Abstract
The circadian clock regulates many aspects of physiology including cardiovascular function. Internal oscillators exist in endothelial, smooth muscle cells and fibroblasts of the vasculature. Vascular tone and thrombus formation – two key elements of vascular function in regard to adverse cardiovascular events - exhibit diurnal rhythmicity. In this review, we describe changes in vascular function that result from genetic disruption of discrete elements of the circadian clock.
Keywords: circadian, vasculature, thrombogenesis, vasoreaction
Introduction
Several aspects of cardiovascular physiology and the incidence of cardiovascular events, such as sudden cardiac death, myocardial infarction, unstable angina, ventricular tachycardia and ischemic and hemorrhagic stroke, are subject to diurnal variation, peaking in the early morning hours.1, 2 The early morning surge in blood pressure, accompanied by a decline in endothelial function, coincides with the peak incidence in clinical cardiovascular events.3, 4 The corresponding oscillations in gene and protein expression of known regulators of vascular physiology highlights the potential importance of the vascular clock in the described diurnal variation of the incidence of cardiovascular events.5, 6
Blood vessels are composed of three major layers. The inner layer is composed of a monolayer of endothelial cells that forms a barrier between the artery wall and the circulating blood. Endothelial cells determine blood-tissue permeability, control vascular tone and regulate the properties of the vascular surface with regard to hemostasis. Endothelial cells release nitric oxide (NO), which activates an enzymatic cascade in the smooth muscle cell that results in smooth muscle relaxation and reduced vascular tone. Other molecules produced by the endothelial cells, such as endothelin-1 and angiotensin-II, act to contract smooth muscle cells. The endothelial surface contains a set of factors that regulate platelet adhesion, coagulation (thrombomodulin) and fibrinolysis (plasminogen activators, inhibitors). The medium layer of the blood vessel consists mainly of smooth muscle cells, which are responsible for vasoconstriction and vasodilation. Endothelial cells, circulating mediators and the sympathetic nervous system, regulate smooth muscle tone. Finally, the outer layer is a connective tissue structure that fuses the vessel with connective tissue from the surrounding organs. The outer layer contains fibroblasts, which, together with endothelial cells, plays a critical role in the angiogenic process.
The oscillator exists as a self sustained transcriptional-translational circuit consisting of positive and negative loops. This circuit creates a rhythm in gene expression with a period of approximately (circa-) one day (dies), which drives circadian rhythms and adapts the physiology of an organism to its needs in an anticipatory manner. The organization of physiology appropriately to adapt to changes in the timing of recurring events (e.g. sunrise, the time of food availability) lies in the ability of the circadian oscillator to synchronize its phase in response to external cues. Importantly, the increased frequency of disorders such as obesity and the metabolic syndrome among night-shift workers and humans with sleeping disorders suggest broader involvement of the circadian clock in chronic disorders of physiology.7 The molecular core of the circadian clock consists of a negative feedback loop comprised of a positive limb of basic helix loop helix (bHLH) transcription factors Bmal1, Clock and Npas2 and a negative limb of regulatory proteins period (Per) 1,2,3 and cryptochrome (Cry) 1,2.8 Heterodimers of Bmal1 with Clock or Npas2 act as activators and drive transcription through E-boxes located within the promoters of Per and Cry genes. Post-translational modifications regulate Per and Cry proteins which then feedback and inhibit the positive limb, resulting in rhythmic oscillation of clock components. At the same time, additional feedback loops participate in the core of the circadian clock. Bmal1, Clock/Npas2 heterodimers drive the transcription of the nuclear receptors Rev-Erbα and ROREα, which in turn repress or activate Bmal1 transcription respectively. The positive limb of the oscillator regulates not only the transcription of clock components, but also a significant percentage of the transcriptome, imposing a rhythm in cellular physiology by creating a rhythm in gene expression.9
The oscillator exists in every cell and tissue examined with the exception of the testis.8, 10 The circadian system is largely organized in a hierarchical manner. Surgical ablation of the suprachiasmatic nucleus (SCN) in the hypothalamus ablates all hormonal and activity rhythms, suggesting the existence of a master circadian pacemaker located in the SCN.11 Circadian clocks have been described in many different peripheral tissues and evidence for the tissue-specific role of the oscillator is beginning to accumulate. In this review we describe aspects of vascular physiology that display circadian rhythms and provide the evidence that support a role for the circadian clock in the regulation of vascular function.
Vascular function exhibits diurnal rhythmicity
The existence of a circadian rhythm in the function of human blood vessels has long been recognized.12 Two aspects of vascular function, vascular tone and thrombus formation have been studied in respect to the daily cycle. We have previously described a circadian variability in both sympathetic tone, and vascular reactivity to adrenergic receptor agonists.13 Other studies have shown that vascular tone exhibits a circadian variation in humans.14 Blood flow and vascular resistance in the forearm and the vasodilator response to phentolamine (an α-adrenergic-antagonist) but not to sodium nitroprusside (a direct vasodilator) varies at different times of the day.14 Flow-mediated dilation of brachial artery, a widely used clinical index of endothelial function, displays diurnal variation when examined in healthy young men.15 The sensitivity of aortic preparations to either vasoconstrictor or vasorelaxing agents is different at different times of the daily cycle, with both endothelium-dependent and -independent relaxations being more pronounced at 03:00 than at other times of the day.16 In support of the latter, endothelial nitric oxide synthase (eNOS) activity exhibits circadian variation, possibly as a result of a variation in its state of phosphorylation.17-20
Platelet aggregation and the thrombus formation subsequent to plaque rupture may precipitate vascular occlusion and resultant tissue infarction and ischemia. Although there have been reports of diurnal variation in platelet aggregability ex vivo,21 the relationship of these observations (prone as they are to artifact ex vivo, attributable in part to diurnal variation in extracellular fluid volume in vivo) to actual platelet activation in vivo is unknown. However, factors external to the platelet—such as plasminogen activator inhibitor (PAI)-1 and tissue plasminogen activator (tPA), which are produced by the vascular endothelium and do indeed oscillate—may influence platelet activation in vivo. PAI-1 and tPA are produced by the vascular endothelium and show diurnal variability throughout the day-night cycle. Other mediators of the hemostatic system display diurnal variation, including coagulation factors (II, VII, X, and tissue factor pathology inhibitor [TFPI]).22-24 Fibrinogen, the circulating precursor of fibrin (a clot stabilizing protein), displays circadian variation in humans,25 with a peak in the early morning. We recently showed that the time to thrombotic vascular occlusion in response to a photochemical injury displays diurnal variation.26
