Abstract
Myocardial ischemia and cardiac dysfunction have been known to follow ischemic heart diseases (IHDs). Despite a plethora of conventional treatment options, their efficacies are associated with skepticism. Cell therapies harbor a promising potential for vascular and cardiac repair, which is corroborated by adequate preclinical evidence. The underlying objectives behind cardiac regenerative therapies subsume enhancing angiomyogenesis in the ischemic myocardium, ameliorating cellular apoptosis, regenerating the damaged myocardium, repopulating the lost resident myocardial cells (smooth muscle, cardiomyocyte, and endothelial cells), and finally, decreasing fibrosis with a consequent reduction in ventricular remodeling. Although-cell based cardiomyoplasty approaches have an immense potential, their clinical utilization is limited owing to the increased need for better candidates for cellular cardiomyoplasty, better routes of delivery, appropriate dose for efficient engraftment, and better preconditioning or genetic-modification strategies for the progenitor and stem cells. Mesenchymal stem cells (MSCs) have emerged as powerful candidates in mediating myocardial repair owing to their unique properties of multipotency, transdifferentiation, intercellular connection with the resident cardiomyocytes via connexin 43 (Cx43)-positive gap junctions in the myocardium, and most important, immunomodulation. In this review, we present an in-depth discussion on the complexities associated with stem and progenitor cell therapies, the potential of preclinical approaches involving MSCs for myocardial repair, and an account of the past milestones and ongoing MSC-based trials in humans. Antioxid. Redox Signal. 11, 1841–1855.
Introduction
Complicated comorbidities of ischemic heart diseases (IHDs) have resulted in a major worldwide death toll (109). As the regenerative capacity of the myocardium is extremely limited, negative remodeling and myocardial scar leads to cardiac decompensation and a gradual terminal failure (99). Contemporary pharmacologic and interventional strategies for myocardial repair [like surgical cardiomyoplasty, ventricular resynchronization, and heart transplantation (81)] fail to ameliorate these pathophysiologic complications and have unveiled numerous flaws, thereby paving a way for the global era of cell-based therapeutics.
The bone marrow acts as a reservoir, housing diverse types of progenitor cells (e.g., endothelial progenitor cells, mesenchymal stem cells, and hematopoietic stem cells), which are differentially regulated by growth factors and cytokines, affecting their retention, self-renewal, cell-cycle status, and mobilization (88). These distinct subsets of progenitor cells are multipotent, although more committed, arising from a parent stem cell, and developmentally, hold a hierarchy between stem cell and a fully differentiated adult cell. On the contrary, stem cells define a class of multipotent/pluripotent cells fully capable of self-renewal and clonal expansion (36). Although, literature is replete with instances of both autologous stem and progenitor cell therapies for the treatment of ischemic cardiomyopathies and their improved therapeutic outcomes have been widely accepted, these trials have led to a less precise use of terms to identify multipotent cells and true stem cells. For example, the terms mesenchymal stem cells (MSCs) and mesenchymal progenitor cells are used interchangeably.
Preclinical studies have shown evidence for a beneficial effect of the transplanted progenitor and stem cells on the enhanced recovery of cardiac functions, which in turn is mediated by replacement of the scarred tissue, reduced fibrosis, restoration of the myocyte contractile activity, and formation of a vascular meshwork for nourishing the newly formed tissue. These therapies participate in myocardial repair via three basic mechanisms: enhanced angiogenesis, decreased cellular apoptosis, and improved myocardial regeneration.
Myocardial infarction engenders an irreversible loss of cardiomyocytes (CMs). It has been reported that a typical human infarct involves a loss of 1–1.5 billion CMs with a consequent loss in myocardial cell mass (approximately one third of the total volume) (14, 92, 97). Therefore, progenitor cell therapies that can contribute to an increase in the number of viable and functional CM population behold considerable clinical significance. As a consequence, many stem and progenitor cell types (including skeletal myoblasts, ESCs, EPCs, HSCs, MSCs, iPSCs, etc.) have been subjected to challenges to demonstrate a CM-differentiation potential. However, the true potential of any of these cell types in defining a CM lineage is controversial.
MSCs, also defined as the multipotent subset of marrow stromal cells (48), comprise a rare population of multipotent progenitor cells in the adult bone marrow (89) and are considered as promising candidates for myocardial repair owing to their unique properties of plasticity, efficient self-renewal, immune tolerance, release of paracrine effectors, trophic support, and establishment of Cx43-positive intercellular connections with the functional CMs. Furthermore, MSCs are omnipresent in virtually all postnatal organs and tissues as pericytes (26), afford easy handling, possess enormous expansion potential, and comply with allotransplantation. MSCs have been shown to acquire the characteristics of osteoblast, chondrocyte, adipocyte, neuron, CM, smooth muscle, and endothelial cells, thus affording diverse clinical utilization for cell-based therapeutics (13, 82, 84, 127, 132). Most important, they have been termed “universal cells,” cells that can work in any host owing to the lack of MHC class II molecules and B7 costimulatory molecules for MHC class I (17).
However, hypoxia, deficiency of survival factors, ischemia, and inflammatory cytokines resulting from oxidative stress and CMs apoptosis in the infarcted myocardium limit the longevity of the transplanted MSCs. Therefore, approaches to ameliorate the harsh microenvironment in the ischemic myocardium and to promote the longevity of engrafted cells via preconditioning (hypoxic, anoxic, pharmacologic, heat shock) the MSCs or genetically modifying them (overexpressing Akt, CXCR4, SDF-1, IGF-1, HGF, etc.) before their transplantation have yielded promising results. These modified or preconditioned MSCs have been proclaimed to stimulate the survival and cardioprotective pathways in the infarcted myocardium. Moreover, the translation of MSC therapy to clinical cardiology has demonstrated some promising improvements in the cardiac functions of patients with acute myocardial infarction (AMI) along with a significant increase in left ventricular ejection fraction (LVEF) after 3 months. In addition, perfusion defects detected by positron emission tomography were significantly ameliorated after MSC transplantation. The study also demonstrated an increased cardiac mechanical capability and electrical property, as measured by real-time cardiac electromechanical mapping and lack of any occurrences of arrhythmia in the MSC-administered patients during electrocardiographic monitoring (23).
The future of MSC-based cellular cardiomyoplasty approaches is encouraging, although further standardization of the delivery strategies and a better understanding of their transdifferentiation, niche regulation, and homing are required.
Candidates for Cell Therapy in Myocardial Repair
Nonresident cells of the myocardium
Skeletal myoblasts
Initial pioneering attempts by C. E. Murry (75) for cell-based myocardial regeneration began with the injection of 3 × 106 skeletal myoblasts (SkMs) superficially into the center of cryoinjured myocardium, which formed myotubes after 3 days of transplantation and myofibers by 2 weeks. These myofibers showed the characteristics of slow-twitch muscle that could be stimulated to contract ex vivo.
Following this, autologous skeletal myoblasts (SkMs) were used to ameliorate the ischemic myocardium (118, 119), owing also to their increased resistance to an ischemic microenvironment and the ability to form myotubes in vivo (79, 112). Interestingly, modified human SkMs expressing human VEGF-165 were found to increase the capillary density and LVEF in a porcine model of myocardial infarction (47, 111). Moreover, diazoxide-preconditioned SkMs were found to release paracrine factors that promoted cardiac angiomyogenesis in Fischer-344 rats (79).
However, it was soon realized that these SkMs fail to couple electromechanically with the resident CMs in the myocardium. Although, after the coculture of SkMs (N-cadherin and Cx43 positive) with neonatal or adult cardiomyocytes, the resulting myotubes contract in synchrony with the cardiomyocytes, this electromechanical coupling was disrupted following the in vivo engraftment, consistent with the lack of expression of N-cadherin and Cx43 in vivo (96). This may contribute to electrical heterogeneity in hearts treated with SkMs, which in turn may cause the increased ventricular arrhythmia as seen in clinical studies of myoblast transfer, thus limiting their therapeutic use (72).
Embryonic stem cells
Unlike most adult stem cells, embryonic stem cells (ESCs) clearly have a cardiomyogenic potential. These cells were successfully isolated from human blastocysts by Thomson et al. (120), after the initial attempts of Evans et al. (39) and later by G. Martin (70) in the same year. ESCs posses all the qualities of a prototypical stem cell: clonal expansion, self-renewal and multipotentiality (41). In addition, they can transdifferentiate into myocardial cell types; endothelial cells, and CMs (27, 58, 65, 95). Transcriptional and functional profiling of human ES cell (hESC)-derived CMs revealed a similarity to those found in 20-week fetal heart cells, thereby appearing as a strong candidate for the in vivo replacement of apoptotic CMs in the failing myocardium (16). Furthermore, ESC-derived cardiomyocytes (ESC-CM) elicit electromechanical coupling via Cx43-positive gap junctions (27, 143).
Unfortunately, despite this huge potential, the transplantation of undifferentiated ES cells into immunodeficient or syngeneic hosts can bring about teratomas, induction of arrhythmias, and allorejection (20, 143).
Hematopoietic stem cells
HSCs probably define a class of the oldest progenitor cells that are still in use for cell-based therapeutics. Initial studies using HSCs can be traced back to the 1970s. Studies involving an injection of Lin−/c-Kit+ HSCs in the periinfarct area of female C57BL/6 mice showed de novo cardiac regeneration (68% of the infarct volume) (83). In another attempt, intravenous injection of GFP-tagged Lin−ScaI+ HSCs in murine model revealed intercellular connections of the administered cells with the resident CMs. In the same study, Lin−CD34+CD38− human HSCs demonstrated intercellular connections with the resident cardiomyocytes in immunodeficient mice (NOD/SCID/IL2rγnull) (51).
However, subsequent studies failed to show any differentiation of the labeled HSCs to CMs or endothelial cells in the infarcted myocardium (80). Furthermore, HSC transplantation has been reported to be associated with some postoperative complications (107).
Mesenchymal stem cells
MSCs are found in many adult tissues like bone marrow [<0.001% of all bone marrow mononuclear cells (BMNCs)], adipose (abundant source), placenta, and umbilical cord blood, exhibiting unique properties like easy procurement and handling, multilineage potential, capability of mediating vascular and myocardial repair, and, most important, immunomodulation. Since the pioneering discovery of bone marrow stromal cells (BMSCs) by Alexander Friedenstein (40), MSC-based cellular cardiomyoplasty approaches have come a long way. MSCs-based therapies herald a great potential in rejuvenating the failing heart through its paracrine effects. In addition, they clearly demonstrate an ability to acquire some phenotypic characteristics and markers of CMs (81) and mediate intercellular connections (associated with exchange of quasi-symmetric junctional current) with the resident CMs, through Cx43-positive gap junctions (9, 11, 87, 91, 126).
