Abstract
Postnatal cortical circuit development is characterized by windows of heightened plasticity that contribute to the acquisition of mature connectivity and function. What drives the transition between different critical plasticity windows is not known. Here we show that a switch in sign of inhibitory plasticity correlates with the reported transition between the precritical period (pre-CP) and the critical period (CP) for ocular dominance plasticity (ODP). In layer 4 of binocular visual cortex (V1b), depression of inhibitory synapses onto pyramidal neurons is induced when rats are monocularly deprived for 2 d at the end of the third postnatal week (pre-CP), whereas potentiation is induced if the monocular deprivation is started in the fourth postnatal week (CP). The magnitude of potentiation increases with deprivations started close to the peak of the CP for ODP. The direction of inhibitory plasticity depends on the differential manipulation of circuits activated by the two eyes. During development, these two forms of plasticity shift the balance between excitation and inhibition of the circuit in opposite directions, whereas the excitatory synaptic drive remains unaffected. Inhibitory plasticity is thus fundamental in modulating cortical circuit refinement and might be one of the mechanisms promoting ocular dominance shifts.
Introduction
The maturation of visual cortical circuitry depends on visual experience during critical periods (CPs) of postnatal development (Fagiolini et al., 1994; Tagawa et al., 2005). The CP for ocular dominance plasticity (ODP), the best studied of such plasticity windows, begins in rodents at the end of the third postnatal week and extends until the end of the fifth postnatal week (Fagiolini et al., 1994). If one eye is deprived of vision during this time window, neurons driven by it lose visual responsiveness (Frenkel and Bear, 2004), shifting the normal contralateral bias of visual cortical neurons toward the ipsilateral eye and generating a shift in ocular dominance (OD). Visual deprivation activates a number of plasticity mechanisms within visual cortex, including synaptic scaling (Desai et al., 2002; Maffei et al., 2004), long-term depression (LTD) of excitatory synapses (Crozier et al., 2007), and plasticity of inhibitory synapses (Maffei et al., 2006), but their relative contribution to ODP remains unclear.
Inhibitory circuits are crucial regulators of the opening of the OD CP (Hensch and Fagiolini, 2005). The maturation of inhibitory synapses is driven by visual activity and continues throughout the first five postnatal weeks. Monocular deprivation (MD) started at different times during development induces different forms of inhibitory plasticity in the monocular portion of rat primary visual cortex (V1m): it weakens unitary synapses from fast spiking (FS) interneurons onto pyramidal neurons when started before the CP (Maffei et al., 2004) and potentiates them if started during the CP (Maffei et al., 2006). Whether MD induces developmentally regulated inhibitory plasticity in V1b, where competitive interactions influence the contralateral or ipsilateral bias of visual cortical neurons, has not been investigated.
Here we address three outstanding questions about the development and plasticity of V1b: (1) if and how competitive interactions between the two eyes affect the balance between excitation and inhibition onto pyramidal neurons; (2) whether there is any correlation between the maturation of inhibition and the direction of its experience-dependent plasticity; and (3) whether the maturation of GABAergic synapses is affected by brief MD. Our findings show that MD at the transition between pre-CP and CP leaves the excitatory drive onto pyramidal neurons unaffected. The inhibitory circuit, however, responds to short MD with plasticity of the opposite sign if started right before or right after the beginning of the CP. We propose that the shift in inhibitory plasticity marks the transition between pre-CP and CP and may regulate the onset of ODP.
Materials and Methods
Monocular deprivation.
Monocular eyelid suture of different duration [postnatal day 18 (P18) until P21 and P25; P21–P23; P25–P28] was performed on Long–Evans rats. All experimental procedures were approved by the Brandeis Animal Use Committee and followed the guidelines of the National Institutes of Health. MD by eyelid suture was performed as described previously (Maffei et al., 2004, 2006, 2008). Dark treatment (DT) was started at P18 and was maintained until P22, P25, P30, or P35 (Desai et al., 2002).
Electrophysiology.
