Abstract
We investigated the role of protein phosphatases (PP) and protein kinases in tyrosine hydroxylase (TH) activation by two patterns of intermittent hypoxia (IH) in rat brainstem. Rats exposed to either IH15s (15 s, 5% O2; 5 min, 21%O2) or IH90s (90 s each of 10% O2 & 21%O2) for 10 days were used. IH15s but not IH90s caused a robust increase in TH activity, dopamine (DA) level, and TH phosphorylation at Ser-31 and Ser-40 in the medulla but not in the pons. Likewise, IH15s but not IH90s decreased activity and expression of protein phosphatase 2A (PP2A) and increased activity of multiple protein kinases. In vitro dephosphorylation with PP2A nearly abolished IH15s-induced increase in TH activity. IH15s increased generation of reactive oxygen species (ROS) in brainstem medullary regions which was nearly threefold higher than that evoked by IH90s. Antioxidants prevented IH15s-induced downregulation of PP2A and increases in multiple protein kinase activity with subsequent reversal of serine phosphorylation of TH, TH activity, and DA to control levels. These findings demonstrate that IH in a pattern-specific manner activates TH involving ROS-mediated sustained increase in TH phosphorylation via downregulation of PP2A and upregulation of protein kinases. Antioxid Redox Signal 11, 1777–1789.
Introduction
Humans living at sea level experience intermittent hypoxia (IH) under a variety of conditions, including sleep-disordered breathing manifested as recurrent apneas. Studies in humans and animal models showed that IH associated with recurrent apneas leads to autonomic abnormalities, resulting in cardiorespiratory morbidities (6, 10, 13, 28, 32, 38, 43). Brainstem catecholaminergic neurons have been implicated in the regulation of cardiorespiratory systems (9, 12, 18, 25, 34, 35). Consequently, several studies examined the effects of IH on catecholamine metabolism and transmission. Results from these studies showed that IH increases norepinephrine levels in the hypothalamus (33) and alters catecholaminergic neurotransmission in brainstem regions of rat (19, 26). Furthermore, studies in PC12 cells showed that IH increased the activity of TH, the rate-limiting enzyme in the biosynthesis of catecholamines, via increased serine phosphorylation of TH (27). The signaling mechanisms that underlie IH-induced alterations in TH activity, however, remain to be investigated.
Tyrosine hydroxylase (TH; EC 1.14.16.2) catalyzes the conversion of tyrosine into dihydroxyphenylalanine (DOPA) in a molecular oxygen and tetrahydrobiopterin-dependent manner. TH activity in vivo is regulated post-translationally by reversible site-specific serine phosphorylation and therefore depends on the balance between protein kinases-mediated phosphorylation and protein phosphatases-dependent dephosphorylation reactions (1, 4, 11). The NH2-terminal region of TH contains three serine residues (Ser-19, Ser-31, and Ser-40) and increased phosphorylation of one or more of these serine residues leads to TH activation (5, 15, 22, 31). Protein kinases, including protein kinase A (PKA), Ca2+/calmodulin-dependent kinase II (CaMKII), and extracellular signal-regulated kinase (ERK), phosphorylate TH at one or more serine residues, resulting in a more active form of TH (see ref. 11 for references). Available evidence suggests that protein phosphatase 2A (PP2A) is the major serine/threonine phosphatase that dephosphorylates TH at Ser-31 and Ser-40 residues (2, 11, 20), leading to less active form of TH.
Chronic sustained hypoxia increased TH activity in the brainstem which was primarily attributed to upregulation of TH protein (42, 46). On the other hand, studies in PC12 cells (27) showed that IH-induced increase in TH activity is not associated with a concomitant increase in TH protein, suggesting IH-induced TH activation. Contrasting this finding, Gozal et al., (17) using an IH protocol comprising alternating cycles of hypoxia (10% O2) and normoxia (21% O2) of 90 s each (i.e., IH90s) reported a lack of stimulatory effect of IH on TH activity. Given the differences in IH protocols used in these two studies (17, 27), it is likely that the stimulatory effect on TH activity by IH is pattern specific. Therefore, in the present study, we investigated whether IH patterns consisting of alternating cycles of 5%O2 for 15 s and 21% O2 for 5 min (i.e., IH15s) which is similar to that used in our previous cell culture studies (27), and IH90s affect TH activity and catecholamine level in the rat brainstem regions implicated in cardiorespiratory control. Furthermore, we also examined the signaling mechanisms that underlie the differences in the response of TH activity to IH15s and IH90s by focusing on the roles of reactive oxygen species (ROS), PP2A, and protein kinases in IH-evoked TH activation.
Materials and Methods
The Institutional Animal Care and Use Committee of the University of Chicago approved animal handling and experimental protocols. The studies were performed with adult male Sprague–Dawley rats weighing 200–230 g.
Exposure to two patterns of IH
The rats were exposed to two different patterns of IH. For the first IH pattern (IH15s), the animals were placed in an IH chamber and exposed to alternating cycles of hypoxia (15 s of 5% O2) and normoxia (5 min of 21% O2) for 8 h/day between 9:00 AM to 5:00 PM for 10 days, as described previously (40). The second IH protocol (IH90s) consists of alternating cycles of hypoxia (10% O2) and normoxia (21% O2) for 90 s each for 10 days, as described previously (17). Ambient O2 level in the IH chamber was continuously monitored with an O2 analyzer (Beckman model OM-11). Inspired CO2 level was maintained at 0.2–0.5% and monitored continuously by an infrared analyzer (Beckman model LB-2). The duration of the gas flow during each hypoxic and normoxic episode was regulated by timed solenoid valves. Animals exposed to normoxia for 10 days served as controls.
In experiments wherein the role of ROS was assessed, rats were given either manganese (III) tetrakis (1-methyl-4-pyridyl) porphyrin pentachloride (MnTMPyP; Alexis Biochemicals, CA; 5 mg.kg−1.day−1; I.P.) or N-acetyl-L-cysteine (NAC; Sigma; 400 mg.kg−1.day−1; I.P.) daily prior to exposure to IH for 10 days. Rats exposed to normoxia receiving either saline or saline + NAC or saline + MnTMPyP served as controls.
Acute experiments were performed within ∼16 h after terminating IH exposures in rats anesthetized with urethane (1.2 g/kg, Sigma; I.P.), and the surgical plane of anesthesia was assessed by the absence of limb withdrawal reflex in response to noxious pinching of toes.
Isolation of tissues
Whole brain tissues were removed from anesthetized rats, frozen in liquid nitrogen, and stored at −80°C until further analysis. In the experiments involving analysis of various brainstem regions, dorsal and ventral pons and dorsal and ventral medulla were dissected under ice-cold conditions.
Assay of TH activity
For the analysis of TH activity, either whole brainstem or dorsal and ventral medulla or dorsal and ventral pons were homogenized in 10 volumes of 10 mM potassium phosphate buffer, pH 7.4, containing complete protease inhibitor cocktail (EDTA-free; Roche, IN) and phosphatase inhibitor cocktail set I (Calbiochem, CA) at 4°C. The extract was centrifuged at 500 g for 10 min and the pellet containing cell debris and nuclei was discarded. The supernatant fraction was further centrifuged at 13,000 g for 30 min at 4°C and the resulting supernatant was passed through a Sephadex G-25 column to remove low molecular weight inhibitory molecules and immediately used for the assay of TH activity.
TH activity was determined by measuring the amount of L-DOPA formed from L-tyrosine, as described previously (27, 36, 37) with few modifications. Briefly, the reaction mixture (in a total volume of 250 μl) contained the following in final concentration: Tris-acetate, 100 mM, pH 5.8; 100 μM L-tyrosine; 400 μM (6R)-5,6,7,8-tetrahydrobiopterin dihydrochloride (Sigma) in 1 M 2-mercaptoethanol; 2500 units catalase; 50 μM 3-hydroxybenzyl hydrazine (NSD-1015, an inhibitor of L-aromatic amino acid decarboxylase); 400 μM FeSO4.7H2O, and tissue extract (200 ng protein equivalent). The concentration of BH4 (400 μM) was selected based on preliminary studies comparing the effect of varying BH4 concentration on TH activity in normoxic and IH brainstem tissues. To assess the specificity of the assay, control experiment was performed wherein L-tyrosine was replaced with D-tyrosine (100 μM). With D-tyrosine, DOPA formation was not detected in extracts derived from either normoxic or IH brainstem regions. After incubation of the reaction mixture at 37°C for 6 min, the reaction was terminated by the addition of 1 M perchloric acid containing 0.2 M EDTA at 4°C. A known amount of freshly prepared 3,4-dihydroxybenzylamine (DHBA), an internal standard, was added to the reaction medium to assess the efficiency of DOPA recovery. DOPA along with DHBA was extracted from the reaction medium using acid-activated aluminum oxide, and their levels were measured by HPLC combined with electrochemical detection as described below under “Analysis of DOPA”. On average, ∼78% DOPA was recovered during extraction, and all data were corrected for recovery losses during extraction and HPLC analysis and for variation in the amount of proteins. TH activity was expressed as nanomoles of DOPA formed from tyrosine per minute per milligram of protein. Protein concentration in tissue extracts was determined using DC protein assay kit from Bio-Rad using bovine serum albumin as the standard.