Endothelial progenitor cells
Improved neovascularization can play a crucial role in rescuing the failing ischemic myocardium. Endothelial progenitor cells (EPCs) are potential candidates for cardiovascular repair (2% of all BMNCs), as they have been shown to mediate neovascularization at the infarct border zone. Bone marrow–derived hemangioblasts and peripheral blood–derived monocytes can differentiate into EPCs, which in turn can differentiate into functional endothelial cells (135). Soon after the discovery of EPCs by Asahara et al. (6), it was realized that tissue ischemia and cytokines can mobilize EPCs, thereby contributing to neovascularization of the ischemic tissue (115). Many other studies have also demonstrated that EPCs can mediate vasculogenesis under diverse pathophysiologic conditions like wound healing, limb ischemia, and after myocardial infarction, under the influence of the released cytokines (38, 52, 66). These EPCs are characterized by their ability to take up acetylated low-density lipoprotein and are CD34+/CD133+/Flk-1+.
Quite interestingly, EPCs share many common characteristics and overlapping expression of surface markers with diverse cell types. For instance, EPCs derived from the bone marrow as well as the circulating EPCs demonstrate the expression of endothelial markers Flk-1, Tie-2, VE-cadherin, CD34, CD146, and E-selectin, just as do the mature circulating endothelial cells. Moreover, the uptake of acetylated LDL, a property associated with EPCs, can be mimicked by monocytes and certain hematopoietic cells (33, 93). However, despite these complexities related to the purification and characterization of EPCs (121), EPCs are the most commonly studied candidates in clinical trials for reendothelialization and neovascularization of the myocardium (124).
Resident progenitor cells
Cardiac progenitor cells
The paradigm of heart being a postmitotic organ was challenged by the discovery of cardiac progenitor cells (CPCs), which are clustered in specialized microenvironments (niches) throughout the myocardium in the adult heart. CPC populations are classified into different subtypes, based on their surface markers: Sca1+, Isl1+, etc. (8).
Experimental studies using an injection of EGFP-tagged CPCs in the proximity of a chronic infarct revealed enhanced homing and accumulation of CPCs within the scar. Moreover, CPCs were found to effectuate cardiac regeneration when administered either intramyocardially or through an intracoronary route (10, 12, 99, 101). A significant study by Dawn et al. (30) showed that the intravascularly administered CPCs, in a rat model of temporary coronary occlusion followed by reperfusion, migrate to the myocardium and promote myocyte regeneration, form new coronary vasculature, and reduce the infarct size without any event of cell fusion between the administered CPCs and the resident CMs. A recent study revealed that the engrafted CPCs not only mediated an increase in the number of myocytes and coronary vessels, but also replaced almost 42% of the scar with newly formed myocardium, thereby attenuating ventricular dilation and leading to a consequent improvement in cardiac functions. It is argued that this reparative mechanism of CPCs involves the synthesis of MMPs to degrade the collagen proteins and formation of tunnels within the fibrotic tissue during their migration across the scarred myocardium (99).
The unique property of CPCs is that they can be isolated from a patient after endomyocardial biopsy followed by their ex vivo expansion (as cKit+ cardiospheres), as demonstrated by Marbán et al. (8, 106). These culture-expanded cells can then be reinjected into the same patient, thus averting the potential risks of allotransplantation.
Despite these advantages, the therapeutic use of CPCs becomes complicated, owing to the difficulties in acquiring myocardial samples from patients and their expansion in quantities of therapeutic significance (61). Therefore, local injection of growth factors to incite the resident CPCs has been offered as an effective approach to mediate myocardial regeneration. Matrix metalloproteinases (MMPs), hepatocyte growth factor (HGF), insulin growth factor-1 (IGF-1), and stromal cell–derived factor-1 (SDF-1) have been implicated in CPC homing and their differentiation into CMs and vascular cells (99).
The presence of resident CPCs in myocardium has been argued for some time. However, why the resident CPCs do not contribute substantially to the recovery of cardiac functions during pathophysiologic complications still remains a mystery. The decreased oxygen supply in the periinfarct area and reduced vascular perfusion might hinder the activation of the resident CPCs (99). Furthermore, the origin of CPCs is quite unclear. Whether they arise early during embryogenesis or are extracardiac in origin or dedifferentiated from adult CMs has been largely debated (8).
Induced pluripotent stem cells
A major breakthrough in stem cell research came from the discovery that differentiated cells like mouse embryonic or adult fibroblasts can be reprogrammed to an ESC-like state after the retroviral introduction of four factors, Oct3/4, Sox2, c-Myc (dispensable), and Klf4 by Takahashi et al. (114). This direct reprogramming approach might enable researchers to treat patients with their own modified cells, thereby affording a simpler, safer, and time-efficient approach to obtaining the desired cell types (128). Interestingly, these iPSCs have also been derived from single neonatal foreskin (human dermal fibroblasts), and the resultant cell lines are morphologically indistinguishable from human ESCs (hESCs) (67). iPSCs can also acquire the characteristics of functional CMs. Moreover, molecular, structural, and functional comparison of iPSC-derived CMs unveiled striking similarities to those derived from ESCs (71). In addition, studies done by Narazaki et al. demonstrated that iPSCs can differentiate into mesodermal cells; endothelial cells; mural cells; arterial, venous, and lymphatic endothelial cells; and self-beating CMs in vitro (78). Thus, iPSCs pose a potential source of autologous functional CMs for myocardial regeneration (71).
However, just as the other progenitor cell therapy approaches, the clinical use of iPSCs has a long way to go. The mechanistic regulation of this “turning back of an adult cell's developmental clock” to an ES-like state (developmental reprogramming) requires further understanding as well as improved quality control. After the delivery of iPSCs, the residual undifferentiated pluripotent cells can induce teratomas caused by the viral vector. Thus, an increasing need exists for iPSC therapies without the involvement of any integrating virus (123).
Despite the availability of a plentitude of diverse classes of stem cells for the treatment of ischemic cardiomyopathies, the use of each cell type is associated with posttreatment complications. The intricacies associated with stem and progenitor cell therapies, like induction of teratomas by ESCs and the failure of SkMs to couple electromechanically with functional CMs, pose a major challenge for their clinical use. Therefore, an indispensible need exists for a suitable candidate for better cell-based myocardial regenerative therapies.
Allogenic MSCs are promising candidates for alleviating chronic heart diseases. On engraftment, MSCs are known to express myocyte (desmin, troponin T) and vascular (αSMA, factor VIII) markers and to establish Cx43-positive intercellular connections with the resident CMs (5, 45, 136, 139). These properties of in vivo differentiation, immunomodulation (31, 56, 60, 108), and establishment of gap junction (Cx43, Cx40)- and adherens junction (N-cadherin)-based intercellular contacts with native CMs make them the preferred candidates for the treatment of ischemic cardiomyopathies.
MSCs: A New Paradigm for Cell-Based Cardiomyoplasty
It is well known that the capacity of the myocardium to regenerate is extremely limited. Myocardial pathophysiologic comorbidities are often associated with an increased CM apoptosis and replacement fibrosis in the infarcted heart. After the realization of local and systemic postoperative complications associated with some stem and progenitor cell therapies (68, 113), MSCs have emerged as a powerful tool to mediate myocardial repair and regeneration owing to their unique characteristics and have been studied intensively in basic cardiac research as well as in clinical studies.
Occurrence
MSCs are a rare population of cells originating from the bone marrow (∼0.001–0.01%). Although MSCs appear predominantly in the bone marrow, they also prevail in adipose, synovium, periosteum, muscle, dental pulp, periodontal ligament, placenta and umbilical cord blood (81).
Expression of myocardia-specific markers
Several studies have emphasized that MSCs can define a vascular endothelial, osteocyte, chondrocyte, or adipocyte lineage (82, 84). In addition, MSCs have been reported to acquire the phenotype of CMs both in vitro, after 5-azacytidine treatment, and in vivo, in a murine model of myocardial infarction (122). Moreover, in vitro studies done at our laboratory (unpublished) clearly demonstrate that MSCs can acquire the markers not only for CMs, but also for endothelial and smooth muscle lineages in a hypoxic milieu, on exposure to the released cytokines from the respective cell types. This expression of CM lineage–specific markers by MSCs has been corroborated with an increased expression of cardiac-specific transcription factors: Nkx2.5, GATA4, and contractile proteins cardiac myosin heavy chain (MHC), α-sarcomeric actinin, phospholamban, and cTnT during their in vitro coculture with CMs (5, 29, 136, 139). Furthermore, several studies have provided mounting evidence on the ability of MSCs to acquire a CM phenotype in both infarcted and healthy myocardium (29, 102, 122). MSCs also demonstrate an expression of α-subunits of the cardiac-specific L-type calcium channel (α1c); the transient outward potassium channel (Kv4.3), and cardiac atrial natriuretic peptide (142).
However, most of these studies have been done in coculture experiments of MSCs with CMs. Whether similar observations in the in vivo studies can be attributed to the transdifferentiating MSCs or are caused by the proliferating endogenous CMs and cardiac progenitors as a response to the released bioactive factors from MSCs remains controversial (45, 92).
Intercellular connection through gap junctions with resident CMs
On engraftment into the infarcted myocardium, growth factor (FGF-2, IGF-1, BMP-2)-treated MSCs have been reported to form intercellular connections with the host CMs via Cx40 and Cx43-mediated junctions. These heterotypic gap junctional channels formed by cardiac-specific Cx40 and Cx43 are not only associated with the movement of partially asymmetric junctional currents (87, 126), but also allow the exchange of small molecules and secondary messengers between adjacent cells and are believed to play a major role in the cytoprotection of resident CMs (45).
Release of cardioprotective cytokines
MSCs not only modulate the neighboring cells through physical contacts but also via paracrine actions. The paracrine effects are mediated via angiogenic, antiapoptotic, mitogenic, and homing factors. These trophic factors (cytokines and growth factors) include VEGF, HGF, IGF-1, and SDF-1 (85, 138, 141). Genetically modified MSCs overexpressing Akt have been shown to release cardioprotective factors (paracrine hypothesis), affording cytoprotective effects on adult rat ventricular CMs (ARVCs) exposed to hypoxia (43).
Activation of other progenitor cells via paracrine stimulation
Despite the existence of CPCs in the myocardium, and after the finding that they can contribute to improved cardiac performance in a rat myocardial infarction model (12), clinical implications of CPC-based therapeutics remains elusive owing to the hurdles pertaining to CPC isolation and expansion from patients. Recently, it was shown that the paracrine effects of MSCs can be used as an indirect approach to activate the resident endogenous CPCs. Considering the fact that HGF has been clearly shown to instigate the migration and survival of endogenous CPCs, the paracrine effectors released by MSCs like HGF, VEGF, and IGF-1 can trigger the resident CPCs, thereby affording their activation, proliferation, migration, and differentiation (77).
EPCs have long been known for their potential in initiating neovascularization (6). The ligand for chemokine receptor CXCR4, SDF-1α, is capable of effectuating EPC mobilization (44). Several studies have shown that this chemokine also mediates the recruitment of progenitor cells like HSCs (62) and CPCs (125) and provides trophic support to the resident CMs in the myocardium (141). As MSCs have been proposed to secrete SDF-1α (141), indirect mobilization of EPCs via MSCs might pave the way for future therapeutics.