Slices were prepared as described previously (Maffei et al., 2006). Visualized patch-clamp recordings were obtained from layer 4 pyramidal neurons in V1b. The center of V1b was identified using rat brain atlas coordinates adjusted for a lambda–bregma distance of 7.6 ± 0.2 mm as measured for P21–P28 rats. Slices were taken from 6.58 to 5.74 mm posterior to bregma, and neurons were selected at 3.81 mm lateral from the midline. Care was taken to record neurons within 100 μm of the expected center of V1b. The morphology of the white matter (WM) was used as an additional landmark. Identity and location of neurons was further confirmed post hoc. Miniature EPSCs (mEPSCs) or mIPSCs were pharmacologically isolated and recorded in voltage clamp (at −70 mV). Neurons with series resistance below 15 MΩ and changing <10% throughout the recording were used for analysis. Cumulative distributions of mEPSC amplitudes were obtained from 50 events for each neuron. The histograms and ranked distributions of mIPSCs were obtained for each neuron after the collection of 150 events. In experiments in which we tested the sensitivity of GABAA receptors to Zolpidem, slices were perfused with artificial CSF (ACSF) containing 0.2 μm Zolpidem after 10 min baseline acquisition. Five minutes into the perfusion of Zolpidem, mIPSCs were recorded for 10 min. The last 150 mIPSCs before and the 150 mIPSCs at the end of Zolpidem exposure were used for the analysis. To control for changes attributable to whole-cell washout, in a separate set of experiments mIPSCs were recorded for 30 min in ACSF alone. No more than 5% change in amplitude was observed. Kolmogorov–Smirnov tests were used to compare mIPSC amplitudes for baseline and Zolpidem. Neurons with p > 0.05 were considered Zolpidem insensitive. To identify putative populations of mIPSCs, the histogram (bin size, 1 pA) for each neuron included in the analysis was fitted with a Lavenberg–Marquard algorithm using a sum of two Gaussians. The fitting equation was as follows:
where b1 and b2 represent the mean of the two Gaussians. Only distributions with significantly different median values were considered bimodal (χ2 test, p < 0.05).
Statistical analysis.
Data are presented as mean ± SEM for the number of neurons indicated. To determine statistical significance, single-factor ANOVAs were run for each data set; if significant, this was followed by two-tailed Student's t tests and/or corrected for multiple comparisons using the Bonferroni method. For distributions with large deviations from normality, the nonparametric Mann–Whitney test was used. The Pearson χ2 test was used to determine significant differences in drug sensitivity among population of neurons. P values ≤0.05 were considered significant.
Solutions.
Regular ACSF included (in mm) 126 NaCl, 3 KCl, 2 MgSO4, 1 NaHPO4, 25 NaHCO3, 2 CaCl2, and 25 dextrose. AMPA-mediated mEPSCs were recorded in the presence of TTX, APV, and picrotoxin. mIPSCs were sampled in TTX, DNQX, and APV. The internal solution for mEPSCs contained 20 mm KCl, 100 mm K-gluconate, 10 mm HEPES, 0.2% biocytin, 4 mm Mg-ATP, 0.3 mm Na-GTP, and 10 mm Na-phosphocreatine. A symmetric chloride internal solution (in mm) was used for recording mIPSCs: 120 KCl, 10 HEPES, 4 Mg-ATP, 0.3 Na-GTP, 10 Na-phosphocreatine, and 0.5 EGTA.
Drugs.
To isolate mEPSCs, the ACSF contained 50 μm APV, 20 μm picrotoxin, and 0.1 μm TTX. For mIPSCs, picrotoxin was substituted with 20 μm DNQX.
Results
MD during the CP decreases visual responsiveness of neurons driven by the deprived eye (Frenkel and Bear, 2004), but the underlying cellular mechanisms are incompletely understood. Here we examined changes in excitatory and inhibitory drive onto pyramidal neurons within layer 4 in V1b after MD, just before and at the peak of the OD CP. AMPA mEPSCs and GABAA mIPSCs onto layer 4 pyramidal neurons were used to quantify the average postsynaptic strength of excitatory and inhibitory synapses. In different sets of experiments, sensory drive was reduced using either DT, to decrease visual drive uniformly to both eyes, or monocular eyelid suture (MD), to induce competitive rearrangements of cortical circuits. mIPSCs recorded from DT rats were compared with those from age-matched controls (control). mEPSCs and mIPSCs from neurons in slices contralateral to the deprived eye (Contra) were compared with those from sham-operated animals, littermates that were anesthetized but not monocularly deprived (sham).
