Abstract
The conventional therapeutic modalities for myocardial infarction have limited success in preventing the progression of left ventricular remodeling and congestive heart failure. The heart cell therapy and therapeutic angiogenesis are two promising strategies for the treatment of ischemic heart disease. After extensive assessment of safety and effectiveness in vitro and in experimental animal studies, both of these approaches have accomplished the stage of clinical utility, albeit with limited success due to the inherent limitations and problems of each approach. Neomyogenesis without restoration of regional blood flow may be less meaningful. A combined stem-cell and gene-therapy approach of angiomyogenesis is expected to yield better results as compared with either of the approaches as a monotherapy. The combined therapy approach will help to restore the mechanical contractile function of the weakened myocardium and alleviate ischemic condition by restoration of regional blood flow. In providing an overview of both stem cell therapy and gene therapy, this article is an in-depth and critical appreciation of combined cell and gene therapy approach for myocardial repair. Antioxid. Redox Signal. 11, 1929–1944.
Introduction
Ischemic heart disease is a leading worldwide cause of morbidity and death (1). Therapeutic interventions include behavioral and dietary modifications, pharmacotherapy, and invasive surgical interventions, such as coronary artery bypass grafting (CABG) and percutaneous transluminal coronary angioplasty (PTCA). Prophylaxis and conventional therapeutic interventions are conservative and can provide only symptomatic relief without addressing the major issue of massive loss of functioning cardiomyocytes, which significantly influences the long-term beneficial effects of treatment in terms of heart function (27, 51, 95). Moreover, for a large number of patients, these treatment modalities are not feasible for one or more reasons. The approach of substituting the damaged heart of the patient with a donor heart is constrained by the problems of availability and graft rejection (6). This is being replaced by the modern approach of regenerating the damaged myocardium through heart cell therapy.
Transplantation of stem cells from various sources has been carried out in animal studies and clinical trials. Mostly, the donor stem cells have shown the ability to improve global heart function through one or more mechanisms including (a) myogenesis (including their ability to adopt smooth muscle, skeletal, or cardiac phenotypes); (b) support of neovascularization (3, 13, 40, 99, 175); and (c) the release of paracrine factors with pleiotropic properties (141, 147, 149).
However, many obstacles arise, such as low survival rate of transplanted cells, immune reaction to allogenic stem cells, and changes in the number and function of stem cells with aging and disease. For an efficient and effective treatment by stem and progenitor cell engraftment, the technique remains to be optimized for its progress as a routinely used clinical modality.
Neomyogenesis alone, however, may not suffice. The poorly vascularized scar tissue does not fully support the survival and engraftment of the donor stem cells, which negatively influences the therapeutic outcome. Moreover, stem cells are highly sensitive to tissue ischemia (114). To ensure optimal therapeutic effectiveness of heart cell therapy, it is therefore imperative to grow a vascular network that would ably and adequately nourish the transplanted stem cells as well as the newly formed myofibers. Therefore, stem cell therapy for ischemic hearts must be combined with additional therapeutic interventions to give adequate vascular support to the newly formed myocardial tissue. Therapeutic angiogenesis, in this regard, represents a potential concomitant treatment modality hat may be accomplished by angiogenic growth factor protein or gene delivery (7, 22). SkMs and BMSCs have been explored as delivery vehicles for therapeutic genes encoding one or more angiogenic growth factors to achieve a myoangiogenic response (55, 167, 168). Transplantation of genetically modified stem cells for overexpression of appropriate angiogenic factor(s) may be more advantageous as compared to either stem cell transplantation or gene therapy alone.
The current review summarizes the latest developments in stem cell and gene therapy with special focus on angiomyogenesis for myocardial regeneration and repair. The advantages, the inherent limitations, and future directions of stem cell–based gene delivery are discussed to improve the results of cellular cardiomyoplasty.
Stem Cell Therapy at a Glance
Stem cell therapy is fast developing as a potential strategy in cardiovascular therapeutics. It involves repopulation of the infarcted myocardium with cells having myogenic or angiogenic potential or both. Mitotic activity of the cardiomyocyte (14, 56), mobilization and homing-in of the BMSCs in response to myocardial ischemia (159), and the existence of resident cardiac stem cells (13, 58) are the recently reported intrinsic repair mechanisms in the infarcted heart. However, inadequacy of the intrinsic repair mechanisms must be supported by outside intervention. Heart cell therapy involving transplantation of stem and progenitor cells with or without genetic manipulation has been shown to supplement efficiently the natural repair mechanism. The transplanted cells replenish the postinfarction scar tissue with neofibers that eventually are structurally and functionally integrated with the host tissue (55, 99, 120, 121). In the search for an ideal cell type with optimal characteristics (i.e., ease of availability, in vitro expansion, response to physiologic and pathologic stimuli, myogenic differentiation potential, ability to integrate functionally with the host myocytes, and release of paracrine factors), cells from various sources with differing potential have been used. Because of the diversity in the nature and sources of the cells used for the heart cell therapy, no definite method of their classification exists. These cell types can be grossly divided as autologous, allogenic, and xenogenic on the basis of their source: neonatal, adult, and embryonic on the basis of the age of the donor, and nonmyogenic and myogenic on the basis of their ability to differentiate and adopt a myogenic phenotype. The nonmyogenic cells, including endothelial cells and endothelial progenitor cells (8, 63, 64), and the genetically modified fibroblasts (97) have been implanted into the heart to assess their feasibility and effectiveness in cardiac repair. However, more-encouraging results came from the studies that used cells with an inherent ability to undergo myogenic differentiation and replace the noncontractile scar tissue with a kind of muscle. These include fetal and adult cardiomyocytes (77, 112, 161, 172), smooth muscle cells (76, 173), cardiac stem and progenitor cells (19, 121), embryonic stem cells (ESCs) and ESC-derived cardiomyocytes (134), umbilical cord derived stem cells (62, 75, 82), BM and its sublineages (57, 105, 120, 158), and SkMs (16, 61, 99, 101, 143). Each donor cell type mentioned has shown limited beneficial effects after engraftment into the heart, and their suitability for clinical use has been restricted because of various ethical, biologic, and technical concerns.
Among the donor-cell types, cardiomyocytes may be the most suitable choice for heart cell therapy. The donor cardiomyocytes have been shown to form a stable graft in the recipient heart, notwithstanding their availability, which remains a drawback in their clinical application. From a practical standpoint, the use of both BMSCs and SkMs is attractive because of their myogenic potential and ease of availability from an autologous source without any ethical or religious issues. Despite being effective, both of these cell types reportedly act through different mechanisms. BMSC-mediated myocardial repair has been shown to result from either one or more mechanisms involving neomyogenesis (33, 144, 146), angiogenesis (33, 41, 93, 176), and paracrine release of multiple cytokines and growth factors (32, 145, 149, 166). Likewise, myogenically differentiated SkMs provide a scaffold-like effect through myogenic differentiation, thus replacing the noncontractile and stiff scar tissue with more-flexible muscle fibers (5). These changes prevent expansion and remodeling of the left ventricle. Additionally, the release of paracrine factors promotes the survival of host myocytes, attenuating the infarct size expansion.
Besides other factors, the choice between SkMs and BMSCs for transplantation is greatly influenced by the required outcome of the procedure. Whereas the transplanted SkMs form muscle fibers that provide a tenacious support for the weakened myocardium and enhance diastolic cardiac function (5), BMSC transplantation mainly induces angiomyogenesis and improves systolic function (13, 18, 120). Keeping in view the varied nature of the myocardial repair process, a more prudent and relevant approach would be a simultaneous transplantation of the different cell types to achieve the benefits associated with the use of each cell type (144). Moreover, the engraftment of SkMs and BMSCs must be combined with other strategies to generate “super stem cells” that have been “preformatted” to survive, engraft, differentiate, and functionally integrate in the ischemic heart and support the inadequate intrinsic repair mechanisms.
With encouraging results from preclinical experimental studies, the safety and feasibility of BMSCs and SkMs also have been assessed in human patients. The first clinical study was performed by Menasche et al. (90) and involved autologous SkM transplantation in a 57-year-old patient. The uneventful cell transplantation was successfully performed as an adjunct to coronary artery bypass grafting. After successful and uneventful SkMs engraftment, Menasche et al. (12) reported 10 more patients who received autologous SkMs engraftment as an adjunct procedure to CABG. The patients' left ventricle ejection fractions increased from 23.8 to 32.1% with concomitant systolic wall thickening and improved New York Heart Association class from 2.7 to 1.6 on average. One of the 10 patients died of complications unrelated to SkM injection. No cell transplantation-related complications were reported other than unwanted ventricular tachycardia in four patients. Since then, a large number of clinical studies have been performed in various medical centers around the globe with both SkMs (20, 30, 49, 89, 106) and BMSCs (135, 137, 148, 162, 163). Most of these clinical studies to date have been carried out as an adjunct procedure with one of the routinely performed revascularization procedures such as CABG (29, 42, 91, 136), placement of left ventricular assist device (106), and percutaneous transluminal angioplasty (4, 18), or used as a sole therapy (27, 108). More important, the use of autologous cells in these studies alleviated the need for immunosuppression. Without exception, cell transplantation during these studies remained uneventful and without any cell transplantation–related mortality, which is vivid proof of the safety of the procedure.
Even with these extreme safety observations, the development of arrhythmias, although pharmacologically treatable, was observed in the patients receiving SkMs engraftment, with no such occurrences in cases of BMSCs engraftment. Even though the arrhythmias have been observed in patients receiving SkMs, no clear indication may be attributed to any particular cell type (26).
The first and the only clinical studies using nonautologous cells were performed by our collaborators, Law et al. (71). They extrapolated our animal model study results to transplant allogenic SkMs from young donors into three patients by using transient immunosuppression (36). The first clinical study involving cell transplantation on a beating heart was performed by Sim et al. in Singapore (131). Under direct vision and stabilization with the Octopus III tissue stabilizer, 3.70 × 108 autologous SkMs in 3 ml of the patient's own serum were injected into the myocardium at 20 sites in and around the infarcted myocardium. The cell-engraftment procedure was well tolerated by the patient and showed improved left ventricle ejection fraction.
The strategy of heart cell therapy has met with multiple controversies. Reports have voiced skepticism vis-à-vis the milieu-dependent differentiation potential of BMSCs after engraftment (9, 98). The authors found no evidence of neomyogenesis after BMSC engraftment and called for a more cautious interpretation of the results (12). Others have shown serious concerns about the safety of the cell-engraftment approach by using both SkMs and BMSCs. Yoon et al. (174) reported intramyocardial calcification subsequent to engraftment of unselected bone marrow cells. They observed the formation of a bright echogenic mass with acoustic shadowing in almost one third of the surviving rat hearts. Likewise, transplantation of undifferentiated mesenchymal stem cells (MSCs) developed into fibroblastic scar tissue (157). The lack of ability of SkMs to integrate functionally with host myocytes remains a major concern (113). Furthermore, the proarrhythmogenic nature of SkMs has severely slowed the progress in their clinical application, although the underlying mechanism of ventricular arrhythmias after SkMs engraftment remains undefined (20, 91).
Although unconfirmed, it is generally anticipated that the injection of cells with different depolarization and repolarization currents increases electrical heterogeneity at the site of SkMs grafts and increase the risk of arrhythmias. A recent report raised serious concern about BMSCs causing arrhythmia after engraftment. This has further complicated the situation and has raised a serious question whether the arrhythmia that was previously attributed to SkMs transplantation is a general problem of stem cell therapy (154).
All these issues need careful consideration, and researchers engaged in this cutting-edge area of research must look into the factors that may be responsible for the altered growth and differentiation characteristics of stem and progenitor cells. The purity of the stem and progenitor cell culture, ex vivo expansion, and the use of animal serum proteins during cell culture may lead to cellular senescence, as compared with the freshly isolated cells, with resultant loss in myogenic and angiogenic potential (109). Similarly, evidence suggests that functional characteristics of the stem and progenitor cells are under the influence of redox regulation, and therefore, their differentiation potential may be significantly influenced by redox changes (43). Therefore, it is imperative to address these issues by optimization of cell-processing conditions and by the reprogramming of stem and progenitor cells to achieve the desired outcome.
An Overview of Angiogenic Therapy for the Infarcted Heart
Angiogenic response in the body is controlled by a delicate balance maintained between a large number of pro- and antiangiogenic factors and the involvement of redox-sensing transcription factors such as NF-κB that regulate blood vessel formation (86). Disruption of the natural balance between the pro- and antiangiogenic factors results in pathologic consequences, with abnormal blood vessel formation. Angiogenic protein and gene-delivery strategies are potential treatment options for ischemic tissues. The effectiveness of angiogenic therapy has been extensively studied in the small and the large experimental animal models (85, 167, 168). These studies have paved the way for clinical application of therapeutic angiogenesis in human patients (115). The choice of an experimental animal model is critical in assessing the applicability of a therapeutic agent for clinical use. Some of the experimental animals have preexisting arteriolar collaterals and, therefore, are not the best models with which to study myocardial angiogenesis (128).