A functional circadian clock exists within different cellular elements of the vasculature
The first molecular evidence of the existence of a circadian clock in the vasculature came with the identification of rhythmic oscillation in gene expression of clock components in mouse aortas isolated at different times throughout the 24-hour period.27-30 The existence of a functional clock was consequently demonstrated by Davidson et. al.31 who discovered rhythmic luciferase activity driven by the Per1 gene promoter in veins and arteries cultured from the transgenic Per1-luciferase rat. This in vivo identification of a circadian clock was accompanied by in vitro evidence of the existence of the clock in vascular fibroblasts, smooth muscle and endothelial cells. Fibroblasts in culture show a circadian pattern of expression for all circadian clock components after synchronization with serum that phase aligns the cells in culture.32, 33 The rhythmic pattern of expression persists for more than 20 days in culture, suggesting the existence of self-sustained circadian clocks in fibroblasts.34 Dramatic oscillations in circadian clock components have been observed in vascular smooth muscle cells (VSMC) throughout the 24-hour period.27, 28, 35 Synchronization of hemangioendothelioma cells in culture with serum resulted in circadian expression of clock genes, providing evidence for the existence of a circadian clock in vascular endothelial cells.36 Although we identified rhythmic expression of Bmal2 and Per2 in VSMC in culture following serum shock,27 we failed to detect rhythmic oscillations of clock components in endothelial cells isolated from aorta after serum shock.26
The oscillations in Per1/2, Cry1/2 mRNA levels in the mouse aorta peak during early circadian night (the time period during constant darkness that corresponds to the night period of a regular day-night cycle), whereas Bmal1 and Npas2 peak at the beginning of circadian day (the time period under constant darkness corresponding to the day period of a regular day-night cycle). These findings indicate that the circadian oscillator in aorta is in temporal phase alignment with the master circadian oscillator in the SCN in mice. Cultures of vascular smooth muscle cells also show an inverse phase between Per, Cry and Bmal1 gene expression (Figure 1). It is still unknown whether individual cells in the vasculature communicate timing information to each other. Single cell recordings and co-culture experiments indicate that cultured fibroblasts do not influence each other's rhythms to any measurable degree.33 However, different tissue explants harvested from the same SCN-lesioned animal display circadian oscillations with different phases and period lengths.34 This suggests that intercellular communication within an organ may exist. Further evidence of intercellular communication of timing information comes from the intercellular coupling of SCN neurons that is essential for synchronization and robustness of cellular oscillators.37
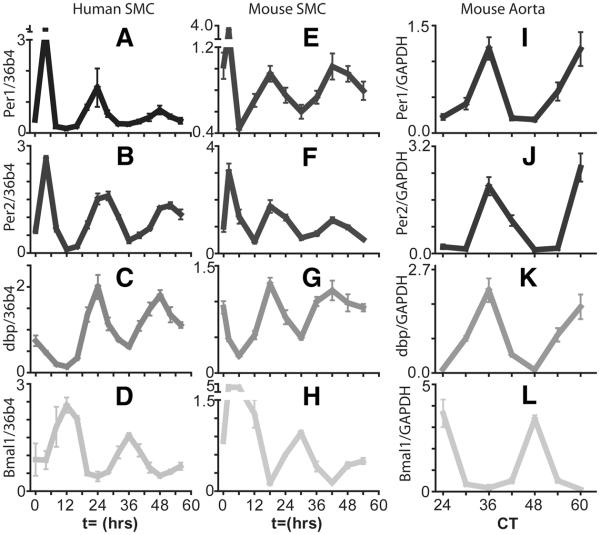
Figure 1. Accumulation of circadian clock gene transcripts in human and mouse aortic smooth muscle cells (SMC) and mouse aorta.
In the mouse aorta, clock gene mRNA transcripts Per2, Bmal1, and clock output gene dbp display circadian oscillations. Clock and Bmal1 heterodimers drive transcription of Per2 and dbp by binding to E box consensus sequences in their promoters. The peak in Per2 and dbp expression is observed around CT36 corresponding to the transition between the light and dark period. Bmal1 expression is driven by RORα acting at RRE sequences and subsequently repressed by RevErbα. The peak in Bmal1 expression is observed at CT24 corresponding to the transition from the dark phase to the light phase. Mouse aortas were harvested in constant darkness. Clock gene mRNA transcripts similarly display circadian rhythmicity in human and mouse aortic smooth muscle cells after treatment with 50% serum. The phase of the rhythms of these cells cultured in vitro is expected to be different to the in vivo rhythm due to the effect of the autonomous nervous system. Oscillation of the clock output gene dbp in smooth muscle cells is in phase with Per2 with a peak at t=24 hour post serum shock in human cells. Per2 and dbp peak slightly earlier (close to t=18 hours) in mouse smooth muscle cells. Expression of Per2, Bmal1, and dbp was monitored by qPCR. From Ref. 35 with permission.
As we already mentioned, the circadian oscillator of the aorta is in temporal phase alignment with the SCN, suggesting that as with the other peripheral oscillators, the SCN provides synchronizing cues to the circadian clock of the vasculature. However, the oscillator of the vasculature responds to signals other than the ones coming from the SCN. These signals may entrain the oscillators in the vasculature out of phase with the SCN. We have shown previously that retinoic acid and the synthetic glucocorticoid, dexamethasone, phase shift Per2 mRNA rhythms in human vascular smooth muscle cells.27 Our findings suggest that periodic availability in steroid hormones and vitamins, such as retinoids, might phase shift or reset the peripheral clocks of cells in the vasculature.
Vascular clock output
The circadian oscillator of any cell regulates the expression of a number of genes - called circadian clock output genes - resulting in rhythms in physiological processes. It is quite possible that molecular clocks within the different cell types of a vessel may express different circadian output genes. According to several microarray studies38-40 clock output genes vary widely between different cell types in different tissues, suggesting a tissue-specific role of clocks. This is consistent with the possibility that the genes under circadian control may differ between the different cells types of the vascular bed.
We examined the rhythmic expression of genes in the thoracic aorta using high density arrays.40 Genes relevant to protein folding, protein degradation, glucose and lipid metabolism, adipocyte maturation, vascular integrity and the response to injury demonstrated a circadian pattern of oscillation in mouse aorta. The number of genes exhibiting circadian expression (307 genes out of approximately 8000 probe sets examined) corresponds to the 5-10% of the transcriptome reported to oscillate in other tissues. The genes with an oscillating pattern of expression in aorta provide a list of candidate genes under the control of the vascular clock. Studies in vascular endothelial cells have identified Pai-1 and thrombomodulin as targets of the circadian clock of the vascular endothelium.36, 41, 42 The Pai-1 gene contains two consensus E-boxes in its promoter, and is driven by the endothelial specific circadian heterodimer Bmal2:Clock,42 and also Bmal1:Clock.41 The Bmal2:Clock heterodimer binds directly to the E-box of the thrombomodulin promoter and drives its rhythmic expression in vascular endothelial cells.36 The above findings suggest a role of the vascular clock in a variety of vascular functions including pressor responses and thrombogenesis.
