Abstract
This review addresses the complexity of coronary collateral growth from the aspect of redox signaling and introduces the concept of a “redox window” in the context of collateral growth. In essence, the redox window constitutes a range in the redox state of cells, which not only is permissive for the actions of growth factors but also amplifies their actions. The interactions of redox-dependent signaling with growth factors are well established through the actions of many redox-dependent kinases (e.g., Akt and p38 mitogen-activated protein kinase). The initial changes in cellular redox can be induced by a variety of events, from the oxidative burst during reperfusion after ischemia, to recruitment of various types of inflammatory cells capable of producing reactive oxygen species. Any event that “upsets” the normal redox equilibrium is capable of amplifying growth. However, extremes of the redox window, oxidative and reductive stresses, are associated with diminished growth-factor signaling and reduced activation of redox-dependent kinases. This concept of a redox window helps to explain why the clinical trials aimed at stimulating coronary collateral growth, the “therapeutic angiogenesis trials,” failed. However, understanding of redox signaling in the context of coronary collateral growth could provide new paradigms for stimulating collateral growth in patients. Antioxid. Redox Signal. 11, 1961–1974.
Introduction
Formation of the collateral network in many organ systems is an enigmatic process. Under normal conditions, as in the absence of pathology, little to no net blood flow occurs through a collateral anastomosis because flow waxes and wanes in a bidirectional manner (103) (refer to Fig. 1). Moreover, in their innate, native state, collateral vessels are very small, and because of their sparse numbers (and thus small total cross-sectional area), resistance to net blood flow is high. Thus, under normal conditions, little purpose appears for their existence. However, if challenged with appropriate stimuli, collaterals can greatly expand their caliber and serve as conduits offering little resistance to blood flow (87). Collateral growth is a remodeling process that represents organized abluminal expansion of preexisting vessels, involving mitosis of endothelial and smooth muscle cells (and likely other vascular cells; e.g., fibroblasts). What engenders this “physiologic” remodeling in an outward direction versus that of “pathologic” remodeling in which cell proliferation is involved in the development of a neointima and atherosclerotic plaque formation remains unknown. The enigma—why do native collateral vessels form when they appear to have little net flow—may be best solved from the perspective of natural principles. The thoughts of Sir Isaac Newton bear on this argument:
FIG. 1.
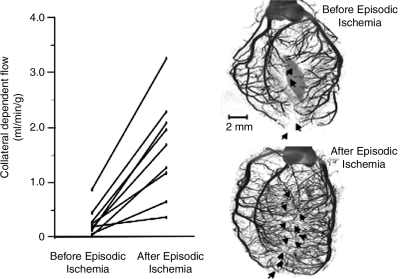
Growth of the collateral circulation in the rat heart. (Left) After a 10-day protocol for episodic ischemia, blood flow to the collateral-dependent zone was increased approximately fivefold over the native flow before the episodic ischemia. (Right) MicroCT images of a rat heart not subjected to repetitive ischemia (top right) and one exposed to the episodic ischemia protocol (bottom right). Numbers of visible collaterals increased from four to 11. [Adapted from Toyota et al. (102) with permission.]
“ … Nature does nothing in vain, and more is in vain when less will serve; for Nature is pleased with simplicity, and affects not the pomp of superfluous causes.” Sir Isaac Newton in Rule I of Rules of Reasoning in Natural Philosophy, Mathematical Principles of Natural Philosophy.
The extension of this reasoning supports the view that collateral connections between arteries develop for a reason; otherwise, they should not develop at all! Collateral vessels may serve as a preemptive adaptive mechanism to ameliorate the effects of vascular pathology. Although this is teleologic reasoning, it is not inconceivable to recognize collateral formation as a selection pressure, insofar as those with a preexisting collateral supply would be better able to circumvent the effects of vascular pathology and perhaps even acute traumatic vascular injury than would those without collaterals. Survival after the course of vascular pathology would be better in individuals with collaterals than in those without. Because of this purpose, these vessels can assume a very important role, especially when carrying flow to an area of ischemia. Understanding the factors responsible for, and the mechanisms of, collateral vessel enlargement is very important. The remainder of this review focuses on experimental approaches and models used to measure collateral growth, initiating factors in collateral development, redox-sensitive mechanisms that produce growth and enlargement of coronary collateral vessels, and how redox-dependent signaling should be considered in putative therapies designed to stimulate collateral growth. In this review, we use collateral growth and enlargement interchangeably.
Measurements of Collateral Growth
Collateral growth has been derived from many indirect and direct measures. Table 1 summarizes the major models used to stimulate collateral vessel growth, in species ranging from mice to horses, and highlights our analysis of advantages and disadvantages of using the various models. In our opinion, most models fail with respect to two important factors: one must know accurately the time when the stimulus for growth is initiated; and (in our opinion) it becomes very important to initiate the process (if possible) when the inflammation and trauma from the surgical procedures have abated after healing. For example, previously we reported that, on the implantation of a catheter in the myocardium from which samples of interstitial fluid were obtained, very high levels of growth factors and cytokines were found immediately after the surgery until healing was completed (115). Thus, if we were to have initiated our sampling from the first day after surgery, substantial artifact would be found with our conclusions about the role of certain growth factors in collateral growth. In contrast, the ligation model is subject to this criticism because the vessel is ligated during the initial surgery. Table 2 illustrates a summary of the measurements and our views on limitations inherent in the measurements. In our opinion, the best measure for the growth of the collaterals is blood flow, because that is the critical variable for the tissue, and that flow represents a composite of the total growth of the vascular bed. However, one should realize that flow measurements may be affected by vascular tone (not a factor in the ischemic heart) and contributions of resistance upstream and downstream from the collateral bed (assumed to be constant in the estimation of collateral growth from flow). Visualization by using angiographic techniques is hindered by the resolution of the method, because some collaterals may be too small to visualize or because of their size, they may not fill well with contrast. Measurement of blood flow to the collateral-dependent zone circumvents this criticism.
Table 1.
Models for Stimulation of Collateral Growth
| Model | Advantage | Disadvantage |
|---|---|---|
| Ameroid occluder (16, 17, 34, 91) | 1. Growth of large collaterals 2. Large body of literature 3. Low mortality 4. Simple surgical procedure |
1. Variable rates of closure 2. Undefined time whengrowth is initiated |
| Repetitive occlusion (23, 59, 63, 102) | 1. Growth of large collaterals 2. Large body of literature 3. Low mortality 4. Known time when growth is initiated 5. Collateral growth is stimulated when surgical trauma and inflammation have subsided |
1. Labor intensive 2. Occasional-to-frequent instrumentation failure |
| Surgical ligation/arterectomy (1, 11, 12, 38, 41, 42, 92, 93) | 1. Growth of large collaterals 2. Large body of literature 3. Low mortality (in organ systems other than heart) 4. Known time when growth is initiated 5. Simple surgical procedure |
1. Collateral growth is stimulated when surgical trauma and inflammation are present |
| Distal embolization (14) | 1. Low mortality 2. Known time when growth is initiated 3. Simple surgical procedure |
1. Collateral growth is stimulated when surgical trauma and inflammation are present 2. Collateral development is not robust 3. Small body of literature |
Table 2.
Measurements of Collateral Growth
| Measurement | Principle/assumption | Limitations |
|---|---|---|
| Blood flow (48, 59, 102, 114) | Collaterals represent a series-coupled resistance to upstream conduit vessels and downstream microvessels/resistance vessels in the area of risk Because native collateral resistance is substantially higher than the microvascular or conduit resistance, flow to the collateral-dependent zone is dependent primarily-to-exclusively (depending on the organ system) on the growth of collateral vessels | Empiric measurements of collateral resistance are difficult |
| Retrograde flow (88–90) | Measurement of flow through the collateral circulation without influences of the downstream microvascular resistance | Terminal experiment |
| Perfusion ratio (collateral-dependent flow/normal flow) (35, 82) | Same principle as for blood flow, but absolute measurements of volume flow are not necessary | No empiric measurements of collateral flow. Ratio can be influenced by changes in flow to the normal zone or region and/or flow to the collateral-dependent region |
| Distal pressure/pressure ratio (68, 72, 110, 123) | During an occlusion, pressure measured downstream from the occlusion reflects pressure inputs from the downstream microcirculation and the collateral vessels. As collateral growth is stimulated, downstream pressures increase, which reflects increased transmission of pressure through these vessels because of lower resistance | Distal pressure (that below the occlusion is a function of both collateral (indirect) and microvascular (direct) resistances. An increase in distal pressure may be related to higher microvascular resistance, (e.g., embolization recovery of tone), in addition to lower collateral resistance |
| Rentrop score/angiography (49, 50, 71, 81, 98, 110 | Characteristics of retrograde “filling” of conduit vessels in the collateral-dependent region during angiography, where retrograde filling is equated to collateral-dependent flow | Collateral-dependent flow is poorly correlated with Rentrop scores. Somewhat subjective categories of results. Not quantitative |
| Imaging/tomography (8, 19, 57, 75, 86, 116 | Visualization of collateral vessels by using angiographic imaging, computerized tomography, etc. Allows measurement and quantification of collateral vessels | With fluorescence angiography, collaterals below the surface are not visualized. Accurate measurements of diameter are difficult. Improper or inadequate filling also corrupts the measurement. Difficult to ascertain total cross-sectional area of the collateral bed, which affects total collateral resistance |
| Function/pathology (15, 24, 49) | Function in most organ systems (e.g., cardiac contraction, limb movement) dependent on perfusion. If perfusion is too low (as after an occlusion), severe pathology (e.g., infarction, gangrene) may ensue. Growth of the collateral circulation allows more perfusion to the area at risk, thereby improving function and ameliorating the pathology | Not an empiric measurement of collateral flow, except in the myocardium, where flow and function are associated in a quantitative manner. Scoring of the pathology is often not quantitative |
General Concepts in the Initiation of Collateral Growth
Collateral growth occurs in a variety of organ systems and vascular beds, including the heart (23,114,115), hindlimb (11,12), mesentery (69), and the brain (11). In the rat heart, the response is extraordinary, with substantial increases in collateral growth in as little as 10 days (Fig. 1) (102). The causal factors that initiate collateral vessel enlargement are still subject to debate, and generally, two hypotheses are cited. One hypothesis maintains that shear stress is an initiating factor (78); whereas the other holds that ischemia initiates the process (14).