Absence of MD-dependent changes in excitatory drive
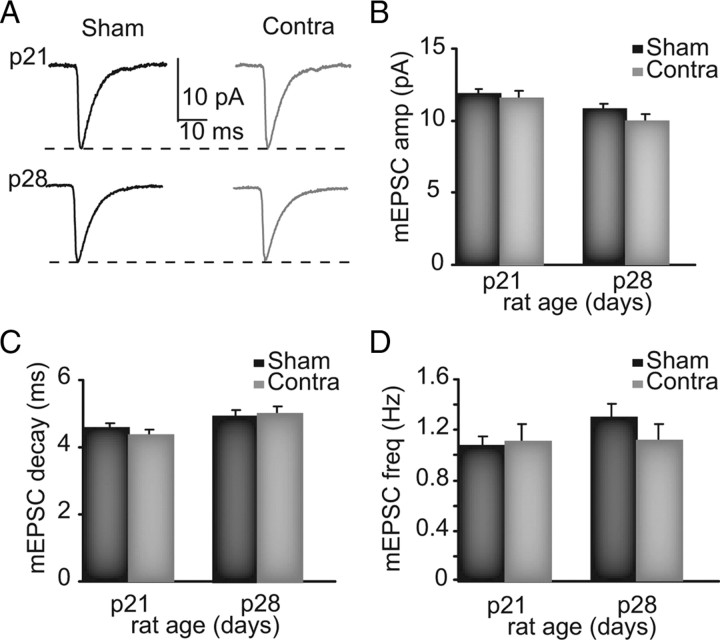
Current thinking about the effect of experience on cortical circuits is that MD induces postsynaptically expressed LTD of excitatory synapses in layer 4 (Crozier et al., 2007; Yoon et al., 2009). We tested this possibility by measuring amplitude, frequency, and kinetics of AMPA mEPSCs, to quantify the postsynaptic strength of the entire distribution of excitatory synapses onto pyramidal neurons. Lid suture was begun at P18 or at P25 and maintained for 2 full days, after which acute slices were prepared and the effect of development and MD at the beginning (P21) and at the peak (P28) of the CP for ODP was compared (Fagiolini et al., 1994). No significant changes dependent either on development or on experience were observed in mEPSC amplitude, frequency, decay, or charge between P21 (sham, n = 20) and P28 (sham, n = 20) (Fig. 1) and between sham and monocularly deprived (P21, Contra, n = 18; P28, Contra, n = 18) (Fig. 1). The large, unbiased sampling of these experiments suggests that brief MD at the beginning and at the peak of the CP does not affect quantal excitatory synaptic drive onto pyramidal neurons in the input layer of V1b.
Figure 1.
MD does not affect mEPSCs in V1b. A, Sample traces of sham and monocularly deprived (Contra) mEPSCs. B–D, Cumulative data for mEPSC amplitude, decay, and frequency from sham (black) and Contra (gray) neurons.
Developmental changes in inhibitory synaptic transmission
Inhibitory synapses are significantly affected by manipulations of sensory drive (Maffei and Turrigiano, 2008) and are known to regulate the beginning of the CP for ODP (Fagiolini and Hensch, 2000). In view of the lack of changes in excitatory drive, we turned our attention to the inhibitory circuit and its plasticity.
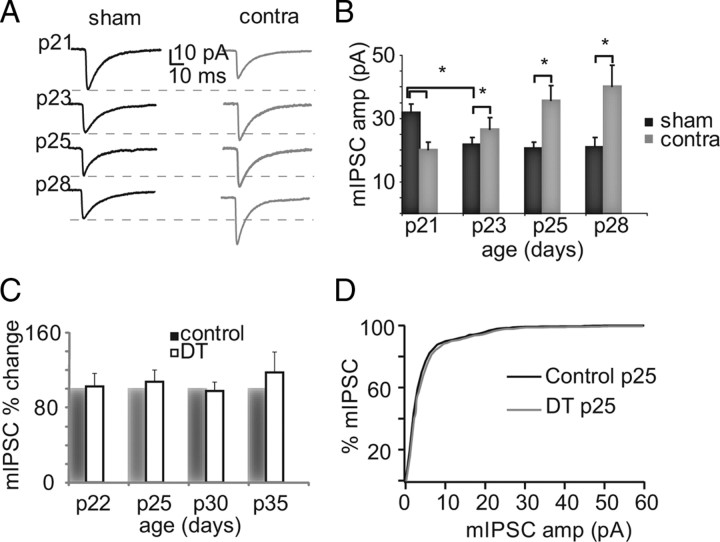
We first looked at developmental changes in mIPSCs from the beginning to the height of the OD CP (Fagiolini et al., 1994). There was a significant reduction in mIPSC amplitude (one-way ANOVA, p < 0.007) between P21 and P28 because of an initial decrease of 31.9 ± 7.2% between P21 and P23, after which amplitudes remained constant up to P28 (Fig. 2A,B), consistent with previous results (Heinen et al., 2004). There was also a small but significant increase in decay kinetics with a similar temporal pattern (one-way ANOVA, p < 0.02). No significant differences emerged in access and input resistance, mIPSC rise time, or frequency, suggesting that the changes in decay are not caused by electrotonic filtering. The changes in mIPSC amplitude suggest that inhibitory synapses undergo a developmental maturation right at the transition between the pre-CP and CP.
Figure 2.
