Abstract
Thrombosis — localized clotting of the blood — can occur in the arterial or the venous circulation and has a major medical impact. Acute arterial thrombosis is the proximal cause of most cases of myocardial infarction (heart attack) and of about 80% of strokes, collectively the most common cause of death in the developed world. Venous thromboembolism is the third leading cause of cardiovascular-associated death. The pathogenic changes that occur in the blood vessel wall and in the blood itself resulting in thrombosis are not fully understood. Understanding these processes is crucial for developing safer and more effective antithrombotic drugs.
The pathophysiology of arterial thrombosis differs from that of venous thrombosis, as reflected by the different ways in which they are treated. In broad terms, arterial thrombosis is treated with drugs that target platelets, and venous thrombosis is treated with drugs that target proteins of the coagulation cascade. The available antithrombotic drugs are effective at reducing arterial thrombosis and venous thrombosis in patients with cardiovascular disease. However, the main side effect of these drugs is bleeding, which limits their use. To develop a new generation of safe and effective antithrombotic drugs with larger therapeutic windows (that is, a larger difference between the dose that prevents thrombosis and the dose that induces bleeding), a better understanding of the pathogenic processes that lead to thrombotic occlusion of blood vessels is needed. In this article I describe the pathological mechanisms and the risk factors that are known to lead to arterial thrombosis and venous thrombosis, and discuss the development of new approaches for antithrombotic therapy.
Arterial thrombosis
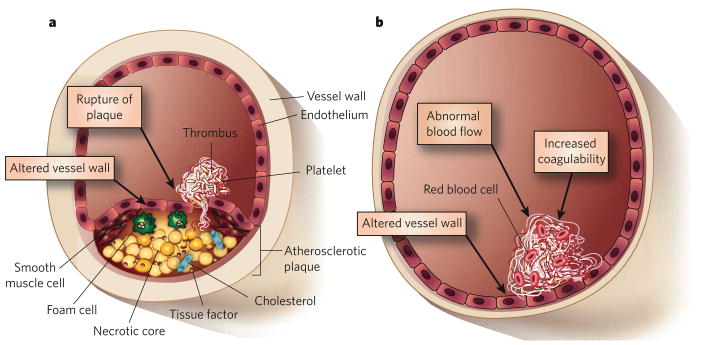
The primary trigger for arterial thrombosis is the rupture of an atherosclerotic plaque (Fig. 1a), which develops through the accumulation of lipid deposits and lipid-laden macrophages (foam cells) in the artery wall (see page 904). The thrombi that form at ruptured plaques are rich in platelets, which are small (about 1 μm in diameter) anucleate cells produced by megakaryocytes in the bone marrow1. These disc-shaped cells circulate in the blood as sentinels of vascular integrity and rapidly form a primary haemostatic plug at sites of vascular injury 2. When an atherosclerotic plaque ruptures, platelets are rapidly recruited to the site, through the interaction of specific platelet cell-surface receptors with collagen and von Willebrand factor3,4 (Fig. 2). After this adhesion to the vessel wall, the receptor-mediated binding of additional platelets (termed platelet aggregation) then results in rapid growth of the thrombus. Platelets also become activated at this stage. A major pathway of activation involves the cleavage and, consequently, the activation of the platelet receptor PAR1 (protease-activated receptor 1; also known as the thrombin receptor) by the protease thrombin (also known as factor II)5, which is activated by the blood coagulation cascade. Activated platelets then release the contents of granules, which further promote platelet recruitment, adhesion, aggregation and activation.
Figure 1. Triggers of arterial and venous thrombosis.
a, Artery. The primary trigger of arterial thrombosis is rupture of an atherosclerotic plaque. This involves disruption of the endothelium and release of constituents of the plaque into the lumen of the blood vessel. b, Vein. By contrast, in venous thrombosis, the endothelium remains intact but can be converted from a surface with anticoagulant properties to one with procoagulant properties. Venous thrombosis can be triggered by several factors: abnormal blood flow (such as the absence of blood flow); altered properties of the blood itself (thrombophilia); and alterations in the endothelium.
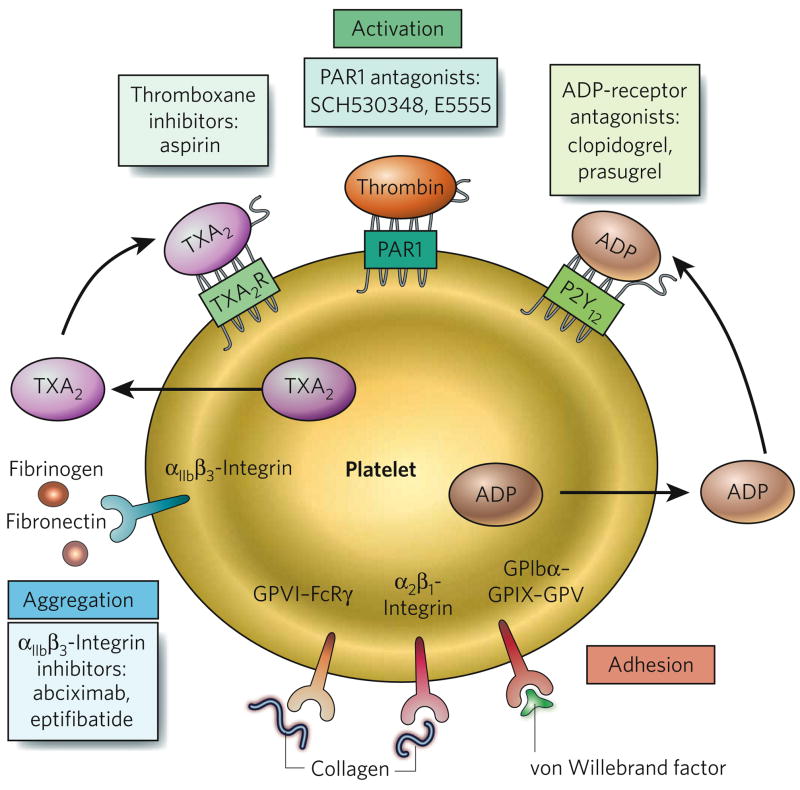
Figure 2. Targets of antiplatelet drugs.