Before we discuss the evidence in favor of and against these two hypotheses, it is important to recognize the anatomic features of an organ system that may provide insight into the factors responsible for growth. For this, a comparison of collateral vessel growth in skeletal muscle versus the heart illustrates the basis of the argument. In the event of a coronary artery occlusion, the ischemic zone is adjacent to, and even interdigitating with, the normally perfused zone of the myocardium (21). Because of the sparing of the epicardium in most species and animal models, epicardial arteries may actually course above ischemic areas of the myocardium (83). Thus, the ischemic area of the heart is within a few to a few hundred microns of the area where collateral growth occurs. In contrast with occlusion of a femoral or iliac artery, the major site of ischemia is remote from the area of occlusion. For example, the occlusion is located in the region of the groin, but the level of ischemia is most intense in the toes—an area far removed from the site of vascular growth. Accordingly, we are compelled to emphasize that collateral growth in different organ systems may be stimulated by unique complements of factors, which is ultimately related to the anatomic relations between the site of vascular growth and the site of ischemia. One caveat is that a complete understanding of vascular communication or even communication between remote tissue areas does not exist. In the preconditioning literature, the observation of remote preconditioning has been well appreciated for a number of years (6, 27, 80). These observations show that ischemia in one region of an organ system, or even within the body, can induce a protective effect within that same or another organ system. Thus, it may be presumptuous to conclude that because the zone of ischemia is far removed from the site of growth, ischemia is not involved.
The role for shear stress in collateral growth has been discussed extensively (18, 29, 38, 39, 78) and has been accepted by many as the factor that drives this process. In our opinion, the role for shear stress in collateral growth remains a hypothesis more than a fact. The vast majority of investigators concluding a role for shear stress in collateral growth in their particular model have not even measured shear stress (40, 78)! Some experimental preparations that are aimed at showing the effects of shear stresses in the process have not eliminated other factors, such as ischemia. For example, Schaper's group (40) reported that shear stress was involved in collateral growth because creation of an arterial venous shunt downstream of the collaterals (that was designed to increase flow and shear stress in the collaterals) enhanced collateral growth. However, an issue not considered by the investigators is that imposition of such a shunt would also worsen downstream ischemia (i.e., collateral-dependent flow that normally would perfuse the tissue at risk is now shunted into the venous system). Thus, this experiment is not a definitive test for shear stress because it did not exclude the effects of ischemia.
In the mesenteric circulation, Unthank (106) argued that shear stress is the critical factor in mesenteric collateral growth. However, some of the upregulated genes in their model (e.g., enolase) have an HIF-binding site in their promoter; thus, the exclusive regulation by shear is questionable. Several years ago, Chilian et al. (14) attempted to resolve the contributions of shear stress from ischemia in the coronary circulation by distally embolizing the microcirculation of the heart with microspheres (thus producing ischemia, but without pressure gradients across upstream collaterals). These investigators observed initiation of collateral growth, but collateral growth was not nearly so robust as with other models. Thus, at least in the heart, ischemia can be thought of as an initiating factor, but shear stress is likely a factor that contributes to remodeling during the continuation of this process.
Finally, collateral growth occurs between vascular territories that are not directly connected to the area at risk, thus casting further doubt that shear stress is the single factor responsible for collateral growth. Several years ago, Schaper (74, 87) reported the presence of mitotic cells in blood vessels far removed from the ischemic territory, and Scheel's laboratory (89, 91) made several contributions showing that collateral growth increased between the right coronary artery and the left anterior descending artery, when an ameroid was placed on the left circumflex. Although both the right coronary artery and the left anterior descending artery are connected to the circumflex artery, it is difficult to envision how shear stress would be elevated in collateral connections between these two territories in an area far removed from the zone of ischemia. Again, the caveat we reemphasize is that ischemia can have influences in remote areas, and based on the current evidence for coronary collateral growth, ischemia is likely the initiating factor.
Several observations support the contention that the process of collateral growth is multifactorial. Matsunaga et al. (61) observed that vascular endothelial growth factor (VEGF) was expressed early in the process of collateral growth but waned during the mid-to-late stages of maturation. Because VEGF has a HIF element in the promoter, such an observation is consistent with the early initiation of collateral growth being regulated by ischemia (and tissue hypoxia), but as collaterals develop, tissue hypoxia is ameliorated because the collaterals enable the delivery of more oxygenated blood. Interestingly, the expression of angiopoietin-2, which also has a HIF promoter element, parallels the expression of VEGF during collateral growth (62). The process of growth and enlargement continues as ischemia/hypoxia wanes, which suggests that other factors, perhaps shear stress, are involved in the continued remodeling. One point worth emphasizing to illustrate the importance of growth factors resulting from ischemic tissue is the observation that if the actions of growth factors are prevented, it appears, at least in the heart, that collateral growth does not occur. Toyota et al. (102) demonstrated the causal role of VEGF in this manner. Specifically, neutralizing antibodies to VEGF prevented coronary collateral growth. Thus, if the initiation of collateral development is prevented, then shear-induced remodeling is of no consequence.
The Role of Reactive Oxygen Species in Coronary Collateral Growth
Reactive oxygen species (ROS), such as O2•−, H2O2, and OH•, play a key role in many cardiovascular pathologies, such as atherosclerosis and ischemia/reperfusion injury (51, 64, 67). Moreover, patients with congestive heart failure (CHF) show signs of oxidative stress, and the link between this stress and the progression of CHF is being explored (70). However, a growing consensus suggests that lower levels of ROS (especially O2•− and H2O2) play pivotal roles in collateral growth, neovascularization, and myocardial adaptations to ischemia. The hydroxyl radical (OH·) is not generally viewed as having a beneficial role because of its high reactivity (with any molecule at diffusion-limited kinetics), which leads to protein and lipid modifications that are deleterious.
Ischemia/reperfusion injury is a biphasic process, in which exposure of the myocardium to prolonged reductions in blood flow (ischemia) elicits a variety of events, which initiate cell death (51). This is followed by further injury with reestablishment of blood flow (reperfusion), which leads to cellular destruction, leading to stunning and necrosis of tissue still alive at the onset of reperfusion (73). ROS are known to cause cell death by lipid peroxidation, interruption of key survival signaling pathways by protein modification, and DNA damage (22). Massive amounts of ROS released during reperfusion have been shown to be the major cause of myocardial tissue death (43, 51, 102). ROS are known to cause cell death by lipid peroxidation, interruption of key survival signaling pathways by protein modification, and DNA damage (22). Treatment with SOD and catalase at the onset of reperfusion is cardioprotective (60). However, transient and repetitive ischemia renders the myocardium tolerant to ischemia/reperfusion, in part through promoting collateral development (13). It has been shown that ROS generated by repetitive ischemia are critical mediators of coronary collateral growth because treatment with an ROS scavenger, N-acetyl cysteine, abrogated coronary collateral growth in a canine model (30). Moreover, ROS also are thought to be involved in ischemia-induced angiogenesis (64). We recently showed that repetitive ischemia induced O2•− and H2O2 production, and that complete blockade of O2•− production abrogated coronary collateral growth in a rat model of repetitive ischemia (85). This suggests an important role for some critical amount of ROS in coronary collateral growth, because these interventions would have shifted the redox state to a reductive milieu. Components of collateral growth include cell survival, proliferation, and migration, and ROS are implicated in the regulation of all three processes. Numerous reports demonstrate that complete ROS depletion/inhibition leads to cell-cycle arrest, cell death, and inhibition of cell migration. Inhibition of NAD(P)H oxidase attenuated collateral growth and neovascularization in a model of hindlimb ischemia (101). Conversely, diseases characterized by elevated oxidative stress, including diabetes and hypertension, are typically characterized by mitigated collateral growth (105). We recently showed that elevation of myocardial O2•− concentrations by SOD inhibition resulted in abrogated coronary collateral growth (85), suggesting that high basal oxidative stress impairs growth-factor production and signaling during ischemia. These observations strongly imply that high levels of ROS inhibit coronary collateral growth. Taken together, this gives a strong suggestion that ROS play a key role in coronary collateral growth: too little ROS, and a redox state that is overly reductive impairs vascular growth; and too many ROS, and a redox state that is overly oxidative likewise impairs collateral growth. There appears to be a redox state that not only is permissive for vascular growth, but that also magnifies vascular growth. These ideas prompt a new concept about collateral growth, in that a “redox window” exists in which ROS-dependent signaling can occur.
The “redox window” concept was in part based on our demonstration that production of an overly reductive redox state impaired collateral growth. Specifically we observed, in a canine model of repetitive ischemia, that a series of 2-min, repetitive left anterior descending coronary artery (LAD) occlusions over a period of 21 days increased coronary collateral blood flow to a level approximating that in the normal zone, thus indicating robust stimulation of coronary collateral growth (30, 61). However, administration of the glutathione precursor, N-acetylcysteine (which would shift the redox state to a more-reductive environment) prevented the increase in collateral blood flow (30). This observation directly suggests an important role for some critical level in the redox state that is necessary for coronary collateral development. It is important to note that the redox state is reflected by ratios of oxidized to reduced constituents within the cell (e.g., oxidized to reduced glutathione, oxidized to reduced thiols, NAD to NADH, NAD(P) to NAD(P)H), in which these ratios are markedly affected by production of ROS. Too many ROS shift the redox state toward oxidative stress, whereas too few ROS shift the redox state toward a reductive stress.
ROS have been implicated in the regulation of cell survival, cell division, and cell migration (64). More significantly, a picture emerges that a critical or threshold amount of ROS is necessary for these events to occur; a concept supported by our previous data (85). Moreover, numerous reports demonstrate that ROS scavenging, under normal oxygen conditions, also leads to cell-cycle arrest, cell death, and inhibition of cell migration (109). N-Acetylcysteine and inhibition of NAD(P)H oxidase with diphenylene iodonium (DPI), as well as administration of antisense oligonucleotides to knock down gp91phox, a critical component of NADPH oxidase, significantly attenuated endothelial cell proliferation and migration in culture (109). However, too many ROS clearly lead to cell death or inhibition of collateral growth and angiogenesis.