Angiogenic growth-factor protein therapy
The administration of recombinant growth factor proteins achieved a significant angiogenic response and resulted in improved myocardial perfusion. Among these growth factors, fibroblast growth factor (FGF) was the first to be used for myocardial neovascularization in a canine model (150). Together with vascular endothelial growth factor (VEGF), these two major proangiogenic factors have been extensively studied. The growth-factor protein administration, however, manages only a transient effect because of the relatively short biologic half-life of these growth factors. Additionally, high-dose bolus delivery of growth-factor proteins adds a potential risk of systemic toxicity through cytokine diffusion into circulation and into the nontargeted organ. Besides the selection of an appropriate vector, targeted delivery of the angiogenic growth-factor protein, genes, and the genetically modified cells to the heart is essential to contain their nonspecific distribution and to achieve localized effects. Intravenous delivery, therefore, is not the best choice in most cases because of the potential systemic response of the vectors. Intracoronary delivery has been found to be appropriate for both protein and gene transfer, but chances of systemic distribution are not fully curtailed (31). The epicardial intramyocardial injection of either angiogenic growth factors or their genetic forms has significantly higher angiogenesis around the ischemic myocardium (83). However, it is an invasive route of administration. Catheter-based trans-endocardial intramyocardial-injection delivery provides equivalent benefits without the need for surgery (69). Intra-pericardial delivery of angiogenic factors may offer a theoretic advantage of prolonged exposure of myocardial tissue to the administered drug, as result of a reservoir function of the pericardium (66). Therefore, the strategy of growth-factor protein treatment warrants multiple dose administrations or a special delivery system that may ensure a prolonged effect of the growth factor. The delivery of gene encoding for the growth factor of interest thus is a more practicable and clinically relevant option.
Angiogenic gene-delivery strategy
Gene therapy involves transfer of naked DNA or a vector constructed to encode a single or multiple growth factors of interest into the targeted organ. Unlike protein therapy, the main advantage of gene-transfer therapy is that it provides a more sustained effect after a single administration, in comparison with protein therapy. Notwithstanding the involvement and a critical role of angiogenesis in various physiologic and pathologic processes, transgene expression in vivo requires careful regulation in a spatial and temporal manner. A variety of methods are available to deliver angiogenic growth-factor genes (39). Transfer of naked DNA, due to its low transfection efficiency, is not the preferred option for gene delivery. Nonviral delivery vectors (i.e., nanoparticles, liposomes) have been extensively studied (17, 21, 93, 171). Lack of safety concerns and ease of production has extended their use to human studies. Nevertheless, low transfection efficiency and short duration of gene expression are the weaker links in their clinical application. Replication-deficient viral vectors are more efficient in gene delivery to the target organ and provide a higher and more prolonged expression of the transgene (74, 123, 170). However, induction of host immune and inflammatory response and the safety concerns after prolonged gene expression, in some cases with deleterious effects, are of major concern with regard to their clinical use. Recombinant adenoassociated viruses have been used for angiogenic gene delivery to the heart for long-term gene expression with minimal inflammatory response (164). The vector is, however, complex and has limited packaging capacity. Furthermore, the long-term sustained expression achieved by adenoassociated viruses may not be required in some cases, such as myocardial revascularization.
Angiogenic gene therapy in experimental animal models
VEGF and bFGF are the most efficient and well-studied growth factors from among the current array of growth factors (37, 52, 73, 103, 168). A comparative study in a porcine model showed that both VEGF and bFGF are equally effective in the promotion of myocardial angiogenesis (53) and may be delivered together to have their combined effects in blood vessel formation and maturation (102). During most of the preclinical studies, the delivery of bFGF and VEGF to the heart, either singly or in conjunction with other growth factors, resulted in significant collateral formation and well-preserved left ventricle function (53, 102). However, a few exceptional reports wherein lack of histologic evidence for improved angiogenesis, or even in the presence of histologic evidence for angiogenesis was seen, no preservation of left ventricle function was observed after angiogenic gene therapy (10, 72).
VEGF, a heparin-binding glycoprotein having specific receptors on the endothelial cells, is an inducer of vascular permeability and plays an essential role in angiogenesis (23). VEGF165 and VEGF121 are the most efficient stimulants of angiogenesis and have been widely studied for preclinical and clinical applications (92). Conversely, bFGF is a member of a large FGF family that is structurally related to heparin-binding growth factors (59). bFGF has the ability to induce endothelial cell proliferation and migration and has been extensively studied in myocardial angiogenesis and protection (15, 44, 78). Both VEGF and bFGF activate the Erk-1/2 pathway via the canonic Grb2–SOS–Ras pathway (87, 133). Various delivery vectors have been used to achieve their transgene overexpression in the ischemic heart; these include naked plasmid (47, 73, 110), adenoviral vector (37, 104, 168), adenoassociated viral vector (24), and nonviral vectors (17, 171).
Clinical studies
Encouraged by the preclinical study results, angiogenic gene therapy has advanced to clinical trials (28). A number of phase I studies were reported, focused mainly on protein administration of VEGF or FGF (70, 119, 126, 139, 151). Moreover, these studies were performed as an adjunct procedure with conventional coronary-revascularization methods (126, 129). Since the reporting of phase I studies, a few phase II trials have begun (34, 35, 46, 48, 68). However, these studies have failed to reveal any substantial benefit from the treatment, and similar improvement was observed in the treated and untreated patients. The failure to achieve therapeutic benefits was attributed to the short biologic half-life of the growth-factor proteins, which sent a reminder to the researchers to develop some alternative modalities for maintenance of optimal serum level of the angiogenic growth factors for a desired therapeutic response. A gene-delivery approach in this regard was expected to make a difference and involved the delivery of naked-plasmid DNA encoding for angiogenic growth factor (80, 152), their viral vector constructs (34, 46, 119), or their plasmid complex with nonviral vectors (17, 171). Whereas the viral-vector delivery of a growth factor gene gave symptomatic relief, the nonviral vector method was less efficient because of low transfection efficiency.
Alternatively, intramyocardial VEGF gene transfer has been combined with granulocyte colony-stimulating factor (GCSF)-induced stem cell mobilization (118). The strategy was aimed to enhance the availability of stem and progenitor cells in the peripheral circulation to promote their participation in the angiogenic cascade. However, the strategy failed to bring any noticeable improvement in the therapeutic outcome. The primary concern with therapeutic angiogenesis is the growth of new blood vessels in the nontargeted tissues, which may precipitate various pathologies and lead to the exacerbation of the disease condition (54, 132). A localized and targeted delivery of the recombinant growth factor or the relevant gene delivery may help in alleviating the associated risks.
The other concern is that most of the potent angiogenic factors, such as VEGF, are found closely related to tumor genesis (100). The approach of therapeutic angiogenesis therefore needs further refinement in methodologic and procedural aspects to become safer and more effective before its adoption in routine clinical application.
The Novel Approach of Cellular Angiogenesis
Preclinical studies in both small- and large-animal models have confirmed that the transplantation of autologous BMSCs or their purified subpopulations restores regional blood flow by angiogenesis, reduces infarction size and fibrosis, improves regional wall thickness, and enhances the contractile function of the left ventricle wall through myogenesis. Besides myogenic differentiation, SkMs also release multiple growth factors, especially under ischemic stress, which act in a paracrine fashion and recruit resident cardiac stem cells and stem and progenitor cells from peripheral circulation with contributory effects on myocardial regeneration and angiogenesis (156). Thus, significantly improved regional and global heart function was noted.
Transplantation of BMSCs can induce both angiogenesis and myogenesis (24, 41, 67, 141). This ability of the BMSCs has been attributed to the subpopulation of cells in the bone marrow with inherent cytokine-producing potential (94). These growth factors and cytokines, after their release, act in a paracrine fashion to induce functional improvement through angiomyogenesis (141).
Angiogenesis has also been achieved by implantation of endothelial and endothelial progenitor cells (125, 141, 147), adipose-derived stem cells (88, 153), embryonic stem cells (122), and umbilical cord–derived stem cells (50, 82). The preclinical results have also been extrapolated into clinical studies with the aim of achieving angiogenesis from the engrafted cells (42, 148). Unlike other studies that use the whole unselected mononuclear cell fraction of bone marrow cells, the AC133+ subpopulation of the mononuclear cells was purified and used for transplantation. The rationale for the use of purified AC133+ cells was to avoid injection of large number of leukocytes and their progenitors, which have limited plasticity, and the presence of which in large numbers may give rise to an unwanted inflammatory response at the site of the graft. A total of 1 × 106 cells was injected at 10 sites (0.2 ml per injection site). All patients survived the procedure without any complications (135, 136).
Stem cell transplantation has also been combined with growth-factor administration to achieve their combined effect in terms of myocardial regeneration and angiogenesis (116, 124). We are the first to exploit the concept of preconditioning to promote stem cell survival and engraftment in the infarcted heart (101). Since the pioneering work of Murry et al. (96), the cytoprotective effects of ischemic preconditioning have been repeatedly shown in the heart as well as in cells. The same results have been duplicated by pharmacologic treatment with preconditioning mimetics (2, 142). We extrapolated these results to stem cell therapy and observed that preconditioning of SkMs enhanced their resistance to oxidant stress in vitro (101). The preconditioned cells showed enhanced proliferation as compared with the non-preconditioned cells (Fig. 1). Besides improvement of cell survival, we observed that SkMs and MSCs preconditioned by diazoxide treatment were able to release copious amounts of cytokines and growth factors at the site of the cell graft. The paracrine factors acted locally in a paracrine fashion and were not released into systemic circulation because the cells were transplanted by intramyocardial injection and therefore did not pose any systemic threat. Furthermore, preconditioned cells showed improved angiomyogenic potential. Immunostaining of the histologic tissue sections of the heart for myosin heavy chain (slow isoform) showed extensive myogenesis at the site of the cell graft (Fig. 2). Double-fluorescent immunostaining of the infarcted rat heart for von Willebrand factor VIII and smooth muscle actin showed significantly higher blood-vessel density in preconditioned cell-transplanted hearts as compared with non-preconditioned cell-transplanted hearts (Fig. 3). These are significant results and have great clinical relevance because of the proven safety and effectiveness of both stem and progenitor cells and of preconditioning mimetics as monotherapies.
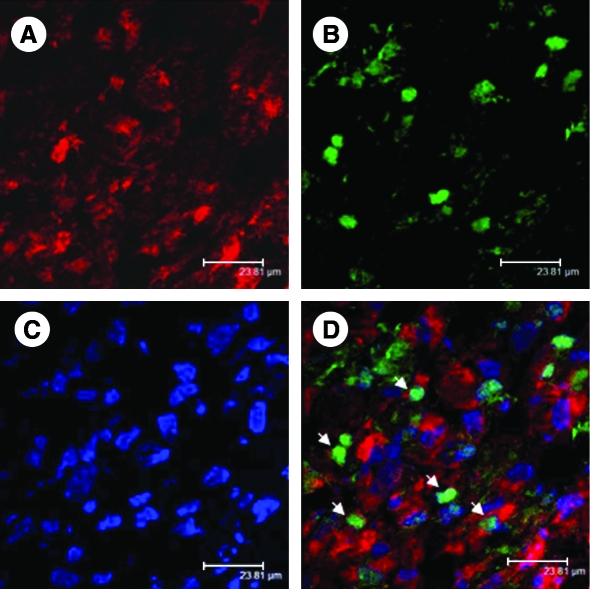
FIG. 1.
Confocal images of the infarcted rat heart histologic sections 7 days after transplantation of the preconditioned SkMs. The cells were labeled with cell-tracker dye PKH26 (A; red). The tissue sections were immunostained for Ki67 expression, a marker for cell proliferation (B; green). The nuclei were visualized with DAPI staining (C; blue). (D) The merged image. The number of Ki67+ cells (white arrows) was significantly higher in the preconditioned cell-engrafted hearts as compared with the non-preconditioned cell-transplanted hearts (p < 0.001) (original magnification, ×630). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
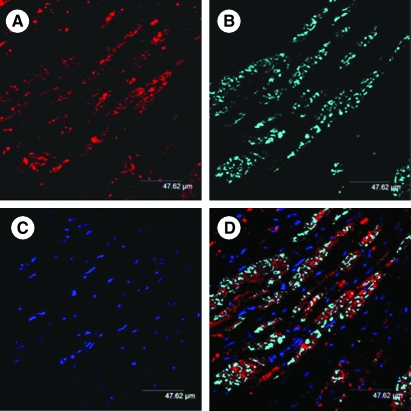
FIG. 2.