Analysis of DOPA
DOPA was analyzed by HPLC-ECD (Bioanalytical Systems) as described previously (27). Briefly, DOPA and DHBA (internal standard) were separated on a Prodigy ODS reverse phase column (Phenomenex) by isocratic elution with a mobile phase consisting of 4% (vol/vol) acetonitrile, 0.1 M sodium nitrate, 0.08 M sodium dihydrogen phosphate, 200 mg/l sodium octyl sulfate, and 0.01% disodium EDTA adjusted to pH 2.7 with phosphoric acid. Under the experimental conditions, DOPA and DHBA were eluted at ∼12 and 14 min, respectively, with an average recovery of ∼78%. The chromatograms were recorded and analyzed using a Hitachi D-2500 Chromato-Integrator. The concentrations of DOPA and DHBA were determined using standard curves correlating their respective amounts to the integrated peak areas, corrected for recovery. The detection limit for DOPA was 50 picomoles.
Analysis of dopamine content
Brainstems from the control and IH rats were homogenized in 500 μl of 0.1 N HClO4 containing 0.25 mM disodium EDTA, and the homogenates were centrifuged at 18,000 g for 15 min at 4°C. The clear supernatant was used for the analysis of catecholamines by HPLC-ECD as described previously (27).
Assay of protein phosphatase 2A (PP2A)
PP2A activity in the cytosolic and membrane fractions was assayed using EnzChek Phosphatase assay Kit (Invitrogen, OR) following the protocol provided by the manufacturer.
The dorsal and ventral medullae of the brainstem were homogenized in 25 mM Tris-HCl buffer, pH 7.4, containing 1 mM EDTA and complete protease inhibitor cocktail (Roche; homogenization buffer). Tissue extracts were centrifuged at 500 g for 15 min to remove the cell debris and nucleus and then at 16,000 g for 1 h at 4°C to separate cytosolic (supernatant) and membrane-enriched (pellet) fractions. Both the cytosolic and membrane-enriched fractions were stored at −80°C until further analysis. Prior to PP2A assay, the membrane fraction was thawed and extracted in 200 μl of the homogenization buffer containing 0.5% (vol/vol) Triton X-100, at 4°C overnight. The membrane extract was spun at 16,000 g for 30 min and the supernatant was used for PP2A assay.
PP2A activity was determined by measuring the amount of 6, 8-difluoro-7-hydroxy-4-methyl-coumarin formed from 6, 8-difluoro-4-methylumbelliferyl phosphate in a continuous fluorescence based assay in the presence and absence of 2 nM okadaic acid. The reaction mixture in a total volume of 200 μl contains Tris-Cl, 50 mM, pH 7.4; 6,8-difluoro-4-methylumbelliferyl phosphate, 50 μM and soluble or membrane fractions (10 μg protein). The formation of 6,8-difluoro-7-hydroxy-4-methyl-coumarin was continuously monitored for 10 min in a Shimadzu spectrofluorometer using excitation and emission wavelengths of 358 and 455 nm, respectively. The concentration of 6,8-difluoro-7-hydroxy-4-methyl-coumarin was determined from a standard curve correlating the concentration of the product to the corresponding fluorescence intensity values. PP2A activity is defined as the amount of okadaic acid (2 nM) inhibitable phosphatase activity and expressed as micromoles per minute per milligram protein.
Assay of protein kinases
For the assay of protein kinases, dorsal and ventral medullae from control and IH exposed rats were homogenized in 25 mM Tris-Cl, pH 7.4, containing complete protease inhibitor cocktail (Roche) and phosphatase inhibitor cocktail set I (Calbiochem) at 4°C. The homogenate was centrifuged at 13,000 g for 30 min at 4°C and the resulting clear supernatant was used for protein kinase assay.
Protein kinase A (PKA) activity was measured using commercially available PKA kinase activity assay kit (EKS-390A; Assay Designs, MI). Phosphorylation of PKA-specific synthetic peptide substrate was measured using a polyclonal antibody specific to a phosphorylated form of the substrate. Using a standard curve relating the amount of active PKA and the level of substrate phosphorylation, PKA activity was determined and expressed as nanogram of active PKA per mg of protein.
For the determination of Ca2+/calmodulin-dependent kinase II (CaMKII) activity, a commercially available CycLex® CaMKII Assay Kit (CY-1173; MBL Corporation, MA) was used as per the manufacturers' instructions. To quantify CaMKII activity, a standard curve correlating the amount of active CaMKII and the level of phosphorylation of Syntide-2, a CaMKII specific substrate was constructed and enzyme activity was expressed as nanogram of active CaMKII per mg of protein.
To assay MAP kinase/extracellular signal-regulated kinase 1 and 2 (ERK1/2) activity, the level of phosphorylated ERK1/2 was determined by immunoblot analysis of tissue extracts as described in the following section. For immunolabeling of ERK1/2, antibodies specific to total and phosphorylated forms of ERK1/2 [phospho-p44/42 (Thr202/Tyr204)] were used (Cell Signaling Technology, MA; each at a dilution of 1:1,000).
Immunoblot analysis of TH and PP protein expressions
Equal amounts of protein (20 μg) from the control and IH tissue extracts were separated on 10% polyacrylamide slab gels containing sodium dodecyl sulfate and transferred to PVDF membrane. The transfer efficiency of proteins was assessed using brief Ponceau S staining. Nonspecific binding sites on the membrane were blocked by incubation with 5% milk in Tris-buffer containing saline and 0.1% (vol/vol) Tween-20 for 1 h. Blots were subsequently incubated with appropriate antibody for 18 h at 4°C. The following antibodies were used: anti-TH (Cell Signaling Technology; at 1:2,000 dilution); anti-phospho TH (Ser-19 and Ser-31, Chemicon International Inc, CA; each at 1:2,500 dilution); anti-phospho TH Ser-40 (Cell Signaling Technology; at 1: 2,500 dilution); anti-PP2A (Cell Signaling Technology; at 1:1,000 dilution); anti-PP2C (Epitomics, CA; at 1:1,000 dilution); anti-PP4 (Santa Cruz Biotech Inc., CA; at 1:1,000 dilution); anti-PP5 (BD Biosciences, CA; at 1:2,000 dilution); anti-PP6 (Exalpha Biologicals Inc., MA; at 1:1,000 dilution). After washing steps, the blots were incubated with horseradish peroxidase-conjugated goat anti-rabbit or goat anti-mouse IgG (Santa Cruz Biotech Inc.; each at 1:10,000) for 1 h at 4°C. The immunoreactive total and phosphorylated forms of TH and various protein phosphatases were identified using ECL detection kit from Amersham Biosciences. The relative intensity of major immunoreactive protein band, as indicated by arrow in the figure legends was quantified using the Image J 1.37v software and the values were expressed as fold change with respect to normoxic control (i.e., Normoxia = 1). As a loading control, the expression level of β-actin (42-kDa) was monitored.
In vitro dephosphorylation of TH by PP2A
Brainstem extracts from the control and IH rats were incubated in the presence of 0.2 units of the catalytic subunit of PP2A (Calbiochem) in 25 mM Tris-Cl buffer, pH 7.0, for 15 min at 25°C. For control samples, the extracts were incubated with buffer. Following dephosphorylation, the reaction mixture was assayed for TH activity as described above.
Assay of Thio-Barbituric Acid Reactive Substances (T-BARS)
The brainstem medullary regions were homogenized in 20 mM phosphate buffer, pH 7.4 and the homogenates were centrifuged in an Eppendorf microcentrifuge at 500 g for 10 min at 4°C. The level of lipid oxidation was determined as T-BARS, following the procedure as described (28, 44). Briefly, 100 μl of either sample or suitable concentrations of malondialdehyde (MDA) standard was added to 50 μl of SDS (8.1%), 375 μl of acetic acid (20%), and 375 μl of thiobarbituric acid (0.8%). The samples were incubated at 90°C for 60 min followed by incubation at 4°C for 10 min. The reaction mixture was centrifuged at 800 g for 15 min and the absorbance of the resulting supernatant was monitored at 532 nm. The level of T-BARS was expressed as nanomoles of MDA formed per mg protein.