Evidence for the importance of circadian clocks in vascular function
The evidence to support a role of the circadian clock in vascular function comes primarily from animal models with genetic deletion of components of the circadian clock. The sensitivity of aorta to vasoactive agents is described to vary according to time of day.16 This variation is altered in mice with mutated circadian clock elements, while the downstream effector response to nitric oxide, through guanylyl cyclase, remains intact.17, 43 The latter is consistent with observations in humans demonstrating that the response to sodium nitroprusside does not vary according to time of day.14 In individuals with compromised endothelial function, this diurnal variation in vascular endothelium-dependent vasodilatation is blunted.44 Endothelium-dependent relaxations are reduced in Per2 mutant mice and the diurnal variation that the relaxations normally exhibit is absent in the mutant mice. Aortic rings isolated from Per2 mutant mice exhibit impaired endothelium-dependent relaxations to acetylcholine,43 which stimulates NO release from the endothelium via activation of endothelial acetylcholine-M3 receptor.45 The expression of endothelial acetylcholine-M3 receptor or eNOS is not altered in the aortas of Per2 mutant mice. Moreover, the endothelium-dependent relaxations to ionomycin, which stimulates eNOS enzymatic activity via non-receptor-mediated increase in intracellular Ca2+ concentration, are also reduced significantly in the Per2 mutant mice during the inactive phase of the daily cycle. This reduced response to ionomycin is improved by the cyclooxygenase (COX) inhibitor indomethacin, suggesting an increase in COX-derived vasoconstrictors in the Per2 mutants. Aortas from Per2 mutant mice express significantly higher COX-1 and indomethacin is able to abolish the differences in endothelium-dependent responses to ATP described in aortic rings from Per2 mutant mice.43 Recent data are also consistent with impaired eNOS signaling and endothelial function in mice with dysfunctional circadian clocks.17, 19 eNOS expression is not modified in these mice, but there is evidence that post-translational mechanisms regulating eNOS activity are compromised, consistent with observations demonstrating that eNOS activity exhibits a circadian variation,20 which maybe a consequence of its phosphorylation state.17-19
Changes in catecholamine production in mice with dysfunctional circadian oscillators may also contribute to the abnormal vasoactive responses described in these mice. Bmal1 knockout and Npas2 mutant mice46 and endothelial cell-specific PPARγ (Peroxisome Proliferator-Activated Receptor γ) knockout mice which have reduced Bmal147 all have substantially reduced levels of norepinephrine and epinephrine in plasma, both at night and during the day. The catecholamine-mediated vasoconstriction response to an α-1-adrenergic receptor agonist is suppressed in Cry-deficient mice.48 This suppression of the α-adrenoceptor response may be caused by an impaired intracellular pathway for α-adrenoceptor-mediated contraction of vascular smooth muscle cells, including reduced expression of α-adrenoceptors. This result is consistent with the possibility that Cry genes in the vasculature contribute to circadian changes in arterial blood pressure regulation by modulating α-adrenoceptor-mediated vasoconstriction in the peripheral vessels. However, it is possible that dysregulation of the clock in the vasculature results from a depletion of sympathetic nervous input after disruption of the central circadian clock in the Cry knockout mice.
The impaired vascular responses in mice with dysfunctional clocks are reflected in the blood pressure of these mice. Transgenic mouse models with disrupted circadian clocks exhibit alterations in blood pressure and its variability over time.46, 48 Bmal1 knockout mice have reduced mean arterial pressure especially during the active phase of the day.46 Bmal1 deletion alters the expression of catechol-O-methyl transferase (comt) in aorta, an enzyme that accounts for an extraneuronal low-affinity high-capacity “sink” for clearance of catecholamines.46 Comt participates in the regulation of blood pressure, with comt-deficient mice being resistant to salt induced hypertension.49 Kininogen, another gene of potential relevance to blood pressure control,50 was also dysregulated in the aorta of Bmal1 knockout mice.46 Similar to the universal Bmal1 knockout mouse, deletion of Bmal1 specifically in vascular endothelium leads to a reduction of blood pressure during the active phase of the day and increased heart rate throughout the 24 hr cycle without changes in plasma catecholamines, nitric oxide biosynthesis or fibrinolytic efficiency.26 In agreement with the findings from Bmal1 deletion, Npas2 mutant mice46 and Per2 mutant mice43 had reduced mean arterial pressure, irrespective of clock time. On the other hand, the circadian variation in blood pressure was lost in Cry1/Cry2 double knockout mice48 and Clock mutants46 without changes in overall pressure levels. In addition to those models of disrupted clock function, deletion of PPARγ in vascular endothelial and smooth muscle cells resulted in a reduction of Bmal1 expression and blunting of the diurnal variation of blood pressure.47 The endothelial cell-specific deletion of PPARγ led to a reduction in blood pressure only during the active phase similar to the reduction described in the endothelial-specific knockout of Bmal1. Interestingly, the smooth muscle cell-specific deletion of PPARγ resulted in an increase of the blood pressure during the resting phase with no change during the active phase.47
The endothelial dysfunction observed in mice with dysfunctional clocks may reflect increased vascular senescence.19 The ability of endothelial cells from Per2 mutant mice to proliferate and form vascular networks is substantially reduced.19 Reduced proliferative capacity is a marker of increased senescence of endothelial cells. Moreover, aortas and endothelial cells isolated from Per2 mutant mice show increased senescence as assessed by β-galactosidase activity, accompanied by increased Akt signaling. Inhibition of Akt signaling restored the impaired vascular network formation and endothelial cell proliferation in Per2 mutant mice. On the other hand, overactivity of Akt has been linked to increased vascular senescence through increased reactive oxygen species generation and decreased NO bioavailability, as well as impaired angiogenesis.51, 52 Per2 mutant mice show decreased angiogenesis in two different in vivo experimental models. Incorporation of hemoglobin, which correlates with vessel formation, after subcutaneous implantation of matrigel is reduced in Per2 mutant mice compared to wild-type mice.19 In a second model, Per2 mutants show autoamputation and impaired blood flow recovery in response to ischemia characterized by a smaller increase in endothelial progenitor cells.19 Bone marrow transplantation experiments showed reduced blood flow recovery to ischemia in mice receiving bone marrow from Per2 mutants, suggesting a role of bone marrow derived endothelial progenitor cells in ischemia-induced neovascularization.19 However, blood flow recovery in Per2 mutant mice receiving bone marrow from wild-type mice was only partially restored to wild-type levels, suggesting that impaired intrinsic vascular function, in addition to impaired endothelial progenitor cells function, contributes to the altered angiogenic response and autoamputation in Per2 mutants. In support of a role for endothelial progenitor cells in ischemia-induced neovascularization, infusion of wild-type endothelial progenitor cells in Per2 mutant mice restores ischemia-induced neovascularization and blood flow recovery. It is known that circadian rhythms affect stem cell function, thus disruption of circadian genes is likely to affect the function of bone marrow-derived cells, including endothelial progenitor cells.53, 54 Both increased endothelial senescence and decreased endothelial progenitor cell mobilization may contribute to the autoamputation observed in Per2 mutant mice.