Confocal images of the rat heart-tissue section at 6 weeks after transplantation of SkMs labeled with PKH26 cell tracker dye (A; red). The histologic sections were immunostained for myosin heavy chain (slow-isoform) expression (B; cyan). The nuclei were visualized with DAPI staining (C; blue). (D) Merged image showing extensive myogenic differentiation of the transplanted cells in the infarcted myocardium (original magnification, × 630). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
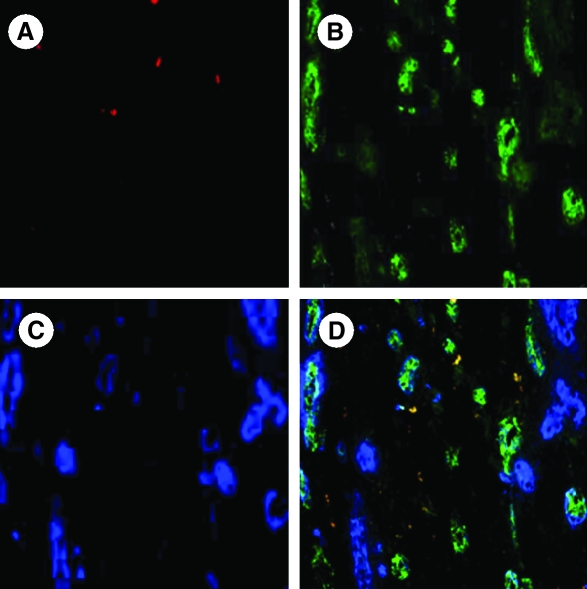
FIG. 3.
Fluorescent photomicrographs of the infarcted rat heart at 6 weeks after engraftment of diazoxide-preconditioned SkMs labeled with PKH26 (red; A). The tissue sections were double immunostained for von Willebrand factor VIII (green; B) and smooth muscle actin (blue; C). (D) Merged image showed extensive angiogenic response; the majority of the blood vessels formed were mature, as indicated by their positivity for smooth muscle actin (magnification, × 400). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Combining Angiogenic Gene Therapy with Stem Cell Transplantation
Transplantation of genetically engineered cells is an attractive option to combine stem cell therapy with angiogenesis and represents a leap forward from the past strategies in achieving the desired outcome of myoangiogenesis (155). Cells from various sources having different characteristics have been used as the delivery vehicles of exogenous angiogenic gene transfer to the heart (45, 55, 85, 103, 122, 168). With the encouraging results from the heart cell therapy in experimental animal models and in patients, combining angiogenesis with cell transplantation has multiple advantages. The potential advantage of this technique is to create a reservoir of myogenic cells that would differentiate to achieve a myogenic phenotype and be established as a part of the myocardial tissue. Concomitantly, these cells provide a localized and sustained source of growth factors to initiate neovascularization after one-time administration. Furthermore, this would alleviate host inflammatory and immune responses, which are the potential disadvantage of direct adenoviral vector administration. This will have a salutary effect on the survival of the donor cells in early stages after transplantation and a subsequent alleviation of myocardial ischemia due to improved regional blood flow. Earlier after engraftment, the donor stem and progenitor cells are greatly stressed by the in vitro manipulations, engraftment procedure, and by post-engraftment exposure to the microenvironment of the infarcted heart, which initiates a vicious cycle of cell apoptosis (38, 51). The overexpression of angiogenic growth-factor proteins initiates survival signaling in the donor stem and progenitor cells, promoting their post-engraftment survival (165). Additionally, these stem and progenitor cells release growth factors that act in paracrine fashion and have cytoprotective effects on stem and progenitor cells themselves and on the host cardiomyocytes. The coronary microvessel dilation achieved by the effect of the growth factors at the site of cell engraftment enhances the regional blood flow, thus alleviating local ischemia. Indeed, the myogenic transdifferentiation of donor cells will generate a muscle-fiber scaffold that will prevent a vicious cycle of left ventricular remodeling and will restore left ventricle diastolic function (5). Induction of angiogenesis may further improve the efficacy of cellular cardiomyoplasty. To achieve these effects independent of angiogenesis, the genetically modified stem cells must be in a progressive state of growth-factor expression at the time of implantation. A combined stem cell and gene therapy approach, therefore, will be a step forward to concomitantly achieving cytoprotection, angiogenesis, and myogenesis, the areas of research currently being probed as 21st-century remedial measures for the failing heart.
One critical aspect in stem cell–based gene delivery that requires special consideration is the duration of the transgene overexpression after engraftment into the infarcted heart. As the shorter time duration of overexpression may hinder the achievement of the desired outcome, longer and persistent overexpression of potent growth factors may also lead to undesired and deleterious effects. Depending on the mode of gene transfer into cells, gene expression has been reported to persist from a few days (with most of the nonviral vectors) to 5 weeks (109), 3 months (65), 7 months (our unpublished data), and for up to a year (111). Lee et al. (74) reported angioma formation in the nonischemic murine myocardium after VEGF expression from retrovirally transduced SkMs transplantation. We have already shown that SkMs transduced with lac-z reporter gene are capable of persistent expression of the transgene on a longer-term basis in the host myocardium (our unpublished data).
SkMs as angiogenic gene-delivery vehicles
With a few exceptions (103), the cells used as the carriers of transgene to the heart are inherently myogenic, and SkMs, in this regard, have been extensively studied for their ability as therapeutic gene-delivery vehicles to different organs, including the heart (37, 138). SkMs have been purified from various species, including mouse, rat, rabbit, sheep, pig, and human, and characterized for their potential to improve the deteriorating heart function and attenuation of left ventricular remodeling after myocardial infarction.
The practicality and effectiveness of SkMs as a vehicle for ex vivo delivery of therapeutic genes has also been well documented (25, 107). The pioneering contribution of Kho et al. (65) highlighted the ability of SkMs to deliver TGF-β to the myocardium (65). The authors observed regions of enhanced neovascularization in and around the area of cell graft. Rinch et al. (117) reported genetically engineered SkMs carrying FGF2 delivery for revascularization in a model of acute skin-flap ischemia. Similar reports have also been published for angiogenic gene delivery to the myocardium by using SkMs (138, 167). We determined the effect of ex vivo human VEGF165 gene transfer to the heart in a porcine heart model. SkMs were transduced with replication-deficient adenoviral and retroviral vectors carrying human VEGF165 and lac-z genes, respectively (37). The transduced human SkMs were characterized for VEGF overexpression in vitro and were evaluated for their engraftment potential and human VEGF165 expression in the infarcted myocardium. We observed increased neovascularization after transduced SkMs transplantation. This was a continuation of the natural endogenous compensatory response. The significantly improved perfusion in the periinfarct region was attributed to the enhanced cellularity of that area that better supported the formation of new blood vessels.
In another study, we also delivered angiopoietin-1 to the infarcted heart in a rodent heart model and observed that angiopoietin, which is generally considered the maturation factor, has the ability to initiate angiogenic response on its own (170).
Despite these positive results, formation of a stable and functional blood vessel involves interplay between several pro- and antiangiogenic factors and vascular modulators, which stimulate vessel sprouting and remodeling through a coordinated targeting of various cells (11). Therefore, single angiogenic factor administration may be insufficient and sometimes may lead to the formation of morphologically and functionally inefficient blood vessels. In addition, uncontrolled and upregulated expression of a single growth factor may cause complications (74, 127). To address this issue, we reported the first bicistronic vector co-overexpressing two of the main players of the angiogenic cascade, VEGF and angiopoietin-1 (169). Angiopoietin-1 efficiently increases angiogenesis in conjunction with VEGF released intrinsically in the infracted myocardium. The presence of angiopoietin-1 ensures leak-resistant, mature, stable, and functioning blood vessels with smooth muscle cell covering (130, 140). The bicistronic vector was used for transduction of human SkMs, which were later transplanted into a porcine myocardial infarction model to achieve angiomyogenesis. Double fluorescent immunostaining for von Willebrand factor VIII and smooth muscle actin showed that at 6 weeks after human SkMs transplantation, a significant increase occurred in blood vessel density in the bicistronic vector–treated SkMs group as compared with control groups. At 12 weeks, blood vessel density improved further, with a concomitantly enhanced maturity index. More than 93% of blood vessels were mature (with smooth muscle cell covering) in the bicistronic vector–treated SkMs group as compared with 86% at 6 weeks, thus indicating slow but progressive maturation of the newly formed capillary network due to the synergistic influence of human VEGF165 and angiopoietin-1.
As discussed earlier, regardless of their low transfection efficiency and short-term expression, the use of a nonviral gene-delivery system is safe and flexible in terms of the plasmid DNA size that can be delivered (38). We have successfully designed a polyethyleneimine (PEI)-based nanoparticle system for transfection of human SkMs for VEGF165 overexpression (171). PEI has a cationic nature, a strong DNA-compaction capacity, effective DNA protection, and an intrinsic endosomolytic activity that contributes to superior gene-transfection efficiency (81). We achieved more than 11% transfection efficiency of SkMs and peak level expression (25 ng/ml) on day 4 to 6 after transfection. In vivo studies in a rodent heart model of myocardial infarction showed higher blood vessel density and regional blood flow (milliliters per minute per gram) in the left ventricle in the transfected cell–transplanted group of animals as compared with the control group. Our results clearly signify the safety and effectiveness of nanoparticle-based nonviral vectors for gene delivery to the heart.
In another interesting study, we used nonviral liposome-mediated delivery of SDF-1α into SkMs, which were later transplanted in a rodent heart model of acute myocardial infarction (21). Our working hypothesis was to develop an SDF-1α gradient in favor of the infarcted heart, which may trigger BMSCs mobilization because of SDF-1α/CXCR4 ligand/receptor interaction. We observed enhanced mobilization of BMSCs to the heart, which resulted in improved an angiogenic response in the SDF-1α-overexpressing SkMs–transplanted heart as compared with nontransfected SkMs–transplanted hearts. Put together, these studies clearly signify the potential of SkMs as transgene-delivery vehicles for the infarcted heart.
BMSCs as the angiogenic gene-delivery vehicles
Besides SkMs, BMSCs have been successfully studied for transgene delivery to the infarcted heart (55, 84). In a recent report, Matsumoto et al. (85) showed that cell transplantation using VEGF-expressing MSCs could enhance the cardioprotective effects of MSCs, followed by angiogenesis effects in salvaging host myocardium. These results indicate a key role for the transplantation of VEGF-expressing MSCs as a strategy for cellular cardiomyoplasty after myocardial infarction. Kawamoto et al. (60) demonstrated the superiority of combining human VEGF therapy together with cytokine-induced BMSCs mobilization in terms of neovascularization and heart-function improvement as compared with monotherapy (60). Similarly, a combination of hepatocyte growth factor (HGF) gene transfer and neonatal rat cardiomyocyte transplantation had more potent therapeutic efficacy in a model of rat myocardial infarction as compared with either of the single treatments (93). These favorable outcomes support the hypothesis that progenitor cells play a key role in human VEGF-induced local tissue revascularization and that the combination of bone marrow mobilization and gene therapy can achieve superior therapeutic neovascularization. In an attempt to examine intramyocardial delivery of genetically modified MSCs overexpressing VEGF, we combined pharmacologic mobilization of BMSCs from their bone marrow niches (160). By tagging intramyocardially transplanted and mobilized BMSCs with two different fluorescent dyes, we studied the fate of these cells in the infarcted myocardium. Our rationale was that the combined therapeutic consequence of intramyocardially delivered BMSCs and local expression of human VEGF165 would be amplified by increasing the supply of circulating progenitor cells via pharmacologic mobilization and elevated serum levels of granulocyte colony-stimulating factor (GCSF) and stem cell factor (SCF). We observed significantly higher angiogenic response in animal heart that received ex vivo delivered human VEGF165 along with pharmacologic mobilization therapy as compared with animals with either of the treatments as monotherapy. Yau et al. (167) attempted a multimodal-therapy approach with ex vivo delivery of VEGF and bFGF gene delivery (167). Their results showed a synergistic effect of their multimodal approach in terms of angiogenesis and regional blood-flow improvement.
Besides using SkMs, we extensively studied MSCs as transgene carriers, and our results clearly showed that MSCs are equally efficient and effective in therapeutic gene delivery to the heart (55).
In a recent study, we showed that angiogenic gene delivery can be combined with the overexpression of Akt. The rationale of our study was to ensure maximal cell survival and enhanced angiogenesis in the infarcted heart. Akt occupies a central position in survival signaling, and its transgene overexpression in the heart has been shown to result in low collagen deposition and attenuated infarct-size expansion (79, 84). Keeping in consideration an emerging role for angiopoietin-1 in angiogenesis and a central role for Akt downstream of angiopoietin-1 in cell-survival signaling, we hypothesized that these two molecules together form an optimal combination of transgenes for simultaneous expression to achieve successful engraftment of stem cells and increased angiogenesis. Our results showed that doubly transduced cells for angiopoietin-1 and Akt overexpression (Fig. 4) were significantly more resistant to ischemic stress in vitro and survived and engrafted better in the infarcted rat heart as compared with the cells with the control MSCs. Moreover, an increased myocardial angiogenic response was stable until 3 months of observation (38). All these results signify that the combined gene- and cell-therapy approach has many advantages and may be a superior strategy for myocardial regeneration as compared with either of the two strategies alone.