Experimental protocols
Series 1
TH activity was determined in the whole brainstem of rats exposed to 10 days of either normoxia or IH15s or IH90s (n = 7 rats in each group). TH activity was measured in triplicate as described above.
Series 2
TH activity as well as the total as well as Ser-19, Ser-31, and Ser-40 phosphorylated forms of TH protein expression were analyzed in triplicate and duplicate, respectively, in the extracts of dorsal and ventral medullary regions of brainstem from rats exposed to 10 days of normoxia or IH15s or IH90s or daily treated with either MnTMPyP or NAC prior to IH15s for 10 days (n = 7 rats in each group). TH activity in the dorsal and ventral pons of rats was also assayed in rats exposed to 10 days of either normoxia or IH15s. The effect of in vitro PP2A treatment on TH activity was determined in the dorsal and ventral medullary extracts of rats exposed to 10 days of either normoxia or IH15s (n = 7 rats in each group).
Series 3
PP2A activity and protein expression of PP2A, PP2C, PP4, PP5, and PP6 were assayed in triplicate and duplicate, respectively, in the dorsal and ventral medulla of rats exposed to 10 days of either normoxia or IH15s or IH90s or daily treated with MnTMPyP prior to IH15s for 10 days (n = 7 rats in each group).
Series 4
Enzyme activities of PKA, CaMKII, and ERK1/2 were assayed in triplicate in the dorsal and ventral medulla of rats exposed to 10 days of either normoxia or IH15s or IH90s or daily treated with MnTMPyP prior to normoxia or IH15s for 10 days (n = 7 rats in each group).
Series 5
Thio-barbituric acid reactive substances (T-BARS) level in the dorsal and ventral medullary brainstem regions was determined in rats exposed to 10 days of either normoxia or IH15s or IH90s or daily treated with MnTMPyP or NAC prior to normoxia or IH15s for 10 days (n = 7 rats in each group).
Series 6
DA content in the whole brainstem was determined in triplicate in rats exposed to 10 days of normoxia or IH15s or daily treated with either MnTMPyP or NAC prior to normoxia or IH15s for 10 days (n = 7 rats in each group).
Data analysis
All enzyme activity measurements were made in triplicate whereas immunoblot analyses were made in duplicate. Data derived from 7 independent experiments (n = 7 rats in each group) in each one of the 6 series as outlined above were expressed as mean ± SE. Statistical significance was evaluated by unpaired t-test or one-way ANOVA for repeated measures. p Values < 0.05 were considered significant.
Results
Effect of IH on TH activity
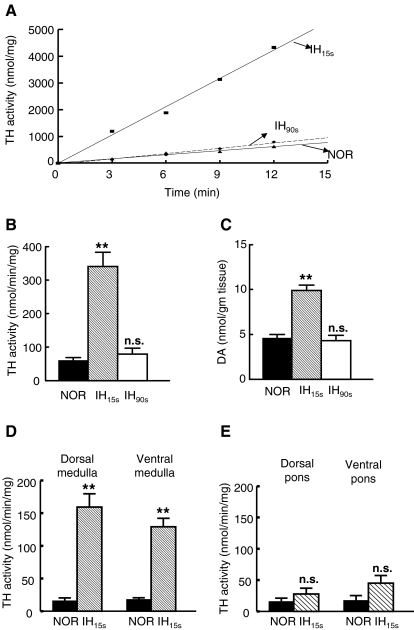
In the normoxic, IH15s, and IH90s brainstem extracts, TH activity, as measured by the formation of DOPA, increased linearly as a function of reaction time (Fig. 1A). TH activity in the normoxic brainstem was 60.6 ± 8.3 nmol/min/mg protein. TH activity, following 10 days of IH15s, increased by 5.6-fold compared to normoxic control (NOR, p < 0.01; Fig. 1B). The IH15s-induced increase in TH activity was associated with a significant elevation in DA level in the brainstem (p < 0.01; Fig. 1C). By contrast, exposure of rats to 10 days of IH90s did not significantly alter either TH activity or DA level in the brainstem (p > 0.05; Fig. 1B and C).
FIG. 1.
Effects of two patterns of intermittent hypoxia (IH) on tyrosine hydroxylase (TH) activity and dopamine (DA) levels in the rat brainstem. Changes in TH activity as a function of reaction time are shown (A). TH activity was measured in the whole brainstem extracts of rats exposed to either IH15s (alternating cycles of 15 s of 5% O2 and 5 min of 21% O2) or IH90s (alternating cycles of 90 s of 10% O2 and 90 s of 21% O2) or normoxia (NOR) for 10 days, as described under “Methods”. Effects of IH15s and IH90s on TH activity (B) and on DA levels (C) in the whole brainstem are shown. For DA analysis, brainstems were extracted with 0.1 N HCLO4. DOPA formed during TH reaction and DA level in the whole brainstem were determined by HPLC combined with electrochemical detection as described in “Methods”. TH activity is expressed as nanomoles of DOPA formed per min per mg protein, and DA as nanomoles per gram tissue. A comparison of the effect of IH15s on TH activity in the dorsal and ventral medullary (D; left panel) and pontine (E; right panel) brainstem regions is shown. All measurements were made in triplicate and average data are shown as mean ± S.E. (n = 7 rats in each group). Asterisks denote p < 0.01. n.s., not significant (p > 0.05).
To assess regional variations, if any, in IH-evoked increase in TH activity, the effects of IH15s on TH activity in the dorsal and ventral medulla as well as dorsal and ventral pons were determined. The basal TH activity in the dorsal medulla was 16.2 ± 4.3 nmol/min/mg protein and was comparable to the activity seen in the dorsal pons as well as ventral pons and medulla. IH15s increased TH activity in the dorsal and ventral medulla by 9.8- and 7.1-fold, respectively, compared to normoxic control (NOR, p < 0.01; Fig. 1D), whereas TH activity in either dorsal or ventral pons was not significantly altered (p > 0.05; Fig. 1E).
Effect of IH on TH protein level
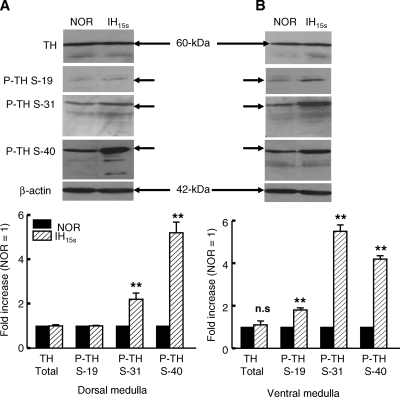
To determine whether IH15s-induced increase in TH activity is due to increase in TH protein, dorsal and ventral medullary extracts from normoxic and IH exposed rats were resolved by SDS-PAGE and probed with anti-TH antibody. Immunoblot analysis showed that IH15s had no significant effect on TH protein expression in the dorsal and ventral medullae (p > 0.05; Fig. 2A and B), suggesting that the increase in TH activity by IH15s is not due to an increase in TH protein level.
FIG. 2.
Effects of IH15s on total and serine phosphorylated forms of TH protein expression in the dorsal and ventral medullary brainstem regions. Equal amounts of proteins (20 μg) from normoxic and IH15s extracts were used for immunoblot analysis. Upper panels: Representative examples of immunoblots of total and serine phosphorylated forms of TH (P-TH). Bottom panels: IH-induced fold change in total or serine phosphorylated forms of TH relative to normoxic control. Other experimental details are as described in “Methods”. All measurements were made in duplicate and average data are shown as mean ± S.E. (n = 7 rats in each group). Asterisks denote p < 0.01; n.s., not significant (p > 0.05).
IH15s increases serine phosphorylation of TH
Because alterations in serine phosphorylation of TH have been shown to markedly affect TH activity (4, 11), the role of serine phosphorylation in IH15s-induced increase in TH activity was investigated by immunoblot analysis using antibodies specific to Ser-19-, Ser-31-, and Ser-40 phosphorylated forms of TH. In the normoxic dorsal and ventral medulla, low levels of serine phosphorylated forms of TH were detected (Fig. 2A and B) and IH15s evoked a robust increase in phosphorylation of TH at multiple serine residues. For instance, phosphorylation of TH at Ser-40 and Ser-31 was increased by 5.2- and 2.2-fold, respectively, in the IH dorsal medulla (Fig. 2A, left panels). On the other hand, IH15s increased the level of phosphorylation of Ser-19, Ser-31, and Ser-40 of TH, albeit to different degrees in the ventral medulla. The rank order of IH-evoked increase in phosphorylation of TH was ser-19 < ser-40 < ser-31 (Fig. 2B; right panels). Taken together, the above findings suggest that IH markedly elevated phosphorylation of TH at Ser-31 and Ser-40 in the dorsal and ventral medulla with a concomitant robust increase in TH activity.