Hyperoxia, a condition also associated with an excess of ROS, induced retinal vascular endothelial cell apoptosis. Importantly, apoptosis was prevented by treatment with catalase and SOD (31). Hyperoxia also blocked cell division by preventing the progression of the cell cycle into the S phase and mitosis through expression of proteins that regulate the G1/S checkpoint, including p21WAF and p53, and the progression of the cell cycle into the S phase and mitosis (25). Addition of glutathione peroxidase in these hyperoxic conditions alleviated the cell-cycle arrest (5). From these observations that too many or too few ROS can inhibit collateral growth, we postulate that, at least on the cellular level in vitro, a critical “redox window” allows the cellular processes that are key components of collateral growth to occur.
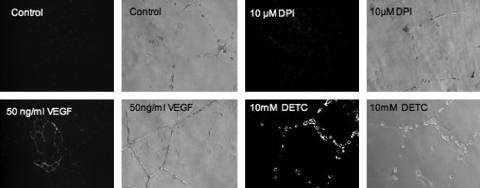
The existence of a redox window for coronary collateral growth was more strongly suggested after demonstration of how altering levels of O2•− affected collateral growth (85) and in vitro endothelial tube formation. Figures. 2 and 3 illustrate these points. Figure 2 provides an example of the impact that ROS make on in vitro endothelial tube formation. Treatment of the endothelial cells (seeded on Matrigel) with VEGF induced tube formation, but administration of either DPI (to block O2•− formation and shift the redox state to a reductive environment) or DETC (SOD inhibitor that will shift the redox state to oxidative stress) both inhibited tube formation. This observation is consistent with the idea that too many or too few ROS have a negative effect on in vitro endothelial cell tube formation, which may share some common mechanisms with angiogenesis and collateral growth.
FIG. 2.
An example of the impact that ROS make on in vitro endothelial tube formation in response to VEGF treatment. Either decreasing or increasing ROS [dark images, with the amount of white color (DHE fluorescence) proportional to ROS] with DPI (flavin-containing oxidase inhibitor) or DETC (SOD1 and SOD3 inhibitor), respectively, blunts VEGF-induced tube formation on Matrigel. VEGF increased ROS production from controls (untreated cells). These observations are consistent with the redox-window hypothesis for growth-factor signaling, in that a shift toward either a reductive or oxidative state will corrupt redox-dependent growth-factor signaling. [Adapted from Rocic et al. (85), with permission.]
FIG. 3.
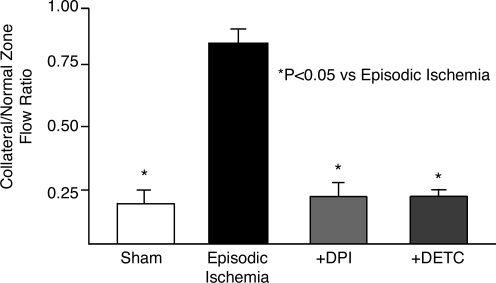
Effects of manipulating the redox state on in vivo coronary collateral growth. Coronary collateral growth (collateral flow is expressed as a ratio of flow to that of the normal zone) during a 10-day protocol of brief episodic ischemia in a sham group, a control group receiving repetitive ischemia, and two experimental groups in which O2•− production was decreased by administration of DPI (flavin-containing oxidase inhibitor) or increased through the inhibition SOD1 and 3 by DETC. Increasing oxidative stress (DETC) or reductive stress (DPI) showed similar inhibitory effects on collateral growth. These results support the concept that a critical redox state is requisite for the growth of coronary collaterals. [Adapted from Rocic et al. (85), with permission.]
To establish whether this in vitro suggestion of a redox window for angiogenesis would extend to in vivo coronary collateral growth, we increased and reduced oxidative stress in vivo by using pharmacologic agents. Figure 3 shows coronary collateral growth. Collateral flow is expressed as a ratio of flow to the collateral and normal zones during a 10-day protocol of brief episodic ischemia in a sham group, a control group receiving episodic ischemia, and two experimental groups in which O2•− production was decreased by administration of DPI or increased through the inhibition SOD1 and 3 by DETC. The major point illustrated by this figure is that the robust growth of the collateral circulation induced by repetitive ischemia (control RI group), was abrogated by either too little or too much O2•− (i.e., overly reductive or overly oxidative state, respectively). To confirm that the agents were producing the desired effect on superoxide levels, we administered a superoxide spin trap in vivo, and then harvested tissues from animals in the various groups. O2•− was measured with X-band electron paramagnetic resonance spectroscopy in myocardial tissue samples. Compared with sham, RI produced a large increase in O2•−, which was blocked by DPI, but augmented by DETC. These results provide a compelling argument for a permissive redox state (perhaps a redox window is a better term) that allows growth-factor signaling and coronary collateral growth.
The question of what is the mediator that connects redox and growth factor signaling is still yet largely unanswered, but the finding of Rocic et al. (85) shed some light on this question. These investigators reported that the redox-sensitive enzyme MAP kinase p38 was activated during collateral growth. They also reported that p38 inhibition blocks collateral growth in response to episodic ischemia. Thus, p38 likely plays a critical role in the connection of redox-dependent and growth factor–dependent signaling, resulting in collateral growth.
Redox-Dependent Signaling in Collateral Growth
Collateral growth, also known as arteriogenesis, represents a complex mosaic of highly coordinated signaling cascades. Vascular endothelial growth factor (VEGF) mediates many cellular responses involved in collateral growth, including cell survival, proliferation, and migration (121). The actions of VEGF on collateral growth appear to be mediated by signaling through the VEGFR1 (Flt-1) and VEGFR2 (Flk-1, KDR) receptors (2, 11, 44, 121). Although more is known about Flk than Flt signaling, recently the role of Flt has been appreciated in ischemic preconditioning (66, 100). Moreover, it was recently suggested that Flk functions are modulated by Flt (84), suggesting an interesting cross-talk between these two receptors.
When VEGF binds to the Flk-1 receptor, it induces phosphorylation of tyrosine residues (Y951, Y996, Y1054, and Y1059), and after phosphorylation, SH2-domain–containing adapter molecules, including Grb2, Nck, Shc, and SHP-2 phosphatase bind to the intracellular domain (121). These steps initiate activation of ERK1/2(97), JNK (76), and p38(28) mitogen-activated protein kinases (MAP kinases), the phosphatidylinositol 3-kinase (PI3-kinase)/Akt pathway(26), the nonreceptor tyrosine kinases c-Src(37), focal adhesion kinase (FAK) (122), and Pyk2 (58). ERK1/2, JNK, and PI3-kinase/Akt signaling appear to regulate cell proliferation and survival, whereas p38 MAP kinase and FAK have been implicated in endothelial cell adherence and migration (121). Importantly, p38 MAP kinase (53, 107), Akt (53), and c-Src (108) are known to be ROS dependent, which then makes the connection to redox-dependent signaling and to our concept of a redox window that is permissive for growth-factor signaling.
Previously we demonstrated the importance of the p38 MAP kinase in collateral growth (85). We found that antagonism of p38 MAP kinase inhibited VEGF-induced tube formation in vitro and collateral growth in vivo. Because VEGF is essential for coronary collateral growth (102), this finding extends well into the role that p38 plays in the process of collateral development in response to ischemia. The redox sensitivity of p38 was also shown by Rocic et al. (85) in which either reductive or oxidative stress prevented p38 activation. Thus, it would appear that the redox window is at least partially mediated by the activity of p38 MAP kinase, which connects to the processes of arteriogenesis and angiogenesis.
Coronary collateral development in the rat model of repetitive ischemia was found to be critically dependent on an optimal concentration of ROS (85). We observed that a specific concentration range of superoxide was required for coronary collateral growth, and that elevation of superoxide levels by pharmacologic treatment with an SOD inhibitor was detrimental to the process (85). Furthermore, we found that, in another rat model in which coronary collateral growth is compromised, the Zucker obese prediabetic rat, reduction of myocardial oxidative stress was required for restoration of coronary collateral growth in response to treatment with VEGF, when treatment with VEGF alone was not sufficient to restore coronary collateral growth (35). Thus, we believe that the oxidative stress induced by excessive production of superoxide in the JCR compromises redox-dependent signaling of growth factors, which is necessary for coronary collateral growth.
Angiotensin II and Redox-Dependent Collateral Growth
The role of angiotensin II (Ang II) in vascular growth is controversial. Angiotensin-converting enzyme (ACE) inhibitors retard angiogenesis in various types of tumors (119). Moreover, AT1-receptor blockers inhibited vascular growth in hindlimb ischemia (20), whereas the ACE inhibition or AT1 antagonism increased angiogenesis in vivo (113). Perhaps some of this controversy is related to the level of oxidative stress induced by angiotensin, or the background redox state existing before administration of angiotensin, the ACE inhibitor, or the AT1 antagonist. In this context, it is well documented that angiotensin increases the production of O2•−, by activation of NAD(P)H oxidase, in a variety of cell types in the vascular wall, including smooth muscle, adventitial fibroblasts, and endothelial cells (32, 33). Moreover, signaling of vascular endothelial growth factor (VEGF) appears to be at least partially mediated by production of O2•− by NAD(P)H oxidase, because DPI or inhibiting gp91phox blocked VEGF-induced superoxide production (109). We reasoned that coronary collateral growth would be dependent on redox signaling because of these findings and of our finding that VEGF plays a requisite role in coronary collateral growth (102).
Reed et al. (82) examined the role of redox signaling in coronary collateral growth by using angiotensin II administration or ATR1 blockade to alter the redox state from oxidative to reductive environments, respectively. These investigators reported that coronary collateral growth in the JCR rat, a model of vascular disease, was less than that observed in WKY controls. The investigators also found that the level of oxidative stress, as evidenced by superoxide production, was elevated in the JCR compared with WKY rats. Interestingly, ATR1 blockade in WKY rats reduced coronary collateral growth in response to repetitive episodes of myocardial ischemia, whereas the same antagonist increased coronary collateral flow in JCR rats. Administration of a subpressor (nonhypertensive) dose of Ang II increased coronary collateral growth in control WKY rats; in contrast, treatment with a hypertensive dose of Ang II abrogated collateral growth.
These findings raise several issues pertaining to the effects of angiotensin II and angiotensin receptor-1 blockade on coronary collateral growth. Ang II has multiple effects, including vasoconstriction and hypertension and activation of myocardial and vascular NAD(P)H oxidases (9), which are major producers of ROS in the vascular wall and the myocardium (117, 118). A subpressor dose of Ang II in WKY animals increased ischemia-induced coronary collateral growth (82). Under these conditions, a slight increase in superoxide production was noted; however, large increases in superoxide produced by administration of a high (hypertensive) dose of Ang II in WKY rats attenuated collateral growth to levels observed in the JCR animals. The authors speculated that the contrasting effects of high and low doses of angiotensin in the WKY are related to the redox state produced by the different levels of superoxide production at the different doses. The finding that the low dose of angiotensin did not stimulate coronary collateral growth in the JCR rat supported this notion. Because the basal redox state in JCR rats is already shifted toward oxidative stress, administration of even the subpressor dose of angiotensin will not have a positive effect on vascular growth, presumably because of worsening of oxidative stress.