Opposite plasticity of mIPSCs in pre-CP and the CP. A, Sample traces for sham and monocularly deprived (Contra) neurons at different ages. B, Bar plot summarizing the effect of MD in development. Asterisks indicate significant differences. C, Bar plot summarizing the effect of DT on mIPSCs. D, Cumulative distribution of mIPSC amplitudes for P25 control and DT rats.
To investigate the role of experience in regulating inhibitory synapses, we quantified the effect of 2 d MD just before or during the CP, to straddle the period when there is a developmental decrease in mIPSC amplitude. Two days of MD started at P18 reduced mIPSC amplitude by 29.8 ± 6.7% (sham, n = 40; Contra, n = 35; p < 0.001) (Fig. 2A,B), while leaving mIPSC frequency unaffected. This effect is in the same direction as the change in inhibition we observed in V1m when MD was started at P14 and might represent a compensatory response to lowered sensory drive (Maffei et al., 2004). Starting MD at P21 for 2 d produced a strikingly different effect: there was a significant increase in mIPSC amplitude (22.1 ± 13.7%; sham, n = 13; Contra, n = 11; p < 0.01) (Fig. 2A,B). A larger increase was observed if MD was started at P18 and protracted for 7 d until P25 (72.8 ± 13.0%; sham, n = 8; Contra, n = 8; p < 0.01) (Fig. 2A,B). The largest increase in mIPSC amplitude was observed when MD was started at P25 and maintained until the peak of the CP, at P28 (91.2 ± 16.7%; sham, n = 12; Contra, n = 11; p < 0.02) (Fig. 2A,B). No changes in mIPSC frequency were observed for any of the tested MD periods. Our results show that there is a sharp transition in the sign of inhibitory plasticity that coincides with the opening of the CP.
In addition to changes in amplitude, MD produced subtle changes in mIPSC decay at some developmental stages, but not others (ANOVA, p = 0.02). Two days of MD started at P18 increased the decay by 18.1 ± 6.3% (p < 0.025), whereas the time constant was not significantly affected for MD begun at P18 and maintained until P25 or started at P21 and maintained until P23. In contrast, a significant decrease in decay was observed for MD between P25 and P28 (34.4 ± 5.7%; p < 0.001). These changes are likely different from the developmental reduction in kinetics observed in layer 5 pyramidal neurons between P8 and P23 and attributed to an increased α1-containing GABAA receptor (Dunning et al., 1999), so the significance of these changes is not clear.
To determine whether experience-dependent plasticity of mIPSC amplitude in V1b requires competitive interactions between the two eyes, we tested the effects of uniform visual deprivation by keeping animals in the dark (DT) for different periods of time. mIPSC amplitude, frequency, and kinetics were compared between control and age-matched DT rats for increasing time intervals starting at P18. Recordings were performed at P22 (control, n = 8; DT, n = 8; p = 0.2), P25 (control, n = 9; DT, n = 9; p = 0.3), P30 (control, n = 6; DT, n = 6; p = 0.3), and P35 (control, n = 8; DT, n = 8; p = 0.9). No changes in mIPSCs were observed for DT of any duration (Fig. 2C,D), suggesting that, in V1b, inhibitory plasticity may require an imbalance in activity between the eyes.
MD and maturation of inhibitory synapses
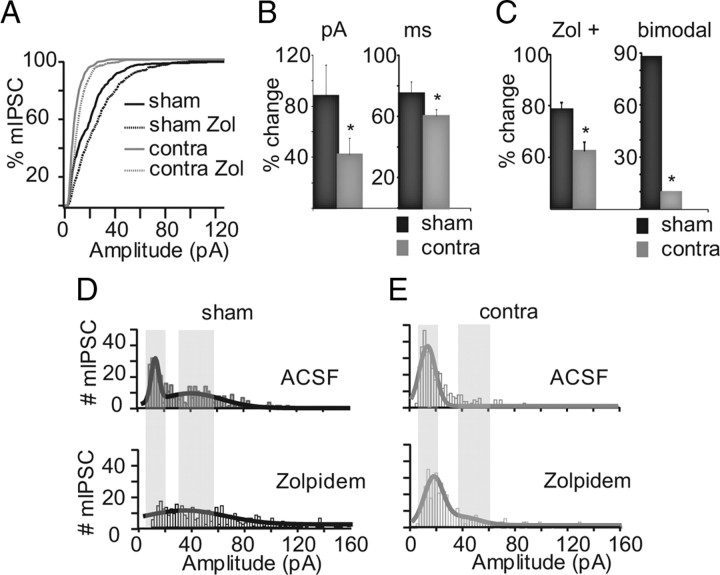
mIPSCs onto pyramidal neurons arise from a diverse set of inputs. To see whether we could identify distinct populations of mIPSCs, we examined the distribution of mIPSC amplitudes in sham and monocularly deprived animals. Our analysis focused on the distributions of mIPSCs obtained at P21 and P25, ages at which MD has opposite effects (Maffei et al., 2004, 2006). Distributions from sham neurons showed two clearly identifiable peaks both at P21 and P25 (Fig. 3D, 4D). At P21, the smaller peak was centered at 22.07 ± 5.6 pA, whereas the second peak was at 47.7 ± 7.8 pA (p < 0.01). Two-day MD shifted the distribution of mIPSCs toward smaller amplitudes (Fig. 3A,D,E); the first peak was centered at 13.5 ± 1.4 pA and was significantly smaller than the first peak in sham neurons (p < 0.01). No second peak was detected, and the MD histogram showed fewer large mIPSCs. Analysis of the distribution of events from individual neurons revealed that MD decreased the percentage of bimodal neurons (sham, 85.7%; Contra, 9.09%; Pearson χ2 test, p < 0.02) (Fig. 3C). These data suggest that MD between P18 and P21 reduces the strength of mIPSCs either by preventing the normal development of a second larger population of inhibitory synaptic inputs, or by selectively depressing this population.