Recently, we showed that deletion of Bmal1 specifically in the vascular endothelium results in loss of the temporal pattern in susceptibility to thrombotic vascular occlusion, as assessed by a vessel injury model.26 The diurnal variation in the time to thrombotic vascular occlusion in response to photochemical injury was also completely abolished in Clock mutant mice, further supporting a role of the circadian clock.26 The deletion or mutation of clock components not only abolished the diurnal variation in thrombogenesis but also had an impact on the functional response of the endothelium. The time to thrombotic vascular occlusion (TTVO) was shorter in mice with endothelial deletion of Bmal1. A shorter TTVO was also observed in global Bmal1 knockout mice. The observed difference in TTVO does not appear to be due to alterations in arterial blood flow because overall baseline blood flow was not altered in mice with both global and endothelial specific deletion of Bmal1. On the other hand, Clock mutant and Npas2 knockout mice had a significantly longer time to thrombotic vascular occlusion. The difference between Clock mutant, Npas2 knockout mice and mice with endothelial specific deletion of Bmal1 in TTVO may be attributed to the lower plasma PAI-1 levels in Clock mutant and Npas2 knockout mice. Mice with endothelial specific deletion of Bmal1 have normal PAI-1 and tPA plasma levels.26
The role of the circadian clock in vascular function was recently investigated in a model of blood flow reduction. Arteries of young Bmal1 knockout mice were unable to adapt to chronic reduction of blood flow by inward luminal remodeling.17 This response of the arteries of Bmal1 knockout mice was accompanied by a substantial increase in collagen deposition in the medial layer and increased thickening of the arterial wall. Older Bmal1 knockout mice exhibited a significant susceptibility to thrombosis in response to vessel ligation. The thrombosis was accompanied by tissue remodeling around the site of thrombus formation. In arterial regions that were thrombus-free, aged Bmal1 knockout mice revealed a paradoxical enlargement of lumen diameter after ligation.17 Both Clock mutant and Bmal1 knockout mice exhibited a significant increase in neointimal area and intima to medial ratio in response to mechanical injury of the vascular endothelium under constant darkness.17 However, under a regular light dark daily cycle, the wall thickening and inward remodeling induced by arterial ligation were not different between wild-type and Clock mutant mice, providing strong evidence for a direct link between light cycle- and circadian rhythm-dependent changes in vascular function. Moreover, PAI-1 was upregulated in the remodeled vascular endothelium of Bmal1 knockout mice. Aortas from both Bmal1 knockout and Clock mutant mice exhibited a severely impaired vasorelaxant response to acetylcholine relative to wild-type mice under constant darkness. This impaired response in Clock mutant mice was again not apparent under light-dark conditions, providing further evidence in support of a direct link between the biological clock and vascular integrity. The impairment in aortic relaxation in Bmal1 knockout mice was improved by administration of the superoxide oxygen radical scavenger superoxide dismutase, without changes in the smooth muscle cell responses to nitric oxide. The endothelial dysfunction and pathological vascular remodeling observed in Bmal1 knockout mice was attributed to reduced Akt signaling. In addition, attenuation of phosphorylated eNOS – a target for Akt to exert its regulatory role in vascular function - was also described in the arteries of Bmal1 knockout mice. The effects of circadian clocks in the regulation of vascular function are summarized in Figure 2. Table 1 provides a summary of the vascular phenotypes observed in genetic models of dysfunctional clocks.
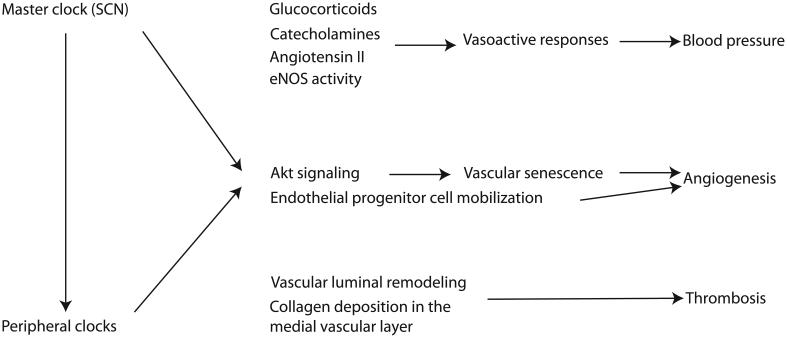
Figure 2.
Overview of the importance of circadian clocks in vascular function. Both the master clock in the suprachiasmatic nucleus (SCN) and the peripheral clocks, including the ones within the vasculature, impose a rhythm in several mediators of vascular function. The SCN can exert its effect both directly into the vasculature and indirectly by synchronizing peripheral clocks. Glucocorticoids, catecholamines, angiotensin II and endothelial nitric oxide synthase (eNOS) activity vary with time within the day. This temporal variation is responsible for a diurnal variation in vasoacting responses resulting in the diurnal rhythm of blood pressure. Moreover, circadian clock function is necessary for physiological angiogenesis and thrombogenesis. Circadian clocks inhibit Akt signaling and resulting vascular senescence and promote endothelial progenitor cell mobilization to maintain angiogenesis. Vascular luminal remodeling and composition requires functional circadian clocks for physiological thrombogenesis.
Table 1.
