Invasion by cancer cells requires proteases such as the serine protease plasmin to degrade the cellular matrix. Plasmin is formed from its zymogen, plasminogen, a reaction catalysed by urokinase type plasminogen activator—which is implicated in invasion1—and partly regulated by plasminogen activator inhibitors. The active form of the inhibitor complexes with free and receptor bound active urokinase plasminogen activator and is bound by vitronectin in plasma and extracellular matrix.2
A high concentration of plasminogen activator inhibitor-1 in biopsy specimens from tumours is associated with a poor prognosis,3 and some patients with ovarian cancer have raised plasma concentrations of plasminogen activator inhibitor-1.4 We studied the prognostic importance of plasma concentrations of plasminogen activator inhibitor-1 in patients with colorectal cancer.
Subjects, methods, and results
Plasma was collected preoperatively as previously described5 from 609 patients having elective surgery for colorectal cancer. Plasma concentrations of plasminogen activator inhibitor-1 were measured by a sandwich enzyme linked immunosorbent assay (ELISA) using two monoclonal antibodies.3 The concentration was expressed as interim units of plasminogen activator inhibitor-1/mg protein.3
All patients had histologically verified colorectal cancer and complete clinical data. The median follow up time was 25 months (range 13-40). Patients were randomised into two groups. Data on 293 patients (optimisation group) were used to determine the optimal cut off value for plasminogen activator inhibitor-1 in relation to survival using Cox’s proportional hazard model, and data on 316 patients (validation group) were used to validate the results obtained from the optimisation group.
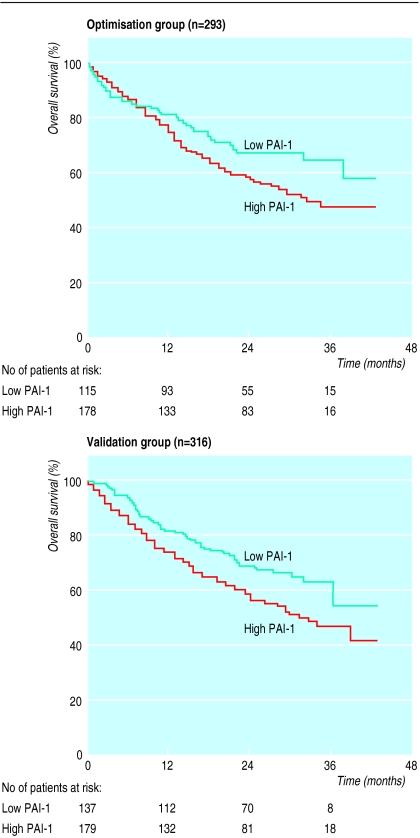
High plasma concentrations of plasminogen activator inhibitor-1 were associated with increasing severity of disease (Dukes’s stage; χ2 test, P=0.001). The best cut off value for plasminogen activator inhibitor-1 was 0.5 interim units/mg of protein. With this value the hazard ratio was 1.5 for patients with high concentrations of plasminogen activator inhibitor-1 (178/293 (61%)) compared with those with low concentrations (115/293 (39%)). Applying this value to the validation group gave similar results (hazard ratio 1.5 (95% confidence interval 1.1 to 2.2); P=0.02; 179/316 (57%) v 137/316 (43%)) (figure). Cox analysis of the 316 patients in the validation group showed that Dukes’s stage was the strongest prognostic variable (hazard ratio 2.9 (2.3 to 3.7)), followed by age (hazard ratio 1.5 (1 to 2.1)).
Comment
This study shows that high preoperative plasma concentrations of plasminogen activator inhibitor-1 are associated with shorter survival in patients with colorectal cancer. The validity of this result is strongly supported by the fact that the best cut off value for plasminogen activator inhibitor-1 obtained from one patient population gave similar prognostic information about a second independent population. It is further supported by the close correlation between high plasma concentrations of plasminogen activator inhibitor-1 and increasing severity of disease according to Dukes’s stage, which is an established predictor of poor prognosis in patients with colorectal cancer.
Figure.
Survival in patients with colorectal cancer according to preoperative plasma concentrations of plasminogen activator inhibitor-1 (PAI-1). See text for definition of groups
Acknowledgments
We thank the RANX05 Colorectal Cancer Study Group for the collection of the plasma samples.
Editorial by Verspaget
Footnotes
Funding: This study received financial support from Glaxo Group Research, United Kingdom; the Kornerup Foundation; the Ingeborg Roikjer Foundation; the University of Copenhagen; the Gerda and Aage Haench Foundation; and the Danish Cancer Society.
Conflict of interest: None.
References
- 1.Danø K, Andreasen PA, Grøndahl-Hansen J, Kristensen P, Nielsen LS, Skriver L.Plasminogen activators, tissue degradation and cancer. Adv Cancer Res 1985;44:139-266. [DOI] [PubMed]
- 2.Andreasen PA, Georg B, Lund LR, Riccio A, Stacey SN. Plasminogen activator inhibitors: hormonally regulated serpins. Mol Cell Endocrinol. 1990;68:1–19. doi: 10.1016/0303-7207(90)90164-4. [DOI] [PubMed] [Google Scholar]
- 3.Grøndahl-Hansen J, Christensen IJ, Rosenquist C, Brünner N, Mouridsen HT, Danø K, et al. High level of urokinase-type plasminogen activator and its inhibitor PAI-1 in cytosolic extracts of breast carcinomas are associated with poor prognosis. Cancer Res. 1993;53:2513–2521. [PubMed] [Google Scholar]
- 4.Casslen B, Bossmar T, Lecander I, Åstedt B. Plasminogen activators and plasminogen activator inhibitors in blood and tumour fluids of patients with ovarian cancer. Eur J Cancer. 1994;30A:1302–1309. doi: 10.1016/0959-8049(94)90178-3. [DOI] [PubMed] [Google Scholar]
- 5.Meijer P, Pollet DE, Wauters J, Kluft C. Specificity of antigen assays of plasminogen activator inhibitor in plasma: Innotest plasminogen activator inhibitor-1 immunoassay evaluated. Clin Chem. 1994;40:110–115. [PubMed] [Google Scholar]