Figure 3.
Pre-CP effect of MD on mIPSCs. A, Ranked distribution of mIPSC amplitude in sham (black solid), sham-Zolpidem (sham Zol; black dotted), monocularly deprived (contra; gray solid), and monocularly deprived-Zolpidem (contra Zol; gray dotted) animals. B, Bar plots summarizing changes in mIPSC amplitude (in picoamperes) and decay (in milliseconds) induced by Zolpidem in sham (black) and monocularly deprived (gray). C, Bar plot showing the percentage of neurons sensitive to Zolpidem (Zol+) and of bimodal neurons in sham (black) and monocularly deprived (gray). D, Distribution of mIPSC amplitude in sham (bin, 2 pA; top) and sham-Zolpidem (bottom). E, Distribution of mIPSC amplitude in monocularly deprived (top) and monocularly deprived-Zolpidem (bottom). Gray shades indicate peaks in the sham distribution in D. Asterisks indicate significant differences.
In cortical circuits, a switch in α subunit composition (from a prevalence of α3 and α5 to α1) is a hallmark of GABAA receptor maturation (Dunning et al., 1999), and α1-containing inhibitory synaptic transmission regulates the opening of the CP (Fagiolini et al., 2004). To ask whether MD affects the acquisition of α1 subunits, we took advantage of the well described effect of Zolpidem, a benzodiazepine with high affinity for GABAA receptor containing the α1 subunit (Mohler et al., 1992). Acute application of 0.2 μm Zolpidem is expected to increase mIPSC amplitude and decay kinetics at α1-containing inhibitory synapses. In sham neurons, Zolpidem increased the amplitude by 87.5 ± 24.9% (n = 14; p < 0.001) and decay by 75.1 ± 7.8% (p < 10−9), as expected. A close look at the distribution of mIPSC amplitudes showed that Zolpidem reduced greatly the leftmost peak of the distribution. Zolpidem had a smaller effect on mIPSC amplitude and decay in monocularly deprived neurons (amplitude: n = 12; 41.7 ± 13.5% increase; p < 0.04; decay: 60.36 ± 4.1% increase; p < 0.04). The first peak in the distribution showed a significant widening (p < 0.0001), mostly attributable to the emergence of a second peak in a small percentage of neurons (bimodality increased from 9.1 ± 0.3 to 27.3 ± 1.5%). Besides reducing the strength of inhibitory synapses, MD from P18 to P21 reduces mIPSC sensitivity to Zolpidem.