Platelets have a variety of cell-surface receptors that mediate their activation (green shading), their adhesion to the blood vessel wall (red) and their aggregation with each other (blue). The ligands for various receptors are shown. Antiplatelet drugs and their targets are also indicated; targets include thromboxane A2 (TXA2), protease-activated receptor 1 (PAR1), the ADP receptor P2Y12 and αIIbβ3-integrin.
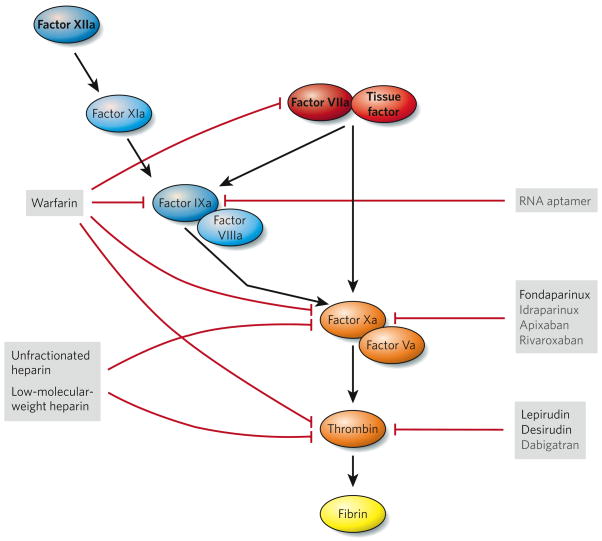
The coagulation cascade (Fig. 3) is the sequential process by which coagulation factors of the blood interact and are activated, ultimately generating fibrin, the main protein component of the thrombus, and this cascade operates in both arterial and venous thrombosis. The cascade is initiated by exposure of the blood to tissue factor (also known as factor III), a protein that is present at high concentrations in atherosclerotic plaques6,7. Circulating tissue factor is also present at increased concentrations in patients with cardiovascular disease and might contribute to thrombosis after plaque rupture8,9.
Figure 3. Targets of anticoagulant drugs.
Tissue factor is present at high concentrations in atherosclerotic plaques. When exposed to the blood — for example, when a plaque ruptures — tissue factor binds to the plasma protein factor VIIa (the extrinsic pathway, red), and this complex triggers activation of the coagulation cascade through the proteolytic cleavage of both factor X and factor IX. This cascade ultimately generates fibrin (through the common pathway, orange), which (on polymerization) stabilizes platelet thrombi. The coagulation cascade is amplified by the tenase complex, which consists of factor VIIIa and factor IXa (components of the intrinsic pathway, blue). Factor XIa and factor XIIa might also help to activate the coagulation cascade under pathological conditions. Triggers of thrombosis are shown in bold face. Anticoagulant drugs that are in current use (black) and in development (grey) are listed, and their targets are also indicated (red blocking arrows).
In the case of acute thrombotic events, drugs that reduce the growth of a thrombus can be administered; the main target of these drugs is platelets. Antiplatelet drugs are also used prophylactically to reduce the incidence of arterial thrombosis in patients with cardiovascular disease10. The primary targets of antiplatelet therapy are molecules involved in platelet activation and aggregation (Box 1). At present, there are no drugs in clinical use that block the binding of platelets to collagen and von Willebrand factor and hence their adhesion to the blood vessel wall. In theory, inhibition of this early step in thrombus formation is more likely to disrupt the primary role of platelets in normal blood clotting (haemostasis) and therefore to increase the risk of bleeding. Nevertheless, such inhibitors are in development10–12.
Box 1. Antiplatelet therapy.
Antiplatelet drugs are used for both the prevention and the acute treatment of arterial thrombosis. These drugs target the activation and the aggregation of platelets. The advantages and disadvantages of the main types of antiplatelet drug are described below.
Cyclooxygenase inhibitors
Aspirin has been used clinically for more than 40 years and is the most commonly used antiplatelet drug. It inhibits platelet cyclooxygenase 1, which is required for the synthesis of thromboxane A2 (TXA2), a potent activator of platelets (Fig. 2). Aspirin not only significantly reduces the incidence of a first myocardial infarction in men at risk of cardiovascular disease (primary prevention) but also reduces the risk in patients who have had a myocardial infarction (secondary prevention)46,47. Aspirin therapy, however, is not without risk and can cause stomach ulcers and bleeding.