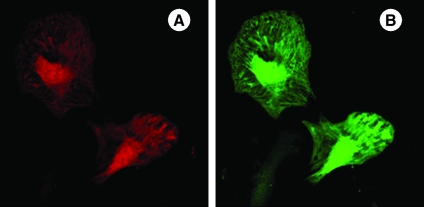
FIG. 4.
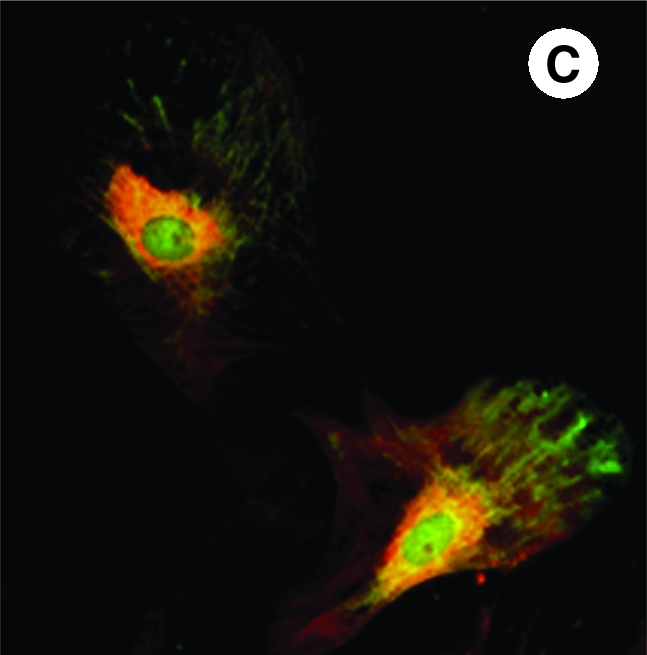
Fluorescent immunostaining of mesenchymal stem cells on day 4 after transduction with adenoviral vectors encoding for (A) Akt (red fluorescence) and (B) angiopoietin-1 (green fluorescence). (C) Merged image showing mesenchymal stem cells simultaneously overexpressing both Akt and angiopoietin-1 (original magnification, × 630). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Concluding Remarks
Heart-cell therapy and angiogenic gene therapy have always been considered to be strategies in competition with each other. With the level of progress made in both of these approaches and with the realization of their strengths and weaknesses, it would be prudent to combine both these approaches and develop a consensus approach to exploit the better aspects of the two approaches. Any such development will help to expedite their establishment for routine clinical applications and lead to better prognosis (87). One, however, should be careful in extrapolating the optimal conditions set for cell engraftment and gene therapy as monotherapies, because gene-modified cells will behave absolutely differently as compared with the nontransduced stem cells. The combined-therapy approach will require that the factors that affect the performance and effectiveness of each of these approaches should be reoptimized. For example, in heart-cell therapy, the general consensus is that the outcome of the procedure is directly related with the number of transplanted stem and progenitor cells (109). However, this statement require rephrasing when genetically modified stem and progenitor cells would be transplanted in the heart because of their ability to express copious amounts of growth factors. The gene expression in the cells may be controlled by insertion of regulatable promoters, which would be sensitive to the lack of oxygen and nutrients in the ischemic myocardial environment. Such manipulation would help in controlling an unbridled transgene overexpression after engraftment of the cells, thus addressing the safety concerns regarding the new arrangement. The development of an optimal nonviral vector will further address the safety concerns. The cells can be transduced to overexpress multiple genes for a multimodal-therapy approach and to achieve a synergism between different growth factors in conjunction with stem cell engraftment to maximize their interplay for an angiomyogenic response (167).
Acknowledgments
This work was supported by National Institutes of Health grants R37-HL074272, HL-080686, and HL087246 to (M.A) and HL087288 and HL089535 to (Kh.H.H).
Abbreviations
bFGF, basic fibroblast growth factor; BMSCs, bone marrow stem cells; CABG, coronary artery bypass graft surgery; ESCs, embryonic stem cells; GCSF, granulocyte colony-stimulating factor; HGF, hepatocyte growth factor; MSCs, mesenchymal stem cells; PEI, polyethyleneimine; PTCA, percutaneous transluminal coronary angioplasty; SCF, stem cell factor; SDF-1α, stromal cell–derived factor 1-α; SkMs, skeletal myoblasts; VEGF, vascular endothelial growth factor.
References
- 1.AHA. Heart Disease and Stroke Statistics, 2008 Update. http://www.americanheart.org/presenter.jhtml?identifier=3037327 http://www.americanheart.org/presenter.jhtml?identifier=3037327
- 2.Ahmad N. Wang Y. Haider KH. Wang B. Pasha Z. Uzun O. Ashraf M. Cardiac protection by mitoKATP channels is dependent on Akt translocation from cytosol to mitochondria during late preconditioning. Am J Physiol Heart Circ Physiol. 2006;290:H2402–H2408. doi: 10.1152/ajpheart.00737.2005. [DOI] [PubMed] [Google Scholar]
- 3.Asahara T. Takahashi T. Masuda H. Kalka C. Chen D. Iwaguro H. Inai Y. Silver M. Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Assmus B. Schachinger V. Teupe C. Britten M. Lehmann R. Dobert N. Grunwald F. Aicher A. Urbich C. Martin H. Hoelzer D. Dimmeler S. Zeiher AM. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI) Circulation. 2002;106:3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 5.Atkins BZ. Hueman MT. Meuchel J. Hutcheson KA. Glower DD. Taylor DA. Cellular cardiomyoplasty improves diastolic properties of injured heart. J Surg Res. 1999;85:234–242. doi: 10.1006/jsre.1999.5681. [DOI] [PubMed] [Google Scholar]
- 6.Atluri P. Hiesinger W. Gorman RC. Pochettino A. Jessup M. Acker MA. Morris RJ. Woo YJ. Cardiac retransplantation is an efficacious therapy for primary cardiac allograft failure. J Cardiothorac Surg. 2008;3:26. doi: 10.1186/1749-8090-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atluri P. Woo YJ. Pro-angiogenic cytokines as cardiovascular therapeutics: assessing the potential. BioDrugs. 2008;22:209–222. doi: 10.2165/00063030-200822040-00001. [DOI] [PubMed] [Google Scholar]
- 8.Badorff C. Brandes RP. Popp R. Rupp S. Urbich C. Aicher A. Fleming I. Busse R. Zeiher AM. Dimmeler S. Transdifferentiation of blood-derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation. 2003;107:1024–1032. doi: 10.1161/01.cir.0000051460.85800.bb. [DOI] [PubMed] [Google Scholar]
- 9.Balsam LB. Wagers AJ. Christensen JL. Kofidis T. Weissman IL. Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 10.Battler A. Scheinowitz M. Bor A. Hasdai D. Vered Z. Di Segni E. Varda-Bloom N. Nass D. Engelberg S. Eldar M. Intracoronary injection of basic fibroblast growth factor enhances angiogenesis in infarcted swine myocardium. J Am Coll Cardiol. 1993;22:2001–2006. doi: 10.1016/0735-1097(93)90790-8. [DOI] [PubMed] [Google Scholar]
- 11.Beck L., Jr D'Amore PA. Vascular development: cellular and molecular regulation. FASEB J. 1997;11:365–373. [PubMed] [Google Scholar]
- 12.Bel A. Messas E. Agbulut O. Richard P. Samuel JL. Bruneval P. Hagege AA. Menasche P. Transplantation of autologous fresh bone marrow into infarcted myocardium: a word of caution. Circulation. 2003;108(suppl 1):II247–H252. doi: 10.1161/01.cir.0000089040.11131.d4. [DOI] [PubMed] [Google Scholar]
- 13.Beltrami AP. Barlucchi L. Torella D. Baker M. Limana F. Chimenti S. Kasahara H. Rota M. Musso E. Urbanek K. Leri A. Kajstura J. Nadal-Ginard B. Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 14.Beltrami AP. Urbanek K. Kajstura J. Yan SM. Finato N. Bussani R. Nadal-Ginard B. Silvestri F. Leri A. Beltrami CA. Anversa P. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 15.Bougioukas I. Didilis V. Ypsilantis P. Giatromanolaki A. Sivridis E. Lialiaris T. Mikroulis D. Simopoulos C. Bougioukas G. Intramyocardial injection of low-dose basic fibroblast growth factor or vascular endothelial growth factor induces angiogenesis in the infarcted rabbit myocardium. Cardiovasc Pathol. 2007;16:63–68. doi: 10.1016/j.carpath.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Brasselet C. Morichetti MC. Messas E. Carrion C. Bissery A. Bruneval P. Vilquin JT. Lafont A. Hagege AA. Menasche P. Desnos M. Skeletal myoblast transplantation through a catheter-based coronary sinus approach: an effective means of improving function of infarcted myocardium. Eur Heart J. 2005;26:1551–1556. doi: 10.1093/eurheartj/ehi151. [DOI] [PubMed] [Google Scholar]
- 17.Bull DA. Bailey SH. Rentz JJ. Zebrack JS. Lee M. Litwin SE. Kim SW. Effect of Terplex/VEGF-165 gene therapy on left ventricular function and structure following myocardial infarction: VEGF gene therapy for myocardial infarction. J Control Release. 2003;93:175–181. doi: 10.1016/j.jconrel.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Chen SL. Fang WW. Ye F. Liu YH. Qian J. Shan SJ. Zhang JJ. Chunhua RZ. Liao LM. Lin S. Sun JP. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92–95. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 19.Dawn B. Stein AB. Urbanek K. Rota M. Whang B. Rastaldo R. Torella D. Tang XL. Rezazadeh A. Kajstura J. Leri A. Hunt G. Varma J. Prabhu SD. Anversa P. Bolli R. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc Natl Acad Sci U S A. 2005;102:3766–3771. doi: 10.1073/pnas.0405957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dib N. Michler RE. Pagani FD. Wright S. Kereiakes DJ. Lengerich R. Binkley P. Buchele D. Anand I. Swingen C. Di Carli MF. Thomas JD. Jaber WA. Opie SR. Campbell A. McCarthy P. Yeager M. Dilsizian V. Griffith BP. Korn R. Kreuger SK. Ghazoul M. MacLellan WR. Fonarow G. Eisen HJ. Dinsmore J. Diethrich E. Safety and feasibility of autologous myoblast transplantation in patients with ischemic cardiomyopathy: four-year follow-up. Circulation. 2005;112:1748–1755. doi: 10.1161/CIRCULATIONAHA.105.547810. [DOI] [PubMed] [Google Scholar]
- 21.Elmadbouh I. Haider H. Jiang S. Idris NM. Lu G. Ashraf M. Ex vivo delivered stromal cell-derived factor-1alpha promotes stem cell homing and induces angiomyogenesis in the infarcted myocardium. J Mol Cell Cardiol. 2007;42:792–803. doi: 10.1016/j.yjmcc.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emanueli C. Madeddu P. Angiogenesis gene therapy to rescue ischaemic tissues: achievements and future directions. Br J Pharmacol. 2001;133:951–958. doi: 10.1038/sj.bjp.0704155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrara N. Vascular endothelial growth factor: molecular and biological aspects. Curr Top Microbiol Immunol. 1999;237:1–30. doi: 10.1007/978-3-642-59953-8_1. [DOI] [PubMed] [Google Scholar]
- 24.Ferrarini M. Arsic N. Recchia FA. Zentilin L. Zacchigna S. Xu X. Linke A. Giacca M. Hintze TH. Adeno-associated virus-mediated transduction of VEGF165 improves cardiac tissue viability and functional recovery after permanent coronary occlusion in conscious dogs. Circ Res. 2006;98:954–961. doi: 10.1161/01.RES.0000217342.83731.89. [DOI] [PubMed] [Google Scholar]
- 25.Floyd SS., Jr Clemens PR. Ontell MR. Kochanek S. Day CS. Yang J. Hauschka SD. Balkir L. Morgan J. Moreland MS. Feero GW. Epperly M. Huard J. Ex vivo gene transfer using adenovirus-mediated full-length dystrophin delivery to dystrophic muscles. Gene Ther. 1998;5:19–30. doi: 10.1038/sj.gt.3300549. [DOI] [PubMed] [Google Scholar]
- 26.Fouts K. Fernandes B. Mal N. Liu J. Laurita KR. Electrophysiological consequence of skeletal myoblast transplantation in normal and infarcted canine myocardium. Heart Rhythm. 2006;3:452–461. doi: 10.1016/j.hrthm.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 27.Fuchs S. Satler LF. Kornowski R. Okubagzi P. Weisz G. Baffour R. Waksman R. Weissman NJ. Cerqueira M. Leon MB. Epstein SE. Catheter-based autologous bone marrow myocardial injection in no-option patients with advanced coronary artery disease: a feasibility study. J Am Coll Cardiol. 2003;41:1721–1724. doi: 10.1016/s0735-1097(03)00328-0. [DOI] [PubMed] [Google Scholar]
- 28.Fukuda S. Yoshii S. Kaga S. Matsumoto M. Kugiyama K. Maulik N. Angiogenic strategy for human ischemic heart disease: brief overview. Mol Cell Biochem. 2004;264:143–149. doi: 10.1023/b:mcbi.0000044383.01785.05. [DOI] [PubMed] [Google Scholar]