Phenotypes relevant to vascular function in genetic models of dysfunctional circadian clock
| Mouse model | Observed phenotype |
|---|---|
| Bmal1 knockout | Reduced endothelial-dependent vascular relaxation |
| Reduced plasma catecholamines | |
| Reduced mean arterial pressure | |
| Loss of circadian variation of arterial pressure | |
| Shorter time to thrombotic vascular occlusion | |
| Accelerated thrombosis in response to vessel ligation |
|
| No adaptation to chronic reduction of blood flow |
|
| Increase in neointimal area in response to mechanical injury of the vascular endothelium |
|
| Endothelial cell-specific Bmal1 knockout |
Reduced mean arterial pressure during the active phase of the day |
| Loss of circadian variation in susceptibility to thrombotic vascular occlusion |
|
| Shorter time to thrombotic vascular occlusion | |
| Clock mutant | Reduced endothelial-dependent vascular relaxation |
| Loss of circadian variation of arterial pressure | |
| Loss of circadian variation in susceptibility to thrombotic vascular occlusion |
|
| Longer time to thrombotic vascular occlusion | |
| Increase in neointimal area in response to mechanical injury of the vascular endothelium |
|
| Npas2 knockout | Longer time to thrombotic vascular occlusion |
| Npas2 mutant | Reduced plasma catecholamines |
| Reduced mean arterial pressure | |
| Phase shift of circadian variation of arterial pressure |
|
| Per2 mutant | Reduced endothelial-dependent vascular relaxation |
| Reduced mean arterial pressure | |
| Increased vascular senescence | |
| Decreased angiogenesis | |
| Cry1/Cry2 double knockout | Reduced catecholamine-mediated vasoconstriction |
| Loss of circadian variation of arterial pressure | |
| Increased baroreflex sensitivity |
The only study not using genetic deletion of clock components to investigate the effect of the disruption of the circadian clock on vascular function was performed in a mouse model of cardiac hypertrophy. Disruption of the circadian clock by altering the light cycle to a 10h light: 10h dark phase scheme resulted in abnormal thinning as opposed to hyperplasia of the aorta vessel wall and less hypertrophy of vascular muscle cells.55
Circadian clocks residing outside the vasculature and vascular function
In all of the previous studies, with the exception of the one involving mice with endothelial deletion of Bmal1, it is unclear whether the effect on the vasculature comes from a circadian oscillator residing inside or outside the vasculature. Although our study on the deletion of endothelial Bmal1 strongly suggests a role of the vascular clock in the physiology of the vasculature, we may not exclude a role for ectopic circadian oscillators. Several lines of evidence indicate the participation of the master oscillator in the SCN and other peripheral oscillators in the production and circadian variation of potent vasoactive proteins. The SCN impinges on the circadian rhythm in glucocorticoid release by regulating both the hypothalamic-pituitary-adrenal axis and the autonomic nervous system.56, 57 Recently, a SCN-dependent light-induced release of corticosterone in the adrenal gland has been described without an accompanying activation of the hypothalamo-adenohypophysial axis. This highlights the importance of the circadian clock in the phenomenon.58 Glucocorticoids, of which cortisol in humans59 and corticosterone in rodents60 are the most potent, have robust diurnal rhythms. Glucocorticoids can suppress the induced production of vasodilators, such as prostacyclin and NO in the endothelium.61 The arterial contractile sensitivity to catecholamines is potentiated by glucocorticoids. Glucocorticoids are also known to suppress inflammation and regulate vascular permeability through control on tight junction proteins.61
Another set of vascular effectors regulated by the circadian clock are the catecholamines, epinephrine and norepinephrine. Catecholamines are potent vasoactive hormones that contribute acutely to vasoconstriction, endothelial dysfunction and platelet activation. Both epinephrine and norepinephrine oscillate with a diurnal rhythm.62 Amplification of the diurnal variation of catecholamines may influence atherogenesis and the response to vascular injury.63 Animal models suggest that the positive components of the molecular clock can affect expression of key rate-limiting enzymes in catecholamine generation such as tyrosine hydroxylase,64 comt, pheylethanolamine – n – methyl transferase (pnmt) and monoamine oxidase (mao)B.46 Interestingly, catecholamines are potent synchronizers of the circadian clock, raising the possibility that their effect on vascular function is mediated, at least in part, through the vascular clock. Depletion of both norepinephrine and epinephrine, by genetic deletion of dopamine β-hydroxylase, results in loss of the diurnal oscillation in blood pressure.35 Norepinephrine and epinephrine acting via α1 and β2 adrenergic receptors phase shift the circadian clock in mouse and human vascular smooth muscle cells in vitro.35 However, aortic clocks in dopamine β-hydroxylase knockout mice that cannot synthesize either norepinephrine or epinephrine, can be entrained by food restriction.35 Similar to catecholamines, we found glucocorticoids to be potent synchronizers of the circadian oscillator in the VSMCs.27 A third potent synchronizer of the oscillator in VSMCs is angiotensin II. Angiotensin II exhibits considerable diurnal variation and plays a central role in the regulation of systemic blood pressure through multiple effects, some of which are exerted on the vasculature.65, 66 Treatment of VSMCs with angiotensin II induces significant oscillation in bmal1, per2 and dbp.28 Overexpression of the renin II gene in the rat is associated with a phase delayed or inverted circadian rhythm of blood pressure, attenuated circadian and photic induction of c-fos gene in SCN neurons, and attenuated phase shifting of behavioral and cardiovascular rhythms in response to light.67, 68 Studies in AT2 receptor knockout mice reveal disrupted circadian rhythms in blood pressure and heart rate compared with wild type mice.69 All the above findings raise the possibility that glucocorticoids, catecholamines and angiotensin II may contribute to regulation of vascular function including integration of the SCN with the peripheral oscillators in the vasculature (Figure 3).
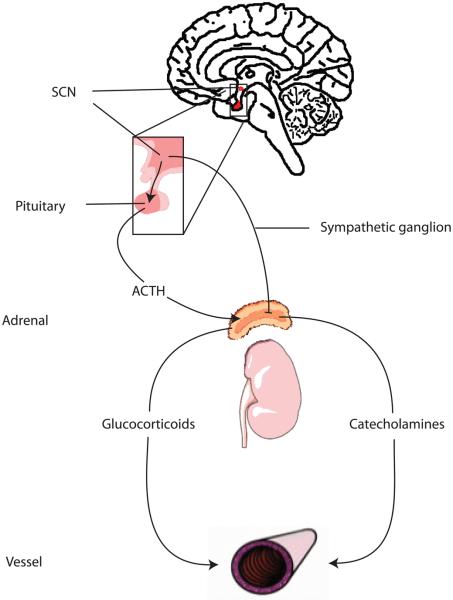
Figure 3.
A network of circadian clocks regulates vascular function. The master oscillator in the suprachiasmatic nucleus (SCN) stimulates the release of glucocorticoids and catecholamines by the adrenal glands. Two pathways mediate the stimulation of the adrenals. The first involves activation of the pituitary and the second the autonomic nervous system. Glucocorticoids and catecholamines exert pleiotropic effects on the vasculature including the synchronization of the vascular oscillator. Oscillators, not only in SCN, but also in pituitary and adrenals participate in the regulation of glucocorticoid and catecholamine oscillations. ATCH: adrenocorticotropic hormone
Conclusion
Although diurnal variation in vascular physiology has been long recognized, many aspects of how the the molecular clock influences vascular function remain unknown. During the past decade, a model of function for the circadian clock has been developed. Disruption of this model by genetic manipulation suggests an influence of the molecular clock on diverse aspects of cardiovascular function. However, it is known that many of the circadian clock genes have non-clock related actions; therefore careful interpretation of those studies is needed. Future use of tissue specific knockout models of circadian clock components and the use of diversified models of clock dysfunction will hopefully prove helpful in unraveling the network of circadian oscillators residing both inside and outside the vasculature that regulate cardiovascular biology. Understanding the role of the clock in vascular function should eventually lead to mechanistic explanations of the temporal incidence in adverse cardiovascular events and present novel therapeutic opportunities. One of the major challenges in this effort will be the identification of mechanisms underlying the oscillations in vascular physiology. This knowledge will potentially enable prospective studies of therapies targeting vascular pathology in respect to diurnal variation. Furthermore, therapeutic approaches may be designed to act in an anticipatory way. Already, antihypertensive drug therapy is timed to intercept the morning surge in blood pressure while mindful of diurnal variation in drug metabolism. However, the ultimate goal is to identify and target for correction the fundamental defects leading to dysregulated rhythms of vascular physiology.
Acknowledgements
Work pertinent to this review is supported by the National Institutes of Health (HL097800). G.A.F. is the McNeill Professor of Translational Medicine and Therapeutics.