Recent studies of the selective cyclooxygenase-2 inhibitors rofecoxib and valdecoxib have shown that inhibiting the ‘wrong’ cyclooxygenase can lead to a significant increase in the incidence of myocardial infarction and stroke, resulting in the withdrawal of these drugs from the market48. It has been proposed that these inhibitors reduce cyclooxygenase-2-dependent synthesis of prostacyclin (also known as PGI2), an inhibitor of platelet activation48.
ADP-receptor antagonists
Another class of antiplatelet drug targets the ADP receptor P2Y12, also reducing platelet activation49 (Fig. 2). Clopidogrel, the most widely used drug in this class, is used to treat patients with acute coronary syndromes (which include various conditions, such as unstable angina, that are associated with chest pain as a result of reduced blood supply to the heart)50,51. Clopidogrel is also administered to those undergoing percutaneous coronary intervention (a procedure in which blood flow is restored to a coronary artery by using a catheter to remove an occlusive atherosclerotic plaque and position a stent). A recent study compared clopidogrel with another P2Y12 antagonist, prasugrel, for the treatment of thrombosis in patients with acute coronary syndromes who are undergoing percutaneous coronary intervention23. Prasugrel significantly reduced the rate of ischaemic events (12.1% for clopidogrel versus 9.9% for prasugrel) but increased the risk of major bleeding (1.8% for clopidogrel versus 2.4% for prasugrel); these results suggest that prasugrel is a more potent P2Y12 inhibitor than clopidogrel.
Protease-activated-receptor-1 inhibitors
The cleavage of protease-activated receptor 1 (PAR1) by thrombin activates platelets5 (Fig. 2). PAR1 is a new target for antiplatelet therapy. Two PAR1 antagonists, E5555 and SCH 530348, are currently in phase II clinical trials10.
αIIbβ3-Integrin inhibitors
The aggregation of platelets is a crucial step in the growth of a thrombus. Inhibitors of αIIbβ3-integrin are designed to reduce platelet aggregation by inhibiting the binding of activated platelets to fibrinogen and other ligands (Fig. 2). Intravenously delivered inhibitors of αIIbβ3-integrin, such as abciximab and eptifibatide, are used for the short-term treatment of patients with acute coronary syndromes who are undergoing percutaneous coronary intervention52. However, the use of oral αIIbβ3-integrin inhibitors is associated with increased mortality, possibly as a result of the longer-term use of these agents and/or their unwanted ability to act as partial agonists of αIIbβ3-integrin.
Another important treatment for acute thrombotic events is the degradation of fibrin, which stabilizes the structure of a thrombus13, by using activators of the fibrinolytic system: namely ‘clot busters’, such as tissue plasminogen activator and streptokinase. However, the success of such treatment depends crucially on the timing of intervention, with earlier intervention generally having a better outcome. For example, for acute myocardial infarction, fibrinolytic therapy seems to be beneficial for at least 12 hours after the onset of symptoms. By contrast, fibrinolytic therapy for stroke has proven beneficial only when used within 3 hours14 and can have the side effect of inducing brain haemorrhage. Therefore, researchers are focusing on strategies that protect the vasculature but have a lower incidence of brain haemorrhage than is induced by current fibrinolytic therapy.
In the past few years, studies identifying platelet receptors and signalling mechanisms have yielded a trove of new targets for antiplatelet therapy. For example, recent studies have shown that several cell-surface receptor–ligand interactions occur on close contact between platelets, such as the binding of the ligand semaphorin 4D to its receptors, CD72 and plexin B1 (ref. 15). These receptors mediate platelet–platelet interactions and thrombus retraction and hence are attractive therapeutic targets. Another example is the receptor CD36. It is well established that CD36 functions as a scavenger receptor at the surface of macrophages: it binds to oxidized low-density lipoproteins, resulting in the formation of foam cells and therefore contributing to the development of atherosclerotic plaques16. CD36 is also present at the surface of platelets. A recent study showed that oxidized low-density lipoproteins activate platelets by binding to CD36 and that the prothrombotic phenotype of mice deficient in apolipoprotein E, which have high concentrations of low-density lipoprotein in the circulation, is reduced by deletion of Cd36 (ref. 17), suggesting that this interaction could explain the increased platelet reactivity and thrombosis associated with hyperlipidaemia. Finally, the complexities of ‘outside-in’ and ‘inside-out’ signalling in platelets (that is, signalling that originates extracellularly or intracellularly, respectively) have begun to be unravelled. However, redundancy in signalling pathways makes it difficult to identify therapeutic targets. An exception seems to be the essential role of the cytoskeletal protein talin 1 (ref. 18). The binding of talin 1 to the cytoplasmic domain of β3-integrin was shown to be required for activation of αIIbβ3-integrin (also known as glycoprotein IIB (GPIIB)–GPIIIA)19. Moreover, changing a single amino acid in the cytoplasmic domain of β3-integrin selectively disrupted talin-1 binding and reduced arterial thrombosis in an animal model19, suggesting that blockade of this interaction could be a new antithrombotic strategy.