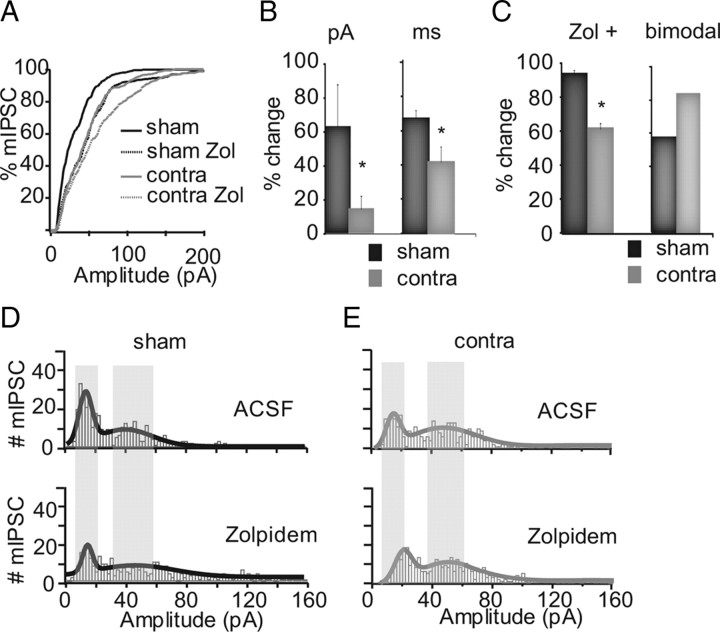
The same experimental approach was used to test the effect of development and experience on mIPSCs at P25 (MD started at P18). At P25, mIPSCs from both sham and monocularly deprived neurons had bimodal amplitude distributions (Fig. 4D,E). In sham neurons, the median of the small peak was at 12.4 ± 2.6 pA and of the larger peak at 39.2 ± 7.6 pA; the small peak of monocularly deprived neurons was at 15.7 ± 4.9 pA, and the larger peak was at 50.1 ± 9.3 pA. The MD-dependent increase in mIPSC amplitude at P25 depended on a significant decrease in the fraction of small events and the increase in that of large events (Fig. 4D,E, top). Acute application of 0.2 μm Zolpidem showed that 93.3 ± 3.4% of sham neurons were affected by the drug, suggesting that most layer 4 pyramidal neurons had α1-containing GABAA receptor. Zolpidem increased mIPSC amplitude by 61.7 ± 23.4% (n = 8; p < 0.03) and slowed mIPSC decay by 70.7 ± 6.9% (p < 10̂−6). The distribution of mIPSCs was also affected by Zolpidem: the height of the leftmost peak was decreased (p < 0.0008), and the rightmost peak was widened (p < 0.0001). As for 2 d MD from P18 to P21, 7 d MD significantly reduced Zolpidem sensitivity (Fig. 4B). Only 61.1 ± 3.4% of the recorded neurons showed significant changes in amplitude and/or decay after acute application of the drug (amplitude: 13.5 ± 7.6% increase; n = 10; p < 0.045; decay: 41.4 ± 10.4% increase; p < 10̂−7). Zolpidem widened the rightmost peak of the monocularly deprived distribution (Fig. 4D, bottom) (p < 0.02) and shifted the median of the leftmost peak while leaving its height and width unaffected (Fig. 4D,E, bottom). Thus, MD reduces Zolpidem sensitivity independently of the direction of inhibitory plasticity.
Figure 4.
CP effect of MD on mIPSCs. A, Ranked distribution of mIPSC amplitude in sham (black solid), sham-Zolpidem (sham-Zol; black dotted), monocularly deprived (contra; gray), and monocularly deprived-Zolpidem (contra Zol; gray dotted) animals. B, Bar plots with changes in mIPSC amplitude (in picoamperes) and decay (in milliseconds) induced by Zolpidem in sham (black) and monocularly deprived (gray). C, Bar plot showing the percentage of neurons sensitive to Zolpidem (Zol+) and of bimodal neurons in sham (black) and monocularly deprived (gray). D, Distribution of mIPSC amplitude in sham (top) and sham-Zolpidem (bottom). E, Distribution of mIPSC amplitude in monocularly deprived (top) and monocularly deprived-Zolpidem (bottom). Gray shades indicate peaks in the sham distribution in D. Asterisks indicate significant differences.
Discussion
We have shown that in layer 4 of V1b, manipulation of sensory experience induces plasticity of inhibition that is profoundly developmentally regulated. MD during the pre-CP reduces mIPSC amplitude onto pyramidal neurons, whereas MD during the CP increases it. The transition between reduction and potentiation is sharp and coincides with the opening of the OD CP (Fagiolini et al., 1994). These characteristics suggest that changes at inhibitory synapses that determine the switch in sign of inhibitory plasticity also contribute to the initiation of the OD CP.
Similarly to what we observed in V1m (Maffei et al., 2006), brief MD during the CP induced no significant changes in mEPSCs onto layer 4 pyramidal neurons, suggesting that the basal strength of intracortical excitatory synaptic transmission was not affected. This is in marked contrast with the hypothesis that MD induces a postsynaptically expressed LTD (Crozier et al., 2007; Yoon et al., 2009). The evidence in favor of LTD was obtained using WM extracellular stimulation and showed that MD occludes the induction of LTD in layer 4 while leaving basal excitatory synaptic transmission unaffected. It is not clear what inputs are activated by WM stimulation, but if there is a selective depression of thalamic inputs, this population would not necessarily be reflected in our mEPSC recordings, as thalamic synapses represent a small fraction of inputs onto layer 4 pyramidal neurons. The lack of change in mEPSCs, coupled to the increase in mIPSC amplitude, suggests that MD during the CP shifts the net balance between excitation and inhibition toward inhibition. Similarly to what we observed in V1m, the enhancement of inhibition could decrease pyramidal neuron excitability and possibly reduce the amplitude of visually evoked potentials, contributing to the loss of visual responsiveness induced by brief MD (Frenkel and Bear, 2004).