- 29.Galinanes M. Loubani M. Davies J. Chin D. Pasi J. Bell PR. Autotransplantation of unmanipulated bone marrow into scarred myocardium is safe and enhances cardiac function in humans. Cell Transplant. 2004;13:7–13. doi: 10.3727/000000004772664842. [DOI] [PubMed] [Google Scholar]
- 30.Gavira JJ. Herreros J. Perez A. Garcia-Velloso MJ. Barba J. Martin-Herrero F. Canizo C. Martin-Arnau A. Marti-Climent JM. Hernandez M. Lopez-Holgado N. Gonzalez-Santos JM. Martin-Luengo C. Alegria E. Prosper F. Autologous skeletal myoblast transplantation in patients with nonacute myocardial infarction: 1-year follow-up. J Thorac Cardiovasc Surg. 2006;131:799–804. doi: 10.1016/j.jtcvs.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 31.Giordano FJ. Ping P. McKirnan MD. Nozaki S. DeMaria AN. Dillmann WH. Mathieu-Costello O. Hammond HK. Intracoronary gene transfer of fibroblast growth factor-5 increases blood flow and contractile function in an ischemic region of the heart. Nat Med. 1996;2:534–539. doi: 10.1038/nm0596-534. [DOI] [PubMed] [Google Scholar]
- 32.Gnecchi M. He H. Noiseux N. Liang OD. Zhang L. Morello F. Mu H. Melo LG. Pratt RE. Ingwall JS. Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 33.Gojo S. Gojo N. Takeda Y. Mori T. Abe H. Kyo S. Hata J. Umezawa A. In vivo cardiovasculogenesis by direct injection of isolated adult mesenchymal stem cells. Exp Cell Res. 2003;288:51–59. doi: 10.1016/s0014-4827(03)00132-0. [DOI] [PubMed] [Google Scholar]
- 34.Grines CL. Watkins MW. Mahmarian JJ. Iskandrian AE. Rade JJ. Marrott P. Pratt C. Kleiman N. A randomized, double-blind, placebo-controlled trial of Ad5FGF-4 gene therapy and its effect on myocardial perfusion in patients with stable angina. J Am Coll Cardiol. 2003;42:1339–1347. doi: 10.1016/s0735-1097(03)00988-4. [DOI] [PubMed] [Google Scholar]
- 35.Gyongyosi M. Khorsand A. Zamini S. Sperker W. Strehblow C. Kastrup J. Jorgensen E. Hesse B. Tagil K. Botker HE. Ruzyllo W. Teresinska A. Dudek D. Hubalewska A. Ruck A. Nielsen SS. Graf S. Mundigler G. Novak J. Sochor H. Maurer G. Glogar D. Sylven C. NOGA-guided analysis of regional myocardial perfusion abnormalities treated with intramyocardial injections of plasmid encoding vascular endothelial growth factor A-165 in patients with chronic myocardial ischemia: subanalysis of the EUROINJECT-ONE multicenter double-blind randomized study. Circulation. 2005;112:I157–I165. doi: 10.1161/01.CIRCULATIONAHA.105.525782. [DOI] [PubMed] [Google Scholar]
- 36.Haider H. Jiang SJ. Lei Y. Law PK. Sim EKW. Effectiveness of transient immunosuppression using cyclosporine for xenomyoblast transplantation for cardiac repair. Transplant Proc. 2004;36:232–235. doi: 10.1016/j.transproceed.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Haider H. Ye L. Jiang S. Ge R. Law PK. Chua T. Wong P. Sim EK. Angiomyogenesis for cardiac repair using human myoblasts as carriers of human vascular endothelial growth factor. J Mol Med. 2004;82:539–549. doi: 10.1007/s00109-004-0546-z. [DOI] [PubMed] [Google Scholar]
- 38.Haider HK. Ashraf M. Strategies to promote donor cell survival: combining preconditioning approach with stem cell transplantation. J Mol Cell Cardiol. 2008;45:554–566. doi: 10.1016/j.yjmcc.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haider HK. Elmadbouh I. Jean-Baptiste M. Ashraf M. Nonviral vector gene modification of stem cells for myocardial repair. Mol Med. 2008;14:79–86. doi: 10.2119/2007-00092.Haider. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halkos ME. Zhao ZQ. Kerendi F. Wang NP. Jiang R. Schmarkey LS. Martin BJ. Quyyumi AA. Few WL. Kin H. Guyton RA. Vinten-Johansen J. Intravenous infusion of mesenchymal stem cells enhances regional perfusion and improves ventricular function in a porcine model of myocardial infarction. Basic Res Cardiol. 2008;103:525–536. doi: 10.1007/s00395-008-0741-0. [DOI] [PubMed] [Google Scholar]
- 41.Hamano K. Li TS. Kobayashi T. Kobayashi S. Matsuzaki M. Esato K. Angiogenesis induced by the implantation of self-bone marrow cells: a new material for therapeutic angiogenesis. Cell Transplant. 2000;9:439–443. doi: 10.1177/096368970000900315. [DOI] [PubMed] [Google Scholar]
- 42.Hamano K. Nishida M. Hirata K. Mikamo A. Li TS. Harada M. Miura T. Matsuzaki M. Esato K. Local implantation of autologous bone marrow cells for therapeutic angiogenesis in patients with ischemic heart disease: clinical trial and preliminary results. Jpn Circ J. 2001;65:845–847. doi: 10.1253/jcj.65.845. [DOI] [PubMed] [Google Scholar]
- 43.Haneline LS. Redox regulation of stem and progenitor cells. Antioxid Redox Signal. 2008;10:1849–1852. doi: 10.1089/ars.2008.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hasegawa T. Kimura A. Miyataka M. Inagaki M. Ishikawa K. Basic fibroblast growth factor increases regional myocardial blood flow and salvages myocardium in the infarct border zone in a rabbit model of acute myocardial infarction. Angiology. 1999;50:487–495. doi: 10.1177/000331979905000607. [DOI] [PubMed] [Google Scholar]
- 45.Hattan N. Warltier D. Gu W. Kolz C. Chilian WM. Weihrauch D. Autologous vascular smooth muscle cell-based myocardial gene therapy to induce coronary collateral growth. Am J Physiol Heart Circ Physiol. 2004;287:H488–H493. doi: 10.1152/ajpheart.00145.2004. [DOI] [PubMed] [Google Scholar]
- 46.Hedman M. Hartikainen J. Syvanne M. Stjernvall J. Hedman A. Kivela A. Vanninen E. Mussalo H. Kauppila E. Simula S. Narvanen O. Rantala A. Peuhkurinen K. Nieminen MS. Laakso M. Yla-Herttuala S. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: phase II results of the Kuopio Angiogenesis Trial (KAT) Circulation. 2003;107:2677–2683. doi: 10.1161/01.CIR.0000070540.80780.92. [DOI] [PubMed] [Google Scholar]
- 47.Heilmann C. von Samson P. Schlegel K. Attmann T. von Specht BU. Beyersdorf F. Lutter G. Comparison of protein with DNA therapy for chronic myocardial ischemia using fibroblast growth factor-2. Eur J Cardiothorac Surg. 2002;22:957–964. doi: 10.1016/s1010-7940(02)00577-8. [DOI] [PubMed] [Google Scholar]
- 48.Henry TD. Annex BH. McKendall GR. Azrin MA. Lopez JJ. Giordano FJ. Shah PK. Willerson JT. Benza RL. Berman DS. Gibson CM. Bajamonde A. Rundle AC. Fine J. McCluskey ER. The VIVA trial: vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation. 2003;107:1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 49.Herreros J. Prosper F. Perez A. Gavira JJ. Garcia-Velloso MJ. Barba J. Sanchez PL. Canizo C. Rabago G. Marti-Climent JM. Hernandez M. Lopez-Holgado N. Gonzalez-Santos JM. Martin-Luengo C. Alegria E. Autologous intramyocardial injection of cultured skeletal muscle-derived stem cells in patients with non-acute myocardial infarction. Eur Heart J. 2003;24:2012–2020. doi: 10.1016/j.ehj.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 50.Hirata Y. Sata M. Motomura N. Takanashi M. Suematsu Y. Ono M. Takamoto S. Human umbilical cord blood cells improve cardiac function after myocardial infarction. Biochem Biophys Res Commun. 2005;327:609–614. doi: 10.1016/j.bbrc.2004.12.044. [DOI] [PubMed] [Google Scholar]
- 51.Hodgetts SI. Beilharz MW. Scalzo AA. Grounds MD. Why do cultured transplanted myoblasts die in vivo? DNA quantification shows enhanced survival of donor male myoblasts in host mice depleted of CD4+ and CD8+ cells or Nk1.1+ cells. Cell Transplant. 2000;9:489–502. doi: 10.1177/096368970000900406. [DOI] [PubMed] [Google Scholar]
- 52.Horvath KA. Doukas J. Lu CY. Belkind N. Greene R. Pierce GF. Fullerton DA. Myocardial functional recovery after fibroblast growth factor 2 gene therapy as assessed by echocardiography and magnetic resonance imaging. Ann Thorac Surg. 2002;74:481–486. doi: 10.1016/s0003-4975(02)03736-0. [DOI] [PubMed] [Google Scholar]
- 53.Hughes GC. Biswas SS. Yin B. Coleman RE. DeGrado TR. Landolfo CK. Lowe JE. Annex BH. Landolfo KP. Therapeutic angiogenesis in chronically ischemic porcine myocardium: comparative effects of bFGF and VEGF. Ann Thorac Surg. 2004;77:812–818. doi: 10.1016/j.athoracsur.2003.09.060. [DOI] [PubMed] [Google Scholar]
- 54.Jebreel A. England J. Bedford K. Murphy J. Karsai L. Atkin S. Vascular endothelial growth factor (VEGF), VEGF receptors expression and microvascular density in benign and malignant thyroid diseases. Int J Exp Pathol. 2007;88:271–277. doi: 10.1111/j.1365-2613.2007.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang S. Haider H. Idris NM. Salim A. Ashraf M. Supportive interaction between cell survival signaling and angiocompetent factors enhances donor cell survival and promotes angiomyogenesis for cardiac repair. Circ Res. 2006;99:776–784. doi: 10.1161/01.RES.0000244687.97719.4f. [DOI] [PubMed] [Google Scholar]
- 56.Kajstura J. Leri A. Finato N. Di Loreto C. Beltrami CA. Anversa P. Myocyte proliferation in end-stage cardiac failure in humans. Proc Natl Acad Sci U S A. 1998;95:8801–8805. doi: 10.1073/pnas.95.15.8801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kajstura J. Rota M. Whang B. Cascapera S. Hosoda T. Bearzi C. Nurzynska D. Kasahara H. Zias E. Bonafe M. Nadal-Ginard B. Torella D. Nascimbene A. Quaini F. Urbanek K. Leri A. Anversa P. Bone marrow cells differentiate in cardiac cell lineages after infarction independently of cell fusion. Circ Res. 2005;96:127–137. doi: 10.1161/01.RES.0000151843.79801.60. [DOI] [PubMed] [Google Scholar]
- 58.Kajstura J. Urbanek K. Rota M. Bearzi C. Hosoda T. Bolli R. Anversa P. Leri A. Cardiac stem cells and myocardial disease. J Mol Cell Cardiol. 2008;45:505–513. doi: 10.1016/j.yjmcc.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 59.Kardami E. Detillieux K. Ma X. Jiang Z. Santiago JJ. Jimenez SK. Cattini PA. Fibroblast growth factor-2 and cardioprotection. Heart Fail Rev. 2007;12:267–77. doi: 10.1007/s10741-007-9027-0. [DOI] [PubMed] [Google Scholar]
- 60.Kawamoto A. Murayama T. Kusano K. Ii M. Tkebuchava T. Shintani S. Iwakura A. Johnson I. von Samson P. Hanley A. Gavin M. Curry C. Silver M. Ma H. Kearney M. Losordo DW. Synergistic effect of bone marrow mobilization and vascular endothelial growth factor-2 gene therapy in myocardial ischemia. Circulation. 2004;110:1398–1405. doi: 10.1161/01.CIR.0000141563.71410.64. [DOI] [PubMed] [Google Scholar]
- 61.Khan M. Kutala VK. Vikram DS. Wisel S. Chacko SM. Kuppusamy ML. Mohan IK. Zweier JL. Kwiatkowski P. Kuppusamy P. Skeletal myoblasts transplanted in the ischemic myocardium enhance in situ oxygenation and recovery of contractile function. Am J Physiol Heart Circ Physiol. 2007;293:H2129–H2139. doi: 10.1152/ajpheart.00677.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim BO. Tian H. Prasongsukarn K. Wu J. Angoulvant D. Wnendt S. Muhs A. Spitkovsky D. Li RK. Cell transplantation improves ventricular function after a myocardial infarction: a preclinical study of human unrestricted somatic stem cells in a porcine model. Circulation. 2005;112:I96–104. doi: 10.1161/01.CIRCULATIONAHA.105.524678. [DOI] [PubMed] [Google Scholar]