Late stent thrombosis
In patients with symptomatic coronary artery disease, insertion of a drug-eluting stent (delivering either sirolimus or paclitaxel) in the occluded coronary artery has become a popular treatment. So far, about 6 million people worldwide have received drug-eluting stents. The drugs are designed to prevent smooth muscle cell proliferation and intimal hyperplasia, which lead to re-occlusion of the vessel (that is, to restenosis), and randomized clinical trials have shown that drug-eluting stents reduce the rate of restenosis compared with bare metal stents20. Individuals receiving stents are initially treated with antiplatelet agents, such as clopidogrel (which inhibits the cell-surface receptor P2Y12). However, recent reports have associated the use of drug-eluting stents with thrombosis after discontinuation of the antiplatelet therapy (a phenomenon known as late stent thrombosis)21. In a report examining the results of 14 randomized trials, the rate of late stent thrombosis was found to be fourfold-to-fivefold higher for drug-eluting stents than for bare metal stents, with thrombosis occurring with a median time of 15.5–18 months after stent deployment22. These results imply that patients with drug-eluting stents might benefit from prolonged antiplatelet therapy, although it is uncertain how long such therapy should continue. The triggering events for stent thrombosis are unclear but probably involve incomplete endothelialization of the stent surface21. Interestingly, the incidence of stent thrombosis was significantly lower in patients receiving a recently developed inhibitor of platelets, prasugrel, than in those receiving the more well-established drug in this class, clopidogrel (an incidence of 1.1% with prasugrel and 2.4% with clopidogrel), suggesting that prasugrel could be useful for this indication23. However, a better long-term option for reducing the incidence of late stent thrombosis will be the design of new types of stent able to deliver drugs that can reduce smooth muscle cell proliferation without interfering with endothelialization.
Venous thrombosis
Deep vein thrombosis and pulmonary embolism are collectively referred to as venous thromboembolism, which is the third leading cause of cardiovascular-associated death, after myocardial infarction and stroke. Deep vein thrombosis occurs most often in the large veins of the legs. Pulmonary embolism is a complication of deep vein thrombosis that can occur if part of the thrombus breaks away, travels to the lungs and lodges in a pulmonary artery, resulting in the disruption of blood flow. Thrombi that form in veins are rich in fibrin and trapped red blood cells and are referred to as red clots (as opposed to the platelet-rich thrombi that form in arteries, which are referred to as white clots). Deep vein thrombosis mainly occurs as a result of changes in the composition of the blood that promote thrombosis, changes that reduce or abolish blood flow, and/or changes to the vessel wall24. Both genetic and environmental factors can increase the risk of thromboembolism25,26. In inherited venous thrombotic disease, there can be increased activity or abundance of proteins that promote coagulation and/or decreased abundance of proteins that inhibit coagulation. For example, a specific point mutation (present in about 5% of Caucasians) in the gene encoding factor V results in a variant that is resistant to inactivation by the anticoagulant protease activated protein C and therefore leads to increased clotting27. Acquired risk factors for venous thromboembolism include cancer, obesity and major surgery 25,26. Increased amounts of circulating tissue factor, which sits at the apex of the coagulation cascade (Fig. 3), might also trigger venous thrombosis28–30.
Anticoagulants are used to treat a wide variety of conditions that involve arterial or venous thrombosis, including prevention of venous thromboembolism and long-term prevention of ischaemic stroke in patients with atrial fibrillation. The two main classes of anticoagulant drug are vitamin K antagonists and heparins, which target multiple proteases in the coagulation cascade (Fig. 3 and Box 2). As is the case for antiplatelet drugs, the main side effect of anticoagulant therapy is bleeding. Which targets are best for anticoagulant therapy and whether the anticoagulant drugs under development will have better therapeutic windows than the existing drugs are topics of intense debate31,32. Recent data from the RECORD 1 clinical trial show that the anticoagulant rivaroxaban holds promise33. Rivaroxaban is an orally available inhibitor of activated factor X (factor Xa, a component of the coagulation cascade), and it reduced the incidence of venous thromboembolic events in patients undergoing total hip replacement — from 3.7% in those administered a low-molecular-weight heparin (enoxaparin) to 1.1% (ref. 33). This translates to a 70% reduction in risk without an increase in bleeding.
Box 2. Anticoagulant therapy.
Anticoagulant drugs reduce the activity of various proteases in the coagulation cascade (Fig. 3) by directly inhibiting them, by inhibiting their post-translational modification or by increasing the activity of an anticoagulant. The advantages and disadvantages of the main types of anticoagulant are described below.
Vitamin K antagonists
Vitamin K antagonists are used for long-term anticoagulant therapy. These inhibitors, introduced more than 50 years ago, are the only orally active anticoagulants in clinical use today. They function by inhibiting the enzyme vitamin K epoxide reductase, which uses vitamin K to modify several coagulation proteins (factor VII, factor IX, factor X and prothrombin) post-translationally. Warfarin is the most commonly prescribed vitamin K antagonist; about 1% of the US population is currently being treated with this drug. Despite careful monitoring, the incidence of major bleeding is about 1–3% of warfarin-treated patients per year53. The activity of warfarin is affected by diet and by genetic make-up: polymorphisms in the gene that encodes vitamin K epoxide reductase and in the cytochrome P450 gene CYP2C9 account for up to 50% of the interindividual variability of warfarin dosing54. In August 2007, the US Food and Drug Administration announced a label change for warfarin, advising that pharmacogenetic tests for polymorphisms in these two genes could improve the accuracy of dosing.