Immunomodulation
It has been argued that these multipotent MSCs can mediate an immunosuppressive effect. Although MSCs do express MHC class I, they lack the B7 costimulatory molecule and escape recognition via both CD4+ T helper (TH) (naïve and memory) and CD8+cytotoxic T (TC) cell subsets. Moreover, MSCs can induce a bias toward TH2 rather than TH1 (two stable differentiation states of TH that produce different lymphokines and different effector functions via divergent signaling mechanisms) (56), owing to reduced IFN-γ secretion (31, 60). Surprisingly, the expression of surface markers associated with lymphocyte activation, like CD25, CD38, and CD69, have been shown to decrease in the presence of MSCs (60). Therefore, they can inhibit lymphocyte proliferation by B-cell mitogens. Furthermore, MSCs can self-modulate immune responses by arresting the stimulated T cells at the G0/G1 checkpoint of the cell cycle via inhibiting cyclin D2 (42). MSCs even inhibit the IL-2–mediated activation of natural killer (NK) cells (108).
MSC Niche, Mobilization, and Homing
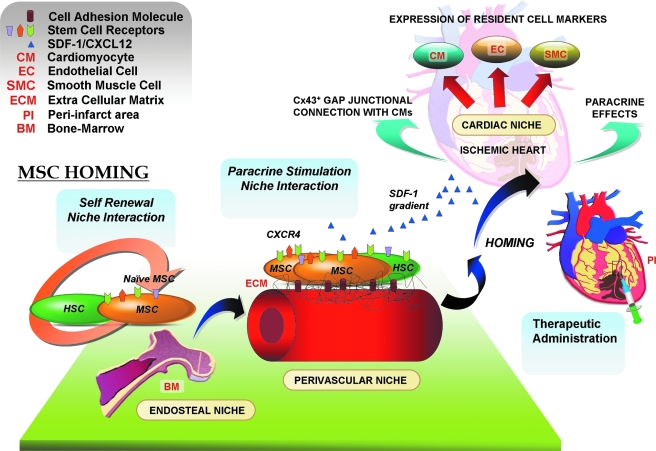
In recent years, research has illuminated the long-lingering question about the localization or niche for marrow stromal cells or MSCs (25). Although MSCs have been isolated from diverse postnatal organs like brain, liver, spleen, kidney, lung, bone marrow, muscle, thymus, and pancreas (26), most of the contemporary research has been concentrated on MSCs isolated primarily from the BM. These progenitor cells reside in predefined niches and home to the target organs upon activation (Fig. 1). Niches are three-dimensional entities composed of supporting cells, extracellular matrix, growth factors, and survival signals (physiochemical microenvironment), and mediate the quiescence, self-renewal, or differentiation of the stem cell population (34). Niches are structurally tailored to meet the requirements of the resident stem cells, and the resident cells in turn play an indispensable role in architectural organization of the niche (61). Furthermore, niche homeostasis is maintained by a complex interplay between cytokines, chemokines, proteases, and adhesion molecules (55).
FIG. 1.
Niche-to-niche migration of MSCs. Niche interaction and paracrine crosstalk play a crucial role in niche homeostasis. After myocardial ischemia, MSCs home from the perivascular niches along the SDF-1/CXCR4 signaling axis to the infarcted myocardium and participate in the repair process.
The endosteal niche
Bone marrow is composed of both endosteal and proximal or interdigitated vascular niches. The endosteal niche is located at the endosteum [inner surface of the bone that interfaces the BM (57) of the trabecular bone] and houses the dormant/naïve MSCs and HSCs. The endosteal surface is rich in calcium ions, and the engraftment of stem cells to the endosteal niche is dictated by a calcium-sensing receptor (CaR) expressed on the surface of these progenitor cells (3). BM-MSCs are believed to play a pivotal role in hematopoiesis through a series of complicated paracrine signaling networks (25). Recent attempts toward the transcriptional profiling of MSCs have unveiled many molecules common to the HSC niche (86). Bioactive factors released by BM-MSCs, like VEGF, SDF-1, Ang-1, Ang-2, M-CSF, Flt-3, IL-3, BMP-2, GM-CSF, and SCF have been implicated in modulating the HSC niche. Furthermore, BM-MSCs also provide the stromal component to the HSC niche (35). Two of the three types of HSCs, those that belong to the osteoblastic niche and those comprising the reticular stromal niche, directly descend from MSCs. A third type of HSCs belong to the vascular endothelium niche (127).
The perivascular or periendothelial niche
MSCs are known to be present throughout the body, housed in the perivascular niche as pericytes, and play a crucial role in stabilizing the blood vessels. MSCs express adhesion molecules P-selectin and VCAM-1, which are vital for their endothelial attachment. The surrounding supporting cells, ECM and signaling molecules act in coordination to activate the naïve MSCs, which can then proliferate, as a consequence of lost attachment with the basement membrane and endothelial cells (25). After the systemic release of chemoattractants (like SDF-1) from the infarcted myocardium (100), the activated MSCs residing in the periendothelial niche (acting as vascular pericytes) are released after digestion of the ECM (by matrix metalloproteinases) (32). After their activation, they egress to the ischemic region, followed by engraftment from local or surrounding sites (18) by using CXCR4 (24) or integrin β1 (50) receptors. It has been observed that a decreased vascular density corresponds to a diminished number of viable MSCs (17), emphasizing the role of perivascular niches in housing and nurturing MSCs as pericytes. In addition, MSCs also comprise an indispensible part of the HSC niche (25). The “paracrine crosstalk” between the MSCs and HSCs affords niche interaction and maintenance in the perivascular milieu (17).
The cardiac niche
The cardiac niche houses the cardiac progenitor cells (CPCs) and is responsible for maintaining homeostasis between the cycling and quiescent CPCs. Although the CPCs are classified into different subtypes based on their surface markers, Sca1+, Isl1+, and so on (8), specific localized niches for the respective CPC subtypes have not yet been clearly defined.
One of the major factors secreted by the myocardium early after MI is stromal cell–derived growth factor-1 (SDF-1), which is released into the circulation after MI, thus creating a systemic SDF-1 gradient (1). SDF-1–CXCR4 signaling plays a crucial role in mobilization of MSCs to the infarcted myocardium. SDF-1 binding to its cognate receptor CXCR4, expressed on MSCs, mediates many complex biologic functions, including proliferation, antiapoptosis, mobilization, and retention of the homed MSCs in the ischemic region to ensure their participation in the repair process (28, 46). During myocardial ischemia, MSCs from the perivascular niche migrate to the cardiac niche. After their niche migration, the released paracrine effectors (antiapoptotic, chemotactic and antifibrotic factors) from MSCs (e.g., VEGF, HGF, IGF-1, or MCP-1), can mediate myocardial angiogenesis, after which MSCs stabilize the degenerating microvessels and render trophic support via reestablishing new perivascular niches, thereby mediating niche homeostasis (25). Recently, it was proclaimed that these bioactive factors released by MSCs are effective and sufficient to activate and protect the endogenous CPCs in the myocardium from hypoxic stress (77). In vitro treatment of CPCs with MSC-conditioned medium caused their enhanced migration (probably via HGF-mediated activation of MMP-2/9) and differentiation into CMs (77). Furthermore, the chemoattractants secreted by MSCs, like SDF-1, can mediate an efficient migration of CPCs (125), as well as the homing of HSCs (37).
MSCs in Cardiovascular Repair
The efficacy of MSC cardiomyoplasty in alleviating the pathophysiology associated with myocardial infarction was realized quite early after the initial attempts to deliver rat MSCs in an infarcted heart with a reperfusion model (116). Pluripotent MSCs used to ameliorate idiopathic dilated cardiomyopathy (DCM) in a swine model demonstrated that the observed improvement in the left ventricular function was associated with the expression of CM and endothelial cell–specific markers by the engrafted MSCs, improved vascular perfusion, reduced fibrosis, and most important, with the release of a plentitude of angiogenic, antiapoptotic, and mitogenic factors (76).
MSCs can acquire cardiomyocyte, endothelial, and smooth muscle markers
As the heart's capacity for self-regeneration is extremely limited, the success of stem and progenitor cell therapies for myocardial repair relies on the three major events: after engraftment, the cells should proliferate and replace the dying cells, electromechanically couple with resident CMs, and finally differentiate into functional CMs, endothelial and smooth muscle cells (92).
DNA demethylating agent 5-azacytidine has long been said to induce cardiac differentiation in MSCs. Initial research revealed that, on treatment with 5-azacytidine, MSCs show an increased current flux across K+ and Ca2+ channels, mimicking CM-like characteristics (7). Much of the subsequent work on MSCs from human bone marrow, fatty tissues, or umbilical cord clearly demonstrated an expression of the markers for cardiac myocytes (myosin heavy chain, α-actinin, vimentin, and troponin-I) (4, 54, 94, 137).
Rat MSCs grown with adult CMs and aortic smooth muscle cells, in direct coculture experiments, acquired markers for CM (α-actin, desmin, cTnT) and smooth muscle (calponin and αSMA) lineage (132), respectively. In addition, the endothelial differentiation of MSCs has been well characterized in the presence of VEGF, IGF-1, and EGF in different experiments. The differentiated cells strongly stain positive for von Willebrand factor (vWF) and VEGF receptor-2 (Flk-1) (53, 63, 84).
Interestingly, the ex vivo treatment of MSCs with a cocktail of insulin, dexamethasone, and ascorbic acid from patients undergoing CABG has been shown to induce the expression of cardiac troponin I, sarcomeric tropomyosin, and cardiac titin, thereby forming CM-like cells (CLCs) (103). Quite interestingly, recent findings have revealed that the proteins expressed in the infarcted myocardium can induce the expression of cardiac-specific markers on the engrafted MSCs in vivo in a TGF-β1– and BMP-2–dependent manner (19).
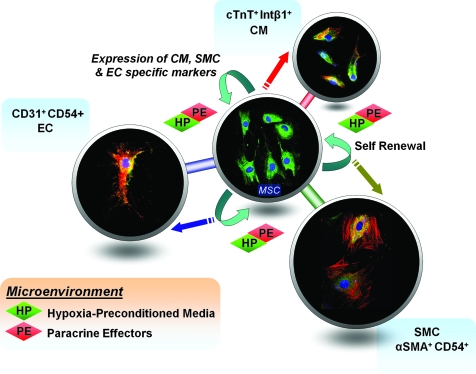
In vitro experiments done in our laboratory revealed that rat MSCs grown in a hypoxia-preconditioned medium from rat cardiomyoblast (H9c2), endothelial (YPEN-1), and smooth muscle cells (A10), demonstrate an enhanced expression of cardiac troponin T (cTnT), platelet–endothelial cell adhesion molecule-1 (PECAM-1), and α-smooth muscle actin (αSMA) markers, respectively, thereby emphasizing their multipotency and a gradual acquisition of the respective cell phenotypes (unpublished data) (Fig. 2).