This effect on the redox state may explain varying observations in previous studies investigating the effect of ACE inhibition or angiotensin-receptor 1 (AT1R) blockade on angiogenesis (20, 113). The results of Reed and colleagues (85) support the thesis that the basal redox state affects the outcome of treatment with AT1R antagonists, which is most likely related to the redox window discussed previously. In this context, reduction of oxidative stress in the JCRs by AT1R blockade produced an effect (increase in coronary collateral growth) similar to that of inhibition of NAD(P)H oxidase by apocynin. Interestingly, treatment with the AT1R antagonist, losartan, or apocynin in the WKY rats decreased superoxide levels and also reduced coronary collateral growth. The authors opined that reductions in the redox state from a “normal” level (in WKYs) by apocynin or ATR1 blockade may have induced reductive stress and corrupted redox signaling in a manner similar to that of oxidative stress. Measurements of p38 and Akt activation supported the authors' contentions that either reductive or oxidative stress corrupts redox-dependent Akt and p38 MAP kinase activation. This concept of redox-dependent signaling in collateral growth corroborated our findings (85), in which we reported that redox dependence of coronary collateral growth parallels an optimal level of reactive oxygen species and p38 MAP kinase activation.
Taken together, a proper redox state is requisite for coronary collateral growth, and this provides at least a partial explanation for the previously observed controversial effects of ACE inhibition and ATR1 blockade on angiogenesis. In the setting of a normal redox state, ACE inhibition and ATR1 blockade inhibit collateral growth because they induce reductive stress. Under conditions of elevated oxidative stress, these interventions will stimulate collateral development because they reduce the oxidative stress to a normal redox state.
Stimulation of Collateral Growth in Patients
A consensus in the literature exists regarding oxidative stress as a corrupting influence on coronary collateral growth in animal models (35, 82, 114) and in patients (10, 104, 120). Yet other literature suggests the opposite, that exposure to ROS produced by hypoxia/reoxygenation induces angiogenesis (47, 65). The critical issue is the level of ROS being produced and how that affects growth factor–dependent redox signaling. It is an oversimplification to think that the relation between ROS production and vascular growth are in direct proportion, because clearly too many ROS have an inhibitory effect on coronary collateral growth. As we discussed previously, oxidative stress can corrupt growth-factor signaling. This was apparent in our previous work in which oxidative stress adversely affected VEGF signaling and blocked the angiogenic actions of this factor (85). The clinical implication of these observations relates to the failed trials of “therapeutic angiogenesis” (the term angiogenesis under these circumstances is a misnomer because the interventions were intended to stimulate collateral growth) (3, 4, 55, 79, 95, 111), where growth-factor gene therapy or administration of recombinant proteins was attempted in patients to stimulate coronary collateral growth. Perhaps the outcome of these trials would have been different had the investigators implemented an intervention to correct the background oxidative stress. The caveat to this statement is the assumption that the oxidative stress was occurring in the various patients, but in all likelihood, this was correct because the development of atherosclerotic lesions usually parallels oxidative stress (33, 46, 96, 112).
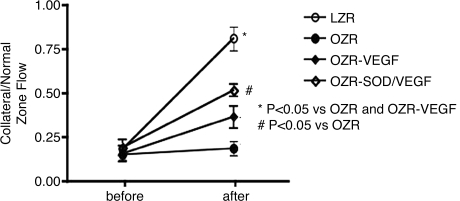
To understand whether amelioration of oxidative stress would confer a positive effect on VEGF gene therapy, we studied coronary collateral growth in the Zucker obese fatty rat. The Zucker obese fatty rat is a model for the human metabolic syndrome, because it shares many of the same afflictions including obesity, insulin resistance, hyperlipidemia, hyperinsulinemia, and hyperphagia. The Zucker rats also demonstrate endothelial dysfunction and oxidative stress (77). We first observed that coronary collateral growth in response to episodic ischemia was markedly compromised in the obese rats (Fig. 4) (35). This observation was confirmed in a different model of the metabolic syndrome, the JCR rat (82). Similar to the clinical trials, VEGF gene therapy, in which the growth factor was administered via transfected smooth muscle cells that were introduced into the coronary circulation, did not significantly improve coronary collateral growth in the obese animals (Fig. 4). However, correction of oxidative stress with ecSOD (SOD3), by using the same smooth muscle–based gene-delivery system as for VEGF, partially restored coronary collateral development (Fig. 4). We note that delivery of VEGF alone in a normal animal greatly magnifies coronary collateral growth (36). These results argue that amelioration of oxidative stress will restore growth-factor redox-dependent signaling and thus enable the VEGF gene therapy to stimulate collateral growth.
FIG. 4.
Coronary collateral growth (as indicated by the flow ratio of the collateral to the normal zone) in response to episodic ischemia was markedly compromised in the obese rats compared with lean controls. VEGF gene therapy did not significantly increase collateral flow; however, combined gene therapy (VEGF + ecSOD) significantly improved collateral flow. The combined therapy would result in a reduction in oxidative stress that occurs in the obese rats. [Adapted from Hattan et al. (35), with permission.]
One critical issue relating to these observations relates to how ecSOD can ameliorate oxidative stress within a cell. A number of possibilities exist. First, O2•− can cross the cell membrane via Cl− channels in a bidirectional manner, so dismutation of this species into H2O2 and subsequent catabolism into H2O and O2 by catalase could reduce internal oxidative stress by preventing O2•− influx (7) from the extracellular space, which could be produced by a variety of inflammatory cells or release by other cell types under oxidative stress.
Second, the metabolism of extracellular O2•− would increase bioavailability of NO, which would also modify oxidative stress. The combined gene therapy of VEGF and ecSOD produced a much more significant increase in collateral flow than did singular VEGF gene therapy. These results may have significant bearing on observations that impaired coronary collateral growth was not significantly improved in the many trials using rhVEGF or VEGF gene therapy (56, 95). Perhaps in conjunction with an antioxidant therapy, stimulation of coronary arteriogenesis with growth factors will become a reality.
An additional explanation for the efficacy of ecSOD gene therapy relates to protection of cells from extracellular superoxide-mediated cell death. In this regard, liver cell apoptosis and necrosis is mitigated by adenoviral ecSOD gene therapy (54). Moreover, it is well known that superoxide produced extracellularly can induce endothelial cell detachment and apoptosis in culture (45). Hypoxia-reoxygenation of cultured endothelial cells induced injury of endothelial cells by extracellular O2•− (99). O2•− induces cell death by lipid peroxidation of cellular membranes. Lipid peroxidation is increased in obese patients (52) and in the plasma of obese Zucker rats (94). Additionally, O2•− is reported to activate proapoptotic signaling pathways in cultured endothelial cells (45). Thus, in addition to facilitation of VEGF-dependent signaling, ecSOD therapy may promote endothelial cell survival and attachment, which would facilitate the actions of VEGF.
Conclusions
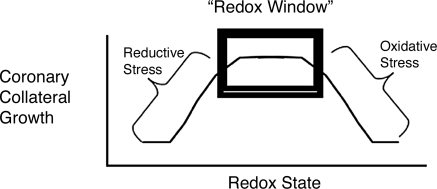
Coronary collateral growth is an important adaptive response of the heart to prevent the pathologic manifestations of ischemia. Collateral growth in the heart is initiated by ischemia, but the role of shear stress in the abluminal expansion of these vessels is still unresolved. Clearly, redox-dependent signaling plays a critical role in collateral growth in the heart, and corruption of this signaling by either reductive or oxidative stress can play a negative part in the influences and actions of growth factors on collateral growth. This concept is illustrated in Fig. 5, which is presents our view of the redox window in collateral growth. This figure illustrates a hypothetical window in which the redox state not only permits, but also can amplify coronary collateral growth. A shift in the redox state to either to an overly reductive environment or an oxidative environment corrupts growth factor–initiated redox-dependent signaling.
FIG. 5.
A diagram of the redox-window hypothesis. On the abscissa is the redox state from reductive state to oxidative state in a rightward direction. On the ordinate is collateral growth. Either reductive or oxidative stress will corrupt collateral growth and redox-dependent growth-factor signaling. The critical point of this figure is that the baseline redox state must be first determined, and only then can one accurately predict how an intervention will affect collateral growth. For example, in a control animal, apocynin inhibits collateral growth (because it shifts the redox state from a normal value to reductive stress), but in a JCR rat, apocynin stimulates collateral growth (because it shifts the redox state from oxidative stress to a normal value).
We are compelled to point out many unknown aspects of the redox-window hypothesis. First, the boundaries of the window are unknown. Clearly oxidative and reductive stresses negatively affect coronary collateral growth, but the exact redox states defining the boundaries are not yet known. The cell type in which the redox window and redox signaling are critical for collateral growth is unknown. Could redox signaling be critical for myocyte expression of growth factors in ischemia? Or perhaps for phenotypic shifts in endothelial and smooth muscle cells from quiescent to migrating and proliferating phenotypes? And what is the initiating signal for the redox signaling? Is it an ROS derived from mitochondrial metabolism or from an oxidative burst from inflammatory cells? Taken together, the concept of the redox window is only a first step in understanding the contributions of redox signaling in coronary collateral growth. However, we can state with conviction that before a regimen or therapy intended to stimulate coronary arteriogenesis, it is important to know the basal redox state to understand whether concomitant therapy to shift the redox to the correct state is required. Quite possibly, the failure to consider the affect that alterations in the redox state make on growth-factor signaling could be a reason that the “therapeutic angiogenesis” trials failed in patients. Finally, perhaps such approaches could be resurrected if correction of the redox state is implemented concomitant with growth-factor gene therapies.
Acknowledgments
This work was supported by NIH grants HL32788 and HL34286.