The transition from pre-CP to CP plasticity of inhibition is accompanied by developmental changes in mIPSCs. mIPSCs undergo a reduction in amplitude and an increase in decay right at CP onset. Together with the change in sign of inhibitory plasticity, these data show that CP onset is associated with profound developmental changes in inhibition onto layer 4 pyramidal neurons. Whether the maturational changes in inhibition are causally related to the change in sign of plasticity is unclear. Recent data show that a determining factor for the opening of the CP is the maturation of inhibitory inputs that express GABAA receptors containing α1 subunits (Fagiolini et al., 2004), a configuration responsible for the sensitivity to Zolpidem of mature GABAergic synapses. Our data show that at the end of the pre-CP, ∼80% of neurons have Zolpidem-sensitive mIPSCs (Fig. 3C), suggesting that most of the transition to α1-containing GABAA receptors has already taken place. The prevalence of Zolpidem-sensitive mIPSCs increases further, to over 90%, well into the CP. Our data show that MD during the pre-CP and CP produced a modest reduction in the proportion of Zolpidem-sensitive neurons. This suggests that activity-dependent changes in Zolpidem sensitivity, and, by extension, changes in subunit composition, are independent of the plasticity of mIPSC amplitude we observe. It is likely that the opening of the ODP CP involves a complex set of changes and may include the switch in sign of mIPSC plasticity we describe here.
We showed previously that in V1m, MD induces bidirectional plasticity at unitary inhibitory synapses from FS inhibitory neurons onto star pyramidal neurons in layer 4: depression if MD was started during the pre-CP and potentiation if MD was started during the CP. While it is tempting to suggest that the bidirectional plasticity of mIPSCs in V1b we document here resembles that observed in V1m, there are important differences. Most strikingly, inhibitory plasticity of FS synapses in V1m was induced by uniform deprivation (Maffei et al., 2004, 2006). In V1b, our data suggest that plasticity of mIPSCs requires an imbalance in activation of the two eyes. Thus, the underlying plasticity mechanisms in V1m and V1b may be different. Furthermore, here we examine mIPSCs that sample postsynaptic strength and arise from a heterogeneous set of inputs, whereas in V1m we sampled from a specific class of inputs, and whether plasticity was expressed presynaptically or postsynaptically was not clear (Maffei et al., 2006). Although the underlying inhibitory plasticity mechanisms may be distinct, what unifies our results from V1m and V1b is that, in both cortical areas, MD before the OD CP shifts the balance between excitation and inhibition toward excitation, whereas MD during the CP shifts this balance toward inhibition.
In V1b, MD affected the mean amplitude and the distribution of mIPSC amplitudes. At both ages examined, sham animals had two clear peaks, with the second peak at roughly double the amplitude of the first. The two peaks could represent inputs arising from different classes of interneuron (Maffei et al., 2004), multiquantal release from some synaptic sites, or other factors. MD during the pre-CP reduced mIPSC amplitude by suppressing the larger peak and enhancing the smaller. Interestingly, MD maintained into the CP had the opposite effect, reducing the fraction of small-amplitude events and enhancing the larger-amplitude peak. MD may bidirectionally regulate average mIPSC amplitude either by shifting inputs between these two pools, or by reciprocally suppressing and enhancing inputs in the two pools.
Classical CP ODP opening correlates well with the timing of MD-dependent potentiation of inhibitory synapses reported here (Fagiolini et al., 1994). First, in rats, no ODP is observed for MD lasting before the fourth postnatal week, a time period during which we observe depression of mIPSCs. Second, OD shifts can be rapidly induced by 2 d MD at the height of the OD CP. Consistent with this, we observed that 7 d of deprivation were necessary to induce potentiation of inhibitory synapses if MD was started before P21, but during the time window between the expected beginning and the peak of the CP, 2 d of MD were sufficient to increase the amplitude of mIPSCs. Furthermore, the magnitude of the potentiation increases closest to the peak of the CP.
Another similarity with ODP is that plasticity of mIPSCs was specific to MD and was not induced by DT. DT started at P18 and maintained for intervals of increasing duration up to P35 did not affect mIPSCs in layer 4, consistent with previous data (Heinen et al., 2004). This suggests that mIPSC plasticity depends either on competitive interactions or on differences in the way these two deprivation paradigms affect cortical activity and thus in the plasticity mechanisms that are engaged. The lack of inhibitory plasticity after DT correlates well with the lack of ODP in similar experimental conditions (Blais et al., 2008), raising the possibility that changes in inhibition may contribute to ODP.