FIG. 2.
Rat MSCs grown in hypoxia-preconditioned media from rat cardiomyoblast (H9c2), endothelial (YPEN-1), and smooth muscle cells (A10) demonstrate enhanced expression of the acquired cardiac troponinT (cTnT), platelet–endothelial cell adhesion molecule-1 (PECAM-1), and α-smooth muscle actin (αSMA) markers, respectively.
Strategic Approaches in MSC-Based Cardiomyoplasty
Genetically modified MSCs
The number of injected stem cells does not necessarily reflect surviving ones in the infarcted myocardium that actually contribute to the cellular cardiomyoplasty. Oxidative stress is a major challenge for the survival of transplanted cells in the ischemic milieu (69). Akt is a serine/threonine kinase downstream of the phosphatidylinositol 3-kinase (PI3K) pathway and has been implicated in conferring protection against oxidative stress–induced apoptosis (133). Therefore, initial attempts to enhance the expression of Akt in the transplanted MSCs, a protein implicated in survival of CMs against ischemia/reperfusion injury, was a major success. On engraftment, these rat MSCs overexpressing Akt-1 revived almost 80–90% of the myocardial volume and normalized cardiac functions (69).
Paracrine effectors are the major players through which the engrafted MSCs exert their beneficial effects (43, 46, 77, 82, 92, 117, 138). Therefore, enhancing the expression of these trophic factors and cytokines or their receptors through genetic modification can afford their efficient survival, homing, and mobilization in the infarcted myocardium. MSCs overexpressing CXCR4, to render an enhanced migration and regeneration effect on binding to its ligand CXCL12 (SDF-1), showed increased neomyoangiogenesis and a decrease in collagen I/III ratio in the infarcted wall, thereby effectuating reduced remodeling (24, 140). Similarly, SDF-1–overexpressing MSCs have yielded beneficial results by preserving the resident CMs and rendering them trophic support (141). Recently, it was demonstrated that MSCs overexpressing insulin-like growth factor (IGF)-1 can improve their survival and engraftment through the release of SDF-1α (46). In addition, the administration of engineered MSCs overexpressing antiapoptotic protein Bcl-2 showed a significant reduction in CM apoptosis (by almost 32%), and it was found to enhance VEGF expression in the myocardium (64). Another very crucial paracrine effector, G-CSF, produced by the infarcted heart, serves as a primitive injury-repair–response mechanism in the body. G-CSF–related therapies for the injured heart certainly are a promising approach (59). For instance, G-CSF has been shown to mediate an increase in the LVEF of patients with AMI (2). Moreover, direct protective effects of G-CSF on CMs and stem cell mobilization from the bone marrow have been reported recently (59).
Combination therapies involving MSCs
The ischemic microenvironment in the infarcted myocardium and the paucity of survival factors pose a limitation on the effectiveness of cell-based therapeutics. A combination of stem and progenitor cells or a combination of angiogenic cytokines with progenitor cells has proved more efficient in ameliorating the myocardium than the administration of MSCs alone. Attempts to coinject MSCs with angiogenic protein VEGF in MI hearts revealed that the microenvironment modulation by VEGF involving reduced cellular stress, and increased prosurvival factors (phospho-Akt and Bcl-xL), resulted in a better engraftment of the transplanted MSCs, and significantly improved cardiac functions (90).
Preconditioned MSCs
Preconditioning MSCs have emerged as an extremely powerful strategy for enhancing the therapeutic efficacy of MSCs. Diverse preconditioning approaches have been shown to render significant cardioprotective effects.
Growth factor–based preconditioning approaches involving the administration of MSCs pretreated with a combination of IGF-1, FGF-2, and BMP-2 in a rat MI model offered a significant upregulation of the Cx43-mediated gap junctions in addition to affording a cytoprotective role on the resident CMs (45). In vivo delivery of the CXCR4 ligand SDF-1 preconditioned MSCs in the myocardium after LAD ligation reduced infarct size and fibrosis after a 4-week period (85).
Heat-shock proteins (HSPs) are molecular chaperones triggered as a response to stress and are involved in cellular homeostasis by aiding proper folding of proteins under diverse pathophysiologic conditions. Initial attempts to realize the effect of heat shock on MSCs revealed elevated levels of HSP 27, HSP70, and HSP90, downregulated proapoptotic protein HSP60 (110), and reduced levels of cellular apoptosis.
Recently, the efficacy of hypoxic preconditioning (HPC) in energizing the naïve MSCs was bolstered through several studies. HPC of BM-MSCs before transplantation into the infarcted heart led to an upregulation of the prosurvival and proangiogenic factors like Hif-1, Ang-1, VEGF, Flk-1, erythropoietin, Bcl-2, and Bcl-xL, thus affording increased angiogenesis and a consequent cardioprotection (49). It has been postulated that hypoxic stress induces Akt and its downstream effectors along with cMet; the major receptor for hepatocyte growth factor (HGF), thereby revealing its mechanistic role (98). Several in vitro studies have demonstrated that MSCs can decrease the apoptotic index, increase survivability, and stabilize the mitochondrial membrane potential via the upregulation of Bcl-2 and VEGF and an increased ERK and Akt phosphorylation (129). Quite surprisingly, administration of anoxic-preconditioned (AP) MSCs also showed a potent improvement in cardiac functions (130). During diabetic cardiomyopathy, AP-MSCs have been shown to afford an antiapoptotic effect, possibly mediated through the upregulation of the Bcl-2/Bax ratio and by inhibition of caspase-3 activation in the myocardium (130).
MSCs in tissue engineering
Lack of organ donors and immunorejection impose limitations on the clinical use of cell-based therapeutics. The advent of engineered tissues has proved an alternative strategy to procure the organs or tissues from a patient's own cells. These cells are then seeded onto biodegradable scaffolds and expanded, followed by implantation in areas of need, thus eliminating the potential drawback of allograft rejection.
MSCs are powerful candidates for tissue engineering because of their ease in isolation, in vitro expansion, and multipotency. As a myocardial infarct leaves a thin scarred area housing the apoptotic CMs, construction of an adequate cardiac mass to replace the scar poses a potential hurdle for the engraftment of engineered tissue/cell-monolayer scaffolds. Initial attempts with a monolayer of adipose-derived MSCs to repair scarred rat myocardium demonstrated a significant reversal in ventricular wall thinning (73). Another attempt, employing porous acellular bovine pericardium sandwiched with multilayered sheets of MSCs, illustrated improved cardiac functions in a syngenic rat MI model (21).
Ex vivo tissue engineering using 3D bioscaffolds seeded with multilayered MSCs have yielded viable cardiac tissues (134). Moreover, cardiac MSC patches were shown to confer superior cardiac function and to generate neomuscle fibers and neomicrovessels in the entire patch, together with an upregulation in cytokines and cardioprotective factors, upon transplantation in syngenic rats with ligated LADs (21). Furthermore, cardiac patches comprising hMSCs embedded in rat-tail type I collagen matrix (collagen hydrogel), and delivered epicardially in LAD-ligated Fischer rats, revealed improved myocardial remodeling. This significant study showed that the improved cardiac functions did not require long-term cell engraftment, emphasizing the paracrine modulatory effects of MSCs (104).
In conclusion, as conventional cell-delivery strategies are blemished by poor cell engraftment, leakage out of the injection sites, and inhomogeneous cell distributions (104), tissue engineering provides an enticing solution for clinical therapeutics.
Preclinic to Clinic: Past, Present, and the Future
Despite the burgeoning progress in preclinical research and after the realization of the great therapeutic potential of MSC-based cardiomyoplasty, its translation to clinical cardiology has not passed beyond phase I trials, for the major part, owing to contrasting results and the lack of stable reproducible therapeutic benefit to the patients.
Initial attempts, involving percutaneous administration of allogenic MSCs in acute myocardial infarction (AMI) patients, followed by analysis with positron emission tomography (PET) and cardiac electromechanical mapping (EMM), showed a significant improvement in LV function after 3 months (23). Furthermore, single-photon-emission computed tomography and echocardiographic analysis, after the transplantation of autologous bone marrow MSCs in patients with old myocardial infarction demonstrated a radical improvement in cardiac functions (74). Interestingly, clinical studies on chronic ischemic cardiomyopathy patients with an occluded left anterior descending artery revealed restored LVEF and improved exercise tolerance with MSC-based cardiomyoplasty (22). In addition, attempts to use MSCs in the treatment of idiopathic dilated cardiomyopathy demonstrated reduced plasma BNP levels, although no significant improvement in LVEF was noticed (131). This opposing observation might be attributed to a plethora of varying factors (e.g., differences in the routes of administration, varying disease models and microenvironments, diverse pathophysiologic backgrounds of the patients, delivery vehicles, and the number of injected cells). Therefore, rigorous endeavors in standardizing the dose, time, delivery strategy, and manipulation of the microenvironment via paracrine effectors (e.g., via engineered cardiac patches) are required in the future.
Our present limitations with respect to the choice of best candidates for cellular cardiomyoplasty, route of delivery, appropriate dose, and better preconditioning or genetic-modification strategies for progenitor and stem cells, influence the long-term safety and efficacy of myocardial regenerative therapies, which are still in an incipient stage. Several clinical attempts are under way to guide us through the realization of the true potential of MSC-based therapeutic cardiomyoplasty (Table 1).
Table 1.
Ongoing MSCs Cardiomyoplasty Clinical Trials*
| Project | Location | Aim | Condition | Intervention |
|---|---|---|---|---|
| MSC therapy in patients undergoing cardiac surgery (PROMETHEUS) | NHLBI, USA | Low and high dose intramyocardial autologous MSC injection in MI patients with chronic ischemic LVD undergoing CABG (Phase I/II) | chronic ischemic LVD | MSCs |
| Transendocardial autologous cells (hMSCs/hBMCs) in ischemic heart failure (TAC-HFT) | University of Miami, USA | Transendocardial delivery of autologous hMSCs and hBMCs for cardiac repair (Phase I/II) | Chronic ischemic LVD; HF | MSCs BMCs |
| Combination stem cell therapy for the treatment of severe CI | TCA Cellular, Therapy, USA | Intracoronary and transendocardial administration of stem cells for CI patients unsuitable for percutaneous revascularization and surgical procedures (Phase II) | Severe CI | BMNCs MSCs |
| MSCs to treat AMI | Orisis Therapeutics, Inc., USA | Intravenous injection of MSCs (Provacel) following AMI | AMI | MSCs |
| Vasculogenesis in patients with severe myocardial ischemia | Rigshospitalet, Denmark | BM MSC stimulated for endothelial differentiation for neovessel formation in ischemic tissue (Phase I/II) | Myocardial ischemia, CHD | BM MSCs |
| Autologous MSC therapy in HF | Rigshospitalet, Denmark | NOGA-guided direct intramyocardial injection of MSCs for cardiomyoplasty and neovessel formation in HF patients (Phase I/II) | HF | MSCs |
| Combined CABG and stem-cell transplantation for HF | Helsinki University, Finland | Intraoperative transmyocardial BM MSCs transplantation for low LVEF patients scheduled to CABG (Phase II) | HF | BM MSCs |
Source: http://clinicaltrials.gov
AMI, acute myocardial infarction; CABG, coronary artery bypass graft surgery; CHD, chronic heart disease; CI, coronary ischemia; HF, heart failure; LVDf, left ventricular dysfunction; LVEF, left ventricular ejection fraction.