- 63.Kim EJ. Li RK. Weisel RD. Mickle DA. Jia ZQ. Tomita S. Sakai T. Yau TM. Angiogenesis by endothelial cell transplantation. J Thorac Cardiovasc Surg. 2001;122:963–971. doi: 10.1067/mtc.2001.117623. [DOI] [PubMed] [Google Scholar]
- 64.Kocher AA. Schuster MD. Szabolcs MJ. Takuma S. Burkhoff D. Wang J. Homma S. Edwards NM. Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–46. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 65.Koh GY. Klug MG. Soonpaa MH. Field LJ. Differentiation and long-term survival of C2C12 myoblast grafts in heart. J Clin Invest. 1993;92:1548–1554. doi: 10.1172/JCI116734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kornowski R. Fuchs S. Leon MB. Epstein SE. Delivery strategies to achieve therapeutic myocardial angiogenesis. Circulation. 2000;101:454–458. doi: 10.1161/01.cir.101.4.454. [DOI] [PubMed] [Google Scholar]
- 67.Kudo M. Wang Y. Wani MA. Xu M. Ayub A. Ashraf M. Implantation of bone marrow stem cells reduces the infarction and fibrosis in ischemic mouse heart. J Mol Cell Cardiol. 2003;35:1113–1119. doi: 10.1016/s0022-2828(03)00211-6. [DOI] [PubMed] [Google Scholar]
- 68.Laham RJ. Chronos NA. Pike M. Leimbach ME. Udelson JE. Pearlman JD. Pettigrew RI. Whitehouse MJ. Yoshizawa C. Simons M. Intracoronary basic fibroblast growth factor (FGF-2) in patients with severe ischemic heart disease: results of a phase I open-label dose escalation study. J Am Coll Cardiol. 2000;36:2132–2139. doi: 10.1016/s0735-1097(00)00988-8. [DOI] [PubMed] [Google Scholar]
- 69.Laham RJ. Post M. Rezaee M. Donnell-Fink L. Wykrzykowska JJ. Lee SU. Baim DS. Sellke FW. Transendocardial and transepicardial intramyocardial fibroblast growth factor-2 administration: myocardial and tissue distribution. Drug Metab Dispos. 2005;33:1101–1107. doi: 10.1124/dmd.104.002774. [DOI] [PubMed] [Google Scholar]
- 70.Laham RJ. Sellke FW. Edelman ER. Pearlman JD. Ware JA. Brown DL. Gold JP. Simons M. Local perivascular delivery of basic fibroblast growth factor in patients undergoing coronary bypass surgery: results of a phase I randomized, double-blind, placebo-controlled trial. Circulation. 1999;100:1865–1871. doi: 10.1161/01.cir.100.18.1865. [DOI] [PubMed] [Google Scholar]
- 71.Law PK. Fang G. Chua F. Kakuchaya T. Bockeria LA. First-in-man myoblast allografts for heart degeneration. Int J Med Implants Devices. 2003;1:100–155. [Google Scholar]
- 72.Lazarous DF. Shou M. Stiber JA. Hodge E. Thirumurti V. Goncalves L. Unger EF. Adenoviral-mediated gene transfer induces sustained pericardial VEGF expression in dogs: effect on myocardial angiogenesis. Cardiovasc Res. 1999;44:294–302. doi: 10.1016/s0008-6363(99)00203-5. [DOI] [PubMed] [Google Scholar]
- 73.Lee JS. Byun J. Kim JM. Kim CY. Kim BM. Chung JH. Jang Y. Kim DK. Cardiac expression profiles of the naked DNA vectors encoding vascular endothelial growth factor and basic fibroblast growth factor. Exp Mol Med. 2005;37:447–456. doi: 10.1038/emm.2005.55. [DOI] [PubMed] [Google Scholar]
- 74.Lee RJ. Springer ML. Blanco-Bose WE. Shaw R. Ursell PC. Blau HM. VEGF gene delivery to myocardium: deleterious effects of unregulated expression. Circulation. 2000;102:898–901. doi: 10.1161/01.cir.102.8.898. [DOI] [PubMed] [Google Scholar]
- 75.Leor J. Guetta E. Chouraqui P. Guetta V. Nagler A. Human umbilical cord blood cells: a new alternative for myocardial repair? Cytotherapy. 2005;7:251–257. doi: 10.1080/14653240510027163. [DOI] [PubMed] [Google Scholar]
- 76.Li RK. Jia ZQ. Weisel RD. Merante F. Mickle DA. Smooth muscle cell transplantation into myocardial scar tissue improves heart function. J Mol Cell Cardiol. 1999;31:513–522. doi: 10.1006/jmcc.1998.0882. [DOI] [PubMed] [Google Scholar]
- 77.Li RK. Weisel RD. Mickle DA. Jia ZQ. Kim EJ. Sakai T. Tomita S. Schwartz L. Iwanochko M. Husain M. Cusimano RJ. Burns RJ. Yau TM. Autologous porcine heart cell transplantation improved heart function after a myocardial infarction. J Thorac Cardiovasc Surg. 2000;119:62–68. doi: 10.1016/s0022-5223(00)70218-2. [DOI] [PubMed] [Google Scholar]
- 78.Liao S. Porter D. Scott A. Newman G. Doetschman T. Schultz JJ. The cardioprotective effect of the low molecular weight isoform of fibroblast growth factor-2: the role of JNK signaling. J Mol Cell Cardiol. 2007;42:106–120. doi: 10.1016/j.yjmcc.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lim SY. Kim YS. Youngkeun AY. Jeong MH. Hong MH. Joo SY. Nam KI. Cho JG. Kang PM. Park JC. The effects of mesenchymal stem cells transduced with Akt in a porcine myocardial infarction model. Cardiovasc Res. 2006;70:530–542. doi: 10.1016/j.cardiores.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 80.Losordo DW. Vale PR. Hendel RC. Milliken CE. Fortuin FD. Cummings N. Schatz RA. Asahara T. Isner JM. Kuntz RE. Phase 1/2 placebo-controlled, double-blind, dose-escalating trial of myocardial vascular endothelial growth factor 2 gene transfer by catheter delivery in patients with chronic myocardial ischemia. Circulation. 2002;105:2012–2018. doi: 10.1161/01.cir.0000015982.70785.b7. [DOI] [PubMed] [Google Scholar]
- 81.Lungwitz U. Breunig M. Blunk T. Gopferich A. Polyethylenimine-based non-viral gene delivery systems. Eur J Pharm Biopharm. 2005;60:247–266. doi: 10.1016/j.ejpb.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 82.Ma N. Stamm C. Kaminski A. Li W. Kleine HD. Muller-Hilke B. Zhang L. Ladilov Y. Egger D. Steinhoff G. Human cord blood cells induce angiogenesis following myocardial infarction in NOD/scid-mice. Cardiovasc Res. 2005;66:45–54. doi: 10.1016/j.cardiores.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 83.Mack CA. Patel SR. Schwarz EA. Zanzonico P. Hahn RT. Ilercil A. Devereux RB. Goldsmith SJ. Christian TF. Sanborn TA. Kovesdi I. Hackett N. Isom OW. Crystal RG. Rosengart TK. Biologic bypass with the use of adenovirus-mediated gene transfer of the complementary deoxyribonucleic acid for vascular endothelial growth factor 121 improves myocardial perfusion and function in the ischemic porcine heart. J Thorac Cardiovasc Surg. 1998;115:168–176. doi: 10.1016/s0022-5223(98)70455-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mangi AA. Noiseux N. Kong D. He H. Rezvani M. Ingwall JS. Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 85.Matsumoto R. Omura T. Yoshiyama M. Hayashi T. Inamoto S. Koh KR. Ohta K. Izumi Y. Nakamura Y. Akioka K. Kitaura Y. Takeuchi K. Yoshikawa J. Vascular endothelial growth factor-expressing mesenchymal stem cell transplantation for the treatment of acute myocardial infarction. Arterioscler Thromb Vasc Biol. 2005;25:1168–1173. doi: 10.1161/01.ATV.0000165696.25680.ce. [DOI] [PubMed] [Google Scholar]
- 86.Maulik N. Redox signaling of angiogenesis. Antioxid Redox Signal. 2002;4:805–815. doi: 10.1089/152308602760598963. [DOI] [PubMed] [Google Scholar]
- 87.Maulik N. Thirunavukkarasu M. Growth factors and cell therapy in myocardial regeneration. J Mol Cell Cardiol. 2008;44:219–227. doi: 10.1016/j.yjmcc.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 88.Mazo M. Planat-Benard V. Abizanda G. Pelacho B. Leobon B. Gavira JJ. Penuelas I. Cemborain A. Penicaud L. Laharrague P. Joffre C. Boisson M. Ecay M. Collantes M. Barba J. Casteilla L. Prosper F. Transplantation of adipose derived stromal cells is associated with functional improvement in a rat model of chronic myocardial infarction. Eur J Heart Fail. 2008;10:454–462. doi: 10.1016/j.ejheart.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 89.Menasche P. Alfieri O. Janssens S. McKenna W. Reichenspurner H. Trinquart L. Vilquin JT. Marolleau JP. Seymour B. Larghero J. Lake S. Chatellier G. Solomon S. Desnos M. Hagege AA. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189–1200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 90.Menasche P. Hagege AA. Scorsin M. Pouzet B. Desnos M. Duboc D. Schwartz K. Vilquin JT. Marolleau JP. Myoblast transplantation for heart failure. Lancet. 2001;357:279–280. doi: 10.1016/S0140-6736(00)03617-5. [DOI] [PubMed] [Google Scholar]
- 91.Menasche P. Hagege AA. Vilquin JT. Desnos M. Abergel E. Pouzet B. Bel A. Sarateanu S. Scorsin M. Schwartz K. Bruneval P. Benbunan M. Marolleau JP. Duboc D. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol. 2003;41:1078–1083. doi: 10.1016/s0735-1097(03)00092-5. [DOI] [PubMed] [Google Scholar]
- 92.Merkle CJ. Montgomery DW. Gene therapy with vascular endothelial growth factor reduces angina. J Cardiovasc Nurs. 2003;18:38–43. doi: 10.1097/00005082-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 93.Miyagawa S. Sawa Y. Taketani S. Kawaguchi N. Nakamura T. Matsuura N. Matsuda H. Myocardial regeneration therapy for heart failure: hepatocyte growth factor enhances the effect of cellular cardiomyoplasty. Circulation. 2002;105:2556–2561. doi: 10.1161/01.cir.0000016722.37138.f2. [DOI] [PubMed] [Google Scholar]
- 94.Miyamoto Y. Suyama T. Yashita T. Akimaru H. Kurata H. Bone marrow subpopulations contain distinct types of endothelial progenitor cells and angiogenic cytokine-producing cells. J Mol Cell Cardiol. 2007;43:627–635. doi: 10.1016/j.yjmcc.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 95.Muller-Ehmsen J. Whittaker P. Kloner RA. Dow JS. Sakoda T. Long TI. Laird PW. Kedes L. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J Mol Cell Cardiol. 2002;34:107–116. doi: 10.1006/jmcc.2001.1491. [DOI] [PubMed] [Google Scholar]
- 96.Murry CE. Jennings RB. Reimer KA. New insights into potential mechanisms of ischemic preconditioning. Circulation. 1991;84:442–445. doi: 10.1161/01.cir.84.1.442. [DOI] [PubMed] [Google Scholar]
- 97.Murry CE. Kay MA. Bartosek T. Hauschka SD. Schwartz SM. Muscle differentiation during repair of myocardial necrosis in rats via gene transfer with MyoD. J Clin Invest. 1996;98:2209–2217. doi: 10.1172/JCI119030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Murry CE. Soonpaa MH. Reinecke H. Nakajima H. Nakajima HO. Rubart M. Pasumarthi KB. Virag JI. Bartelmez SH. Poppa V. Bradford G. Dowell JD. Williams DA. Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 99.Murry CE. Wiseman RW. Schwartz SM. Hauschka SD. Skeletal myoblast transplantation for repair of myocardial necrosis. J Clin Invest. 1996;98:2512–2523. doi: 10.1172/JCI119070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Neufeld G. Kessler O. Pro-angiogenic cytokines and their role in tumor angiogenesis. Cancer Metastasis Rev. 2006;25:373–385. doi: 10.1007/s10555-006-9011-5. [DOI] [PubMed] [Google Scholar]
- 101.Niagara MI. Haider H. Jiang S. Ashraf M. Pharmacologically preconditioned skeletal myoblasts are resistant to oxidative stress and promote angiomyogenesis via release of paracrine factors in the infarcted heart. Circ Res. 2007;100:545–555. doi: 10.1161/01.RES.0000258460.41160.ef. [DOI] [PubMed] [Google Scholar]