Non-standard Abbreviations and Acronyms
- NO
Nitric oxide
- Bmal1
Brain and muscle Arnt-like protein-1
- Clock
Circadian Locomotor Output Cycles Kaput
- Npas2
Neuronal PAS domain-containing protein 2
- Per
Period
- Cry
Cryptochrome
- ROREα
Retinoic acid-related orphan receptor elements α
- SCN
Suprachiasmatic nucleus
- eNOS
Endothelial nitric oxide synthase
- PAI-1
Plasminogen activator inhibitor 1
- tPA
Tissue plasminogen activator
- VSMC
Vascular smooth muscle cells
- COX
Cyclooxygenase
- PPARγ
Peroxisome proliferator-activated receptor γ
- Comt
Catechol-O-methyl transferase
- TTVO
Time to thrombotic vascular occlusion
- Pnmt
Pheylethanolamine – n – methyl transferase
Footnotes
Disclosures
None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cohen MC, Rohtla KM, Lavery CE, Muller JE, Mittleman MA. Meta-analysis of the morning excess of acute myocardial infarction and sudden cardiac death. Am J Cardiol. 1997;79:1512–1516. doi: 10.1016/s0002-9149(97)00181-1. [DOI] [PubMed] [Google Scholar]
- 2.Elliott WJ. Circadian variation in the timing of stroke onset: a meta-analysis. Stroke. 1998;29:992–996. doi: 10.1161/01.str.29.5.992. [DOI] [PubMed] [Google Scholar]
- 3.Bridges AB, McLaren M, Saniabadi A, Fisher TC, Belch JJ. Circadian variation of endothelial cell function, red blood cell deformability and dehydro-thromboxane B2 in healthy volunteers. Blood Coagul Fibrinolysis. 1991;2:447–452. doi: 10.1097/00001721-199106000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Muller JE. Circadian variation and triggering of acute coronary events. Am Heart J. 1999;137:S1–S8. doi: 10.1016/s0002-8703(99)70390-x. [DOI] [PubMed] [Google Scholar]
- 5.Angleton P, Chandler WL, Schmer G. Diurnal variation of tissue-type plasminogen activator and its rapid inhibitor (PAI-1) Circulation. 1989;79:101–106. doi: 10.1161/01.cir.79.1.101. [DOI] [PubMed] [Google Scholar]
- 6.Andreotti F, Kluft C. Circadian variation of fibrinolytic activity in blood. Chronobiol Int. 1991;8:336–351. doi: 10.3109/07420529109059170. [DOI] [PubMed] [Google Scholar]
- 7.Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009;5:253–261. doi: 10.1038/nrendo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15(Spec No 2):R271–277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- 9.Hughes ME, DiTacchio L, Hayes KR, Vollmers C, Pulivarthy S, Baggs JE, Panda S, Hogenesch JB. Harmonics of circadian gene transcription in mammals. PLoS Genet. 2009;5:e1000442. doi: 10.1371/journal.pgen.1000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alvarez JD, Chen D, Storer E, Sehgal A. Non-cyclic and developmental stage-specific expression of circadian clock proteins during murine spermatogenesis. Biol Reprod. 2003;69:81–91. doi: 10.1095/biolreprod.102.011833. [DOI] [PubMed] [Google Scholar]
- 11.Weaver DR. The suprachiasmatic nucleus: a 25-year retrospective. J Biol Rhythms. 1998;13:100–112. doi: 10.1177/074873098128999952. [DOI] [PubMed] [Google Scholar]
- 12.Kaneko M, Zechman FW, Smith RE. Circadian variation in human peripheral blood flow levels and exercise responses. J Appl Physiol. 1968;25:109–114. doi: 10.1152/jappl.1968.25.2.109. [DOI] [PubMed] [Google Scholar]
- 13.Hossmann V, Fitzgerald GA, Dollery CT. Circadian rhythm of baroreflex reactivity and adrenergic vascular response. Cardiovasc Res. 1980;14:125–129. doi: 10.1093/cvr/14.3.125. [DOI] [PubMed] [Google Scholar]
- 14.Panza JA, Epstein SE, Quyyumi AA. Circadian variation in vascular tone and its relation to alpha-sympathetic vasoconstrictor activity. N Engl J Med. 1991;325:986–990. doi: 10.1056/NEJM199110033251402. [DOI] [PubMed] [Google Scholar]
- 15.Etsuda H, Takase B, Uehata A, Kusano H, Hamabe A, Kuhara R, Akima T, Matsushima Y, Arakawa K, Satomura K, Kurita A, Ohsuzu F. Morning attenuation of endothelium-dependent, flow-mediated dilation in healthy young men: possible connection to morning peak of cardiac events? Clin Cardiol. 1999;22:417–421. doi: 10.1002/clc.4960220610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keskil Z, Gorgun CZ, Hodoglugil U, Zengil H. Twenty-four-hour variations in the sensitivity of rat aorta to vasoactive agents. Chronobiol Int. 1996;13:465–475. doi: 10.3109/07420529609020917. [DOI] [PubMed] [Google Scholar]
- 17.Anea CB, Zhang M, Stepp DW, Simkins GB, Reed G, Fulton DJ, Rudic RD. Vascular disease in mice with a dysfunctional circadian clock. Circulation. 2009;119:1510–1517. doi: 10.1161/CIRCULATIONAHA.108.827477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunieda T, Minamino T, Miura K, Katsuno T, Tateno K, Miyauchi H, Kaneko S, Bradfield CA, FitzGerald GA, Komuro I. Reduced nitric oxide causes age-associated impairment of circadian rhythmicity. Circ Res. 2008;102:607–614. doi: 10.1161/CIRCRESAHA.107.162230. [DOI] [PubMed] [Google Scholar]
- 19.Wang CY, Wen MS, Wang HW, Hsieh IC, Li Y, Liu PY, Lin FC, Liao JK. Increased vascular senescence and impaired endothelial progenitor cell function mediated by mutation of circadian gene Per2. Circulation. 2008;118:2166–2173. doi: 10.1161/CIRCULATIONAHA.108.790469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tunctan B, Weigl Y, Dotan A, Peleg L, Zengil H, Ashkenazi I, Abacioglu N. Circadian variation of nitric oxide synthase activity in mouse tissue. Chronobiol Int. 2002;19:393–404. doi: 10.1081/cbi-120002915. [DOI] [PubMed] [Google Scholar]
- 21.Dalby MC, Davidson SJ, Burman JF, Davies SW. Diurnal variation in platelet aggregation iwth the PFA-100 platelet function analyser. Platelets. 2000;11:320–324. doi: 10.1080/09537100050144731. [DOI] [PubMed] [Google Scholar]
- 22.Pinotti M, Bertolucci C, Portaluppi F, Colognesi I, Frigato E, Foa A, Bernardi F. Daily and circadian rhythms of tissue factor pathway inhibitor and factor VII activity. Arterioscler Thromb Vasc Biol. 2005;25:646–649. doi: 10.1161/01.ATV.0000153140.13148.e0. [DOI] [PubMed] [Google Scholar]