Evidence for the involvement of protein phosphatase 2A (PP2A) in IH15s-induced activation of TH
The steady state level of serine phosphorylation of TH in vivo is regulated by the combined activities of protein kinases and protein phosphatases (see Ref. 11 for references). Either downregulation of TH dephosphorylation or upregulation of TH phosphorylation may contribute to increased serine phosphorylation of TH. In the following experiments, we tested the above possibilities.
Available evidence suggests that PP2A, a serine/threonine protein phosphatase that is sensitive to low concentration of okadaic acid (2 nM) plays a major role in TH dephosphorylation and hence TH activation in the rat adrenal medulla and brain (2, 20, 30). Therefore, we examined whether downregulation of PP2A contributes to IH15s-evoked increase in TH activity in brainstem medullary regions.
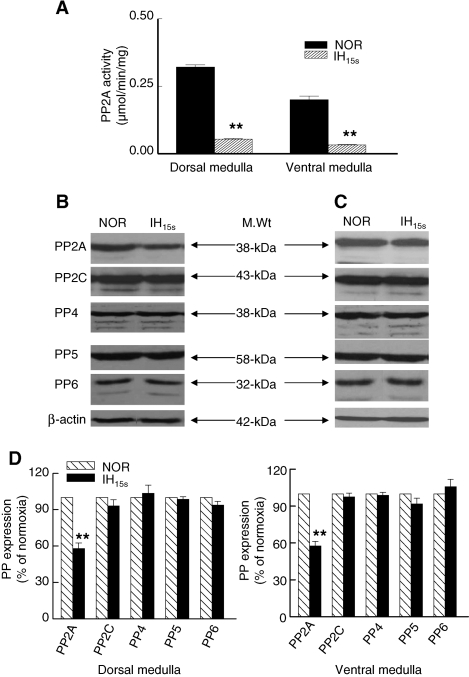
Nearly 70% of PP2A activity was expressed in the cytosolic fractions isolated from the medullary brainstem regions. PP2A activity was relatively higher in the dorsal than in the ventral medulla of normoxic rats (NOR; Fig. 3A). IH15s decreased PP2A activity by nearly 80% in both dorsal and ventral medulla (p < 0.01; Fig. 3A). The IH15s-induced decrease in PP2A activity was, in part, due to reduced PP2A protein expression (38 kDa; p < 0.01; Fig. 3B, C, and D). On the other hand, PP2A activity in the membrane-enriched fraction was unaffected by IH15s (data not shown).
FIG. 3.
Changes in PP2A enzyme activity and protein phosphatase (PP) expression in the dorsal and ventral medulla by IH15s. Effect of IH on PP2A enzyme activity is shown (A). PP2A activity was determined by procedures as described under “Methods” and is expressed as micromoles per minute per mg protein. Representative immunoblots showing IH15s-induced changes in PP expression in the dorsal (B) and ventral (C) medulla are presented. Equal amounts of proteins (20 μg) from normoxic (NOR) and IH15s medullary extracts were used for immunoblot analysis. Changes in PP expression as percent of normoxia are shown in (D). All measurements were made in triplicate and average data are shown as mean ± S.E. (n = 7 rats in each group). Asterisks denote p < 0.01.
To assess whether IH15s affect protein phosphatases other than PP2A that are sensitive to low concentration of okadaic acid (2 nM), we determined expression of PP4, PP5, and PP6, as well as PP2C which is insensitive to low concentration of okadaic acid in the extracts of normoxic and IH15s brainstem medullary regions. Immunoblot analysis showed that IH15s has no significant effect on the level of PP2C, PP4, PP5, and PP6 proteins (p > 0.05; Fig. 3B–D).
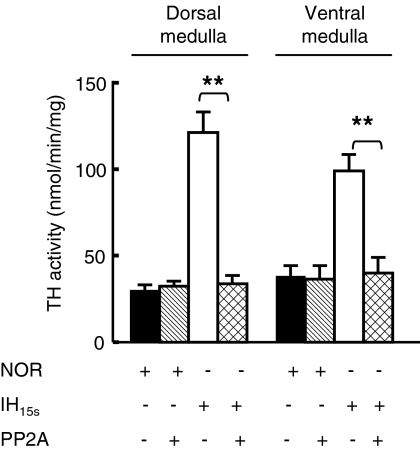
The results presented above suggest that IH15s downregulates selectively PP2A in the medullary regions of the brainstem. To establish whether the downregulation of PP2A contributes to IH15s-evoked TH activation, TH activity in the normoxic and IH15s extracts of dorsal and ventral medulla was measured after incubating the extracts with the catalytic subunit of PP2A (0.2 units) for 15 min, and the results were compared with those derived from untreated controls. Pre-treatment with the catalytic subunit of PP2A had no significant effect on TH activity in the normoxic controls, whereas it markedly reduced IH15s-evoked increase in TH activity in the dorsal and ventral medulla (Fig. 4). Further increases in PP2A concentration had no additional effect on TH activity (data not shown).
FIG. 4.
Reversal of IH15s-induced increase in TH activity by PP2A. TH activity was determined in the extracts of dorsal and ventral medulla derived from rats exposed to normoxia (NOR) or IH15s for 10 days before and after dephosphorylation. For dephosphorylation, aliquots of the medullary extracts were treated with 0.2 units of the catalytic subunit of PP2A for 15 min, as described in the “Methods”. All measurements were made in triplicate and average data are shown as mean ± S.E. (n = 7 rats in each group). Asterisks denote p < 0.01.
IH15s upregulates multiple protein kinases
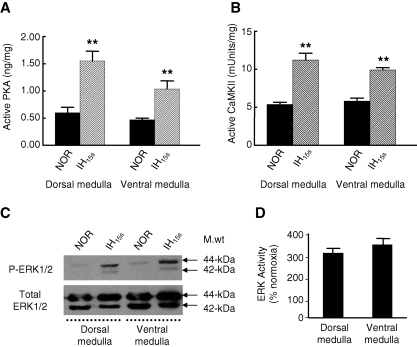
In vitro and in situ studies showed that protein kinase A (PKA), Ca2+/calmodulin-dependent kinase II (CaMKII), and MAP kinase/extracellular-signal regulated kinases 1 and 2 (ERK1/2) are the major protein kinases that phosphorylate TH at Ser-40, Ser-19, and Ser-31, respectively, (see ref. 11 for references). We determined whether IH15s altered the activity of one or more of these protein kinases. Basal levels of active PKA, CaMKII, and ERK1/2 were comparable between dorsal and ventral medulla of brainstem (Fig. 5A–C). IH15s upregulated active forms of PKA, CaMKII, and ERK1/2 in the dorsal and ventral medulla, albeit to different degrees. The rank order of increase in protein kinase activity by IH15s was: ERK1/2 > PKA > CaMKII (Fig. 5A–D).
FIG. 5.
Activation of protein kinases by IH15s. Effects of IH15s on the activity of protein kinase A (PKA; A), Ca2+/calmodulin-dependent kinase II (CaMKII; B), and MAP kinase/extracellular signal regulated kinase 1 and 2 (ERK1/2; C) in the dorsal and ventral medulla are shown. Active PKA and CaMKII were determined using commercially available Kinase Assay Kit as per manufacturer's instructions. Activity of ERK1/2 was assessed by immunoblot analysis using antibodies specific for total- and phospho-ERK1/2 (P-ERK1/2). All measurements were made in triplicate and average data are shown as mean ± S.E. (n = 7 rats in each group). Asterisks denote p > 0.01.
Effect of IH90s on TH phosphorylation, protein phosphatase 2A, and protein kinases
As mentioned earlier, IH15s but not IH90s, evoked a robust increase in TH activity in brainstem medullary regions. The increase in TH activity by IH15s was, in part, due to increased TH serine phosphorylation via mechanisms involving downregulation of protein phosphatase 2A and upregulation of protein kinases including PKA, CaMKII, and ERK1/2. We examined whether IH90s can evoke a similar effect in dorsal and ventral medulla. In sharp contrast to IH15s, neither TH phosphorylation (data not shown) nor PP2A activity nor levels of active PKA and CaMKII were affected by IH90s (Table 1). Likewise, the level of phospho-ERK1/2 was also unaffected (data not shown).
