Abstract
Despite significant improvements in the medical, percutaneous, and surgical management, numerous patients are first seen with non-revascularizable coronary artery disease (CAD). The growth of new blood vessels to improve myocardial perfusion (i.e., therapeutic angiogenesis) is an attractive treatment option for these patients. However, the successes of angiogenic therapy, observed in preclinical studies, have not been realized in clinical trials. Increasing evidence suggests that this discrepancy between animal and human studies may be due to the nature of the substrate, or the molecular and cellular environment within which the angiogenic agent acts. Antiangiogenic influences, including endothelial dysfunction, hypercholesterolemia, and diabetes, are present in virtually all patients with advanced CAD. Recent studies have better characterized the abnormalities associated with these disease states, providing novel targets for intervention. These substrate-modifying interventions can potentially enhance the response to protein-, gene-, or cell-based angiogenic therapy. In this review, we discuss key aspects of the angiogenic process and the pathophysiologic and molecular mechanisms that contribute to an impaired angiogenic response in the setting of endothelial dysfunction, hypercholesterolemia, and diabetes, with a focus on the role of oxidative stress. Last, we briefly explore substrate modifying agents that have been evaluated in preclinical and clinical studies to improve the angiogenic response. Antioxid. Redox Signal. 11, 1945–1959.
Introduction
Cardiovascular disease not only is the leading cause of death, disability, and health care expenditure in the United States, but also is the leading cause of mortality around the world. The principal cardiovascular disorder responsible for increases in cardiovascular mortality is no longer rheumatic disease, but rather ischemic cardiovascular disease (10). The prevalence of its risk factors (i.e., physical inactivity, obesity, diabetes, hypercholesterolemia and smoking) continues to increase worldwide. It is not surprising, therefore, that despite improvements in the management of these cardiovascular risk factors and advances in percutaneous and surgical revascularization methods, coronary artery disease (CAD) affects more than 13 million people in the United States and is responsible for one of every five deaths (44). In a large number of patients, CAD can be of such a diffuse and severe nature that repeated attempts at catheter-based interventions and surgical bypass may be unsuccessful at restoring normal myocardial blood flow. Up to 20–37% of patients with ischemic heart disease cannot undergo coronary artery bypass surgery (CABG) or percutaneous coronary intervention (PCI), or they receive incomplete revascularization with these standard revascularization strategies (35, 50, 67, 69, 92). Furthermore, incomplete revascularization has been associated with increased mortality and poorer clinical outcome (32, 55).
The goal of therapeutic angiogenesis, with growth factor– or cell-based therapies, is to restore perfusion to chronically ischemic myocardium without intervening on the epicardial coronary vasculature. Early experiments in myocardial angiogenesis with recombinant growth factors or gene-based delivery led to great enthusiasm about their therapeutic potential. However, subsequent application in phase I to III clinical studies has demonstrated limited clinical benefit, and therapeutic angiogenesis remains an experimental treatment for patients for whom conventional therapies have failed (Table 1).
Table 1.
Placebo-controlled Clinical Trials of Growth-factor Therapy
| Author | Year | N | Growth Factor | Vehicle | Delivery |
|---|---|---|---|---|---|
| Laham (58) | 1999 | 24 | FGF-2 | Protein | IM (surgical) |
| Unger (113) | 2000 | 25 | FGF-2 | Protein | IC |
| Pecher (82) | 2000 | 40 | FGF | Protein | IM |
| Vale (115) | 2001 | 6 | VEGF | Plasmid | IM (perc) |
| Simons (98) | 2002 | 337 | FGF-2 | Protein | IC |
| Losordo (60) | 2002 | 19 | VEGF | Plasmid | IM (perc) |
| Grines (37) | 2002 | 79 | FGF-4 | Adenovirus | IC |
| Grines (38) | 2003 | 52 | FGF-4 | Adenovirus | IC |
| Henry (45) | 2003 | 178 | VEGF | Protein | IC + IV |
| Hedman (43) | 2003 | 103 | VEGF | Plasmid + adenovirus | IC |
| Tio (108) | 2004 | 23 | VEGF | Plasmid | IM (perc) |
| Kastrup (53) | 2005 | 80 | VEGF | Plasmid | IM (perc) |
| Ruel (86) | 2008 | 19 | VEGF ± l-arginine | Plasmid | IM (surgical) |
| Total | 984 |
IM, intramyocardial; IC, intracoronary; IV, intravenous; perc, percutaneously delivered; year, year of publication.
The discordance between successful preclinical studies and disappointing clinical trials may be explained by a number of factors (99). First, angiogenesis is a complex process that involves interactions between a number of pro- and antiangiogenic mediators, the endothelium, and the extracellular matrix. It is therefore not surprising that single-agent growth-factor therapy has not led to large functional improvements in patients. Second, patients with end-stage coronary disease are vastly different from the young and healthy animals in whom preclinical testing is typically conducted. The presence of diabetes, hypercholesterolemia, and endothelial dysfunction can significantly limit the effect of growth factors on the angiogenic response (87, 118). Third, the optimal delivery strategy, one that provides local delivery and prolonged exposure to a sufficient dose of growth factor without causing unwanted effects, remains to be discovered. Last, the lack of sensitive assays of myocardial angiogenesis limits our ability to detect small, subclinical changes that may be occurring in response to growth-factor delivery. Despite these limitations, angiogenesis is a critical process that occurs in all humans and, if appropriately modulated, can provide therapeutic benefit to the large population of patients with ischemic coronary artery disease.