Heparins
The anticoagulant properties of unfractionated heparin were first described in 1916. Since then, it has become evident that heparin binds to the protein antithrombin and markedly increases the ability of this protein to inhibit factor Xa and thrombin (Fig. 3). Unfractionated heparin is currently used for cardiovascular surgery and for the prevention of venous thromboembolism. Fractionated heparin, in the form of low-molecular-weight heparins, was introduced more than 15 years ago. These molecules also target both factor Xa and thrombin, but their administration results in a lower incidence of bleeding than does unfractionated heparin (1.4% for low-molecular-weight heparins versus 2.3% for unfractionated heparin)55. Synthetic pentasaccharides, such as fondaparinux and idraparinux, have been designed with a structure based on the antithrombin-binding sequence of heparin56. Owing to their small size, these drugs target factor Xa but not thrombin in an antithrombin-dependent manner (as they are too short to stabilize the interaction between antithrombin and thrombin). A complication of administering unfractionated heparin is the syndrome of heparin-induced thrombocytopenia, which is associated with high rates of both arterial thrombosis and venous thrombosis. More specifically, heparin administration can result in the generation of antibodies specific for heparin–platelet-factor-4 complexes; these antibodies can then activate platelets, generating thrombin and leading to thrombosis57. The incidence of heparin-induced thrombocytopenia is reduced when low-molecular-weight heparins are used, and thrombocytopenia is rarely observed when synthetic pentasaccharides are used.
Direct inhibitors of factor Xa and thrombin
Direct thrombin inhibitors, such as lepirudin and desirudin, are used for anticoagulant therapy and for the treatment of patients with heparin-induced thrombocytopenia. Several orally administered agents are in development, including: the thrombin inhibitor dabigatran, which is as effective as the low-molecular-weight heparin enoxaparin at reducing the risk of venous thromboembolism after hip-replacement surgery and has a similar safety profile58; and the factor-Xa inhibitor rivaroxaban, which has a favourable balance of efficacy and safety for preventing venous thromboembolism after major orthopaedic surgery33,59. Several other orally administered direct inhibitors of factor Xa are also in the pipeline40. Further studies are required to determine whether oral inhibitors of thrombin or factor Xa can replace the use of heparins and warfarin for both short-term and long-term anticoagulant therapy.
When targeting factors in the coagulation cascade, it is important to consider that the sequential activation of factors by proteolytic cleavage results in an amplification of each step. Therefore, a drug that targets an upstream component of the cascade, such as tissue factor, might be more potent than one that targets a downstream component, such as thrombin. However, the tissue factor and factor VIIa complex, which initiates the coagulation cascade, is essential for haemostasis, and inhibition of this complex can lead to considerable bleeding34. Indeed, gene-knockout experiments in mice have shown that tissue factor, as well as factor VII, factor X and prothrombin, are essential for haemostasis and for life35.
It is also important to consider that the coagulation cascade can be subdivided into three pathways (Fig. 3): the extrinsic pathway (tissue factor and factor VIIa), which is the primary activator of the cascade; the intrinsic pathway (factor XIIa, factor XIa, factor IXa and factor VIIIa), which amplifies the cascade; and the common pathway (factor Xa, factor Va and thrombin), which generates thrombin and fibrin. In contrast to the critical nature of the extrinsic pathway, mice can survive without components of the intrinsic pathway35. Humans deficient in factor VIII, factor IX or factor XI have mild haemostatic defects, whereas those deficient in factor XII have normal haemostasis36. Intrinsic-pathway components might therefore be usefully targeted for therapy. Factor XIIa is of particular interest in this regard. A recent study with factor-XII-deficient mice confirmed that factor XIIa is not required for haemostasis; however, it was shown to be important for thrombosis and thus seems an inviting target for antithrombotic therapy37. Factor IXa, part of the intrinsic pathway, has also been proposed as a target38. Despite the possibility that the risk of bleeding is lower after inhibition of components of the intrinsic pathway than of the common coagulation pathway, most pharmaceutical companies have chosen to focus on inhibition of factor Xa and thrombin39,40 (Fig. 3). This might be because inhibition of the intrinsic pathway is expected to have less impact on ongoing thrombosis than would inhibition of the downstream proteases.
An important concern about antithrombotic drugs is how to reverse their effects in the event of bleeding. A new approach that addresses this concern uses aptamers, which are composed of modified oligonucleotides. The first aptamer developed as an anticoagulant was targeted to thrombin and was shown to inhibit the activity of clot-bound thrombin and to reduce the formation of platelet-rich arterial thrombi41. More recently, an RNA aptamer that inhibits factor IXa has been developed42. By elegant design, an ‘antidote’ oligonucleotide was also developed, to reverse the anticoagulant activity of the inhibitory aptamer rapidly in the event of bleeding43. The factor-IXa aptamer–antidote pair was well tolerated in a phase Ia clinical trial with healthy volunteers44. In another approach using oligonucleotides, antisense therapy has been used to block not the activity of the target but its production (in this case, targeting prothrombin)45.