Table 1.
Effects of IH90s on PP2A, PKA and CaMKII Activities in the Rat Dorsal and Ventral Medullary Brainstem Regions
| |
PP2A activity* (μmol/min/mg) |
PKA activity (ng of active PKA/mg) |
CaMKII activity (ng of activeCaMKII/mg) |
|||
|---|---|---|---|---|---|---|
| Gas challenge | Dorsal medulla | Ventral medulla | Dorsal medulla | Ventral medulla | Dorsal medulla | Ventral medulla |
| Control (NOR) | 0.34 ± 0.08 | 0.22 ± 0.02 | 0.6 ± 0.2 | 0.4 ± 0.1 | 5.4 ± 0.3 | 5.8 ± 0.4 |
| IH90s | 0.31 ± 0.11 (n.s.) | 0.21 ± 0.04 (n.s.) | 0.7 ± 0.3 (n.s.) | 0.5 ± 0.4 (n.s.) | 6.1 ± 0.6 (n.s.) | 5.3 ± 0.5 (n.s.) |
Okadaic (2nM) acid inhibitable; All measurements were made in triplicate and average data are shown as mean ± S.E. (n = 7 rats in each group). n.s.-not significant, p > 0.05.
Analysis of ROS generation by IH15s and IH90s and effects of antioxidants on IH15s-induced functional alterations in TH
ROS generation is increased in central and peripheral tissues of rodents exposed to IH (28, 40, 44, 52). The following experiments were performed to determine whether IH15s and IH90s both facilitate ROS generation equally in the dorsal and ventral medulla and to assess whether ROS contributes to IH15s-induced TH activation and the ensuing elevation in dopamine level, as well as the simultaneous upregulation of protein kinases and downregulation of PP2A and the resultant increase in serine phosphorylation of TH in the medullary brainstem regions.
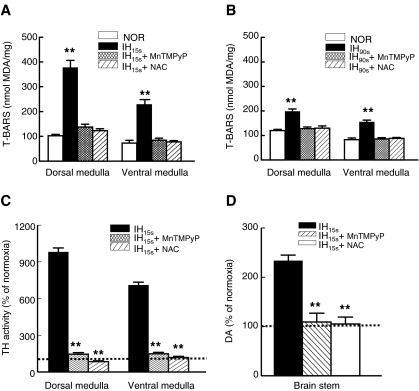
An increase in the level of MDA was used as an index of ROS generation (28, 44). In the dorsal and ventral medulla, IH15s increased MDA level by nearly 3.8-, and 2.5-fold, respectively compared to normoxic control (Fig. 6A). Although MDA level was elevated in brainstem medullary regions of rats exposed to IH90s, the increase was much lower than that seen with IH15s (Fig. 6B).
FIG. 6.
IH-evoked ROS generation and effects of antioxidants on IH-induced ROS generation, increase in TH activity, and elevation in DA level in the dorsal and ventral medulla. The effects of IH15s (A) and IH90s (B) on T-BARS level in dorsal and ventral medullary homogenates are shown. MnTMPyP, a membrane permeable superoxide anion mimetic and N-acetyl-L-cysteine (NAC; a scavenger of reactive oxygen species) were administered (i.p.) daily prior to exposure of rats to either normoxia (NOR) or IH as described in “Methods”. T-BARS level was expressed as nanomoles of malondialdehyde (MDA) formed per mg protein. Effects of MnTMPyP and NAC on IH15s-induced increase in TH activity (C) and elevation of DA (D) are shown. DA was measured in whole brainstem extracts. All measurements were made in triplicate and average data are shown as mean ± S.E. (n = 7 rats in each group). Asterisks denote p < 0.01.
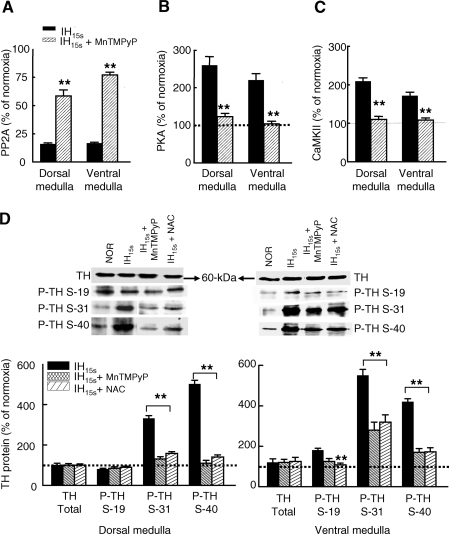
To determine whether antioxidants prevent IH15s-, and IH90s-induced increase in MDA level as well as IH15s-evoked structural and functional changes in TH, rats were treated daily either with MnTMPyP, a membrane permeable O2•− scavenger or with NAC, an antioxidant and then exposed to IH or normoxia for 10 days. MnTMPyP and NAC treatments nearly abolished IH15s and IH90s-evoked elevation of MDA level (Fig. 6A and B). Notably, these antioxidants markedly attenuated IH15s-induced increases in TH activity (Fig. 6C) and dopamine levels (Fig. 6D). However, neither MnTMPyP nor NAC affected TH activity and DA level in the normoxic controls (data not shown). More importantly, antioxidant reversed IH15s-induced decrease in PP2A activity (IH15s versus IH15s + MnTMPyP; p < 0.01; Fig. 7A), as well as the increases in active PKA and CaMKII (p < 0.01; Fig. 7B and C) and in the level of phospho-ERK1/2 (data not shown) with a parallel attenuation of IH15s-evoked increases in Ser-31 and Ser-40 phosphorylation of TH (IH15s versus IH15s + antioxidants; p < 0.01; Fig. 7D) in the dorsal and ventral medulla.
FIG. 7.
Reversal of IH15s-induced decrease in PP2A activity and the increase in PKA and CaMKII activity and serine phosphorylation of TH by antioxidants. The effects of MnTMPyP on IH15s-evoked decrease in PP2A activity (A) and increases in PKA (B), CaMKII (C), and phosphorylation of TH at S-19, S-31 and S-40 (P-TH; D) in the dorsal and ventral medullary brainstem regions are presented. Other experimental details are as described in the legends of Figs. 3 and 5. The data are expressed as percent of normoxic control. All measurements were made in triplicate and average data are shown as mean ± S.E. (n = 7 rats in each group). Asterisks denote p < 0.01.
Discussion
A major finding of the present study is that IH, in a pattern-specific manner, increased TH activity in the rat brainstem medullary regions; IH15s induced a robust increase in TH activity, whereas in sharp contrast, IH90s had no significant effect. The increase in TH activity by IH15s is consistent with a previously reported increase in TH activity in PC12 cells by an IH pattern similar to that used in the present study (27). The ineffectiveness of IH90s to upregulate TH activity in brainstem medullary regions is in accord with the lack of stimulation of TH activity reported previously in the rat brainstem using a similar IH90s protocol (17). The possible signaling mechanisms that may contribute to this contrasting pattern-specific effect of IH on TH activity will be discussed in a later section.
Interestingly, IH15s exerts a regio-specific effect on TH activity. Despite its expression in both pontine and medullary brainstem regions, TH activity was augmented by IH15s only in the dorsal and ventral medulla. Since a similar increase in TH activity was not seen in pons, it is likely that the potentiating effect of IH15s on TH activity is primarily restricted to the medullary brainstem regions. It is interesting to note that IH15s, in sharp contrast to its effect on TH, uniformly increased the activity of peptidyl glycine α-amidating mono-oxygenase, an O2 requiring, rate-limiting enzyme in the synthesis of C-terminal alpha amidated neuropeptides (e.g., substance P) in both medulla and pons (45). The mechanism(s) that contributes to the differential responses of TH and PAM that are monooxygenases to IH15s in the pontine brainstem region, however, remains to be determined.
IH15s-induced increase in TH activity was accompanied by an increase in DA in the medullary brainstem regions. The IH15s-induced elevation in DA may arise either from an increased synthesis or decreased degradation of DA. Since IH15s also increased the level of dihydroxyphenylacetic acid, a major oxidative degradation product of DA (Kumar et al., unpublished observation), it is likely that IH15s-induced increase in DA in the brainstem is primarily due to increased TH-mediated synthesis.