MSC-Based Cardiomyoplasty: Unresolved Puzzles and Future Insights
Cell-based therapeutic approaches with diverse types of stem and progenitor cells hold a huge potential, not only for the treatment of cardiovascular diseases, but also for a vast array of other degenerative and age-related diseases. Despite this immense efficacy, the success of stem cell therapeutics is being challenged by many obstacles, which must be addressed for diversifying their wider clinical use.
A patient's pathophysiologic profile is crucial in determining the best route for administering progenitor cell therapy (105). Intracoronary infusion of stem and progenitor cells allows them to priority-pass to well-perfused regions in the myocardium, thus ensuring a favorable microenvironment for survival. Unfortunately, preclinical studies have shown that these intracoronary-administered cells often get trapped and aggregate in small capillaries, owing to their adherent nature, or move into systemic circulation, thus diluting the therapeutic effects (92). Other routes of delivery, like intravenous injection, provide an extremely efficient noninvasive route for stem or progenitor cell delivery. However, systemic injection also poses potential flaws owing to the possible homing of the infused cells to other organs or extravasations. Finally, despite the highest cell retention and survivability during intramyocardial delivery, this route has the risk of ventricular perfusion, thus limiting its clinical use. Therefore, an optimal route for delivery must be reconsidered. To overcome these drawbacks, modern delivery strategies, like epicardial administration of MSCs as a cardiac patch, pose a promising approach (134).
Although the therapeutic administration of a large number of stem and progenitor cells (∼1–5 million cells per injection) have often been used in clinical studies, it is still controversial whether a single administration or an injection at multiple sites would bring forth an efficient and stable functional improvement of cardiac functions in the infarcted myocardium (15). There are quite a few hazards with stem cell therapies. Teratoma formation by ESCs and iPSCs, collagen deposition after SkM transplantation, inflammatory reaction and leukocyte infiltration after HSC delivery, and so on, certainly raise a question on the effectiveness of these approaches. Therefore, the choice of a suitable stem cell type is still under an earnest consideration. With the promising properties of immunomodulation, establishment of intercellular connections with the resident CMs, and paracrine effects, MSCs have paved their way in the treatment of myocardial pathophysiologic complications.
The ischemic milieu and the severe paucity of survival factors limit the survival of progenitor and stem cells in the infarcted myocardium. Therefore, better cellular cardiomyoplasty approaches that can mediate a copious release of cardioprotective paracrine effectors to modulate the acerbic microenvironment and to enhance the viability and transdifferentiation of the engrafted progenitor cells still must be addressed. The bioactive factors released by MSCs, which can be enhanced further by genetically modifying these progenitor cells, have been suggested as an alternative strategy affording long-term viability and engraftment of the administered cells.
In conclusion, the success of MSC cardiomyoplasty and the advancements in tissue engineering have opened a new dimension in cellular cardiomyoplasty, conferring novel therapeutic options for the generations yet to come.
Acknowledgments
This publication was supported by grants from the NIH (to N.M.).
Abbreviations
AMI, acute myocardial infarction; Ang, angiopoietin; AP, anoxic preconditioning; BMNC, bone marrow mononuclear cell; BMP, bone morphogenetic protein; BNP, brain natriuretic peptide; CABG, coronary artery bypass graft surgery; CLCs, CM-like cells; CM, cardiomyocyte; CPC, cardiac progenitor cell; cTnT, cTnI, cardiac troponin T, cardiac troponin I; Cx, connexin; CXCR4, chemokine receptor 4; EGF, epidermal growth factor; EPC, endothelial progenitor cell; ESC, embryonic stem cell; FGF, fibroblast growth factor; GM-CSF, granulocyte–macrophage colony-stimulating factor; HGF, hepatocyte growth factor; HPC, hypoxic preconditioning; HSC, hematopoietic stem cell; HSP, heat-shock protein; IFN, interferon; IGF-1, insulin growth factor-1; IHD, ischemic heart disease; IL, interleukin; iPSC, induced pluripotent stem cell; LAD, left anterior descending artery; LVEF, left ventricular ejection fraction; MCP, monocyte chemoattractant protein; MMP, matrix metalloproteinase; MSC, mesenchymal stem cell; NK cell, natural killer cell; PECAM-1, platelet endothelial cell adhesion molecule; SCF, stem cell factor; SDF-1/CXCL-12, stromal cell–derived factor-1/chemokine receptor ligand-12; SkM, skeletal myoblast; VEGF, vascular endothelial growth factor; vWF, von Willebrand factor; αSMA, smooth muscle actin-α chain.
References
- 1.Abbott JD. Huang Y. Liu D. Hickey R. Krause DS. Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 2.Abdel-Latif A. Bolli R. Zuba-Surma EK. Tleyjeh IM. Hornung CA. Dawn B. Granulocyte colony-stimulating factor therapy for cardiac repair after acute myocardial infarction: a systematic review and meta-analysis of randomized controlled trials. Am Heart J. 2008;156:216–226, e9. doi: 10.1016/j.ahj.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams GB. Chabner KT. Alley IR. Olson DP. Szczepiorkowski ZM. Poznansky MC. Kos CH. Pollak MR. Brown EM. Scadden DT. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439:599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- 4.Antonitsis P. Ioannidou-Papagiannaki E. Kaidoglou A. Papakonstantinou C. In vitro cardiomyogenic differentiation of adult human bone marrow mesenchymal stem cells: the role of 5-azacytidine. Interact Cardiovasc Thorac Surg. 2007;6:593–597. doi: 10.1510/icvts.2007.157875. [DOI] [PubMed] [Google Scholar]
- 5.Arminan A. Gandia C. Bartual C. Garcia-Verdugo JM. Lledo E. Mirabet V. Llop M. Barea J. Montero JA. Sepulveda P. Cardiac differentiation is driven by NKX2.5 and GATA4 nuclear translocation in tissue specific mesenchymal stem cells. Stem Cells Dev. 2008. Nov 4 [Epub ahead of print] [DOI] [PubMed]
- 6.Asahara T. Murohara T. Sullivan A. Silver M. van der Zee R. Li T. Witzenbichler B. Schatteman G. Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 7.Balana B. Nicoletti C. Zahanich I. Graf EM. Christ T. Boxberger S. Ravens U. 5-Azacytidine induces changes in electrophysiological properties of human mesenchymal stem cells. Cell Res. 2006;16:949–960. doi: 10.1038/sj.cr.7310116. [DOI] [PubMed] [Google Scholar]
- 8.Barile L. Messina E. Giacomello A. Marban E. Endogenous cardiac stem cells. Prog Cardiovasc Dis. 2007;50:31–48. doi: 10.1016/j.pcad.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Bayes-Genis A. Roura S. Soler-Botija C. Farre J. Hove-Madsen L. Llach A. Cinca J. Identification of cardiomyogenic lineage markers in untreated human bone marrow-derived mesenchymal stem cells. Transplant Proc. 2005;37:4077–4079. doi: 10.1016/j.transproceed.2005.09.103. [DOI] [PubMed] [Google Scholar]
- 10.Bearzi C. Rota M. Hosoda T. Tillmanns J. Nascimbene A. De Angelis A. Yasuzawa-Amano S. Trofimova I. Siggins RW. Lecapitaine N. Cascapera S. Beltrami AP. D'Alessandro DA. Zias E. Quaini F. Urbanek K. Michler RE. Bolli R. Kajstura J. Leri A. Anversa P. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beeres SL. Atsma DE. van der Laarse A. Pijnappels DA. van Tuyn J. Fibbe WE. de Vries AA. Ypey DL. van der Wall EE. Schalij MJ. Human adult bone marrow mesenchymal stem cells repair experimental conduction block in rat cardiomyocyte cultures. J Am Coll Cardiol. 2005;46:1943–1952. doi: 10.1016/j.jacc.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 12.Beltrami AP. Barlucchi L. Torella D. Baker M. Limana F. Chimenti S. Kasahara H. Rota M. Musso E. Urbanek K. Leri A. Kajstura J. Nadal-Ginard B. Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 13.Benvenuti S. Saccardi R. Luciani P. Urbani S. Deledda C. Cellai I. Francini F. Squecco R. Rosati F. Danza G. Gelmini S. Greeve I. Rossi M. Maggi R. Serio M. Peri A. Neuronal differentiation of human mesenchymal stem cells: changes in the expression of the Alzheimer's disease-related gene seladin-1. Exp Cell Res. 2006;312:2592–2604. doi: 10.1016/j.yexcr.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 14.Bonaros N. Rauf R. Schachner T. Laufer G. Kocher A. Enhanced cell therapy for ischemic heart disease. Transplantation. 2008;86:1151–1160. doi: 10.1097/TP.0b013e3181880f9e. [DOI] [PubMed] [Google Scholar]
- 15.Bongso A. Fong CY. Gauthaman K. Taking stem cells to the clinic: major challenges. J Cell Biochem. 2008;105:1352–1360. doi: 10.1002/jcb.21957. [DOI] [PubMed] [Google Scholar]
- 16.Cao F. Wagner RA. Wilson KD. Xie X. Fu JD. Drukker M. Lee A. Li RA. Gambhir SS. Weissman IL. Robbins RC. Wu JC. Transcriptional and functional profiling of human embryonic stem cell-derived cardiomyocytes. PLoS ONE. 2008;3:e3474. doi: 10.1371/journal.pone.0003474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caplan A. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217:318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caplan AI. All MSCs are pericytes? Cell Stem Cell. 2008;3:229–230. doi: 10.1016/j.stem.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Chang SA. Lee EJ. Kang HJ. Zhang SY. Kim JH. Li L. Youn SW. Lee CS. Kim KH. Won JY. Sohn JW. Park KW. Cho HJ. Yang SE. Oh WI. Yang YS. Ho WK. Park YB. Kim HS. Impact of myocardial infarct proteins and oscillating pressure on the differentiation of mesenchymal stem cells: effect of acute myocardial infarction on stem cell differentiation. Stem Cells. 2008;26:1901–1912. doi: 10.1634/stemcells.2007-0708. [DOI] [PubMed] [Google Scholar]