Conclusions
The ‘holy grail’ for antithrombotic therapy — a drug that will prevent coagulation without promoting bleeding — has yet to be found. However, molecules that contribute to thrombosis continue to be identified, and these could be new targets for the next generation of antithrombotic therapy. More immediately, recent studies of new antiplatelet drugs (such as prasugrel) and new anticoagulant drugs (such as rivaroxaban) suggest that more options will soon be available for the treatment of thrombosis. Furthermore, the ability to identify patients with an increased risk of thrombosis by measuring the concentrations of circulating factors, such as tissue factor, might allow more effective use of prophylaxis. Another exciting new approach is combination therapy, administering both antiplatelet drugs and anticoagulant drugs, which might prove more effective than using a single class of drug. Finally, personalized medicine is on the horizon and should allow customized dosing of antithrombotic drugs rather than the current ‘one dose fits all’ strategy.
Acknowledgments
I thank N. Key, R. Kasthuri and R. Stouffer for suggestions during preparation of the manuscript, and F. Church, J. Luyendyk, W. Biosvert and C. Mackman for critical reading of the manuscript.
Footnotes
Reprints and permissions information is available at npg.nature.com/reprints. The author declares competing financial interests: details accompany the full-text HTML version of the paper at www.nature.com/nature.
References
- 1.Hartwig J, Italiano J. The birth of the platelet. J Thromb Haemost. 2003;1:1580–1586. doi: 10.1046/j.1538-7836.2003.00331.x. [DOI] [PubMed] [Google Scholar]
- 2.Ruggeri ZM, Mendolicchio GL. Adhesion mechanisms in platelet function. Circ Res. 2007;100:1673–1685. doi: 10.1161/01.RES.0000267878.97021.ab. [DOI] [PubMed] [Google Scholar]
- 3.Denis CV, Wagner DD. Platelet adhesion receptors and their ligands in mouse models of thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:728–739. doi: 10.1161/01.ATV.0000259359.52265.62. [DOI] [PubMed] [Google Scholar]
- 4.Savage B, Almus-Jacobs F, Ruggeri ZM. Specific synergy of multiple substrate–receptor interactions in platelet thrombus formation under flow. Cell. 1998;94:657–666. doi: 10.1016/s0092-8674(00)81607-4. [DOI] [PubMed] [Google Scholar]
- 5.Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost. 2005;3:1800–1814. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- 6.Marmur JD, et al. Identification of active tissue factor in human coronary atheroma. Circulation. 1996;94:1226–1232. doi: 10.1161/01.cir.94.6.1226. [DOI] [PubMed] [Google Scholar]
- 7.Tremoli E, Camera M, Toschi V, Colli S. Tissue factor in atherosclerosis. Atherosclerosis. 1999;144:273–283. doi: 10.1016/s0021-9150(99)00063-5. [DOI] [PubMed] [Google Scholar]
- 8.Misumi K, et al. Comparison of plasma tissue factor levels in unstable and stable angina pectoris. Am J Cardiol. 1998;81:22–26. doi: 10.1016/s0002-9149(97)00801-1. [DOI] [PubMed] [Google Scholar]
- 9.Mackman N. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscler Thromb Vasc Biol. 2004;24:1015–1022. doi: 10.1161/01.ATV.0000130465.23430.74. [DOI] [PubMed] [Google Scholar]
- 10.Meadows TA, Bhatt DL. Clinical aspects of platelet inhibitors and thrombus formation. Circ Res. 2007;100:1261–1275. doi: 10.1161/01.RES.0000264509.36234.51. This recent review describes the targets of the various antiplatelet drugs and their clinical use. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert JC, et al. First-in-human evaluation of anti von Willebrand factor therapeutic aptamer ARC1779 in human volunteers. Circulation. 2007;116:2678–2686. doi: 10.1161/CIRCULATIONAHA.107.724864. [DOI] [PubMed] [Google Scholar]
- 12.Oney ES, et al. Antidote-controlled platelet inhibition targeting von Willebrand factor with aptamers. Oligonucleotides. 2007;17:265–274. doi: 10.1089/oli.2007.0089. [DOI] [PubMed] [Google Scholar]
- 13.Lord ST. Fibrinogen and fibrin: scaffold proteins in hemostasis. Curr Opin Hematol. 2007;14:236–241. doi: 10.1097/MOH.0b013e3280dce58c. [DOI] [PubMed] [Google Scholar]
- 14.Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke. 1997;28:2109–2118. doi: 10.1161/01.str.28.11.2109. [DOI] [PubMed] [Google Scholar]