How does IH15s increase TH activity? TH activity is known to be regulated in vivo by several factors that include altered protein expression, serine phosphorylation, and feed-back product inhibition. Since the total TH protein expression per se remained the same between the normoxic and IH15s medullae, the increase in TH activity by IH15s is likely due to enzyme activation. Phosphorylation and/or dephosphorylation also contribute to long-term regulation of TH activity. Thus, increased phosphorylation at either Ser-31 or Ser-40, alone or in combination with Ser-19, augmented TH enzyme activity either by affecting the Vmax or Km (5, 50). Our results showed that TH phosphorylation at Ser-40 and Ser-31 is markedly upregulated, paralleling with the robust increase in TH activity seen in the medullary regions of IH15s exposed rats. Taken together, the above findings suggest that increased serine phosphorylation could be a major mechanism by which IH15s evokes TH activation in brainstem medullary regions.
The steady-state level of TH phosphorylation in vivo is altered by protein kinases (via phosphorylation; 1, 4, 11, 15, 16, 29) and protein phosphatases (via dephosphorylation; 2, 3, 7, 14, 20, 30). Either an increase in the activity of protein kinases or downregulation of protein phosphatases would account for IH15s-induced increase in serine phosphorylation of TH. Our results showed that IH15s increased the levels of active forms of PKA, CaMKII, and ERK1/2, albeit to different degree, in the brainstem medullary regions. Since in vitro, as well as in situ, studies showed the involvement of PKA and CaMKII in Ser-40 phosphorylation (11) and ERK1/2 in Ser-31 phosphorylation (11, 22), it is likely that the increased activities of these protein kinases might have contributed to IH15s-mediated robust phosphorylation of TH at Ser-40 and Ser-31. The finding that inhibitors specific to either PKA or CaMKII not only attenuated IH-evoked increase in TH phosphorylation but also TH activation in PC12 cells lends support for such a possibility (27). In addition to activation of protein kinases, IH15s markedly decreased PP2A activity in the medullary brainstem regions which is, in part, due to a concomitant reduction in PP2A protein expression. Previous studies in the adrenal medulla and striatum showed that TH dephosphorylation is primarily mediated by okadaic acid-sensitive PP2A with a minor contribution from okadaic acid-insensitive PP2C (2, 3, 7, 20, 30). The findings that IH15s had no significant effect on the expression of either okadaic acid-insensitive (PP2C) or other okadaic acid-sensitive protein phosphatases including PP4, PP5, and PP6, and that post-IH PP2A treatment reversed IH-induced increase in TH activity provide an indirect evidence for a critical role of PP2A in IH15s-induced facilitation of TH phosphorylation. However, additional experiments involving reversal of IH15s-evoked TH activation via overexpression of PP2A are needed to further confirm the modulatory role of PP2A in TH activation by IH. Furthermore, it is plausible that PP2A downregulation by IH15s is functionally coupled to activation of protein kinases that are implicated in TH phosphorylation. Several studies support such a possibility. For instance, in cerebral cortex of newborn piglets, hypoxia-evoked increase in CaMK IV activity, a modulator of programmed cell death, has been attributed to decrease in PP2A activity (48). Likewise, survival of macrophages during hyperoxia has been shown to involve sustained activation of ERK which is mediated by PP2A downregulation (39). Thus, IH15s-evoked activation of a subset of protein kinases via PP2A downregulation may provide a positive feed forward mechanism for sustained TH phosphorylation, a possibility which needs to be further investigated. Taken together, our results suggest that IH15s via tilting the balance between protein kinases and protein phosphatases facilitates robust TH phosphorylation.
ROS signaling plays a critical role in eliciting systemic and cellular responses to IH (28, 40, 47, 49). The following lines of evidence suggest that ROS is an upstream signaling molecule that plays a central role in IH-evoked TH activation and the resulting increase in DA levels in the rat medullary brainstem regions. First, IH increased ROS in the rat dorsal and ventral medulla as evidenced by increases in MDA level, a marker of increased ROS generation. Second, pre-treatment with two mechanistically distinct antioxidants, viz., MnTMPyP, a SOD mimetic and N-NAC, a precursor of glutathione, not only attenuated IH-induced increases in MDA level, TH activity, DA level, and activities of PKA, CaMKII, and ERK1/2, but also the elevation of serine phosphorylation of TH at Ser-31 and Ser-40 by IH. Interestingly, antioxidant also reversed IH-evoked downregulation of PP2A activity, suggesting a direct role for ROS in post-translational redox regulation of PP2A. Supporting such a possibility are the findings that exposure of PC12 and SH-SY5Y cells to cadmium not only increases ROS but also inhibits PP2A and PP5 and that NAC reverses cadmium-induced PP2A inhibition (8). Taken together, the above observations suggest that ROS-mediated downregulation of PP2A contributes to IH15s-induced post-translational serine phosphorylation and subsequent activation of TH. However, the cellular sources of ROS generation (cytosolic oxidases and complexes of mitochondrial electron transport chain) during IH15s and the chemical identity of the ROS species (O2.− or H2O2 or OH−) that mediate IH-induced functional alteration in TH in brainstem medullary regions, however, remain to be established. Recent studies in rat carotid body (41) and PC12 cells (51) suggest that NADPH oxidase-derived ROS may contribute to IH15s-mediated responses. Furthermore, in vitro studies have shown that TH, under certain reaction conditions, facilitates H2O2 generation (21). Future studies, however, are required to determine whether ROS derived from NADPH oxidase and/or TH catalyzed reaction mediate IH15s-evoked TH activation in the brainstem medullary regions.
It is noteworthy that IH15s, but not IH90s, augments TH activity. Since molecular oxygen is required for the catalytic activity of TH and hypoxia lowers tissue oxygen level, it is rather intriguing that IH with only short (15 s) but not long (90 s) duration of hypoxia facilitates TH activity. It is likely that the signaling mechanism(s) that are necessary for TH activation is fully developed in IH15s whereas it is not sufficiently developed in IH90s. Supporting such a possibility are the findings that the level of ROS which is critical for IH-induced activation of TH, is three times higher in brainstem medullary regions of rats exposed to IH15s than those exposed to IH90s. Moreover, ROS signaling mediated upregulation of PKA/CaMKII and downregulation of PP2A were entirely absent in IH90s brainstem regions. Thus, IH15s was able to induce a robust ROS generation reaching a threshold level necessary for the activation of ROS signaling pathway involving protein kinases and phosphatases for marked elevation in TH activity. The possibility remains that with IH90s such robust ROS generation may be elicited after prolonged exposure to IH lasting several weeks. Thus, it is likely that for a given duration and type of tissue, the magnitude of ROS generation may vary among different IH patterns. The finding that mice exposed to IH consisting of alternating cycles of 4.9% O2 for 30 s and 20.9% O2 for 30 s for 1 or 4 weeks exhibited oxidative stress only in liver but not in the aorta and heart (24) supports such a possibility. Furthermore, IH90s, unlike in the brainstem, was able to increase TH phosphorylation and catecholamines only in the carotid body but not in the adrenal medulla or superior cervical ganglion (23), suggesting that IH90s-evoked responses are tissue specific. In marked contrast, with IH15s, we observed significant elevations in adrenal medullary catecholamines (28) and robust sustained increase in TH activity in the adrenal medulla as well as in the superior cervical ganglion similar to those seen in brainstem medullary regions (Kumar et al., unpublished observation). These observations suggest that IH15s evokes a robust global TH activation in catecholamine expressing tissues.
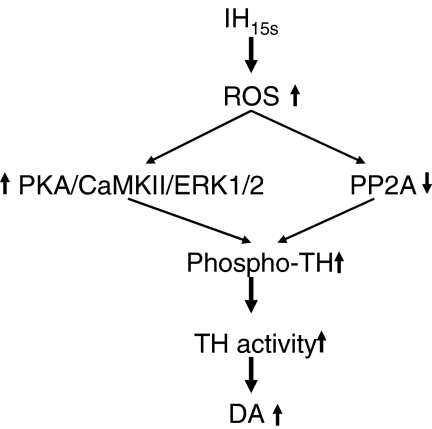
In summary, our results provide evidence that IH15s-induced increase in ROS initiates a cascade of reactions involving downregulation of PP2A and upregulation of protein kinases, resulting in sustained increase in phosphorylation at multiple serine residues and the ensuing activation of TH and increase in DA synthesis (Fig. 8). Interestingly, the increase in TH phosphorylation by IH15s occurs in the absence of significant alteration in the overall TH protein expression and therefore constitutes as one of the major post-translational mechanisms activated by IH15s for the regulation of enzymes associated with neurotransmitter synthesis. The finding that IH15s-mediated activation of TH is coupled to increased DA level in the medullary brainstem regions suggests that IH-evoked changes in DA may, in part, contribute to cardiorespiratory abnormalities associated with recurrent apneas.
