Venous (red) and arterial (yellow) thrombi contain different proportions of fibrin and platelets. Parental direct thrombin inhibitors and factor Xa inhibitors are shown in the light pink boxes. Fondaparinux and idraparinux require antithrombin (AT) for inhibition of factor Xa. Oral inhibitors of thrombin and factor Xa are shown in the dark pink boxes. Other possible targets of anticoagulant drugs include factor VIIa, factor IXa, factor XIa, and factor XIIa (red lines).
Vitamin K antagonists, such as warfarin, and unfractionated heparin have been the mainstay of anticoagulant therapy for >50 years. Vitamin K antagonists are oral drugs that interfere with a posttranslational modification of several coagulation proteins (factor VII, factor IX, factor X, and thrombin) and anticoagulant proteins (protein C and protein S) that is required for functional activity. The broad range of targets may be viewed as a disadvantage of these drugs. Initiation of vitamin K antagonist therapy requires short-term coverage with heparin because of a slow onset of action. Although vitamin K antagonist therapy is inexpensive, there are several problems, including a poor pharmacokinetics, a narrow therapeutic index, and numerous drug and dietary interactions. This means that long-term monitoring is required, which is expensive and inconvenient to the patient. Unfractionated heparin has a rapid onset of action but must be delivered intravenously or parenterally. Unfractionated heparin binds to antithrombin and enhances its ability to inactivate factor Xa and thrombin. A major side effect of unfractionated heparin is life-threatening heparin-induced thrombocytopenia. More recently, low-molecular-weight heparins have been developed. These drugs are delivered via subcutaneous injection and have a more predictable anticoagulant response than unfractionated heparin. They preferentially inhibit factor Xa over thrombin in an antithrombindependent manner and are associated with a lower incidence of heparin-induced thrombocytopenia. Low-molecular-weight heparins are used in the initial treatment of patients with VTE. The review by Key and Kasthuri8 in this issue of Arteriosclerosis, Thrombosis, and Vascular Biology describes the use of the currently available anticoagulant drugs in the treatment of patients with VTE.
The development of target-specific anticoagulants has mainly focused on components of the common pathway, ie, factor Xa and thrombin. Fondaparinux is a synthetic pentasaccharide that selectively inhibits factor Xa in an antithrombin-dependent manner because of its small size. It is Food and Drug Administration-approved for prophylaxis and treatment of VTE. Idraparinux is a long-acting version of fondaparinux (Figure). Direct thrombin inhibitors do not require antithrombin for inhibition (Figure). This includes argatroban, bivalirudin, lepirudin, and desirudin. These are reversible inhibitors of thrombin that can inhibit free and clot-bound thrombin, and they have less bleeding than the first generation of irreversible direct thrombin inhibitors. The reversible nature is beneficial because it allows escape of small amounts of thrombin that can help maintain hemostasis. These drugs are mainly used in acute coronary syndrome patients to prevent arterial thrombosis, or in VTE patients with heparin-induced thrombocytopenia.
The new development of orally available, small-molecule inhibitors of either factor Xa or thrombin is extremely exciting.9 Dabigatran directly inhibits free thrombin and clot-bound thrombin. Dabigatran etexilate is a prodrug that is rapidly converted to dabigatran and has a half-life of 14 to 17 hours. Relatively high doses are needed because it has a low bioavailability (6%). It is approved in Canada and Europe for VTE prevention after orthopedic surgery. In a recent phase III trial of stroke prevention in patients with nonvalvular atrial fibrillation, a twice-daily dose of 150 mg of dabigatran resulted in a lower rate of stroke/systemic embolism compared with warfarin (1.11% vs 1.69%; P<0.003), with a similar risk of major bleeding.10 A review by Eisert et al11 describes the development of dabigatran and will be published in an upcoming issue of Arteriosclerosis, Thrombosis, and Vascular Biology. Other orally available direct thrombin inhibitors in development include AZD0837 and MCC 977.
Rivaroxaban is an orally available factor Xa inhibitor that is in the most advanced stage of development among this class of drug. Rivaroxaban is a small-molecule inhibitor of free factor Xa and factor Xa in the prothrombinase complex. It has a rapid onset of action, a high bioavailability (80%), and a half-life of 4 to 12 hours. Results from 4 phase III clinical trials with knee and hip replacement patients indicated that a 10-mg oral dose of rivaroxaban reduced VTE compared with the low-molecular-weight heparin enoxaparin, with similar rates of major bleeding (although there was a trend toward increased bleeding with rivaroxaban). This led to its approval in Canada, Europe, and several other countries for VTE prevention after orthopedic surgery. Perzborn et al12 describe the development of rivaroxaban in this issue of Arteriosclerosis, Thrombosis, and Vascular Biology. There are a number of other orally available antifactor Xa drugs in development, including apixaban, edoxaban, betrixaban, eribaxaban, LY517717, YM150, and TAK-442.9 Apixaban and edoxaban are currently being evaluated in phase III clinical trials.