Abbreviations
AII, angiotensin II; ACE, angiotensin-converting enzyme; Akt, protein kinase B; ATR1, angiotensin receptor 1; CHF, congestive heart failure; c-Src, cellular-src or cellular Schmidt-Ruppin sarcoma gene; DETC, diethyldithiocarbamic acid; DNA, deoxyribonucleic acid; DPI, diphenylene iodonium; ERK1/2, extracellular signal regulated mitogen-activated protein; FAK, focal adhesion kinase; G1/S, synthesis phase of cell cycle; Grb2, growth-factor receptor–bound protein 2; H2O2, hydrogen peroxide; HIF, hypoxia-inducible factor; JCR, rat characterized by the metabolic syndrome; JNK, c-jun N-terminal kinase; LAD, left anterior descending coronary artery; MAP, mitogen-activated kinase; NAD, nicotinamide adenine dinucleotide; NAD(P)H oxidase, nicotinamide adenine dinucleotide phosphate oxidase; Nck, Src homology domain-containing adaptor; O2•−, superoxide; OH·, hydroxyl radical; p21WAF, cyclin-dependent kinase inhibitor; p38, p38 mitogen- activated kinase; p53, protein 53; PI3-kinase, phosphatidylinositol 3-kinase; Pyk2, proline-rich tyrosine kinase 2; rhVEGF, recombinant human vascular endothelial growth factor; RI, repetitive ischemia; ROS, reactive oxygen species; Shc, src homology 2 domain-containing; SHP, small heterodimer partner protein kinase 1 and 2; SOD, superoxide dismutase; SOD1, cytosolic or Cu/Zn superoxide dismutase; SOD3 or ecSOD, extracellular superoxide dismutase; VEGF, vascular endothelial growth factor; VEGFR1 or Flt-1, vascular endothelial growth factor receptor 1; VEGFR2 (or Flk-1 or KDR), vascular endothelial growth factor receptor 2; WKY, Wistar–Kyoto rat; Y, symbol for amino acid tyrosine.
References
- 1.Arras M. Ito WD. Scholz D. Winkler B. Schaper J. Schaper W. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J Clin Invest. 1998;101:40–50. doi: 10.1172/JCI119877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babiak A. Schumm AM. Wangler C. Loukas M. Wu J. Dombrowski S. Matuschek C. Kotzerke J. Dehio C. Waltenberger J. Coordinated activation of VEGFR-1 and VEGFR-2 is a potent arteriogenic stimulus leading to enhancement of regional perfusion. Cardiovasc Res. 2004;61:789–795. doi: 10.1016/j.cardiores.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 3.Baklanov D. Simons M. Arteriogenesis: lessons learned from clinical trials. Endothelium. 2003;10:217–223. doi: 10.1080/10623320390246397. [DOI] [PubMed] [Google Scholar]
- 4.Berglund JD. Galis ZS. Designer blood vessels and therapeutic revascularization. Br J Pharmacol. 2003;140:627–636. doi: 10.1038/sj.bjp.0705457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilodeau JF. Patenaude A. Piedboeuf B. Carrier C. Petrov P. Faure R. Mirautt ME. Glutathione peroxidase-1 expression enhances recovery of human breast carcinoma cells from hyperoxic cell cycle arrest. Free Radic Biol Med. 2002;33:1279–1289. doi: 10.1016/s0891-5849(02)01013-4. [DOI] [PubMed] [Google Scholar]
- 6.Birnbaum Y. Hale SL. Kloner RA. Ischemic preconditioning at a distance: reduction of myocardial infarct size by partial reduction of blood supply combined with rapid stimulation of the gastrocnemius muscle in the rabbit. Circulation. 1997;96:1641–1646. doi: 10.1161/01.cir.96.5.1641. [DOI] [PubMed] [Google Scholar]
- 7.Brzezinska AK. Lohr N. Chilian WM. Electrophysiological effects of O2*- on the plasma membrane in vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2005;289:H2379–H2386. doi: 10.1152/ajpheart.00132.2005. [DOI] [PubMed] [Google Scholar]
- 8.Buschmann IR. Busch HJ. Mies G. Hossmann KA. Therapeutic induction of arteriogenesis in hypoperfused rat brain via granulocyte-macrophage colony-stimulating factor. Circulation. 2003;108:610–615. doi: 10.1161/01.CIR.0000074209.17561.99. [DOI] [PubMed] [Google Scholar]
- 9.Cai H. Griendling KK. Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci. 2003;24:471–478. doi: 10.1016/S0165-6147(03)00233-5. [DOI] [PubMed] [Google Scholar]
- 10.Celik T. Berdan ME. Iyisoy A. Kursaklioglu H. Turhan H. Kilic S. Gulec M. Ozturk S. Isik E. Impaired coronary collateral vessel development in patients with proliferative diabetic retinopathy. Clin Cardiol. 2005;28:384–388. doi: 10.1002/clc.4960280808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chalothorn D. Clayton JA. Zhang H. Pomp D. Faber JE. Collateral density, remodeling and VEGF-A expression differ widely between mouse strains. Physiol Genomics. 2007;30:179–191. doi: 10.1152/physiolgenomics.00047.2007. [DOI] [PubMed] [Google Scholar]
- 12.Chalothorn D. Moore SM. Zhang H. Sunnarborg SW. Lee DC. Faber JE. Heparin-binding epidermal growth factor-like growth factor, collateral vessel development, and angiogenesis in skeletal muscle ischemia. Arterioscler Thromb Vasc Biol. 2005;25:1884–1890. doi: 10.1161/01.ATV.0000175761.59602.16. [DOI] [PubMed] [Google Scholar]
- 13.Chen W. Gabel S. Steenbergen C. Murphy E. A redox-based mechanism for cardioprotection induced by ischemic preconditioning in perfused rat heart. Circ Res. 1995;77:424–429. doi: 10.1161/01.res.77.2.424. [DOI] [PubMed] [Google Scholar]
- 14.Chilian WM. Mass HJ. Williams SE. Layne SM. Smith EE. Scheel KW. Microvascular occlusions promote coronary collateral growth. Am J Physiol. 1990;258:H1103–H1111. doi: 10.1152/ajpheart.1990.258.4.H1103. [DOI] [PubMed] [Google Scholar]
- 15.Clayton JA. Chalothorn D. Faber JE. Vascular endothelial growth factor-A specifies formation of native collaterals and regulates collateral growth in ischemia. Circ Res. 2008;103:1027–1036. doi: 10.1161/CIRCRESAHA.108.181115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen MV. Chukwuogo N. Yarlagadda A. Heparin does not stimulate coronary-collateral growth in a canine model of progressive coronary-artery narrowing and occlusion. Am J Med Sci. 1993;306:75–81. doi: 10.1097/00000441-199308000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Crottogini A. Meckert PC. Vera Janavel G. Lascano E. Negroni J. Del Valle H. Dulbecco E. Werba P. Cuniberti L. Martinez V. De Lorenzi A. Telayna J. Mele A. Fernandez JL. Marangunich L. Criscuolo M. Capogrossi MC. Laguens R. Arteriogenesis induced by intramyocardial vascular endothelial growth factor 165 gene transfer in chronically ischemic pigs. Hum Gene Ther. 2003;14:1307–1318. doi: 10.1089/104303403322319390. [DOI] [PubMed] [Google Scholar]
- 18.Deindl E. Schaper W. The art of arteriogenesis. Cell Biochem Biophys. 2005;43:1–15. doi: 10.1385/CBB:43:1:001. [DOI] [PubMed] [Google Scholar]
- 19.Duvall CL. Taylor WR. Weiss D. Guldberg RE. Quantitative microcomputed tomography analysis of collateral vessel development after ischemic injury. Am J Physiol Heart Circ Physiol. 2004;287:H302–H310. doi: 10.1152/ajpheart.00928.2003. [DOI] [PubMed] [Google Scholar]
- 20.Ebrahimian TG. Tamarat R. Clergue M. Duriez M. Levy BI. Silvestre JS. Dual effect of angiotensin-converting enzyme inhibition on angiogenesis in type 1 diabetic mice. Arterioscler Thromb Vasc Biol. 2005;25:65–70. doi: 10.1161/01.ATV.0000149377.90852.d8. [DOI] [PubMed] [Google Scholar]
- 21.Eng C. Cho S. Factor SM. Sonnenblick EH. Kirk ES. Myocardial micronecrosis produced by microsphere embolization: role of an alpha-adrenergic tonic influence on the coronary microcirculation. Circ Res. 1984;54:74–82. doi: 10.1161/01.res.54.1.74. [DOI] [PubMed] [Google Scholar]
- 22.Erdogan H. Fadillioglu E. Yagmurca M. Ucar M. Irmak M. Protein oxidation and lipid peroxidation after renal ischemia-reperfusion injury: protective effects of erdosteine and N-acetylcysteine. Urol Res. 2006;34:41–46. doi: 10.1007/s00240-005-0031-3. [DOI] [PubMed] [Google Scholar]
- 23.Fujita M. Kihara Y. Hasegawa K. Nohara R. Sasayama S. Heparin potentiates collateral growth but not growth of intramyocardial end arteries in dogs with repeated coronary occlusion. Int J Cardiol. 1999;70:165–170. doi: 10.1016/s0167-5273(99)00080-7. [DOI] [PubMed] [Google Scholar]
- 24.Fujita M. McKown DP. McKown MD. Franklin D. Changes in coronary flow following repeated brief coronary occlusion in the conscious dog. Heart Vessels. 1986;2:87–90. doi: 10.1007/BF02059961. [DOI] [PubMed] [Google Scholar]
- 25.Gansauge S. Gansauge F. Gause H. Poch B. Schoenberger MH. Beger HG. The induction of apoptosis in proliferating human fibroblasts by oxygen radicals is associated with a p53- and p21WAF1CIP1 induction. FEBS Lett. 1997;404:6–10. doi: 10.1016/s0014-5793(97)00059-8. [DOI] [PubMed] [Google Scholar]
- 26.Gerber HP. McMurtrey A. Kowalski A. Yan J. Keyt BA. Dixit V. Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway: requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 27.Gho BC. Schoemaker RG. van den Doel MA. Duncker DJ. Verdouw PD. Myocardial protection by brief ischemia in noncardiac tissue. Circulation. 1996;94:2193–200. doi: 10.1161/01.cir.94.9.2193. [DOI] [PubMed] [Google Scholar]