- 15.Brass LF, Zhu L, Stalker TJ. Minding the gaps to promote thrombus growth and stability. J Clin Invest. 2005;115:3385–3392. doi: 10.1172/JCI26869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Febbraio M, Hajjar DP, Silverstain RL. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest. 2001;108:785–791. doi: 10.1172/JCI14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Podrez EA, et al. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. J Clin Invest. 2007;13:1086–1095. doi: 10.1038/nm1626. This paper shows that oxidized low-density lipoproteins activate platelets by binding to CD36, providing a possible explanation for the link between hyperlipidaemia and thrombosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ratnikov BI, Partridge AW, Ginsberg MH. Integrin activation by talin. J Thromb Haemost. 2007;3:1783–1790. doi: 10.1111/j.1538-7836.2005.01362.x. [DOI] [PubMed] [Google Scholar]
- 19.Petrich BG, et al. The antithrombotic potential of selective blockade of talin-dependent integrin αIIbβ3 (platelet GPIIb-IIIa) activation. J Clin Invest. 2007;117:2250–2259. doi: 10.1172/JCI31024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babapulle MN, Joseph L, Belisle P, Brophy JM, Eisenberg MJ. A hierarchical Bayesian meta-analysis of randomised clinical trials of drug-eluting stents. Lancet. 2004;364:583–591. doi: 10.1016/S0140-6736(04)16850-5. [DOI] [PubMed] [Google Scholar]
- 21.Finn AV, et al. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007;115:2435–2441. doi: 10.1161/CIRCULATIONAHA.107.693739. [DOI] [PubMed] [Google Scholar]
- 22.Bavry AA, et al. Late thrombosis of drug-eluting stents: a meta-analysis of randomized clinical trials. Am J Med. 2006;119:1056–1061. doi: 10.1016/j.amjmed.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 23.Wiviott S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. This paper describes the results of a recent clinical trial of prasugrel, a new antiplatelet drug. [DOI] [PubMed] [Google Scholar]
- 24.Virchow R. Gesammelte Abhandlungen zur Wissenschaftlichen Medizin. Meidinger; Frankfurt: 1856. [Google Scholar]
- 25.Cushman M. Epidemiology and risk factors for venous thrombosis. Semin Hematol. 2007;44:62–69. doi: 10.1053/j.seminhematol.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heit JA. Venous thromboembolism: disease burden, outcomes and risk factors. J Thromb Haemost. 2007;3:1611–1617. doi: 10.1111/j.1538-7836.2005.01415.x. [DOI] [PubMed] [Google Scholar]
- 27.Segers K, Dahlback B, Nicolaes GA. Coagulation factor V and thrombophilia: background and mechanisms. Thromb Haemost. 2007;98:530–542. [PubMed] [Google Scholar]
- 28.Hron G, et al. Tissue factor-positive microparticles: cellular origin and association with coagulation activation in patients with colorectal cancer. Thromb Haemost. 2007;97:119–123. [PubMed] [Google Scholar]
- 29.Tesselaar ME, et al. Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J Thromb Haemost. 2007;5:520–527. doi: 10.1111/j.1538-7836.2007.02369.x. [DOI] [PubMed] [Google Scholar]
- 30.Polgar J, Matuskova J, Wagner DD. The P-selectin, tissue factor, coagulation triad. J Thromb Haemost. 2005;3:1590–1596. doi: 10.1111/j.1538-7836.2005.01373.x. [DOI] [PubMed] [Google Scholar]
- 31.Weitz JI, Linkins LA. Beyond heparin and warfarin: the new generation of anticoagulants. Expert Opin Investig Drugs. 2007;16:271–282. doi: 10.1517/13543784.16.3.271. [DOI] [PubMed] [Google Scholar]
- 32.Hirsh J, O’Donnell M, Eikelboom JW. Beyond unfractionated heparin and warfarin: current and future advances. Circulation. 2007;116:552–560. doi: 10.1161/CIRCULATIONAHA.106.685974. This review summarizes the anticoagulants that are in clinical use and the development of new anticoagulant drugs. [DOI] [PubMed] [Google Scholar]
- 33.Eriksson BI, et al. Oral rivaroxaban compared with subcutaneous enoxaparin for extended thromboprohylaxis after total hip arthroplasty. Blood. 2007;110 abstr. 6. This abstract describes the recent clinical trial assessing the effect of the factor-Xa inhibitor rivaroxaban on rates of thrombosis. [Google Scholar]
- 34.Snyder LA, et al. Expression of human tissue factor under the control of the mouse tissue factor promoter mediates normal hemostasis in knock-in mice. J Thromb Haemost. 2008;6:306–314. doi: 10.1111/j.1538-7836.2008.02833.x. [DOI] [PubMed] [Google Scholar]
- 35.Mackman N. Tissue-specific hemostasis in mice. Arterioscler Thromb Vasc Biol. 2005;25:2273–2281. doi: 10.1161/01.ATV.0000183884.06371.52. [DOI] [PubMed] [Google Scholar]
- 36.Bolton-Maggs PH, Pasi KJ. Haemophilias A and B. Lancet. 2003;361:1801–1809. doi: 10.1016/S0140-6736(03)13405-8. [DOI] [PubMed] [Google Scholar]
- 37.Galiani D, Renne T. The intrinsic pathway of coagulation: a target for treating thromboembolic disease? J Thromb Haemost. 2007;5:1106–1112. doi: 10.1111/j.1538-7836.2007.02446.x. [DOI] [PubMed] [Google Scholar]
- 38.Howard EL, Becker CD, Rusconi CP, Becker RC. Factor IXa inhibitors as novel anticoagulants. Arterioscler Thromb Vasc Biol. 2007;27:722–727. doi: 10.1161/01.ATV.0000259363.91070.f1. [DOI] [PubMed] [Google Scholar]