FIG. 8.
Schematic presentation of ROS-dependent downregulation of PP2A and upregulation of protein kinases in IH15s-induced activation of TH.
Acknowledgments
This work was supported by grants from the National Heart, Lung, and Blood Institute (PO1HL-90554 and HL-86493 to NRP, and RO1HL-89616 to GKK).
Abbreviations
CaMK, calcium/calmodulin-dependent kinase; DA, dopamine; DHBA, 3,4-dihydroxybenzylamine; DOPA, dihydroxyphenylalanine; ERK, extracellular signal-regulated kinase; IH, intermittent hypoxia; MDA, malondialdehyde; MnTMPyP, manganese (III) tetrakis (1-methyl-4-pyridyl) porphyrin pentachloride; NAC, N-acetyl-L-cysteine; NOR, normoxia; PC12, pheochromocytoma 12; PKA, protein kinase A; PP, protein phosphatase; ROS, reactive oxygen species; T-BARS, thio-barbituric acid reactive substances; TH, tyrosine hydroxylase.
Author Disclosure Statement
The authors have no commercial associations that might create a conflict of interest in connection with the submission of the manuscript.
References
- 1.Ames MM. Lerner P. Lovenberg W. Tyrosine hydroxylase. Activation by protein phosphorylation and end product inhibition. J Biol Chem. 1978;253:27–31. [PubMed] [Google Scholar]
- 2.Berresheim U. Kuhn DM. Dephosphorylation of tyrosine hydroxylase by brain protein phosphatases: a predominant role for type 2A. Brain Res. 1994;637:273–276. doi: 10.1016/0006-8993(94)91244-0. [DOI] [PubMed] [Google Scholar]
- 3.Bevilaqua LR. Cammarota M. Dickson PW. Sim AT. Dunkley PR. Role of protein phosphatase 2C from bovine adrenal chromaffin cells in the dephosphorylation of phospho-serine 40 tyrosine hydroxylase. J Neurochem. 2003;85:1368–1373. doi: 10.1046/j.1471-4159.2003.01792.x. [DOI] [PubMed] [Google Scholar]
- 4.Bobrovskaya L. Gilligan C. Bolster EK. Flaherty JJ. Dickson PW. Dunkley PR. Sustained phosphorylation of tyrosine hydroxylase at serine 40: a novel mechanism for maintenance of catecholamine synthesis. J Neurochem. 2007;100:479–489. doi: 10.1111/j.1471-4159.2006.04213.x. [DOI] [PubMed] [Google Scholar]
- 5.Campbell DG. Hardie DG. Vulliet PR. Identification of four phosphorylation sites in the N-terminal region of tyrosine hydroxylase. J Biol Chem. 1986;261:10489–10492. [PubMed] [Google Scholar]
- 6.Carlson JT. Hedner J. Elam M. Ejnell H. Sellgren J. Wallin BG. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest. 1993;103:1763–1768. doi: 10.1378/chest.103.6.1763. [DOI] [PubMed] [Google Scholar]
- 7.Chen J. Martin BL. Brautigan DL. Regulation of protein serine-threonine phosphatase type-2A by tyrosine phosphorylation. Science. 1992;257:1261–1264. doi: 10.1126/science.1325671. [DOI] [PubMed] [Google Scholar]
- 8.Chen L. Liu L. Huang S. Cadmium activates the mitogen-activated protein kinase (MAPK) pathway via induction of reactive oxygen species and inhibition of protein phosphatases 2A and 5. Free Radic Biol Med. 2008;45:1035–1044. doi: 10.1016/j.freeradbiomed.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Chitravanshi VC. Sapru HN. Chemoreceptor-sensitive neurons in commissural subnucleus of nucleus tractus solitarius of the rat. Am J Physiol Regul Integr Comp Physiol. 1995;268:R851–R858. doi: 10.1152/ajpregu.1995.268.4.R851. [DOI] [PubMed] [Google Scholar]
- 10.Cistulli PA. Sullivan CE. Pathophysiology of sleep apnea. In: Saunders NA, editor; Sullivan CE, editor. Sleep and Breathing. New York, NY: Dekker; 1994. pp. 405–448. [Google Scholar]
- 11.Dunkley PR. Bobrovskaya L. Graham ME. von Nagy–Felsobuki EI. Dickson PW. Tyrosine hydroxylase phosphorylation: Regulation and consequences. J Neurochem. 2004;91:1025–1043. doi: 10.1111/j.1471-4159.2004.02797.x. [DOI] [PubMed] [Google Scholar]
- 12.Finley JCW. Polak J. Katz DM. Transmitter diversity in carotid body afferent neurons: dopaminergic and peptidergic phenotypes. Neuroscience. 1992;51:973–987. doi: 10.1016/0306-4522(92)90534-9. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher EC. Physiological consequences of intermittent hypoxia: Systemic blood pressure. J Appl Physiol. 2001;90:1600–1605. doi: 10.1152/jappl.2001.90.4.1600. [DOI] [PubMed] [Google Scholar]
- 14.Fujisawa H. Okuno S. Regulatory mechanism of tyrosine hydroxylase activity. Biochem Biophys Res Commun. 2005;338:271–276. doi: 10.1016/j.bbrc.2005.07.183. [DOI] [PubMed] [Google Scholar]
- 15.Funakoshi H. Okuno S. Fujisawa H. Different effects on activity caused by phosphorylation of tyrosine hydroxylase at serine 40 by three multifunctional protein kinases. J Biol Chem. 1991;266:15614–15620. [PubMed] [Google Scholar]
- 16.Gonçalves CA. Hall A. Sim AT. Bunn SJ. Marley PD. Cheah TB. Dunkley PR. Tyrosine hydroxylase phosphorylation in digitonin-permeabilized bovine adrenal chromaffin cells: The effect of protein kinase and phosphatase inhibitors on Ser19 and Ser40 phosphorylation. J Neurochem. 1997;69:2387–2396. doi: 10.1046/j.1471-4159.1997.69062387.x. [DOI] [PubMed] [Google Scholar]
- 17.Gozal E. Shah ZA. Pequignot J–M. Pequignot J. Sachleben LR. Czyzyk–Krzeska MF. Li RC. Guo S–Z. Gozal D. Tyrosine hydroxylase expression and activity in the rat brain: Differential regulation after long-term intermittent or sustained hypoxia. J Appl Physiol. 2005;99:642–649. doi: 10.1152/japplphysiol.00880.2004. [DOI] [PubMed] [Google Scholar]
- 18.Granata AR. Woodruff GN. Dopaminergic mechanisms in the nucleus tractus solitarius and effects on blood pressure. Brain Res Bull. 1982;8:483–488. doi: 10.1016/0361-9230(82)90005-3. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg HE. Sica AL. Scharf SM. Ruggiero DA. Expression of c-fos in the rat brainstem after chronic intermittent hypoxia. Brain Res. 1999;816:638–645. doi: 10.1016/s0006-8993(98)01222-0. [DOI] [PubMed] [Google Scholar]
- 20.Haavik J. Schelling DL. Campbell DG. Andersson KK. Flatmark T. Cohen P. Identification of protein phosphatase 2A as the major tyrosine hydroxylase phosphatase in adrenal medulla and corpus striatum: Evidence from the effects of okadaic acid. FEBS Lett. 1989;251:36–42. doi: 10.1016/0014-5793(89)81424-3. [DOI] [PubMed] [Google Scholar]
- 21.Haavik J. Almås B. Flatmark T. Generation of reactive oxygen species by tyrosine hydroxylase: A possible contribution to the degeneration of dopaminergic neurons? J Neurochem. 1997;68:328–332. doi: 10.1046/j.1471-4159.1997.68010328.x. [DOI] [PubMed] [Google Scholar]
- 22.Haycock JW. Phosphorylation of tyrosine hydroxylase in situ at serine 8, 19, 31, and 40. J Biol Chem. 1990;265:11682–11691. [PubMed] [Google Scholar]
- 23.Hui AS. Striet JB. Gudelsky G. Soukhova GK. Gozal E. Beitner–Johnson D. Guo SZ. Sachleben LR Jr. Haycock JW. Gozal D. Czyzyk–Krzeska MF. Regulation of catecholamines by sustained and intermittent hypoxia in neuroendocrine cells and sympathetic neurons. Hypertension. 2003;42:1130–1136. doi: 10.1161/01.HYP.0000101691.12358.26. [DOI] [PubMed] [Google Scholar]
- 24.Jun J. Savransky V. Nanayakkara A. Bevans S. Li J. Smith PL. Polotsky VY. Intermittent hypoxia has organ-specific effects on oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1274–281. doi: 10.1152/ajpregu.90346.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitahama K. Nagatsu I. Geffard M. Maeda T. Distribution of dopamine-immunoreactive fibers in the rat brainstem. J Chem Neuroanat. 2000;18:1–9. doi: 10.1016/s0891-0618(99)00047-2. [DOI] [PubMed] [Google Scholar]