- 102.Nillesen ST. Geutjes PJ. Wismans R. Schalkwijk J. Daamen WF. van Kuppevelt TH. Increased angiogenesis and blood vessel maturation in acellular collagen-heparin scaffolds containing both FGF2 and VEGF. Biomaterials. 2007;28:1123–1131. doi: 10.1016/j.biomaterials.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 103.Ninomiya M. Koyama H. Miyata T. Hamada H. Miyatake S. Shigematsu H. Takamoto S. Ex vivo gene transfer of basic fibroblast growth factor improves cardiac function and blood flow in a swine chronic myocardial ischemia model. Gene Ther. 2003;10:1152–1160. doi: 10.1038/sj.gt.3301984. [DOI] [PubMed] [Google Scholar]
- 104.Ohara N. Koyama H. Miyata T. Hamada H. Miyatake SI. Akimoto M. Shigematsu H. Adenovirus-mediated ex vivo gene transfer of basic fibroblast growth factor promotes collateral development in a rabbit model of hind limb ischemia. Gene Ther. 2001;8:837–845. doi: 10.1038/sj.gt.3301475. [DOI] [PubMed] [Google Scholar]
- 105.Orlic D. Kajstura J. Chimenti S. Jakoniuk I. Anderson SM. Li B. Pickel J. McKay R. Nadal-Ginard B. Bodine DM. Leri A. Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 106.Pagani FD. DerSimonian H. Zawadzka A. Wetzel K. Edge AS. Jacoby DB. Dinsmore JH. Wright S. Aretz TH. Eisen HJ. Aaronson KD. Autologous skeletal myoblasts transplanted to ischemia-damaged myocardium in humans: histological analysis of cell survival and differentiation. J Am Coll Cardiol. 2003;41:879–888. doi: 10.1016/s0735-1097(03)00081-0. [DOI] [PubMed] [Google Scholar]
- 107.Pagel CN. Morgan JE. Myoblast transfer and gene therapy in muscular dystrophies. Microsc Res Tech. 1995;30:469–479. doi: 10.1002/jemt.1070300604. [DOI] [PubMed] [Google Scholar]
- 108.Perin E. Transendocardial injection of autologous mononuclear bone marrow cells in end-stage ischemic heart failure patients: one-year follow-up. Int J Cardiol. 2004;95(suppl 1):S45–S46. doi: 10.1016/s0167-5273(04)90013-7. [DOI] [PubMed] [Google Scholar]
- 109.Pouzet B. Vilquin JT. Hagege AA. Scorsin M. Messas E. Fiszman M. Schwartz K. Menasche P. Intramyocardial transplantation of autologous myoblasts: can tissue processing be optimized? Circulation. 2000;102:III210–III215. doi: 10.1161/01.cir.102.suppl_3.iii-210. [DOI] [PubMed] [Google Scholar]
- 110.Radke PW. Heinl-Green A. Frass OM. Griesenbach U. Ferrari S. Geddes DM. Alton EW. Effects of intramyocardial pVEGF165 delivery on regional myocardial blood flow: evidence for a spatial “delivery-efficacy” mismatch. Gene Ther. 2004;11:1249–1255. doi: 10.1038/sj.gt.3302296. [DOI] [PubMed] [Google Scholar]
- 111.Rando TA. Pavlath GK. Blau HM. The fate of myoblasts following transplantation into mature muscle. Exp Cell Res. 1995;220:383–389. doi: 10.1006/excr.1995.1329. [DOI] [PubMed] [Google Scholar]
- 112.Reffelmann T. Dow JS. Dai W. Hale SL. Simkhovich BZ. Kloner RA. Transplantation of neonatal cardiomyocytes after permanent coronary artery occlusion increases regional blood flow of infarcted myocardium. J Mol Cell Cardiol. 2003;35:607–613. doi: 10.1016/s0022-2828(03)00081-6. [DOI] [PubMed] [Google Scholar]
- 113.Reinecke H. Poppa V. Murry CE. Skeletal muscle stem cells do not transdifferentiate into cardiomyocytes after cardiac grafting. J Mol Cell Cardiol. 2002;34:241–249. doi: 10.1006/jmcc.2001.1507. [DOI] [PubMed] [Google Scholar]
- 114.Reinlib L. Field L. Cell transplantation as future therapy for cardiovascular disease? a workshop of the National Heart, Lung, and Blood Institute. Circulation. 2000;101:E182–E187. doi: 10.1161/01.cir.101.18.e182. [DOI] [PubMed] [Google Scholar]
- 115.Renault MA. Losordo DW. Therapeutic myocardial angiogenesis. Microvasc Res. 2007;74:159–171. doi: 10.1016/j.mvr.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Retuerto MA. Schalch P. Patejunas G. Carbray J. Liu N. Esser K. Crystal RG. Rosengart TK. Angiogenic pretreatment improves the efficacy of cellular cardiomyoplasty performed with fetal cardiomyocyte implantation. J Thorac Cardiovasc Surg. 2004;127:1041–1049. doi: 10.1016/j.jtcvs.2003.09.049. [DOI] [PubMed] [Google Scholar]
- 117.Rinsch C. Quinodoz P. Pittet B. Alizadeh N. Baetens D. Montandon D. Aebischer P. Pepper MS. Delivery of FGF-2 but not VEGF by encapsulated genetically engineered myoblasts improves survival and vascularization in a model of acute skin flap ischemia. Gene Ther. 2001;8:523–533. doi: 10.1038/sj.gt.3301436. [DOI] [PubMed] [Google Scholar]
- 118.Ripa RS. Wang Y. Jorgensen E. Johnsen HE. Hesse B. Kastrup J. Intramyocardial injection of vascular endothelial growth factor-A165 plasmid followed by granulocyte-colony stimulating factor to induce angiogenesis in patients with severe chronic ischaemic heart disease. Eur Heart J. 2006;27:1785–1792. doi: 10.1093/eurheartj/ehl117. [DOI] [PubMed] [Google Scholar]
- 119.Rosengart TK. Lee LY. Patel SR. Sanborn TA. Parikh M. Bergman GW. Hachamovitch R. Szulc M. Kligfield PD. Okin PM. Hahn RT. Devereux RB. Post MR. Hackett NR. Foster T. Grasso TM. Lesser ML. Isom OW. Crystal RG. Angiogenesis gene therapy: phase I assessment of direct intramyocardial administration of an adenovirus vector expressing VEGF121 cDNA to individuals with clinically significant severe coronary artery disease. Circulation. 1999;100:468–474. doi: 10.1161/01.cir.100.5.468. [DOI] [PubMed] [Google Scholar]
- 120.Rota M. Kajstura J. Hosoda T. Bearzi C. Vitale S. Esposito G. Iaffaldano G. Padin-Iruegas ME. Gonzalez A. Rizzi R. Small N. Muraski J. Alvarez R. Chen X. Urbanek K. Bolli R. Houser SR. Leri A. Sussman MA. Anversa P. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci U S A. 2007;104:17783–17788. doi: 10.1073/pnas.0706406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rota M. Padin-Iruegas ME. Misao Y. De Angelis A. Maestroni S. Ferreira-Martins J. Fiumana E. Rastaldo R. Arcarese ML. Mitchell TS. Boni A. Bolli R. Urbanek K. Hosoda T. Anversa P. Leri A. Kajstura J. Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ Res. 2008;103:107–116. doi: 10.1161/CIRCRESAHA.108.178525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rufaihah AJ. Haider HK. Heng BC. Ye L. Toh WS. Tian XF. Lu K. Sim EK. Cao T. Directing endothelial differentiation of human embryonic stem cells via transduction with an adenoviral vector expressing the VEGF(165) gene. J Gene Med. 2007;9:452–461. doi: 10.1002/jgm.1034. [DOI] [PubMed] [Google Scholar]
- 123.Saeed M. Saloner D. Martin A. Do L. Weber O. Ursell PC. Jacquier A. Lee R. Higgins CB. Adeno-associated viral vector-encoding vascular endothelial growth factor gene: effect on cardiovascular MR perfusion and infarct resorption measurements in swine. Radiology. 2007;243:451–460. doi: 10.1148/radiol.2432060928. [DOI] [PubMed] [Google Scholar]
- 124.Sakakibara Y. Nishimura K. Tambara K. Yamamoto M. Lu F. Tabata Y. Komeda M. Prevascularization with gelatin microspheres containing basic fibroblast growth factor enhances the benefits of cardiomyocyte transplantation. J Thorac Cardiovasc Surg. 2002;124:50–56. doi: 10.1067/mtc.2002.121293. [DOI] [PubMed] [Google Scholar]
- 125.Schuh A. Liehn EA. Sasse A. Hristov M. Sobota R. Kelm M. Merx MW. Weber C. Transplantation of endothelial progenitor cells improves neovascularization and left ventricular function after myocardial infarction in a rat model. Basic Res Cardiol. 2008;103:69–77. doi: 10.1007/s00395-007-0685-9. [DOI] [PubMed] [Google Scholar]
- 126.Schumacher B. Pecher P. von Specht BU. Stegmann T. Induction of neoangiogenesis in ischemic myocardium by human growth factors: first clinical results of a new treatment of coronary heart disease. Circulation. 1998;97:645–650. doi: 10.1161/01.cir.97.7.645. [DOI] [PubMed] [Google Scholar]
- 127.Schwarz ER. Speakman MT. Patterson M. Hale SS. Isner JM. Kedes LH. Kloner RA. Evaluation of the effects of intramyocardial injection of DNA expressing vascular endothelial growth factor (VEGF) in a myocardial infarction model in the rat: angiogenesis and angioma formation. J Am Coll Cardiol. 2000;35:1323–1330. doi: 10.1016/s0735-1097(00)00522-2. [DOI] [PubMed] [Google Scholar]
- 128.Schwarz F. Flameng W. Mack B. Turschmann W. Schaper W. Vascular and cardiac contractile reserve in the dog heart with chronic multiple coronary occlusions. Am Heart J. 1976;92:600–608. doi: 10.1016/s0002-8703(76)80079-8. [DOI] [PubMed] [Google Scholar]
- 129.Sellke FW. Laham RJ. Edelman ER. Pearlman JD. Simons M. Therapeutic angiogenesis with basic fibroblast growth factor: technique and early results. Ann Thorac Surg. 1998;65:1540–1544. doi: 10.1016/s0003-4975(98)00340-3. [DOI] [PubMed] [Google Scholar]
- 130.Shyu KG. Manor O. Magner M. Yancopoulos GD. Isner JM. Direct intramuscular injection of plasmid DNA encoding angiopoietin-1 but not angiopoietin-2 augments revascularization in the rabbit ischemic hindlimb. Circulation. 1998;98:2081–2087. doi: 10.1161/01.cir.98.19.2081. [DOI] [PubMed] [Google Scholar]
- 131.Sim EK. Haider HK. Aziz S. Ooi OC. Law PK. Myoblast transplantation on the beating heart. Int Surg. 2005;90:148–50. [PubMed] [Google Scholar]
- 132.Simo R. Carrasco E. Garcia-Ramirez M. Hernandez C. Angiogenic and antiangiogenic factors in proliferative diabetic retinopathy. Curr Diabetes Rev. 2006;2:71–98. doi: 10.2174/157339906775473671. [DOI] [PubMed] [Google Scholar]
- 133.Simons M. Integrative signaling in angiogenesis. Mol Cell Biochem. 2004;264:99–102. doi: 10.1023/b:mcbi.0000044379.25823.03. [DOI] [PubMed] [Google Scholar]
- 134.Siu CW. Moore JC. Li RA. Human embryonic stem cell-derived cardiomyocytes for heart therapies. Cardiovasc Hematol Disord Drug Targets. 2007;7:145–152. doi: 10.2174/187152907780830851. [DOI] [PubMed] [Google Scholar]
- 135.Stamm C. Kleine HD. Choi YH. Dunkelmann S. Lauffs JA. Intramyocardial delivery of CD133+ bone marrow cells and coronary artery bypass grafting for chronic ischemic heart disease: safety and efficacy studies. J Thorac Cardiovasc Surg. 2007;133:717–725. doi: 10.1016/j.jtcvs.2006.08.077. [DOI] [PubMed] [Google Scholar]
- 136.Stamm C. Kleine HD. Choi YH. Dunkelmann S. Lauffs JA. Lorenzen B. David A. Liebold A. Nienaber C. Zurakowski D. Freund M. Steinhoff G. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet. 2003;361:45–46. doi: 10.1016/S0140-6736(03)12110-1. [DOI] [PubMed] [Google Scholar]
- 137.Strauer BE. Brehm M. Zeus T. Bartsch T. Schannwell C. Antke C. Sorg RV. Kogler G. Wernet P. Muller HW. Kostering M. Regeneration of human infarcted heart muscle by intracoronary autologous bone marrow cell transplantation in chronic coronary artery disease: the IACT Study. J Am Coll Cardiol. 2005;46:1651–1658. doi: 10.1016/j.jacc.2005.01.069. [DOI] [PubMed] [Google Scholar]
- 138.Suzuki K. Murtuza B. Smolenski RT. Sammut IA. Suzuki N. Kaneda Y. Yacoub MH. Cell transplantation for the treatment of acute myocardial infarction using vascular endothelial growth factor-expressing skeletal myoblasts. Circulation. 2001;104:I207–1212. doi: 10.1161/hc37t1.094524. [DOI] [PubMed] [Google Scholar]