- 23.Kanabrocki EL, George M, Hermida RC, Messmore HL, Ryan MD, Ayala DE, Hoppensteadt DA, Fareed J, Bremner FW, Third JL, Shirazi P, Nemchausky BA. Day-night variations in blood levels of nitric oxide, T-TFPI, and E-selectin. Clin Appl Thromb Hemost. 2001;7:339–345. doi: 10.1177/107602960100700417. [DOI] [PubMed] [Google Scholar]
- 24.Soulban G, Labrecque G. Circadian rhythms of blood clotting time and coagulation factors II, VII, IX and X in rats. Life Sci. 1989;45:2485–2489. doi: 10.1016/0024-3205(89)90015-5. [DOI] [PubMed] [Google Scholar]
- 25.Bremner WF, Sothern RB, Kanabrocki EL, Ryan M, McCormick JB, Dawson S, Connors ES, Rothschild R, Third JL, Vahed S, Nemchausky BM, Shirazi P, Olwin JH. Relation between circadian patterns in levels of circulating lipoprotein(a), fibrinogen, platelets, and related lipid variables in men. Am Heart J. 2000;139:164–173. doi: 10.1016/s0002-8703(00)90324-7. [DOI] [PubMed] [Google Scholar]
- 26.Westgate EJ, Cheng Y, Reilly DF, Price TS, Walisser JA, Bradfield CA, FitzGerald GA. Genetic components of the circadian clock regulate thrombogenesis in vivo. Circulation. 2008;117:2087–2095. doi: 10.1161/CIRCULATIONAHA.107.739227. [DOI] [PubMed] [Google Scholar]
- 27.McNamara P, Seo SB, Rudic RD, Sehgal A, Chakravarti D, FitzGerald GA. Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a humoral mechanism to reset a peripheral clock. Cell. 2001;105:877–889. doi: 10.1016/s0092-8674(01)00401-9. [DOI] [PubMed] [Google Scholar]
- 28.Nonaka H, Emoto N, Ikeda K, Fukuya H, Rohman MS, Raharjo SB, Yagita K, Okamura H, Yokoyama M. Angiotensin II induces circadian gene expression of clock genes in cultured vascular smooth muscle cells. Circulation. 2001;104:1746–1748. doi: 10.1161/hc4001.098048. [DOI] [PubMed] [Google Scholar]
- 29.Curtis AM, Seo SB, Westgate EJ, Rudic RD, Smyth EM, Chakravarti D, FitzGerald GA, McNamara P. Histone acetyltransferase-dependent chromatin remodeling and the vascular clock. J Biol Chem. 2004;279:7091–7097. doi: 10.1074/jbc.M311973200. [DOI] [PubMed] [Google Scholar]
- 30.Rudic RD, Curtis AM, Cheng Y, FitzGerald G. Peripheral clocks and the regulation of cardiovascular and metabolic function. Methods Enzymol. 2005;393:524–539. doi: 10.1016/S0076-6879(05)93027-9. [DOI] [PubMed] [Google Scholar]
- 31.Davidson AJ, London B, Block GD, Menaker M. Cardiovascular tissues contain independent circadian clocks. Clin Exp Hypertens. 2005;27:307–311. [PubMed] [Google Scholar]
- 32.Welsh DK, Yoo SH, Liu AC, Takahashi JS, Kay SA. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr Biol. 2004;14:2289–2295. doi: 10.1016/j.cub.2004.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagoshi E, Saini C, Bauer C, Laroche T, Naef F, Schibler U. Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell. 2004;119:693–705. doi: 10.1016/j.cell.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 34.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reilly DF, Curtis AM, Cheng Y, Westgate EJ, Rudic RD, Paschos G, Morris J, Ouyang M, Thomas SA, FitzGerald GA. Peripheral circadian clock rhythmicity is retained in the absence of adrenergic signaling. Arterioscler Thromb Vasc Biol. 2008;28:121–126. doi: 10.1161/ATVBAHA.107.152538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takeda N, Maemura K, Horie S, Oishi K, Imai Y, Harada T, Saito T, Shiga T, Amiya E, Manabe I, Ishida N, Nagai R. Thrombomodulin is a clock-controlled gene in vascular endothelial cells. J Biol Chem. 2007;282:32561–32567. doi: 10.1074/jbc.M705692200. [DOI] [PubMed] [Google Scholar]
- 37.Liu AC, Welsh DK, Ko CH, Tran HG, Zhang EE, Priest AA, Buhr ED, Singer O, Meeker K, Verma IM, Doyle FJ, 3rd, Takahashi JS, Kay SA. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell. 2007;129:605–616. doi: 10.1016/j.cell.2007.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 39.Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- 40.Rudic RD, McNamara P, Reilly D, Grosser T, Curtis AM, Price TS, Panda S, Hogenesch JB, FitzGerald GA. Bioinformatic analysis of circadian gene oscillation in mouse aorta. Circulation. 2005;112:2716–2724. doi: 10.1161/CIRCULATIONAHA.105.568626. [DOI] [PubMed] [Google Scholar]
- 41.Schoenhard JA, Smith LH, Painter CA, Eren M, Johnson CH, Vaughan DE. Regulation of the PAI-1 promoter by circadian clock components: differential activation by BMAL1 and BMAL2. J Mol Cell Cardiol. 2003;35:473–481. doi: 10.1016/s0022-2828(03)00051-8. [DOI] [PubMed] [Google Scholar]
- 42.Maemura K, de la Monte SM, Chin MT, Layne MD, Hsieh CM, Yet SF, Perrella MA, Lee ME. CLIF, a novel cycle-like factor, regulates the circadian oscillation of plasminogen activator inhibitor-1 gene expression. J Biol Chem. 2000;275:36847–36851. doi: 10.1074/jbc.C000629200. [DOI] [PubMed] [Google Scholar]
- 43.Viswambharan H, Carvas JM, Antic V, Marecic A, Jud C, Zaugg CE, Ming XF, Montani JP, Albrecht U, Yang Z. Mutation of the circadian clock gene Per2 alters vascular endothelial function. Circulation. 2007;115:2188–2195. doi: 10.1161/CIRCULATIONAHA.106.653303. [DOI] [PubMed] [Google Scholar]
- 44.Shaw JA, Chin-Dusting JP, Kingwell BA, Dart AM. Diurnal variation in endothelium-dependent vasodilatation is not apparent in coronary artery disease. Circulation. 2001;103:806–812. doi: 10.1161/01.cir.103.6.806. [DOI] [PubMed] [Google Scholar]