- 26.Kline DD. Ramirez–Navarro A. Kunze DL. Adaptive depression in synaptic transmission in the nucleus of the solitary tract after in vivo chronic intermittent hypoxia: Evidence for homeostatic plasticity. J Neurosci. 2007;27:4663–4673. doi: 10.1523/JNEUROSCI.4946-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar GK. Kim DK. Lee MS. Ramachandran R. Prabhakar NR. Activation of tyrosine hydroxylase by intermittent hypoxia: Involvement of serine phosphorylation. J Appl Physiol. 2003;95:536–544. doi: 10.1152/japplphysiol.00186.2003. [DOI] [PubMed] [Google Scholar]
- 28.Kumar GK. Rai V. Sharma SD. Ramakrishnan DP. Peng YJ. Souvannakitti D. Prabhakar NR. Chronic intermittent hypoxia induces hypoxia-evoked catecholamine efflux in adult rat adrenal medulla via oxidative stress. J Physiol. 2006;575:229–239. doi: 10.1113/jphysiol.2006.112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumer SC. Vrana KE. Intricate regulation of tyrosine hydroxylase activity and gene expression. J Neurochem. 1996;67:443–462. doi: 10.1046/j.1471-4159.1996.67020443.x. [DOI] [PubMed] [Google Scholar]
- 30.Leal RB. Sim AT. Gonçalves CA. Dunkley PR. Tyrosine hydroxylase dephosphorylation by protein phosphatase 2A in bovine adrenal chromaffin cells. Neurochem Res. 2002;27:207–213. doi: 10.1023/a:1014880403970. [DOI] [PubMed] [Google Scholar]
- 31.Lehmann IT. Bobrovskaya L. Gordon SL. Dunkley PR. Dickson PW. Differential regulation of the human tyrosine hydroxylase isoforms via hierarchical phosphorylation. J Biol Chem. 2006;281:17644–17651. doi: 10.1074/jbc.M512194200. [DOI] [PubMed] [Google Scholar]
- 32.Leuenberger UA. Brubaker D. Quraishi S. Hogeman CS. Imadojemu VA. Gray KS. Effects of intermittent hypoxia on sympathetic activity and blood pressure in humans. Auton Neurosci. 2005;121:87–93. doi: 10.1016/j.autneu.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 33.Li R. Bao G. el–Mallakh RS. Fletcher EC. Effects of chronic episodic hypoxia on monoamine metabolism and motor activity. Physiol Behav. 1996;60:1071–1076. doi: 10.1016/0031-9384(96)00149-7. [DOI] [PubMed] [Google Scholar]
- 34.Li A. Emond L. Nattie E. Brainstem catecholaminergic neurons modulate both respiratory and cardiovascular function. Adv Exp Med Biol. 2008;605:371–376. doi: 10.1007/978-0-387-73693-8_65. [DOI] [PubMed] [Google Scholar]
- 35.Mifflin SW. Arterial chemoreceptor input to nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol. 1992;263:R368–R375. doi: 10.1152/ajpregu.1992.263.2.R368. [DOI] [PubMed] [Google Scholar]
- 36.Moura E. Pinho Costa PM. Moura D. Guimarães S. Vieira–Coelho MA. Decreased tyrosine hydroxylase activity in the adrenals of spontaneously hypertensive rats. Life Sci. 2005;76:2953–2964. doi: 10.1016/j.lfs.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 37.Nagatsu T. Oka K. Kato T. Highly sensitive assay for tyrosine hydroxylase activity by high-performance liquid chromatography. J Chromatogr. 1979;163:247–252. doi: 10.1016/s0378-4347(00)81411-5. [DOI] [PubMed] [Google Scholar]
- 38.Nieto FJ. Young TB. Lind BK. Shahar E. Samet JM. Redline S. D'Agostino RB. Newman AB. Lebowitz MD. Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 39.Nyunoya T. Monick MM. Powers LS. Yarovinsky TO. Hunninghake GW. Macrophages survive hyperoxia via prolonged ERK activation due to phosphatase down-regulation. J Biol Chem. 2005;280:26295–26302. doi: 10.1074/jbc.M500185200. [DOI] [PubMed] [Google Scholar]
- 40.Peng YJ. Overholt JL. Kline DD. Kumar GK. Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci USA. 2003;100:10073–10078. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng YJ. Nanduri J. Yuan G. Wang N. Deneris E. Pendyala S. Natarajan V. Kumar GK. Prabhakar NR. NADPH oxidase is required for the sensory plasticity of the carotid body by chronic intermittent hypoxia. J Neurosci. 2009;29:4903–4910. doi: 10.1523/JNEUROSCI.4768-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pepin JL. Levy P. Garcin A. Feuerstein C. Savasta M. Effects of long-term hypoxia on tyrosine hydroxylase protein content in catecholaminergic rat brainstem areas: A quantitative autoradiographic study. Brain Res. 1996;733:1–8. doi: 10.1016/0006-8993(96)00250-8. [DOI] [PubMed] [Google Scholar]
- 43.Prabhakar NR. Kumar GK. Nanduri J. Semenza GL. ROS signaling in systemic and cellular responses to chronic intermittent hypoxia. Antioxid Redox Signal. 2007;9:1397–1403. doi: 10.1089/ars.2007.1732. [DOI] [PubMed] [Google Scholar]
- 44.Ramanathan L. Gozal D. Siegel JM. Antioxidant responses to chronic hypoxia in the rat cerebellum and pons. J Neurochem. 2005;93:47–52. doi: 10.1111/j.1471-4159.2004.02988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharma SD. Raghuraman G. Lee MS. Prabhakar NR. Kumar GK. Intermittent hypoxia activates peptidylglycine {alpha}-amidating monooxygenase in rat brainstem via reactive oxygen species-mediated proteolytic processing. J Appl Physiol. 2009;106:12–19. doi: 10.1152/japplphysiol.90702.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soulier V. Dalmaz Y. Cottet-Emard JM. Kitahama K. Pequignot JM. Delayed increase of tyrosine hydroxylation in the rat A2 medullary neurons upon long-term hypoxia. Brain Res. 1995;674:188–195. doi: 10.1016/0006-8993(94)01441-j. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki YJ. Jain V. Park AM. Day RM. Oxidative stress and oxidant signaling in obstructive sleep apnea and associated cardiovascular diseases. Free Radic Biol Med. 2006;40:1683–1692. doi: 10.1016/j.freeradbiomed.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Truttmann AC. Ashraf Q. Mishra OP. Delivoria–Papadopoulos M. Effect of hypoxia on protein phosphatase 2A activity, subcellular distribution and expression in cerebral cortex of newborn piglets. Neuroscience. 2004;127:355–363. doi: 10.1016/j.neuroscience.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 49.Veasey SC. Davis CW. Fenik P. Zhan G. Hsu YJ. Pratico D. Gow A. Long-term intermittent hypoxia in mice: Protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep. 2004;27:194–201. doi: 10.1093/sleep/27.2.194. [DOI] [PubMed] [Google Scholar]
- 50.Wu J. Filer D. Friedhoff AJ. Goldstein M. Site-directed mutagenesis of tyrosine hydroxylase. Role of serine 40 in catalysis. J Biol Chem. 1992;267:25754–25758. [PubMed] [Google Scholar]
- 51.Yuan G. Nanduri J. Khan S. Semenza GL. Prabhakar NR. Induction of HIF-1alpha expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J Cell Physiol. 2008;217:674–685. doi: 10.1002/jcp.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhan G. Serrano F. Fenik P. Hsu R. Kong L. Pratico D. Klann E. Veasey SC. NADPH oxidase mediates hypersomnolence and brain oxidative injury in a murine model of sleep apnea. Am J Respir Crit Care Med. 2005;172:921–929. doi: 10.1164/rccm.200504-581OC. [DOI] [PMC free article] [PubMed] [Google Scholar]