- 28.Gratton JP. Morales-Ruiz M. Kureishi Y. Fulton D. Walsk K. WC S. Akt down-regulation of p38 signaling provides a novel mechanism of vascular endothelial growth factor-mediated cytoprotection in endothelial cells. J Biol Chem. 2001;276:30359–30365. doi: 10.1074/jbc.M009698200. [DOI] [PubMed] [Google Scholar]
- 29.Gray C. Packham IM. Wurmser F. Eastley NC. Hellewell PG. Ingham PW. Crossman DC. Chico TJ. Ischemia is not required for arteriogenesis in zebrafish embryos. Arterioscler Thromb Vasc Biol. 2007;27:2135–2141. doi: 10.1161/ATVBAHA.107.143990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu W. Weihrauch D. Tanaka K. Tessmer JP. Pagel PS. Kersten JR. Chilian WM. Warltier DC. Reactive oxygen species are critical mediators of coronary collateral development in a canine model. Am J Physiol Heart Circ Physiol. 2003;285:H1582–H1589. doi: 10.1152/ajpheart.00318.2003. [DOI] [PubMed] [Google Scholar]
- 31.Gu X. El-Remessy AB. Brooks SE. Al-Shabrawey M. Tsai N. Caldwell RB. Hyperoxia induces retinal vascular endothelial cell apoptosis through formation of peroxynitrite. Am J Physiol Cell Physiol. 2003;285:C546–C554. doi: 10.1152/ajpcell.00424.2002. [DOI] [PubMed] [Google Scholar]
- 32.Hanna IR. Taniyama Y. Szocs K. Rocic P. Griendling KK. NAD(P)H oxidase-derived reactive oxygen species as mediators of angiotensin II signaling. Antioxid Redox Signal. 2002;4:899–914. doi: 10.1089/152308602762197443. [DOI] [PubMed] [Google Scholar]
- 33.Harrison D. Griendling KK. Landmesser U. Hornig B. Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91:7A–11A. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 34.Harrison DG. Chilian WM. Marcus ML. Absence of functioning alpha-adrenergic receptors in mature canine coronary collaterals. Circ Res. 1986;59:133–142. doi: 10.1161/01.res.59.2.133. [DOI] [PubMed] [Google Scholar]
- 35.Hattan N. Chilian WM. Park F. Rocic P. Restoration of coronary collateral growth in the Zucker obese rat: impact of VEGF and ecSOD. Basic Res Cardiol. 2007;102:217–223. doi: 10.1007/s00395-007-0646-3. [DOI] [PubMed] [Google Scholar]
- 36.Hattan N. Warltier D. Gu W. Kolz C. Chilian WM. Weihrauch D. Autologous vascular smooth muscle cell-based myocardial gene therapy to induce coronary collateral growth. Am J Physiol Heart Circ Physiol. 2004;287:H488–H493. doi: 10.1152/ajpheart.00145.2004. [DOI] [PubMed] [Google Scholar]
- 37.He H. Venema VJ. Gu X VR. Marrero MB. RB C. Vascular endothelial growth factor signals endothelial cell production of nitric oxide and prostacyclin through flk-1/KDR activation of c-Src. J Biol Chem. 1999;274:25130–25135. doi: 10.1074/jbc.274.35.25130. [DOI] [PubMed] [Google Scholar]
- 38.Heil M. Eitenmuller I. Schmitz-Rixen T. Schaper W. Arteriogenesis versus angiogenesis: similarities and differences. J Cell Mol Med. 2006;10:45–55. doi: 10.1111/j.1582-4934.2006.tb00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heil M. Schaper W. Insights into pathways of arteriogenesis. Curr Pharm Biotechnol. 2007;8:35–42. doi: 10.2174/138920107779941408. [DOI] [PubMed] [Google Scholar]
- 40.Heil M. Schaper W. Influence of mechanical, cellular, and molecular factors on collateral artery growth (arteriogenesis) Circ Res. 2004;95:449–458. doi: 10.1161/01.RES.0000141145.78900.44. [DOI] [PubMed] [Google Scholar]
- 41.Heil M. Ziegelhoeffer T. Wagner S. Fernandez B. Helisch A. Martin S. Tribulova S. Kuziel WA. Bachmann G. Schaper W. Collateral artery growth (arteriogenesis) after experimental arterial occlusion is impaired in mice lacking CC-chemokine receptor-2. Circ Res. 2004;94:671–677. doi: 10.1161/01.RES.0000122041.73808.B5. [DOI] [PubMed] [Google Scholar]
- 42.Hoefer IE. van Royen N. Buschmann IR. Piek JJ. Schaper W. Time course of arteriogenesis following femoral artery occlusion in the rabbit. Cardiovasc Res. 2001;49:609–617. doi: 10.1016/s0008-6363(00)00243-1. [DOI] [PubMed] [Google Scholar]
- 43.Huang S. Zheng R. Biphasic regulation of angiogenesis by reactive oxygen species. Pharmazie. 2006;61:223–229. [PubMed] [Google Scholar]
- 44.Huss R. Heil M. Moosmann S. Ziegelhoeffer T. Sagebiel S. Seliger C. Kinston S. Gottgens B. Improved arteriogenesis with simultaneous skeletal muscle repair in ischemic tissue by SCL(+) multipotent adult progenitor cell clones from peripheral blood. J Vasc Res. 2004;41:422–431. doi: 10.1159/000081441. [DOI] [PubMed] [Google Scholar]
- 45.Jacobi J. Kristal B. Chezar J. Shaul SM. Sela S. Exogenous superoxide mediates pro-oxidative, proinflammatory, and procoagulatory changes in primary endothelial cell cultures. Free Radic Biol Med. 2005;39:1238–1248. doi: 10.1016/j.freeradbiomed.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 46.Keaney JF Jr. Vita JA. Atherosclerosis, oxidative stress, and antioxidant protection in endothelium-derived relaxing factor action. Progr Cardiovasc Dis. 1995;38:129–154. doi: 10.1016/s0033-0620(05)80003-9. [DOI] [PubMed] [Google Scholar]
- 47.Kersten J. McGough M. Pagel P. Tessmer J. Warltier D. Temporal dependence of coronary collateral development. Cardiovasc Res. 1997;34:306–312. doi: 10.1016/s0008-6363(97)00019-9. [DOI] [PubMed] [Google Scholar]
- 48.Kersten JR. Schmeling T. Tessmer J. Hettrick DA. Pagel PS. Warltier DC. Sevoflurane selectively increases coronary collateral blood flow independent of KATP channels in vivo. Anesthesiology. 1999;90:246–256. doi: 10.1097/00000542-199901000-00031. [DOI] [PubMed] [Google Scholar]
- 49.Koerselman J. de Jaegere PP. Verhaar MC. Grobbee DE. der Graaf Y. Cardiac ischemic score determines the presence of coronary collateral circulation. Cardiovasc Drugs Ther. 2005;19:283–289. doi: 10.1007/s10557-005-2919-0. [DOI] [PubMed] [Google Scholar]
- 50.Koerselman J. de Jaegere PP. Verhaar MC. Grobbee DE. van der Graaf Y. Coronary collateral circulation: the effects of smoking and alcohol. Atherosclerosis. 2007;191:191–198. doi: 10.1016/j.atherosclerosis.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 51.Koneru S. Penumathsa SV. Thirunavukkarasu M. Samuel SM. Zhan L. Han Z. Maulik G. Das DK. Maulik N. Redox regulation of ischemic preconditioning is mediated by the differential activation of caveolins and their association with eNOS and GLUT-4. Am J Physiol Heart Circ Physiol. 2007;292:H2060–H2072. doi: 10.1152/ajpheart.01169.2006. [DOI] [PubMed] [Google Scholar]
- 52.Konukoglu D. Serin O. Turhan M. Plasma leptin and its relationship with lipid peroxidation and nitric oxide in obese female patients with or without hypertension. Arch Med Res. 2006;37:602–606. doi: 10.1016/j.arcmed.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 53.Lassegue B. Sorescu D. Szocs K. Yin Q. Akers M. Zhang Y. Grant SL. Lambeth JD. KK G. Novel gp91(phox) homologues in vascular smooth muscle cells : nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res. 2001;88:888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- 54.Laukkanen MO. Leppanen P. Turunen P. Tuomisto T. Naarala J. Yla-Herttuala S. EC-SOD gene therapy reduces paracetamol-induced liver damage in mice. J Gene Med. 2001;3:321–325. doi: 10.1002/jgm.194. [DOI] [PubMed] [Google Scholar]
- 55.Lee SU. Wykrzykowska JJ. Laham RJ. Angiogenesis: bench to bedside, have we learned anything? Toxicol Pathol. 2006;34:3–10. doi: 10.1080/01926230500499415. [DOI] [PubMed] [Google Scholar]
- 56.Lei Y. Haider HKh. Shujia J. Sim ES. Therapeutic angiogenesis: devising new strategies based on past experiences. Basic Res Cardiol. 2004;99:121–132. doi: 10.1007/s00395-004-0447-x. [DOI] [PubMed] [Google Scholar]
- 57.Li W. Shen W. Gill R. Corbly A. Jones B. Belagaje R. Zhang Y. Tang S. Chen Y. Zhai Y. Wang G. Wagle A. Hui K. Westmore M. Hanson J. Chen YF. Simons M. Singh J. High-resolution quantitative computed tomography demonstrating selective enhancement of medium-size collaterals by placental growth factor-1 in the mouse ischemic hindlimb. Circulation. 2006;113:2445–2453. doi: 10.1161/CIRCULATIONAHA.105.586818. [DOI] [PubMed] [Google Scholar]
- 58.Liu ZY. Ganju RK. Wang JF. Schweitzer K. Weksler B. Avraham S. Groopman JE. Characterization of signal transduction pathways in human bone marrow endothelial cells. Blood. 1997;90:2253–2259. [PubMed] [Google Scholar]
- 59.Lohr NL. Warltier DC. Chilian WM. Weihrauch D. Haptoglobin expression and activity during coronary collateralization. Am J Physiol Heart Circ Physiol. 2005;288:H1389–H1395. doi: 10.1152/ajpheart.00938.2004. [DOI] [PubMed] [Google Scholar]