- 139.Symes JF. Losordo DW. Vale PR. Lathi KG. Esakof DD. Mayskiy M. Isner JM. Gene therapy with vascular endothelial growth factor for inoperable coronary artery disease. Ann Thorac Surg. 1999;68:830–836. doi: 10.1016/s0003-4975(99)00807-3. [DOI] [PubMed] [Google Scholar]
- 140.Takahashi K. Ito Y. Morikawa M. Kobune M. Huang J. Tsukamoto M. Sasaki K. Nakamura K. Dehari H. Ikeda K. Uchida H. Hirai S. Abe T. Hamada H. Adenoviral-delivered angiopoietin-1 reduces the infarction and attenuates the progression of cardiac dysfunction in the rat model of acute myocardial infarction. Mol Ther. 2003;8:584–592. doi: 10.1016/s1525-0016(03)00230-2. [DOI] [PubMed] [Google Scholar]
- 141.Takahashi M. Li TS. Suzuki R. Kobayashi T. Ito H. Ikeda Y. Matsuzaki M. Hamano K. Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. Am J Physiol Heart Circ Physiol. 2006;291:H886–H893. doi: 10.1152/ajpheart.00142.2006. [DOI] [PubMed] [Google Scholar]
- 142.Takashi E. Wang Y. Ashraf M. Activation of mitochondrial K(ATP) channel elicits late preconditioning against myocardial infarction via protein kinase C signaling pathway. Circ Res. 1999;85:1146–1153. doi: 10.1161/01.res.85.12.1146. [DOI] [PubMed] [Google Scholar]
- 143.Tambara K. Sakakibara Y. Sakaguchi G. Lu F. Premaratne GU. Lin X. Nishimura K. Komeda M. Transplanted skeletal myoblasts can fully replace the infarcted myocardium when they survive in the host in large numbers. Circulation. 2003;108(Suppl 1):II259–II263. doi: 10.1161/01.cir.0000087430.17543.b8. [DOI] [PubMed] [Google Scholar]
- 144.Thompson RB. van den Bos EJ. Davis BH. Morimoto Y. Craig D. Sutton BS. Glower DD. Taylor DA. Intracardiac transplantation of a mixed population of bone marrow cells improves both regional systolic contractility and diastolic relaxation. J Heart Lung Transplant. 2005;24:205–214. doi: 10.1016/j.healun.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 145.Togel F. Hu Z. Weiss K. Isaac J. Lange C. Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–F42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 146.Toma C. Pittenger MF. Cahill KS. Byrne BJ. Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 147.Tse HF. Siu CW. Zhu SG. Songyan L. Zhang QY. Lai WH. Kwong YL. Nicholls J. Lau CP. Paracrine effects of direct intramyocardial implantation of bone marrow derived cells to enhance neovascularization in chronic ischaemic myocardium. Eur J Heart Fail. 2007;9:747–753. doi: 10.1016/j.ejheart.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 148.Tse HF. Thambar S. Kwong YL. Rowlings P. Bellamy G. McCrohon J. Thomas P. Bastian B. Chan JK. Lo G. Ho CL. Chan WS. Kwong RY. Parker A. Hauser TH. Chan J. Fong DY. Lau CP. Prospective randomized trial of direct endomyocardial implantation of bone marrow cells for treatment of severe coronary artery diseases (PROTECT-CAD trial) Eur Heart J. 2007;28:2998–3005. doi: 10.1093/eurheartj/ehm485. [DOI] [PubMed] [Google Scholar]
- 149.Uemura R. Xu M. Ahmad N. Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98:1414–1421. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 150.Unger EF. Banai S. Shou M. Lazarous DF. Jaklitsch MT. Scheinowitz M. Correa R. Klingbeil C. Epstein SE. Basic fibroblast growth factor enhances myocardial collateral flow in a canine model. Am J Physiol. 1994;266:H1588–H1595. doi: 10.1152/ajpheart.1994.266.4.H1588. [DOI] [PubMed] [Google Scholar]
- 151.Unger EF. Goncalves L. Epstein SE. Chew EY. Trapnell CB. Cannon RO., 3rd Quyyumi AA. Effects of a single intracoronary injection of basic fibroblast growth factor in stable angina pectoris. Am J Cardiol. 2000;85:1414–1419. doi: 10.1016/s0002-9149(00)00787-6. [DOI] [PubMed] [Google Scholar]
- 152.Vale PR. Losordo DW. Milliken CE. Maysky M. Esakof DD. Symes JF. Isner JM. Left ventricular electromechanical mapping to assess efficacy of phVEGF(165) gene transfer for therapeutic angiogenesis in chronic myocardial ischemia. Circulation. 2000;102:965–974. doi: 10.1161/01.cir.102.9.965. [DOI] [PubMed] [Google Scholar]
- 153.Valina C. Pinkernell K. Song YH. Bai X. Sadat S. Campeau RJ. Le Jemtel TH. Alt E. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J. 2007;28:2667–2677. doi: 10.1093/eurheartj/ehm426. [DOI] [PubMed] [Google Scholar]
- 154.Villa A. Sanchez PL. Fernandez-Aviles F. Ventricular arrhythmias following intracoronary bone marrow stem cell transplantation. Europace. 2007;9:1222–1223. doi: 10.1093/europace/eum190. [DOI] [PubMed] [Google Scholar]
- 155.von Degenfeld G. Banfi A. Springer ML. Blau HM. Myoblast-mediated gene transfer for therapeutic angiogenesis and arteriogenesis. Br J Pharmacol. 2003;140:620–626. doi: 10.1038/sj.bjp.0705492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Walgenbach KJ. Gratas C. Shestak KC. Becker D. Ischaemia-induced expression of bFGF in normal skeletal muscle: a potential paracrine mechanism for mediating angiogenesis in ischaemic skeletal muscle. Nat Med. 1995;1:453–459. doi: 10.1038/nm0595-453. [DOI] [PubMed] [Google Scholar]
- 157.Wang JS. Shum-Tim D. Chedrawy E. Chiu RC. The coronary delivery of marrow stromal cells for myocardial regeneration: pathophysiologic and therapeutic implications. J Thorac Cardiovasc Surg. 2001;122:699–705. doi: 10.1067/mtc.2001.116317. [DOI] [PubMed] [Google Scholar]
- 158.Wang M. Tsai BM. Crisostomo PR. Meldrum DR. Pretreatment with adult progenitor cells improves recovery and decreases native myocardial proinflammatory signaling after ischemia. Shock. 2006;25:454–459. doi: 10.1097/01.shk.0000209536.68682.90. [DOI] [PubMed] [Google Scholar]
- 159.Wang Y. Haider H. Ahmad N. Zhang D. Ashraf M. Evidence for ischemia induced host-derived bone marrow cell mobilization into cardiac allografts. J Mol Cell Cardiol. 2006;41:478–487. doi: 10.1016/j.yjmcc.2006.06.074. [DOI] [PubMed] [Google Scholar]
- 160.Wang Y. Haider HK. Ahmad N. Xu M. Ge R. Ashraf M. Combining pharmacological mobilization with intramyocardial delivery of bone marrow cells over-expressing VEGF is more effective for cardiac repair. J Mol Cell Cardiol. 2006;40:736–745. doi: 10.1016/j.yjmcc.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 161.Watanabe E. Smith DM., Jr Delcarpio JB. Sun J. Smart FW. Van Meter CH., Jr Claycomb WC. Cardiomyocyte transplantation in a porcine myocardial infarction model. Cell Transplant. 1998;7:239–246. doi: 10.1177/096368979800700302. [DOI] [PubMed] [Google Scholar]
- 162.Weintraub WS. Sadanandan S. Percutaneous coronary intervention in stable patients after acute myocardial infarction. Circulation. 2003;108:1292–1294. doi: 10.1161/01.CIR.0000088844.63899.78. [DOI] [PubMed] [Google Scholar]
- 163.Wollert KC. Meyer GP. Lotz J. Ringes-Lichtenberg S. Lippolt P. Breidenbach C. Fichtner S. Korte T. Hornig B. Messinger D. Arseniev L. Hertenstein B. Ganser A. Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 164.Wright MJ. Wightman LM. Lilley C. de Alwis M. Hart SL. Miller A. Coffin RS. Thrasher A. Latchman DS. Marber MS. In vivo myocardial gene transfer: optimization, evaluation and direct comparison of gene transfer vectors. Basic Res Cardiol. 2001;96:227–236. doi: 10.1007/s003950170053. [DOI] [PubMed] [Google Scholar]
- 165.Xie X. Cao F. Sheikh AY. Li Z. Connolly AJ. Pei X. Li RK. Robbins RC. Wu JC. Genetic modification of embryonic stem cells with VEGF enhances cell survival and improves cardiac function. Cloning Stem Cells. 2007;9:549–563. doi: 10.1089/clo.2007.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xu M. Uemura R. Dai Y. Wang Y. Pasha Z. Ashraf M. In vitro and in vivo effects of bone marrow stem cells on cardiac structure and function. J Mol Cell Cardiol. 2007;42:441–448. doi: 10.1016/j.yjmcc.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yau TM. Kim C. Li G. Zhang Y. Fazel S. Spiegelstein D. Weisel RD. Li RK. Enhanced angiogenesis with multimodal cell-based gene therapy. Ann Thorac Surg. 2007;83:1110–1119. doi: 10.1016/j.athoracsur.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 168.Ye L. Haider H. Jiang S. Ling LH. Ge R. Law PK. Sim EK. Reversal of myocardial injury using genetically modulated human skeletal myoblasts in a rodent cryoinjured heart model. Eur J Heart Fail. 2005;7:945–952. doi: 10.1016/j.ejheart.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 169.Ye L. Haider H. Jiang S. Tan RS. Ge R. Law PK. Sim EK. Improved angiogenic response in pig heart following ischaemic injury using human skeletal myoblast simultaneously expressing VEGF165 and angiopoietin-1. Eur J Heart Fail. 2007;9:15–22. doi: 10.1016/j.ejheart.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 170.Ye L. Haider H. Jiang S. Tan RS. Toh WC. Ge R. Sim EK. Angiopoietin-1 for myocardial angiogenesis: a comparison between delivery strategies. Eur J Heart Fail. 2007;9:458–465. doi: 10.1016/j.ejheart.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 171.Ye L. Haider H. Tan R. Toh W. Law PK. Tan W. Su L. Zhang W. Ge R. Zhang Y. Lim Y. Sim EK. Transplantation of nanoparticle transfected skeletal myoblasts overexpressing vascular endothelial growth factor-165 for cardiac repair. Circulation. 2007;116:I113–I1120. doi: 10.1161/CIRCULATIONAHA.106.680124. [DOI] [PubMed] [Google Scholar]
- 172.Yoo KJ. Li RK. Weisel RD. Mickle DA. Jia ZQ. Kim EJ. Tomita S. Yau TM. Heart cell transplantation improves heart function in dilated cardiomyopathic hamsters. Circulation. 2000;102:III204–III209. doi: 10.1161/01.cir.102.suppl_3.iii-204. [DOI] [PubMed] [Google Scholar]
- 173.Yoo KJ. Li RK. Weisel RD. Mickle DA. Li G. Yau TM. Autologous smooth muscle cell transplantation improved heart function in dilated cardiomyopathy. Ann Thorac Surg. 2000;70:859–865. doi: 10.1016/s0003-4975(00)01630-1. [DOI] [PubMed] [Google Scholar]
- 174.Yoon YS. Park JS. Tkebuchava T. Luedeman C. Losordo DW. Unexpected severe calcification after transplantation of bone marrow cells in acute myocardial infarction. Circulation. 2004;109:3154–3157. doi: 10.1161/01.CIR.0000134696.08436.65. [DOI] [PubMed] [Google Scholar]
- 175.Zemani F. Silvestre JS. Fauvel-Lafeve F. Bruel A. Vilar J. Bieche I. Laurendeau I. Galy-Fauroux I. Fischer AM. Boisson-Vidal C. Ex vivo priming of endothelial progenitor cells with SDF-1 before transplantation could increase their proangiogenic potential. Arterioscler Thromb Vasc Biol. 2008;28:644–650. doi: 10.1161/ATVBAHA.107.160044. [DOI] [PubMed] [Google Scholar]
- 176.Zen K. Okigaki M. Hosokawa Y. Adachi Y. Nozawa Y. Takamiya M. Tatsumi T. Urao N. Tateishi K. Takahashi T. Matsubara H. Myocardium-targeted delivery of endothelial progenitor cells by ultrasound-mediated microbubble destruction improves cardiac function via an angiogenic response. J Mol Cell Cardiol. 2006;40:799–809. doi: 10.1016/j.yjmcc.2006.03.012. [DOI] [PubMed] [Google Scholar]