Our data show that changes in sign of inhibitory plasticity correlate with the opening of the OD CP and suggest that mechanisms regulating the sign of inhibitory plasticity during development could play an important role in the functional changes in V1 induced by sensory deprivation.
Footnotes
This work was supported by National Institutes of Health (NIH)/National Eye Institute Grant EY 014439 (G.G.T., M.E.L.) and NIH Training Grant T32 NS07292-21 (A.M.). We thank Dr. Alfredo Fontanini for useful comments.
References
- Blais B, Frenkel M, Kuindersma S, Muhammad R, Shouval H, Cooper L, Bear M. Recovery from monocular deprivation using binocular deprivation. J Neurophysiol. 2008;100:2217–2224. doi: 10.1152/jn.90411.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozier R, Wang Y, Liu C, Bear M. Deprivation-induced synaptic depression by distinct mechanisms in different layers of mouse visual cortex. Proc Natl Acad Sci U S A. 2007;104:1383–1388. doi: 10.1073/pnas.0609596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai N, Cudmore R, Nelson S, Turrigiano G. Critical periods for experience-dependent synaptic scaling in visual cortex. Nat Neurosci. 2002;5:783–789. doi: 10.1038/nn878. [DOI] [PubMed] [Google Scholar]
- Dunning D, Hoover C, Soltesz I, Smith M, O'Dowd D. GABA(A) receptor-mediated miniature postsynaptic currents and alpha-subunit expression in developing cortical neurons. J Neurophysiol. 1999;82:3286–3297. doi: 10.1152/jn.1999.82.6.3286. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Hensch T. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404:183–186. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Pizzorusso T, Berardi N, Domenici L, Maffei L. Functional postnatal development of the rat primary visual cortex and the role of visual experience: dark rearing and monocular deprivation. Vision Res. 1994;34:709–720. doi: 10.1016/0042-6989(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Fritschy J, Löw K, Möhler H, Rudolph U, Hensch T. Specific GABAA circuits for visual cortical plasticity. Science. 2004;303:1681–1683. doi: 10.1126/science.1091032. [DOI] [PubMed] [Google Scholar]
- Frenkel M, Bear M. How monocular deprivation shifts ocular dominance in visual cortex of young mice. Neuron. 2004;44:917–923. doi: 10.1016/j.neuron.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Heinen K, Bosman L, Spijker S, van Pelt J, Smit A, Voorn P, Baker R, Brussaard A. GABAA receptor maturation in relation to eye opening in the rat visual cortex. Neuroscience. 2004;124:161–171. doi: 10.1016/j.neuroscience.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Hensch T, Fagiolini M. Excitatory-inhibitory balance and critical period plasticity in developing visual cortex. Prog Brain Res. 2005;147:115–124. doi: 10.1016/S0079-6123(04)47009-5. [DOI] [PubMed] [Google Scholar]
- Maffei A, Turrigiano G. The age of plasticity: developmental regulation of synaptic plasticity in neocortical microcircuits. Prog Brain Res. 2008;169:211–223. doi: 10.1016/S0079-6123(07)00012-X. [DOI] [PubMed] [Google Scholar]
- Maffei A, Nelson S, Turrigiano G. Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nat Neurosci. 2004;7:1353–1359. doi: 10.1038/nn1351. [DOI] [PubMed] [Google Scholar]
- Maffei A, Nataraj K, Nelson S, Turrigiano G. Potentiation of cortical inhibition by visual deprivation. Nature. 2006;443:81–84. doi: 10.1038/nature05079. [DOI] [PubMed] [Google Scholar]
- Mohler H, Benke D, Mertens S, Fritschy J. GABAA-receptor subtypes differing in alpha-subunit composition display unique pharmacological properties. Adv Biochem Psychopharmacol. 1992;47:41–53. [PubMed] [Google Scholar]
- Tagawa Y, Kanold P, Majdan M, Shatz C. Multiple periods of functional ocular dominance plasticity in mouse visual cortex. Nat Neurosci. 2005;8:380–388. doi: 10.1038/nn1410. [DOI] [PubMed] [Google Scholar]
- Yoon B, Smith G, Heynen A, Neve R, Bear M. Essential role for a long-term depression mechanism in ocular dominance plasticity. Proc Natl Acad Sci U S A. 2009;106:9860–9865. doi: 10.1073/pnas.0901305106. [DOI] [PMC free article] [PubMed] [Google Scholar]