- 45.Khurana S, Yamada M, Wess J, Kennedy RH, Raufman JP. Deoxycholyltaurine-induced vasodilation of rodent aorta is nitric oxide- and muscarinic M(3) receptor-dependent. Eur J Pharmacol. 2005;517:103–110. doi: 10.1016/j.ejphar.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 46.Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS, Fitzgerald GA. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci U S A. 2007;104:3450–3455. doi: 10.1073/pnas.0611680104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang N, Yang G, Jia Z, Zhang H, Aoyagi T, Soodvilai S, Symons JD, Schnermann JB, Gonzalez FJ, Litwin SE, Yang T. Vascular PPARgamma controls circadian variation in blood pressure and heart rate through Bmal1. Cell Metab. 2008;8:482–491. doi: 10.1016/j.cmet.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Masuki S, Todo T, Nakano Y, Okamura H, Nose H. Reduced alpha-adrenoceptor responsiveness and enhanced baroreflex sensitivity in Cry-deficient mice lacking a biological clock. J Physiol. 2005;566:213–224. doi: 10.1113/jphysiol.2005.086728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Helkamaa T, Mannisto PT, Rauhala P, Cheng ZJ, Finckenberg P, Huotari M, Gogos JA, Karayiorgou M, Mervaala EM. Resistance to salt-induced hypertension in catechol-O-methyltransferase-gene-disrupted mice. J Hypertens. 2003;21:2365–2374. doi: 10.1097/00004872-200312000-00026. [DOI] [PubMed] [Google Scholar]
- 50.Ura N, Sasaki H. Significance of kallikrein-kinin system on blood pressure regulation. Nippon Rinsho. 2005;63(Suppl 3):392–397. [PubMed] [Google Scholar]
- 51.Nishi J, Minamino T, Miyauchi H, Nojima A, Tateno K, Okada S, Orimo M, Moriya J, Fong GH, Sunagawa K, Shibuya M, Komuro I. Vascular endothelial growth factor receptor-1 regulates postnatal angiogenesis through inhibition of the excessive activation of Akt. Circ Res. 2008;103:261–268. doi: 10.1161/CIRCRESAHA.108.174128. [DOI] [PubMed] [Google Scholar]
- 52.Miyauchi H, Minamino T, Tateno K, Kunieda T, Toko H, Komuro I. Akt negatively regulates the in vitro lifespan of human endothelial cells via a p53/p21-dependent pathway. EMBO J. 2004;23:212–220. doi: 10.1038/sj.emboj.7600045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smaaland R, Sothern RB, Laerum OD, Abrahamsen JF. Rhythms in human bone marrow and blood cells. Chronobiol Int. 2002;19:101–127. doi: 10.1081/cbi-120002594. [DOI] [PubMed] [Google Scholar]
- 54.Tsinkalovsky O, Smaaland R, Rosenlund B, Sothern RB, Hirt A, Steine S, Badiee A, Abrahamsen JF, Eiken HG, Laerum OD. Circadian variations in clock gene expression of human bone marrow CD34+ cells. J Biol Rhythms. 2007;22:140–150. doi: 10.1177/0748730406299078. [DOI] [PubMed] [Google Scholar]
- 55.Martino TA, Tata N, Belsham DD, Chalmers J, Straume M, Lee P, Pribiag H, Khaper N, Liu PP, Dawood F, Backx PH, Ralph MR, Sole MJ. Disturbed diurnal rhythm alters gene expression and exacerbates cardiovascular disease with rescue by resynchronization. Hypertension. 2007;49:1104–1113. doi: 10.1161/HYPERTENSIONAHA.106.083568. [DOI] [PubMed] [Google Scholar]
- 56.Buijs RM, Kalsbeek A, van der Woude TP, van Heerikhuize JJ, Shinn S. Suprachiasmatic nucleus lesion increases corticosterone secretion. Am J Physiol. 1993;264:R1186–1192. doi: 10.1152/ajpregu.1993.264.6.R1186. [DOI] [PubMed] [Google Scholar]
- 57.Ottenweller JE, Meier AH. Adrenal innervation may be an extrapituitary mechanism able to regulate adrenocortical rhythmicity in rats. Endocrinology. 1982;111:1334–1338. doi: 10.1210/endo-111-4-1334. [DOI] [PubMed] [Google Scholar]
- 58.Ishida A, Mutoh T, Ueyama T, Bando H, Masubuchi S, Nakahara D, Tsujimoto G, Okamura H. Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metab. 2005;2:297–307. doi: 10.1016/j.cmet.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 59.Edwards S, Clow A, Evans P, Hucklebridge F. Exploration of the awakening cortisol response in relation to diurnal cortisol secretory activity. Life Sci. 2001;68:2093–2103. doi: 10.1016/s0024-3205(01)00996-1. [DOI] [PubMed] [Google Scholar]
- 60.Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, Fitzgerald GA. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang S, Zhang L. Glucocorticoids and vascular reactivity. Curr Vasc Pharmacol. 2004;2:1–12. doi: 10.2174/1570161043476483. [DOI] [PubMed] [Google Scholar]
- 62.Sauerbier I, von Mayersbach H. Circadian variation of catecholamines in human blood. Horm Metab Res. 1977;9:529–530. doi: 10.1055/s-0028-1095589. [DOI] [PubMed] [Google Scholar]
- 63.Zhang H, Faber JE. Trophic effect of norepinephrine on arterial intima-media and adventitia is augmented by injury and mediated by different alpha1-adrenoceptor subtypes. Circ Res. 2001;89:815–822. doi: 10.1161/hh2101.098379. [DOI] [PubMed] [Google Scholar]
- 64.McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, Nestler EJ. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci U S A. 2005;102:9377–9381. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dinh DT, Frauman AG, Johnston CI, Fabiani ME. Angiotensin receptors: distribution, signalling and function. Clin Sci (Lond) 2001;100:481–492. [PubMed] [Google Scholar]
- 66.Katz FH, Smith JA, Lock JP, Loeffel DE. Plasma vasopressin variation and renin activity in normal active humans. Horm Res. 1979;10:289–302. doi: 10.1159/000179011. [DOI] [PubMed] [Google Scholar]
- 67.Lemmer B, Witte K, Schanzer A, Findeisen A. Circadian rhythms in the renin-angiotensin system and adrenal steroids may contribute to the inverse blood pressure rhythm in hypertensive TGR(mREN-2)27 rats. Chronobiol Int. 2000;17:645–658. doi: 10.1081/cbi-100101071. [DOI] [PubMed] [Google Scholar]
- 68.Lemmer B, Hauptfleisch S, Witte K. Loss of 24 h rhythm and light-induced c-fos mRNA expression in the suprachiasmatic nucleus of the transgenic hypertensive TGR(mRen2)27 rat and effects on cardiovascular rhythms. Brain Res. 2000;883:250–257. doi: 10.1016/s0006-8993(00)02989-9. [DOI] [PubMed] [Google Scholar]
- 69.Gross V, Milia AF, Plehm R, Inagami T, Luft FC. Long-term blood pressure telemetry in AT2 receptor-disrupted mice. J Hypertens. 2000;18:955–961. doi: 10.1097/00004872-200018070-00018. [DOI] [PubMed] [Google Scholar]