- 39.Weitz JI, Buller HR. Direct thrombin inhibitors in acute coronary syndromes: present and future. Circulation. 2002;105:1004–1011. doi: 10.1161/hc0802.104331. [DOI] [PubMed] [Google Scholar]
- 40.Turpie AG. Oral, direct factor Xa inhibitors in development for the prevention and treatment of thromboembolic diseases. Arterioscler Thromb Vasc Biol. 2007;27:1238–1247. doi: 10.1161/ATVBAHA.107.139402. [DOI] [PubMed] [Google Scholar]
- 41.Li WX, Kaplan AV, Grant GW, Toole JJ, Leung LL. A novel nucleotide-based thrombin inhibitor inhibits clot-bound thrombin and reduces arterial platelet thrombus formation. Blood. 1994;83:677–682. [PubMed] [Google Scholar]
- 42.Rusconi CP, et al. RNA aptamers as reversible antagonists of coagulation factor IXa. Nature. 2002;419:90–94. doi: 10.1038/nature00963. [DOI] [PubMed] [Google Scholar]
- 43.Rusconi CP, et al. Antidote-mediated control of an anticoagulant aptamer in vivo. Nature Biotechnol. 2004;22:1423–1428. doi: 10.1038/nbt1023. This paper describes the use of an aptamer targeting factor IXa and an ‘antidote’ oligonucleotide for the treatment of thrombosis. [DOI] [PubMed] [Google Scholar]
- 44.Dyke CK, et al. First-in-human experience of an antidote-controlled anticoagulant using RNA aptamer technology: a phase 1a pharmacodynamic evaluation of a drug–antidote pair for the controlled regulation of factor IXa activity. Circulation. 2006;114:2490–2497. doi: 10.1161/CIRCULATIONAHA.106.668434. [DOI] [PubMed] [Google Scholar]
- 45.Monia B, et al. ISIS 401025, a second generation antisense oligonucleotide targeting prothrombin, inhibits plasma prothrombin level and promotes anticoagulation in mice. Circulation. 2007;116(suppl) abstr. 716. [Google Scholar]
- 46.Berger JS, et al. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. J Am Med Assoc. 2006;295:306–313. doi: 10.1001/jama.295.3.306. [DOI] [PubMed] [Google Scholar]
- 47.Hennekens CH, Sechenova O, Hollar D, Serebruary VL. Dose of aspirin in the treatment and prevention of cardiovascuar disease: current and future directions. J Cardiovasc Pharmacol Ther. 2006;11:170–176. doi: 10.1177/1074248406292263. [DOI] [PubMed] [Google Scholar]
- 48.Grosser T, Fries S, FitzGerald GA. Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. J Clin Invest. 2006;116:4–15. doi: 10.1172/JCI27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gachet C. The platelet P2 receptors as molecular targets for old and new antiplatelet drugs. Pharmacol Ther. 2005;108:180–192. doi: 10.1016/j.pharmthera.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 50.The Clopidogrel in Unstable Angina To Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2007;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 51.Sabatine MS, et al. Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated wilth fibrinolytics. J Am Med Assoc. 2005;294:1224–1232. doi: 10.1001/jama.294.10.1224. [DOI] [PubMed] [Google Scholar]
- 52.Kong DF, et al. Clinical outcomes of therapeutic agents that block the platelet glycoprotein IIb/IIIa integrin in ischemic heart disease. Circulation. 1998;98:2829–2835. doi: 10.1161/01.cir.98.25.2829. [DOI] [PubMed] [Google Scholar]
- 53.Palareti G, et al. Bleeding complications of oral anticoagulant treatment: an inception-cohort, prospective collaborative study (ISCOAT) Lancet. 1996;348:423–428. doi: 10.1016/s0140-6736(96)01109-9. [DOI] [PubMed] [Google Scholar]
- 54.Krynetskly E. Building individualized medicine: prevention of adverse reactions to warfarin therapy. J Pharmacol Exp Ther. 2007;322:427–434. doi: 10.1124/jpet.106.117952. [DOI] [PubMed] [Google Scholar]
- 55.Quinlan DJ, McQuillan A, Eikelboom JW. Low-molecular-weight heparin compared with intravenous unfractionated heparin for the treatment of pulmonary embolism: a meta-analysis of randomized, controlled trials. Ann Intern Med. 2004;140:175–183. doi: 10.7326/0003-4819-140-3-200402030-00008. [DOI] [PubMed] [Google Scholar]
- 56.Turpie AG, Eriksson BI, Lassen MR, Bauer KA. Fondaparinux, the first selective factor Xa inhibitor. Curr Opin Hematol. 2003;10:327–332. doi: 10.1097/00062752-200309000-00001. [DOI] [PubMed] [Google Scholar]
- 57.Cines DB, et al. Heparin-induced thrombocytopenia: an autoimmune disorder regulated through dynamic autoantigen assembly/disassembly. J Clin Apher. 2007;22:31–36. doi: 10.1002/jca.20109. [DOI] [PubMed] [Google Scholar]
- 58.Eriksson BI, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet. 2007;370:949–956. doi: 10.1016/S0140-6736(07)61445-7. [DOI] [PubMed] [Google Scholar]
- 59.Fisher WD, et al. Rivaroxaban for thromboprophylaxis after orthopaedic therapy: pooled analysis of two studies. Thromb Haemost. 2007;97:931–937. [PubMed] [Google Scholar]