- 20.Charwat S. Gyongyosi M. Lang I. Graf S. Beran G. Hemetsberger R. Nyolczas N. Sochor H. Glogar D. Role of adult bone marrow stem cells in the repair of ischemic myocardium: current state of the art. Exp Hematol. 2008;36:672–680. doi: 10.1016/j.exphem.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Chen CH. Wei HJ. Lin WW. Chiu I. Hwang SM. Wang CC. Lee WY. Chang Y. Sung HW. Porous tissue grafts sandwiched with multilayered mesenchymal stromal cell sheets induce tissue regeneration for cardiac repair. Cardiovasc Res. 2008;80:88–95. doi: 10.1093/cvr/cvn149. [DOI] [PubMed] [Google Scholar]
- 22.Chen S. Liu Z. Tian N. Zhang J. Yei F. Duan B. Zhu Z. Lin S. Kwan TW. Intracoronary transplantation of autologous bone marrow mesenchymal stem cells for ischemic cardiomyopathy due to isolated chronic occluded left anterior descending artery. J Invas Cardiol. 2006;18:552–556. [PubMed] [Google Scholar]
- 23.Chen SL. Fang WW. Ye F. Liu YH. Qian J. Shan SJ. Zhang JJ. Chunhua RZ. Liao LM. Lin S. Sun JP. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92–95. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 24.Cheng Z. Ou L. Zhou X. Li F. Jia X. Zhang Y. Liu X. Li Y. Ward CA. Melo LG. Kong D. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther. 2008;16:571–579. doi: 10.1038/sj.mt.6300374. [DOI] [PubMed] [Google Scholar]
- 25.da Silva Meirelles L. Caplan AI. Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26:2287–2299. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- 26.da Silva Meirelles L. Chagastelles PC. Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 27.Dai W. Kloner RA. Myocardial regeneration by embryonic stem cell transplantation: present and future trends. Expert Rev Cardiovasc Ther. 2006;4:375–383. doi: 10.1586/14779072.4.3.375. [DOI] [PubMed] [Google Scholar]
- 28.Dawn B. Bolli R. Bone marrow for cardiac repair: the importance of characterizing the phenotype and function of injected cells. Eur Heart J. 2007;28:651–652. doi: 10.1093/eurheartj/ehm009. [DOI] [PubMed] [Google Scholar]
- 29.Dawn B. Bolli R. Adult bone marrow-derived cells: regenerative potential, plasticity, and tissue commitment. Basic Res Cardiol. 2005;100:494–503. doi: 10.1007/s00395-005-0552-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dawn B. Stein AB. Urbanek K. Rota M. Whang B. Rastaldo R. Torella D. Tang XL. Rezazadeh A. Kajstura J. Leri A. Hunt G. Varma J. Prabhu SD. Anversa P. Bolli R. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc Natl Acad Sci U S A. 2005;102:3766–3771. doi: 10.1073/pnas.0405957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dazzi F. Marelli-Berg FM. Mesenchymal stem cells for graft-versus-host disease: close encounters with T cells. Eur J Immunol. 2008;38:1479–1482. doi: 10.1002/eji.200838433. [DOI] [PubMed] [Google Scholar]
- 32.De Becker A. Van Hummelen P. Bakkus M. Vande Broek I. De Wever J. De Waele M. Van Riet I. Migration of culture-expanded human mesenchymal stem cells through bone marrow endothelium is regulated by matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-3. Haematologica. 2007;92:440–449. doi: 10.3324/haematol.10475. [DOI] [PubMed] [Google Scholar]
- 33.de Muinck ED. Thompson C. Simons M. Progress and prospects: cell based regenerative therapy for cardiovascular disease. Gene Ther. 2006;13:659–671. doi: 10.1038/sj.gt.3302680. [DOI] [PubMed] [Google Scholar]
- 34.Dellatore SM. Garcia AS. Miller WM. Mimicking stem cell niches to increase stem cell expansion. Curr Opin Biotechnol. 2008;19:534–540. doi: 10.1016/j.copbio.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delorme B. Chateauvieux S. Charbord P. The concept of mesenchymal stem cells. Regen Med. 2006;1:497–509. doi: 10.2217/17460751.1.4.497. [DOI] [PubMed] [Google Scholar]
- 36.Dimmeler S. Zeiher AM. Cell therapy of acute myocardial infarction: open questions. Cardiology. 2008;113:155–160. doi: 10.1159/000187652. [DOI] [PubMed] [Google Scholar]
- 37.Eaves C. Hematopoietic stem cells get closer to the bone. Nat Med. 2004;10:1042–1044. doi: 10.1038/nm1004-1042. [DOI] [PubMed] [Google Scholar]
- 38.Eguchi M. Masuda H. Asahara T. Endothelial progenitor cells for postnatal vasculogenesis. Clin Exp Nephrol. 2007;11:18–25. doi: 10.1007/s10157-006-0448-1. [DOI] [PubMed] [Google Scholar]
- 39.Evans MJ. Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 40.Friedenstein AJ. Chailakhyan RK. Latsinik NV. Panasyuk AF. Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues: cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Garry DJ. Olson EN. A common progenitor at the heart of development. Cell. 2006;127:1101–1104. doi: 10.1016/j.cell.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 42.Glennie S. Soeiro I. Dyson PJ. Lam EW. Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–2827. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 43.Gnecchi M. He H. Liang OD. Melo LG. Morello F. Mu H. Noiseux N. Zhang L. Pratt RE. Ingwall JS. Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 44.Gurtner GC. Chang E. “Priming” endothelial progenitor cells: a new strategy to improve cell based therapeutics. Arterioscler Thromb Vasc Biol. 2008;28:1034–1035. doi: 10.1161/ATVBAHA.108.163246. [DOI] [PubMed] [Google Scholar]
- 45.Hahn JY. Cho HJ. Kang HJ. Kim TS. Kim MH. Chung JH. Bae JW. Oh BH. Park YB. Kim HS. Pre-treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction. J Am Coll Cardiol. 2008;51:933–943. doi: 10.1016/j.jacc.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 46.Haider H. Jiang S. Idris NM. Ashraf M. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair. Circ Res. 2008;103:1300–1308. doi: 10.1161/CIRCRESAHA.108.186742. [DOI] [PubMed] [Google Scholar]
- 47.Haider H. Ye L. Jiang S. Ge R. Law PK. Chua T. Wong P. Sim EK. Angiomyogenesis for cardiac repair using human myoblasts as carriers of human vascular endothelial growth factor. J Mol Med. 2004;82:539–549. doi: 10.1007/s00109-004-0546-z. [DOI] [PubMed] [Google Scholar]
- 48.Horwitz EM. Le Blanc K. Dominici M. Mueller I. Slaper-Cortenbach I. Marini FC. Deans RJ. Krause DS. Keating A. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 49.Hu X. Yu SP. Fraser JL. Lu Z. Ogle ME. Wang JA. Wei L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135:799–808. doi: 10.1016/j.jtcvs.2007.07.071. [DOI] [PubMed] [Google Scholar]
- 50.Ip JE. Wu Y. Huang J. Zhang L. Pratt RE. Dzau VJ. Mesenchymal stem cells use integrin beta1 not CXC chemokine receptor 4 for myocardial migration and engraftment. Mol Biol Cell. 2007;18:2873–2882. doi: 10.1091/mbc.E07-02-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ishikawa F. Shimazu H. Shultz LD. Fukata M. Nakamura R. Lyons B. Shimoda K. Shimoda S. Kanemaru T. Nakamura K. Ito H. Kaji Y. Perry AC. Harada M. Purified human hematopoietic stem cells contribute to the generation of cardiomyocytes through cell fusion. FASEB J. 2006;20:950–952. doi: 10.1096/fj.05-4863fje. [DOI] [PubMed] [Google Scholar]
- 52.Iwami Y. Masuda H. Asahara T. Endothelial progenitor cells: past, state of the art, and future. J Cell Mol Med. 2004;8:488–497. doi: 10.1111/j.1582-4934.2004.tb00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jazayeri M. Allameh A. Soleimani M. Jazayeri SH. Piryaei A. Kazemnejad S. Molecular and ultrastructural characterization of endothelial cells differentiated from human bone marrow mesenchymal stem cells. Cell Biol Int. 2008;32:1183–1192. doi: 10.1016/j.cellbi.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 54.Kadivar M. Khatami S. Mortazavi Y. Shokrgozar MA. Taghikhani M. Soleimani M. In vitro cardiomyogenic potential of human umbilical vein-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2006;340:639–647. doi: 10.1016/j.bbrc.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 55.Kaplan RN. Psaila B. Lyden D. Niche-to-niche migration of bone-marrow-derived cells. Trends Mol Med. 2007;13:72–81. doi: 10.1016/j.molmed.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 56.Kawakami K. Parker DC. Differences between T helper cell type I (Th1) and Th2 cell lines in signalling pathways for induction of contact-dependent T cell help. Eur J Immunol. 1992;22:85–93. doi: 10.1002/eji.1830220114. [DOI] [PubMed] [Google Scholar]
- 57.Kiel MJ. Morrison SJ. Maintaining hematopoietic stem cells in the vascular niche. Immunity. 2006;25:862–864. doi: 10.1016/j.immuni.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 58.Kumar D. Kamp TJ. LeWinter MM. Embryonic stem cells: differentiation into cardiomyocytes and potential for heart repair and regeneration. Coron Artery Dis. 2005;16:111–116. doi: 10.1097/00019501-200503000-00006. [DOI] [PubMed] [Google Scholar]
- 59.Kurdi M. Booz GW. G-CSF-based stem cell therapy for the heart-unresolved issues part A: paracrine actions, mobilization, and delivery. Congest Heart Fail. 2007;13:221–227. doi: 10.1111/j.1527-5299.2007.07111.x. [DOI] [PubMed] [Google Scholar]
- 60.Le Blanc K. Ringden O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262:509–525. doi: 10.1111/j.1365-2796.2007.01844.x. [DOI] [PubMed] [Google Scholar]
- 61.Leri A. Kajstura J. Anversa P. Frishman WH. Myocardial regeneration and stem cell repair. Curr Probl Cardiol. 2008;33:91–153. doi: 10.1016/j.cpcardiol.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 62.Levesque JP. Winkler IG. Mobilization of hematopoietic stem cells: state of the art. Curr Opin Organ Transplant. 2008;13:53–58. doi: 10.1097/MOT.0b013e3282f42473. [DOI] [PubMed] [Google Scholar]
- 63.Li Q. Xu X. Wang Z. Liu W. Li Z. [Investigation of canine mesenchymal stem cells differentiation to vascular endothelial cell in vitro] Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2007;24:1348–1351. [PubMed] [Google Scholar]