- 60.Maczewski M. Duda M. Pawlak W. Beresewicz A. Endothelial protection from reperfusion injury by ischemic preconditioning and diazoxide involves a SOD-like anti-O2− mechanism. J Physiol Pharmacol. 2004;55:537–550. [PubMed] [Google Scholar]
- 61.Matsunaga T. Warltier DC. Moniz M. Tessmmer J. Weihrauch D. Chilian WM. Role of nitric oxide and vascular endothelial growth factor in coronary collateral growth. Circulation. 2000;102:3098–3103. doi: 10.1161/01.cir.102.25.3098. [DOI] [PubMed] [Google Scholar]
- 62.Matsunaga T. Warltier DC. Tessmer J. Weihrauch D. Simons M. Chilian WM. Expression of VEGF and angiopoietins-1 and -2 during ischemia-induced coronary angiogenesis. Am J Physiol Heart Circ Physiol. 2003;285:H352–H358. doi: 10.1152/ajpheart.00621.2002. [DOI] [PubMed] [Google Scholar]
- 63.Matsunaga T. Warltier DC. Weihrauch DW. Moniz M. Tessmer J. Chilian WM. Ischemia-induced coronary collateral growth is dependent on vascular endothelial growth factor and nitric oxide. Circulation. 2000;102:3098–3103. doi: 10.1161/01.cir.102.25.3098. [DOI] [PubMed] [Google Scholar]
- 64.Maulik N. Redox signaling of angiogenesis. Antioxid Redox Signal. 2002;4:805–815. doi: 10.1089/152308602760598963. [DOI] [PubMed] [Google Scholar]
- 65.Maulik N. Das D. Potentiation of angiogenic response by ischemic and hypoxic preconditioning of the heart. J Cell Mol Med. 2002;6:13–24. doi: 10.1111/j.1582-4934.2002.tb00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maulik N. Das DK. Potentiation of angiogenic response by ischemic and hypoxic preconditioning of the heart. J Cell Mol Med. 2002;6:13–24. doi: 10.1111/j.1582-4934.2002.tb00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maulik N. Das DK. Potentiation of angiogenic response by ischemic and hypoxic preconditioning of the heart. J Cell Mol Med. 2002;6:13–24. doi: 10.1111/j.1582-4934.2002.tb00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meier P. Zbinden R. Togni M. Wenaweser P. Windecker S. Meier B. Seiler C. Coronary collateral function long after drug-eluting stent implantation. J Am Coll Cardiol. 2007;49:15–20. doi: 10.1016/j.jacc.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 69.Miller SJ. Norton LE. Murphy MP. Dalsing MC. Unthank JL. The role of the renin-angiotensin system and oxidative stress in spontaneously hypertensive rat mesenteric collateral growth impairment. Am J Physiol Heart Circ Physiol. 2007;292:H2523–H2531. doi: 10.1152/ajpheart.01296.2006. [DOI] [PubMed] [Google Scholar]
- 70.Moskowitz R. Kukin M. Oxidative stress and congestive heart failure. Congest Heart Fail. 1999;5:153–163. [PubMed] [Google Scholar]
- 71.Muehling OM. Huber A. Cyran C. Schoenberg SO. Reiser M. Steinbeck G. Nabauer M. Jerosch-Herold M. The delay of contrast arrival in magnetic resonance first-pass perfusion imaging: a novel non-invasive parameter detecting collateral-dependent myocardium. Heart. 2007;93:842–847. doi: 10.1136/hrt.2006.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Muhs A. Lenter MC. Seidler RW. Zweigerdt R. Kirchengast M. Weser R. Ruediger M. Guth B. Nonviral monocyte chemoattractant protein-1 gene transfer improves arteriogenesis after femoral artery occlusion. Gene Ther. 2004;11:1685–1693. doi: 10.1038/sj.gt.3302360. [DOI] [PubMed] [Google Scholar]
- 73.Park JL. Lucchesi B. Mechanisms of myocardial reperfusion injury. Ann Thorac Surg. 1999;68:1905–1912. doi: 10.1016/s0003-4975(99)01073-5. [DOI] [PubMed] [Google Scholar]
- 74.Pasyk S. Schaper W. Schaper J. Pasyk K. Miskiewicz G. Steinseifer B. DNA synthesis in coronary collaterals after coronary artery occlusion in conscious dog. Am J Physiol. 1982;242:H1031–H1037. doi: 10.1152/ajpheart.1982.242.6.H1031. [DOI] [PubMed] [Google Scholar]
- 75.Pearlman JD. Laham RJ. Simons M. Coronary angiogenesis: detection in vivo with MR imaging sensitive to collateral neocirculation: preliminary study in pigs. Radiology. 2000;214:801–807. doi: 10.1148/radiology.214.3.r00mr39801. [DOI] [PubMed] [Google Scholar]
- 76.Pedram A. Razandi M. Levin ER. Extracellular signal-regulated protein kinase/Jun kinase cross-talk underlies vascular endothelial cell growth factor-induced endothelial cell proliferation. J Biol Chem. 1998;273:26722–26728. doi: 10.1074/jbc.273.41.26722. [DOI] [PubMed] [Google Scholar]
- 77.Picchi A. Gao X. Belmadani S. Potter BJ. Focardi M. Chilian WM. Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ Res. 2006;99:69–77. doi: 10.1161/01.RES.0000229685.37402.80. [DOI] [PubMed] [Google Scholar]
- 78.Pipp F. Boehm S. Cai WJ. Adili F. Ziegler B. Karanovic G. Ritter R. Balzer J. Scheler C. Schaper W. Schmitz-Rixen T. Elevated fluid shear stress enhances postocclusive collateral artery growth and gene expression in the pig hind limb. Arterioscler Thromb Vasc Biol. 2004;24:1664–1668. doi: 10.1161/01.ATV.0000138028.14390.e4. [DOI] [PubMed] [Google Scholar]
- 79.Post MJ. Simons M. The rational phase of therapeutic angiogenesis. Minerva Cardioangiol. 2003;51:421–432. [PubMed] [Google Scholar]
- 80.Przyklenk K. Bauer B. Ovize M. Kloner RA. Whittaker P. Regional ischemic “preconditioning” protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893–899. doi: 10.1161/01.cir.87.3.893. [DOI] [PubMed] [Google Scholar]
- 81.Radke PW. Heinl-Green A. Frass OM. Post MJ. Sato K. Geddes DM. Alton EW. Evaluation of the porcine ameroid constrictor model of myocardial ischemia for therapeutic angiogenesis studies. Endothelium. 2006;13:25–33. doi: 10.1080/10623320600660128. [DOI] [PubMed] [Google Scholar]
- 82.Reed R. Kolz C. Potter B. Rocic P. The mechanistic basis for the disparate effects of angiotensin II on coronary collateral growth. Arterioscler Thromb Vasc Biol. 2008;28:61–67. doi: 10.1161/ATVBAHA.107.154294. [DOI] [PubMed] [Google Scholar]
- 83.Reimer KA. Jennings RB. The “wavefront phenomenon” of myocardial ischemic cell death: transmural progression of necrosis within the framework of ischemic bed size (myocardium at risk) and collateral flow. Lab Invest. 1979;40:633–644. [PubMed] [Google Scholar]
- 84.Roberts DM. Kearney JB. Johnson JH. Rosenberg MP. Kumar R. Bautch VL. The vascular endothelial growth factor (VEGF) receptor Flt-1 (VEGFR-1) modulates Flk-1 (VEGFR-2) signaling during blood vessel formation. Am J Pathol. 2004;164:1531–1535. doi: 10.1016/S0002-9440(10)63711-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rocic P. Kolz C. Reed R. Potter B. Chilian WM. Optimal reactive oxygen species concentration and p38 MAP kinase are required for coronary collateral growth. Am J Physiol Heart Circ Physiol. 2007;292:H2729–H2736. doi: 10.1152/ajpheart.01330.2006. [DOI] [PubMed] [Google Scholar]
- 86.Rockstroh J. Brown BG. Coronary collateral size, flow capacity, and growth: estimates from the angiogram in patients with obstructive coronary disease. Circulation. 2002;105:168–173. doi: 10.1161/hc0202.102120. [DOI] [PubMed] [Google Scholar]
- 87.Schaper W. Gorge G. Winkler B. Schaper J. The collateral circulation of the heart. Prog Cardiol Dis. 1988;31:57–77. doi: 10.1016/0033-0620(88)90011-4. [DOI] [PubMed] [Google Scholar]
- 88.Scheel KW. Daulat G. Mass HJ. Williams SE. Intramural coronary collateral flow in dogs. Am J Physiol. 1990;258:H679–H682. doi: 10.1152/ajpheart.1990.258.3.H679. [DOI] [PubMed] [Google Scholar]
- 89.Scheel KW. Galindez TA. Cook B. Rodriguez RJ. Ingram LA. Changes in coronary and collateral flows and adequacy of perfusion in the dog following one and three months of circumflex occlusion. Circ Res. 1976;39:654–658. doi: 10.1161/01.res.39.5.654. [DOI] [PubMed] [Google Scholar]
- 90.Scheel KW. Mass H. Williams SE. Pressure-flow characteristics of coronary collaterals in dogs. Am J Physiol. 1989;256:H441–H445. doi: 10.1152/ajpheart.1989.256.2.H441. [DOI] [PubMed] [Google Scholar]
- 91.Scheel KW. Rodriguez RJ. Ingram LA. Directional coronary collateral growth with chronic circumflex occlusion in the dog. Circ Res. 1977;40:384–390. doi: 10.1161/01.res.40.4.384. [DOI] [PubMed] [Google Scholar]
- 92.Scholz D. Elsaesser H. Sauer A. Friedrich C. Luttun A. Carmeliet P. Schaper W. Bone marrow transplantation abolishes inhibition of arteriogenesis in placenta growth factor (PlGF) −/− mice. J Mol Cell Cardiol. 2003;35:177–184. doi: 10.1016/s0022-2828(02)00304-8. [DOI] [PubMed] [Google Scholar]
- 93.Scholz D. Ito W. Fleming I. Deindl E. Sauer A. Wiesnet M. Busse R. Schaper J. Schaper W. Ultrastructure and molecular histology of rabbit hind-limb collateral artery growth (arteriogenesis) Virchows Arch. 2000;436:257–270. doi: 10.1007/s004280050039. [DOI] [PubMed] [Google Scholar]