Other targets in the clotting cascade for new anticoagulant drugs include components of the intrinsic pathway (factor FIXa, factor XIa, and factor XIIa). As discussed, components of the intrinsic pathway are not “essential” for survival but are required for propagation of the clotting system. This means that, in theory, anticoagulants that target components of the intrinsic pathway may have a wider therapeutic index compared with inhibitors of the common pathway, especially those that target either factor XIa or factor XIIa. Moreover, the fact that mice lacking factor XI or factor XII have normal hemostasis but are protected from thrombosis has led to the suggestion that hemostasis and thrombosis maybe distinct processes.3 However, inhibitors of the intrinsic pathway may be better at preventing thrombosis rather than treating ongoing coagulation. Eikelboom et al13 describe the development of factor IXa inhibitors in this issue of Arteriosclerosis, Thrombosis, and Vascular Biology. One challenge with the development of factor IXa inhibitors is that formation of the active site of this protease requires interaction with its cofactor factor VIIIa. TTP889 is an oral small-molecule inhibitor of factor IXa. Unfortunately, total VTE in patients with hip fracture did not differ between the TTP889 and placebo groups, possibly because of inadequate dosing of the drug. Another factor IXa inhibitor is called RB006, but this drug requires parenteral delivery. This is an aptamer that blocks the associations of factor X with the factor VIIIa–factor IXa complex. An advantage of RB006 is that there is an antidote that can be used to rapidly inactivate the drug. The final review in this issue is by Schumacher et al,14 and this discusses the potential advantages of inhibition of factor XIa as a new approach to anticoagulation. It will be interesting to see if proteases of the intrinsic pathway (factor IXa, factor Xia, and factor XIIa) will prove to be better targets of anticoagulant therapy than proteases of the common pathway (factor Xa and thrombin). Only time will tell.
These are exciting times. After 50 years without the development of new oral anticoagulant drugs, we now have 2 new drugs that selectively inhibit either factor Xa or thrombin, with many more in the pipeline. These drugs should allow better treatment of venous thrombosis.
Acknowledgments
The authors thank Mac Monroe, Raj Kasthuri, and Carolyn Mackman for helpful comments.
Footnotes
Disclosures N.M. has received consultant fees from Bayer and has served on advisory boards for Daiichi Sankyo. R.C.B. has received research support from AstraZeneca, Johnson & Johnson, Bayer, Regado Biosciences, The Medicines Company, and Bristol-Myers-Squibb, and has served on advisory boards for Portola, Merck, Daiichi-Sanyko, and Boehringer-Ingelheim.
Contributor Information
Nigel Mackman, Department of Medicine, McAllister Heart Institute, University of North Carolina at Chapel Hill, Chapel Hill, NC.
Richard C. Becker, Duke University School of Medicine, Duke Clinical Research Institute, Durham, NC
References
- 1.Mackman N. Triggers, targets and treatments for thrombosis. Nature. 2008;451:914–918. doi: 10.1038/nature06797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gross PL, Weitz JI. New anticoagulants for treatment of venous thromboembolism. Arterioscler Thromb Vasc Biol. 2008;28:380–386. doi: 10.1161/ATVBAHA.108.162677. [DOI] [PubMed] [Google Scholar]
- 3.Mackman N. Tissue-specific hemostasis in mice. Arterioscler Thromb Vasc Biol. 2005;25:2273–2281. doi: 10.1161/01.ATV.0000183884.06371.52. [DOI] [PubMed] [Google Scholar]
- 4.Gailani D, Renné T. The intrinsic pathway of coagulation: a target for treating thromboembolic disease? J Thromb Haemost. 2007;5:1106–1112. doi: 10.1111/j.1538-7836.2007.02446.x. [DOI] [PubMed] [Google Scholar]
- 5.Müller F, Mutch NJ, Schenk WA, Smith SA, Esterl L, Spronk HM, Schmidbauer S, Gahl WA, Morrissey JH, Renné T. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139:1143–1156. doi: 10.1016/j.cell.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts HR, Hoffman M, Monroe DM. A cell-based model of thrombin generation. Semin Thromb Hemost. 2006;1:32–38. doi: 10.1055/s-2006-939552. [DOI] [PubMed] [Google Scholar]
- 7.Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost. 2005;3:1800–1804. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- 8.Key NS, Kasthuri RS. Current treatment of venous thromboembolism. Arterioscler Thromb Vasc Biol. 2010;30:372–375. doi: 10.1161/ATVBAHA.109.197145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia D, Libby E, Crowther MA. The new oral anticoagulants. Blood. 2010;115:15–20. doi: 10.1182/blood-2009-09-241851. [DOI] [PubMed] [Google Scholar]
- 10.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L. RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 11.Eisert WG, Ryn JV, Hauel N, Stangier J, Wienen W. Dabigatran—a novel potent non-peptide inhibitor of thrombin. Arterioscler Thromb Vasc Biol. doi: 10.1161/ATVBAHA.110.203604. In press. [DOI] [PubMed] [Google Scholar]
- 12.Perzborn E, Roehrig S, Straub A, Kubitza D, Mueck W, Laux V. Rivoraxaban: a new oral factor Xa inhibitor. Arterioscler Thromb Vasc Biol. 2010;30:376–381. doi: 10.1161/ATVBAHA.110.202978. [DOI] [PubMed] [Google Scholar]
- 13.Eikelboom JW, Zelenkofske SL, Rusconi CP. Coagulation factor IXa as a target for treatment and prophylaxis of venous thromboembolism. Arterioscler Thromb Vasc Biol. 2010;30:382–387. doi: 10.1161/ATVBAHA.110.203117. [DOI] [PubMed] [Google Scholar]
- 14.Schumacher WA, Luettgen JM, Quan ML, Seiffert DA. Inhibition of factor Xia as a new approach to anticoagulation. Arterioscler Thromb Vasc Biol. 2010;30:388–392. doi: 10.1161/ATVBAHA.109.197178. [DOI] [PubMed] [Google Scholar]