- 64.Li W. Ma N. Ong LL. Nesselmann C. Klopsch C. Ladilov Y. Furlani D. Piechaczek C. Moebius JM. Lutzow K. Lendlein A. Stamm C. Li RK. Steinhoff G. Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cell. 2007;25:2118–2127. doi: 10.1634/stemcells.2006-0771. [DOI] [PubMed] [Google Scholar]
- 65.Li Z. Wu JC. Sheikh AY. Kraft D. Cao F. Xie X. Patel M. Gambhir SS. Robbins RC. Cooke JP. Differentiation, survival, and function of embryonic stem cell derived endothelial cells for ischemic heart disease. Circulation. 2007;116:I46–154. doi: 10.1161/CIRCULATIONAHA.106.680561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Losordo DW. Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease, part II: cell-based therapies. Circulation. 2004;109:2692–2697. doi: 10.1161/01.CIR.0000128596.49339.05. [DOI] [PubMed] [Google Scholar]
- 67.Lowry WE. Richter L. Yachechko R. Pyle AD. Tchieu J. Sridharan R. Clark AT. Plath K. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc Natl Acad Sci U S A. 2008;105:2883–2888. doi: 10.1073/pnas.0711983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Majhail NS. Parks K. Defor TE. Weisdorf DJ. Diffuse alveolar hemorrhage and infection-associated alveolar hemorrhage following hematopoietic stem cell transplantation: related and high-risk clinical syndromes. Biol Blood Marrow Transplant. 2006;12:1038–1046. doi: 10.1016/j.bbmt.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 69.Mangi AA. Noiseux N. Kong D. He H. Rezvani M. Ingwall JS. Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 70.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mauritz C. Schwanke K. Reppel M. Neef S. Katsirntaki K. Maier LS. Nguemo F. Menke S. Haustein M. Hescheler J. Hasenfuss G. Martin U. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation. 2008;118:507–517. doi: 10.1161/CIRCULATIONAHA.108.778795. [DOI] [PubMed] [Google Scholar]
- 72.Menasche P. Skeletal myoblasts as a therapeutic agent. Prog Cardiovasc Dis. 2007;50:7–17. doi: 10.1016/j.pcad.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 73.Miyahara Y. Nagaya N. Kataoka M. Yanagawa B. Tanaka K. Hao H. Ishino K. Ishida H. Shimizu T. Kangawa K. Sano S. Okano T. Kitamura S. Mori H. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 74.Mohyeddin-Bonab M. Mohamad-Hassani MR. Alimoghaddam K. Sanatkar M. Gasemi M. Mirkhani H. Radmehr H. Salehi M. Eslami M. Farhig-Parsa A. Emami-Razavi H. Alemohammad MG. Solimani AA. Ghavamzadeh A. Nikbin B. Autologous in vitro expanded mesenchymal stem cell therapy for human old myocardial infarction. Arch Iran Med. 2007;10:467–473. [PubMed] [Google Scholar]
- 75.Murry CE. Wiseman RW. Schwartz SM. Hauschka SD. Skeletal myoblast transplantation for repair of myocardial necrosis. J Clin Invest. 1996;98:2512–2523. doi: 10.1172/JCI119070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nagaya N. Kangawa K. Itoh T. Iwase T. Murakami S. Miyahara Y. Fujii T. Uematsu M. Ohgushi H. Yamagishi M. Tokudome T. Mori H. Miyatake K. Kitamura S. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;112:1128–1135. doi: 10.1161/CIRCULATIONAHA.104.500447. [DOI] [PubMed] [Google Scholar]
- 77.Nakanishi C. Yamagishi M. Yamahara K. Hagino I. Mori H. Sawa Y. Yagihara T. Kitamura S. Nagaya N. Activation of cardiac progenitor cells through paracrine effects of mesenchymal stem cells. Biochem Biophys Res Commun. 2008;374:11–16. doi: 10.1016/j.bbrc.2008.06.074. [DOI] [PubMed] [Google Scholar]
- 78.Narazaki G. Uosaki H. Teranishi M. Okita K. Kim B. Matsuoka S. Yamanaka S. Yamashita JK. Directed and systematic differentiation of cardiovascular cells from mouse induced pluripotent stem cells. Circulation. 2008;118:498–506. doi: 10.1161/CIRCULATIONAHA.108.769562. [DOI] [PubMed] [Google Scholar]
- 79.Niagara MI. Haider H. Jiang S. Ashraf M. Pharmacologically preconditioned skeletal myoblasts are resistant to oxidative stress and promote angiomyogenesis via release of paracrine factors in the infarcted heart. Circ Res. 2007;100:545–555. doi: 10.1161/01.RES.0000258460.41160.ef. [DOI] [PubMed] [Google Scholar]
- 80.Norol F. Bonnet N. Peinnequin A. Chretien F. Legrand R. Isnard R. Herodin F. Baillou C. Delache B. Negre D. Klatzmann D. Vernant JP. Lemoine FM. GFP-transduced CD34+ and Lin- CD34– hematopoietic stem cells did not adopt a cardiac phenotype in a nonhuman primate model of myocardial infarct. Exp Hematol. 2007;35:653–661. doi: 10.1016/j.exphem.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 81.Novotny NM. Ray R. Markel TA. Crisostomo PR. Wang M. Wang Y. Meldrum DR. Stem cell therapy in myocardial repair and remodeling. J Am Coll Surg. 2008;207:423–434. doi: 10.1016/j.jamcollsurg.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 82.Ohnishi S. Nagaya N. Prepare cells to repair the heart: mesenchymal stem cells for the treatment of heart failure. Am J Nephrol. 2007;27:301–307. doi: 10.1159/000102000. [DOI] [PubMed] [Google Scholar]
- 83.Orlic D. Kajstura J. Chimenti S. Jakoniuk I. Anderson SM. Li B. Pickel J. McKay R. Nadal-Ginard B. Bodine DM. Leri A. Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 84.Oswald J. Boxberger S. Jorgensen B. Feldmann S. Ehninger G. Bornhauser M. Werner C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22:377–384. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- 85.Pasha Z. Wang Y. Sheikh R. Zhang D. Zhao T. Ashraf M. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc Res. 2008;77:134–142. doi: 10.1093/cvr/cvm025. [DOI] [PubMed] [Google Scholar]
- 86.Pedemonte E. Benvenuto F. Casazza S. Mancardi G. Oksenberg JR. Uccelli A. Baranzini SE. The molecular signature of therapeutic mesenchymal stem cells exposes the architecture of the hematopoietic stem cell niche synapse. BMC Genomics. 2007;8:65. doi: 10.1186/1471-2164-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pedrotty DM. Klinger RY. Badie N. Hinds S. Kardashian A. Bursac N. Structural coupling of cardiomyocytes and noncardiomyocytes: quantitative comparisons using a novel micropatterned cell pair assay. Am J Physiol Heart Circ Physiol. 2008;295:H390–H400. doi: 10.1152/ajpheart.91531.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pitchford SC. Furze RC. Jones CP. Wengner AM. Rankin SM. Differential mobilization of subsets of progenitor cells from the bone marrow. Cell Stem Cell. 2009;4:62–72. doi: 10.1016/j.stem.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 89.Pittenger MF. Mackay AM. Beck SC. Jaiswal RK. Douglas R. Mosca JD. Moorman MA. Simonetti DW. Craig S. Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 90.Pons J. Huang Y. Arakawa-Hoyt J. Washko D. Takagawa J. Ye J. Grossman W. Su H. VEGF improves survival of mesenchymal stem cells in infarcted hearts. Biochem Biophys Res Commun. 2008;376:419–422. doi: 10.1016/j.bbrc.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 91.Potapova I. Plotnikov A. Lu Z. Danilo P., Jr Valiunas V. Qu J. Doronin S. Zuckerman J. Shlapakova IN. Gao J. Pan Z. Herron AJ. Robinson RB. Brink PR. Rosen MR. Cohen IS. Human mesenchymal stem cells as a gene delivery system to create cardiac pacemakers. Circ Res. 2004;94:952–959. doi: 10.1161/01.RES.0000123827.60210.72. [DOI] [PubMed] [Google Scholar]
- 92.Psaltis PJ. Zannettino AC. Worthley SG. Gronthos S. Concise review: mesenchymal stromal cells: potential for cardiovascular repair. Stem Cells. 2008;26:2201–2210. doi: 10.1634/stemcells.2008-0428. [DOI] [PubMed] [Google Scholar]
- 93.Rafii S. Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 94.Rangappa S. Fen C. Lee EH. Bongso A. Sim EK. Transformation of adult mesenchymal stem cells isolated from the fatty tissue into cardiomyocytes. Ann Thorac Surg. 2003;75:775–779. doi: 10.1016/s0003-4975(02)04568-x. [DOI] [PubMed] [Google Scholar]
- 95.Raya A. Rodriguez-Piza I. Aran B. Consiglio A. Barri PN. Veiga A. Izpisua Belmonte JC. Generation of cardiomyocytes from new human embryonic stem cell lines derived from poor-quality blastocysts. Cold Spring Harb Symp Quant Biol. 2008;24:2669–2676. doi: 10.1101/sqb.2008.73.038. [DOI] [PubMed] [Google Scholar]
- 96.Reinecke H. MacDonald GH. Hauschka SD. Murry CE. Electromechanical coupling between skeletal and cardiac muscle: implications for infarct repair. J Cell Biol. 2000;149:731–740. doi: 10.1083/jcb.149.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reinecke H. Minami E. Zhu WZ. Laflamme MA. Cardiogenic differentiation and transdifferentiation of progenitor cells. Circ Res. 2008;103:1058–1071. doi: 10.1161/CIRCRESAHA.108.180588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rosova I. Dao M. Capoccia B. Link D. Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26:2173–2182. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rota M. Padin-Iruegas ME. Misao Y. De Angelis A. Maestroni S. Ferreira-Martins J. Fiumana E. Rastaldo R. Arcarese ML. Mitchell TS. Boni A. Bolli R. Urbanek K. Hosoda T. Anversa P. Leri A. Kajstura J. Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ Res. 2008;103:107–116. doi: 10.1161/CIRCRESAHA.108.178525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schenk S. Mal N. Finan A. Zhang M. Kiedrowski M. Popovic Z. McCarthy PM. Penn MS. Monocyte chemotactic protein-3 is a myocardial mesenchymal stem cell homing factor. Stem Cell. 2007;25:245–251. doi: 10.1634/stemcells.2006-0293. [DOI] [PubMed] [Google Scholar]
- 101.Segers VF. Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 102.Shake JG. Gruber PJ. Baumgartner WA. Senechal G. Meyers J. Redmond JM. Pittenger MF. Martin BJ. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thorac Surg. 2002;73:1919–1925. doi: 10.1016/s0003-4975(02)03517-8. discussion 1926. [DOI] [PubMed] [Google Scholar]