- 94.Serkova NJ. Jackman M. Brown JL. Liu T. Hirose R. Roberts JP. Maher JJ. CU N. Metabolic profiling of livers and blood from obese Zucker rats. J Hepatol. 2006;44:956–962. doi: 10.1016/j.jhep.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 95.Simons M. Bonow RO. Chronos NA. Cohen DJ. Giordano FJ. Hammond HK. Laham RJ. Li W. Pike M. Sellke FW. Stegmann TJ. Udelson JE. Rosengart TK. Clinical trials in coronary angiogenesis: issues, problems, consensus: an expert panel summary. Circulation. 2000;102:E73–E86. doi: 10.1161/01.cir.102.11.e73. [DOI] [PubMed] [Google Scholar]
- 96.Steinbrecher UP. Zhang H. Lougheed M. Role of oxidatively modified LDL in atherosclerosis. Free Radic Biol Med. 1990;9:155–168. doi: 10.1016/0891-5849(90)90119-4. [DOI] [PubMed] [Google Scholar]
- 97.Takahashi T. Ueno H. Shibuya M. VEGF activates protein kinase C-dependent, but Ras-independent Raf-MEK-MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene. 1999;18:2221–2230. doi: 10.1038/sj.onc.1202527. [DOI] [PubMed] [Google Scholar]
- 98.Tatli E. Yildiz M. Gul C. Birsin A. Karahasanoglu E. Ozcelik F. Ozbay G. Effect of obesity on coronary collateral vessel development in patients with coronary artery disease. Angiology. 2005;56:657–661. doi: 10.1177/000331970505600602. [DOI] [PubMed] [Google Scholar]
- 99.Terada L. Hypoxia-reoxygenation increases O2−. efflux which injures endothelial cells by an extracellular mechanism. Am J Physiol. 1996;270:H945–H950. doi: 10.1152/ajpheart.1996.270.3.H945. [DOI] [PubMed] [Google Scholar]
- 100.Thirunavukkarasu M. Juhasz B. Zhan L. Menon VP. Tosaki A. Otani H. Maulik N. VEGFR1 (Flt-1 + /−) gene knockout leads to the disruption of VEGF-mediated signaling through the nitric oxide/heme oxygenase pathway in ischemic preconditioned myocardium. Free Radic Biol Med. 2007;42:1487–1495. doi: 10.1016/j.freeradbiomed.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tojo T. Ushio-Fukai M. Yamaoka-Tojo M. Ikeda S. Patrushev N. Alexander R. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation. 2005;111:2347–2355. doi: 10.1161/01.CIR.0000164261.62586.14. [DOI] [PubMed] [Google Scholar]
- 102.Toyota E. Warltier DC. Brock T. Ritman E. Kolz C. O'Malley P. Rocic P. Focardi M. Chilian WM. Vascular endothelial growth factor is required for coronary collateral growth in the rat. Circulation. 2005;112:2108–2113. doi: 10.1161/CIRCULATIONAHA.104.526954. [DOI] [PubMed] [Google Scholar]
- 103.Trzeciakowski J. Chilian WM. Chaotic behavior of the coronary circulation. Med Biol Eng Comput. 2008;46:433–442. doi: 10.1007/s11517-008-0329-8. [DOI] [PubMed] [Google Scholar]
- 104.Turhan H. Yasar AS. Erbay AR. Yetkin E. Sasmaz H. Sabah I. Impaired coronary collateral vessel development in patients with metabolic syndrome. Coron Artery Dis. 2005;16:281–285. doi: 10.1097/00019501-200508000-00004. [DOI] [PubMed] [Google Scholar]
- 105.Tuttle JL. Sanders BM. Burkhart HM. Fath SW. Kerr KA. Watson WC. Herring BP. Dalsing MC. Unthank J. Impaired collateral artery development in spontaneously hypertensive rats. Microcirculation. 2002;9:343–351. doi: 10.1038/sj.mn.7800151. [DOI] [PubMed] [Google Scholar]
- 106.Tuttle JL. Hahn TL. Sanders BM. Witzmann FA. Miller SJ. Dalsing MC. Unthank JL. Impaired collateral development in mature rats. Am J Physiol Heart Circ Physiol. 2002;283:H146–H155. doi: 10.1152/ajpheart.00766.2001. [DOI] [PubMed] [Google Scholar]
- 107.Ushio-Fukai M. Alexander RW. Akers M. Griendling KK. p38 Mitogen-activated protein kinase is a critical component of the redox-sensitive signaling pathways activated by angiotensin II: role in vascular smooth muscle cell hypertrophy. J Biol Chem. 1998;273:15022–15029. doi: 10.1074/jbc.273.24.15022. [DOI] [PubMed] [Google Scholar]
- 108.Ushio-Fukai M. Griendling KK. Becker PL. Helenski L. Halleran S. Alexander RW. Epidermal growth factor receptor transactivation by angiotensin II requires reactive oxygen species in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2001;21:489–495. doi: 10.1161/01.atv.21.4.489. [DOI] [PubMed] [Google Scholar]
- 109.Ushio-Fukai M. Tang Y. Fukai T. Dikolov SI. Ma Y. Fujimoto M. Quinn MT. Pagano PJ. Johnson C. Alexander RW. Novel role of gp91(phox)-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res. 2002;91:1160–1167. doi: 10.1161/01.res.0000046227.65158.f8. [DOI] [PubMed] [Google Scholar]
- 110.Verhoye JP. de Latour B. Drochon A. Corbineau H. Collateral flow reserve and right coronary occlusion: evaluation during off-pump revascularization. Interact Cardiovasc Thorac Surg. 2005;4:23–26. doi: 10.1510/icvts.2004.093088. [DOI] [PubMed] [Google Scholar]
- 111.Vincent KA. Jiang C. Boltje I. Kelly RA. Gene therapy progress and prospects: therapeutic angiogenesis for ischemic cardiovascular disease. Gene Ther. 2007;14:781–789. doi: 10.1038/sj.gt.3302953. [DOI] [PubMed] [Google Scholar]
- 112.Walldius G. Regnström J. Nilsson J. Johansson J. Schäfer-Elinder L. Moelgaard J. Hådell K. Olsson AG. Carlson LA. The role of lipids and antioxidative factors for development of atherosclerosis. Am J Cardiol. 1993;71:15B–19B. doi: 10.1016/0002-9149(93)90140-8. [DOI] [PubMed] [Google Scholar]
- 113.Walther T. Menrad A. Orzechowski HD. Siemeister G. Paul M. Schirner M. Differential regulation of in vivo angiogenesis by angiotensin II receptors. FASEB J. 2003;17:2061–2067. doi: 10.1096/fj.03-0129com. [DOI] [PubMed] [Google Scholar]
- 114.Weihrauch D. Lohr NL. Mraovic B. Ludwig LM. Chilian WM. Pagel PS. Warltier DC. Kersten JR. Chronic hyperglycemia attenuates coronary collateral development and impairs proliferative properties of myocardial interstitial fluid by production of angiostatin. Circulation. 2004;109:2343–2348. doi: 10.1161/01.CIR.0000129225.67353.1F. [DOI] [PubMed] [Google Scholar]
- 115.Weihrauch D. Tessmer J. Warltier DC. Chilian WM. Repetitive coronary artery occlusions induce release of growth factors into the myocardial interstitium. Am J Physiol. 1998;275:H969–H976. doi: 10.1152/ajpheart.1998.275.3.H969. [DOI] [PubMed] [Google Scholar]
- 116.Werner GS. Ferrari M. Heinke S. Kuethe F. Surber R. Richartz BM. Figulla HR. Angiographic assessment of collateral connections in comparison with invasively determined collateral function in chronic coronary occlusions. Circulation. 2003;107:1972–1977. doi: 10.1161/01.CIR.0000061953.72662.3A. [DOI] [PubMed] [Google Scholar]
- 117.Wolin MS. Ahmad M. Gupte SA. The sources of oxidative stress in the vessel wall. Kidney Int. 2005;67:1659–1661. doi: 10.1111/j.1523-1755.2005.00257.x. [DOI] [PubMed] [Google Scholar]
- 118.Wolin MS. Ahmad M. Gupte SA. Oxidant and redox signaling in vascular oxygen sensing mechanisms: basic concepts, current controversies, and potential importance of cytosolic NADPH. Am J Physiol Lung Cell Mol Physiol. 2005;289:L159–L173. doi: 10.1152/ajplung.00060.2005. [DOI] [PubMed] [Google Scholar]
- 119.Yasumatsu R. Nakashima T. Masuda M. Ito A. Kuratomi Y. Nakagawa T. Komune S. Effects of the angiotensin-I converting enzyme inhibitor perindopril on tumor growth and angiogenesis in head and neck squamous cell carcinoma cells. J Cancer Res Clin Oncol. 2004;130:567–573. doi: 10.1007/s00432-004-0582-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yilmaz MB. Biyikoglu SF. Akin Y. Guray U. Kisacik HL. Korkmaz S. Obesity is associated with impaired coronary collateral vessel development. Int J Obes Relat Metab Disord. 2003;27:1541–1545. doi: 10.1038/sj.ijo.0802474. [DOI] [PubMed] [Google Scholar]
- 121.Zachary I. Gliki G. Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovasc Res. 2001;49:568–581. doi: 10.1016/s0008-6363(00)00268-6. [DOI] [PubMed] [Google Scholar]
- 122.Zachary I. Rozengurt E. Focal adhesion kinase (p125FAK): a point of convergence in the action of neuropeptides, integrins, and oncogenes. Cell. 1992;71:891–894. doi: 10.1016/0092-8674(92)90385-p. [DOI] [PubMed] [Google Scholar]
- 123.Zbinden R. Zbinden S. Meier P. Hutter D. Billinger M. Wahl A. Schmid JP. Windecker S. Meier B. Seiler C. Coronary collateral flow in response to endurance exercise training. Eur J Cardiovasc Prev Rehabil. 2007;14:250–257. doi: 10.1097/HJR.0b013e3280565dee. [DOI] [PubMed] [Google Scholar]
- 124.Zhang J. Regieli JJ. Schipper M. Entius MM. Liang F. Koerselman J. Ruven HJ. van der Graaf Y. Grobbee DE. Doevendans PA. Inflammatory gene haplotype-interaction networks involved in coronary collateral formation. Hum Hered. 2008;66:252–264. doi: 10.1159/000143407. [DOI] [PubMed] [Google Scholar]
- 125.Zhuang ZW. Gao L. Murakami M. Pearlman JD. Sackett TJ. Simons M. de Muinck ED. Arteriogenesis: noninvasive quantification with multi-detector row CT angiography and three-dimensional volume rendering in rodents. Radiology. 2006;240:698–707. doi: 10.1148/radiol.2403050976. [DOI] [PubMed] [Google Scholar]