- 103.Shim WS. Jiang S. Wong P. Tan J. Chua YL. Tan YS. Sin YK. Lim CH. Chua T. Teh M. Liu TC. Sim E. Ex vivo differentiation of human adult bone marrow stem cells into cardiomyocyte-like cells. Biochem Biophys Res Commun. 2004;324:481–488. doi: 10.1016/j.bbrc.2004.09.087. [DOI] [PubMed] [Google Scholar]
- 104.Simpson D. Liu H. Fan TH. Nerem R. Dudley SC., Jr A tissue engineering approach to progenitor cell delivery results in significant cell engraftment and improved myocardial remodeling. Stem Cells. 2007;25:2350–2357. doi: 10.1634/stemcells.2007-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Singh P. Williams DJ. Cell therapies: realizing the potential of this new dimension to medical therapeutics. J Tissue Eng Regen Med. 2008;2:307–319. doi: 10.1002/term.108. [DOI] [PubMed] [Google Scholar]
- 106.Smith RR. Barile L. Cho HC. Leppo MK. Hare JM. Messina E. Giacomello A. Abraham MR. Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 107.Soubani AO. Critical care considerations of hematopoietic stem cell transplantation. Crit Care Med. 2006;34:S251–S267. doi: 10.1097/01.CCM.0000231886.80470.B6. [DOI] [PubMed] [Google Scholar]
- 108.Spaggiari GM. Capobianco A. Abdelrazik H. Becchetti F. Mingari MC. Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 109.Stewart DJ. Hilton JD. Arnold JM. Gregoire J. Rivard A. Archer SL. Charbonneau F. Cohen E. Curtis M. Buller CE. Mendelsohn FO. Dib N. Page P. Ducas J. Plante S. Sullivan J. Macko J. Rasmussen C. Kessler PD. Rasmussen HS. Angiogenic gene therapy in patients with nonrevascularizable ischemic heart disease: a phase 2 randomized, controlled trial of AdVEGF(121) (AdVEGF121) versus maximum medical treatment. Gene Ther. 2006;13:1503–1511. doi: 10.1038/sj.gt.3302802. [DOI] [PubMed] [Google Scholar]
- 110.Stolzing A. Sethe S. Scutt AM. Stressed stem cells: temperature response in aged mesenchymal stem cells. Stem Cells Dev. 2006;15:478–487. doi: 10.1089/scd.2006.15.478. [DOI] [PubMed] [Google Scholar]
- 111.Suzuki K. Murtuza B. Smolenski RT. Sammut IA. Suzuki N. Kaneda Y. Yacoub MH. Cell transplantation for the treatment of acute myocardial infarction using vascular endothelial growth factor-expressing skeletal myoblasts. Circulation. 2001;104:I207–1212. doi: 10.1161/hc37t1.094524. [DOI] [PubMed] [Google Scholar]
- 112.Suzuki K. Murtuza B. Suzuki N. Smolenski RT. Yacoub MH. Intracoronary infusion of skeletal myoblasts improves cardiac function in doxorubicin-induced heart failure. Circulation. 2001;104:I213–I217. doi: 10.1161/hc37t1.094929. [DOI] [PubMed] [Google Scholar]
- 113.Swijnenburg RJ. Tanaka M. Vogel H. Baker J. Kofidis T. Gunawan F. Lebl DR. Caffarelli AD. de Bruin JL. Fedoseyeva EV. Robbins RC. Embryonic stem cell immunogenicity increases upon differentiation after transplantation into ischemic myocardium. Circulation. 2005;112:I166–I172. doi: 10.1161/CIRCULATIONAHA.104.525824. [DOI] [PubMed] [Google Scholar]
- 114.Takahashi K. Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 115.Takahashi T. Kalka C. Masuda H. Chen D. Silver M. Kearney M. Magner M. Isner JM. Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 116.Tang J. Xie Q. Pan G. Wang J. Wang M. Mesenchymal stem cells participate in angiogenesis and improve heart function in rat model of myocardial ischemia with reperfusion. Eur J Cardiothorac Surg. 2006;30:353–361. doi: 10.1016/j.ejcts.2006.02.070. [DOI] [PubMed] [Google Scholar]
- 117.Tang YL. Zhao Q. Qin X. Shen L. Cheng L. Ge J. Phillips MI. Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann Thorac Surg. 2005;80:229–236. doi: 10.1016/j.athoracsur.2005.02.072. discussion 236–237. [DOI] [PubMed] [Google Scholar]
- 118.Taylor DA. Atkins BZ. Hungspreugs P. Jones TR. Reedy MC. Hutcheson KA. Glower DD. Kraus WE. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med. 1998;4:929–933. doi: 10.1038/nm0898-929. [DOI] [PubMed] [Google Scholar]
- 119.Taylor DA. Silvestry SC. Bishop SP. Annex BH. Lilly RE. Glower DD. Kraus WE. Delivery of primary autologous skeletal myoblasts into rabbit heart by coronary infusion: a potential approach to myocardial repair. Proc Assoc Am Physicians. 1997;109:245–253. [PubMed] [Google Scholar]
- 120.Thomson JA. Itskovitz-Eldor J. Shapiro SS. Waknitz MA. Swiergiel JJ. Marshall VS. Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 121.Timmermans F. Plum J. Yoder MC. Ingram DA. Vandekerckhove B. Case J. Endothelial progenitor cells: identity defined? J Cell Mol Med. 2008;13:87–102. doi: 10.1111/j.1582-4934.2008.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Toma C. Pittenger MF. Cahill KS. Byrne BJ. Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 123.Tulloch NL. Pabon L. Murry CE. Get with the (re)program: cardiovascular potential of skin-derived induced pluripotent stem cells. Circulation. 2008;118:472–475. doi: 10.1161/CIRCULATIONAHA.108.791442. [DOI] [PubMed] [Google Scholar]
- 124.Umemura T. Higashi Y. Endothelial progenitor cells: therapeutic target for cardiovascular diseases. J Pharmacol Sci. 2008;108:1–6. doi: 10.1254/jphs.08r01cp. [DOI] [PubMed] [Google Scholar]
- 125.Unzek S. Zhang M. Mal N. Mills WR. Laurita KR. Penn MS. SDF-1 recruits cardiac stem cell-like cells that depolarize in vivo. Cell Transplant. 2007;16:879–886. doi: 10.3727/096368907783338271. [DOI] [PubMed] [Google Scholar]
- 126.Valiunas V. Doronin S. Valiuniene L. Potapova I. Zuckerman J. Walcott B. Robinson RB. Rosen MR. Brink PR. Cohen IS. Human mesenchymal stem cells make cardiac connexins and form functional gap junctions. J Physiol. 2004;555:617–626. doi: 10.1113/jphysiol.2003.058719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Valtieri M. Sorrentino A. The mesenchymal stromal cell contribution to homeostasis. J Cell Physiol. 2008;217:296–300. doi: 10.1002/jcp.21521. [DOI] [PubMed] [Google Scholar]
- 128.Vogel G. Breakthrough of the year: reprogramming cells. Science. 2008;322:1766–1767. doi: 10.1126/science.322.5909.1766. [DOI] [PubMed] [Google Scholar]
- 129.Wang JA. Chen TL. Jiang J. Shi H. Gui C. Luo RH. Xie XJ. Xiang MX. Zhang X. Hypoxic preconditioning attenuates hypoxia/reoxygenation-induced apoptosis in mesenchymal stem cells. Acta Pharmacol Sin. 2008;29:74–82. doi: 10.1111/j.1745-7254.2008.00716.x. [DOI] [PubMed] [Google Scholar]
- 130.Wang JA. He A. Hu X. Jiang Y. Sun Y. Jiang J. Gui C. Wang Y. Chen H. Anoxic preconditioning: a way to enhance the cardioprotection of mesenchymal stem cells. Int J Cardiol. 2008;133:410–412. doi: 10.1016/j.ijcard.2007.11.096. [DOI] [PubMed] [Google Scholar]
- 131.Wang JA. Xie XJ. He H. Sun Y. Jiang J. Luo RH. Fan YQ. Dong L. [A prospective, randomized, controlled trial of autologous mesenchymal stem cells transplantation for dilated cardiomyopathy] Zhonghua Xin Xue Guan Bing Za Zhi. 2006;34:107–110. [PubMed] [Google Scholar]
- 132.Wang T. Xu Z. Jiang W. Ma A. Cell-to-cell contact induces mesenchymal stem cell to differentiate into cardiomyocyte and smooth muscle cell. Int J Cardiol. 2006;109:74–81. doi: 10.1016/j.ijcard.2005.05.072. [DOI] [PubMed] [Google Scholar]
- 133.Wang X. McCullough KD. Franke TF. Holbrook NJ. Epidermal growth factor receptor-dependent Akt activation by oxidative stress enhances cell survival. J Biol Chem. 2000;275:14624–14631. doi: 10.1074/jbc.275.19.14624. [DOI] [PubMed] [Google Scholar]
- 134.Wei HJ. Chen CH. Lee WY. Chiu I. Hwang SM. Lin WW. Huang CC. Yeh YC. Chang Y. Sung HW. Bioengineered cardiac patch constructed from multilayered mesenchymal stem cells for myocardial repair. Biomaterials. 2008;29:3547–3556. doi: 10.1016/j.biomaterials.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 135.Wojakowski W. Kucia M. Kazmierski M. Ratajczak MZ. Tendera M. Circulating progenitor cells in stable coronary heart disease and acute coronary syndromes: relevant reparatory mechanism? Heart. 2008;94:27–33. doi: 10.1136/hrt.2006.103358. [DOI] [PubMed] [Google Scholar]
- 136.Xie XJ. Wang JA. Cao J. Zhang X. Differentiation of bone marrow mesenchymal stem cells induced by myocardial medium under hypoxic conditions. Acta Pharmacol Sin. 2006;27:1153–1158. doi: 10.1111/j.1745-7254.2006.00436.x. [DOI] [PubMed] [Google Scholar]
- 137.Xu W. Zhang X. Qian H. Zhu W. Sun X. Hu J. Zhou H. Chen Y. Mesenchymal stem cells from adult human bone marrow differentiate into a cardiomyocyte phenotype in vitro. Exp Biol Med (Maywood) 2004;229:623–631. doi: 10.1177/153537020422900706. [DOI] [PubMed] [Google Scholar]
- 138.Xu YX. Chen L. Wang R. Hou WK. Lin P. Sun L. Sun Y. Dong QY. Mesenchymal stem cell therapy for diabetes through paracrine mechanisms. Med Hypotheses. 2008;71:390–393. doi: 10.1016/j.mehy.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 139.Zeng J. Wang Y. Wei Y. Xie A. Lou Y. Zhang M. Co-culture with cardiomyocytes induces mesenchymal stem cells to differentiate into cardiomyocyte-like cells and express heart development-associated genes. Cell Res. 2008;18:562. [Google Scholar]
- 140.Zhang D. Fan GC. Zhou X. Zhao T. Pasha Z. Xu M. Zhu Y. Ashraf M. Wang Y. Over-expression of CXCR4 on mesenchymal stem cells augments myoangiogenesis in the infarcted myocardium. J Mol Cell Cardiol. 2008;44:281–292. doi: 10.1016/j.yjmcc.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang M. Mal N. Kiedrowski M. Chacko M. Askari AT. Popovic ZB. Koc ON. Penn MS. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. 2007;21:3197–3207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- 142.Zhao P. Ise H. Hongo M. Ota M. Konishi I. Nikaido T. Human amniotic mesenchymal cells have some characteristics of cardiomyocytes. Transplantation. 2005;79:528–535. doi: 10.1097/01.tp.0000149503.92433.39. [DOI] [PubMed] [Google Scholar]
- 143.Zhu WZ. Hauch KD. Xu C. Laflamme MA. Human embryonic stem cells and cardiac repair. Transplant Rev (Orlando) 2008;23:53–68. doi: 10.1016/j.trre.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]