Processes involved in Blood Vessel Formation
An understanding of the biology of growth factors and their therapeutic potential requires the understanding of the processes involved in new blood vessel formation and has been previously reviewed (21, 22). Vasculogenesis, angiogenesis, and arteriogenesis are three processes that may contribute to the growth of blood vessels (57).
Vasculogenesis is the formation of new vessels from pluripotent stem cells, as seen in embryonic development. Increasing evidence suggests that vasculogenesis may also occur in the adult, as seen in the mobilization of endothelial progenitor cells from bone marrow and the incorporation of these cells into foci of neovascularization.
Angiogenesis refers to the growth of capillaries from enlarged venules that sprout capillary buds, become divided by periendothelial cells (intussusception), or are separated by transendothelial cell bridges (bridging) to form capillaries. The process starts with vasodilation and increased permeability to allow extravasation of proteins that modify the extracellular matrix. This is followed by endothelial cell proliferation and migration and tube formation with endothelial cell differentiation in response to the local tissue environment. Angiogenesis is the manner by which capillaries proliferate in healing wounds, along the border of myocardial infarctions, as well as in neoplasms. Whether these vessels are capable of producing physiologically relevant increases in tissue perfusion is debated.
Arteriogenesis is the process that results in the appearance of arteries possessing a fully developed tunica media by proliferation of preexisting arterioles into true collateral arteries. Smooth muscle cells may differentiate from various cell types, including endothelial cells and bone marrow precursors. Arteriogenesis involves smooth muscle cell growth and proliferation, migration, and differentiation to a contractile phenotype (23). An example of arteriogenesis is the development of angiographically visible collaterals in patients with advanced obstructive coronary or peripheral vascular disease.
Angiogenic Signaling
The formation of new blood vessels involves a complex molecular signaling cascade. A significant number of cytokines involved in this process have been identified, including members of the fibroblast growth factor (FGF) family, the vascular endothelial growth factor (VEGF) family, the platelet-derived growth factor (PDGF) family, and angiopoietins (127). VEGFs and FGFs are the most widely studied and the only ones used for clinical studies.
Vascular endothelial growth factors are a family of heparin-binding glycoproteins shown to act as mitogens for vascular endothelial cells as well as to stimulate endothelial progenitor cell mobilization from the bone marrow (5). The family of VEGF molecules includes VEGF [A-D] as well as placental growth factor (PIGF). These ligands interact with a number of different tyrosine kinase receptors (flt-1, flk-1, and flt-4) (15). VEGFs are expressed in cardiac myocytes and vascular smooth muscle cells, with increased expression in the setting of vascular injury, acute and chronic ischemia, and hypoxia (109). VEGFs bind to their tyrosine kinase receptor, which activates PI3 kinase, leading to the phosphorylation of Akt (protein kinase B). Phosphorylation of Akt has numerous downstream effects, among which is the phosphorylation and activation of endothelial nitric oxide synthase (eNOS), eventually leading to the production and release of nitric oxide (NO) (110), Downstream effects of VEGFs include vascular permeability, increased endothelial cell growth and survival, and formation of tubular structures (127). The VEGF family of growth factors has been demonstrated to be a crucial component of redox cell signaling that occurs in response to ischemia and reperfusion and provides the stimulus for neovascularization (64, 65).
The FGF family consists of 23 proteins that are classified by their expression pattern, receptor-binding preference, and protein sequence (29, 34). FGF is present in the normal myocardium (24). Its expression is stimulated by hypoxia (9) and hemodynamic stress (89). FGF-2 is a pluripotent molecule and modulates numerous cellular functions in multiple cell types. In the context of angiogenesis, it induces endothelial cell proliferation, survival, and differentiation, and also is involved in the migration of endothelial cells, smooth muscle cells, macrophages, and fibroblasts (29). These effects are mediated through its interaction with the tyrosine kinase receptor FGFR1 (29, 131), leading to the activation of protein kinase C (α and ɛ isoforms) and also involves syndecan-4 as a downstream mediator. Although FGF signaling also involves NO release (100), in contrast to VEGF, a lesser number of studies have tied the angiogenic effects of FGF-2 directly to NO. Additionally, FGF-2 stimulates endothelial cells to produce a variety of proteases, including plasminogen activator and matrix metalloproteinases (27, 120), promoting chemotaxis.
Role of Substrate in Determining Effects of Therapeutic Angiogenesis
As discussed earlier, one of the major reasons for the discordant results between successful animals models and less efficacious clinical studies is the presence of important pathophysiologic changes in patients with end-stage coronary artery disease in whom angiogenic therapy has been attempted. Despite important advances in risk factor management and medical therapy, patients with advanced coronary artery disease have a number of influences that can impair their response to therapeutic angiogenic therapy. Cardiovascular risk factors, such as hypertension, hypercholesterolemia, diabetes, metabolic syndrome, and smoking all have independent effects on vascular function. However, in addition to these independent effects, a common pathway in which this vascular impairment is manifest is the presence of endothelial dysfunction. Increasing awareness of these antiangiogenic influences in patients with coronary disease has led to the emergence of the notion that the substrate, or the molecular, cellular, and microvascular environment, on which the therapeutic angiogenic agent acts is as important, if not more important, than the agent itself. In the following sections, we discuss the link between endothelial dysfunction, hypercholesterolemia, and diabetes, and the response to therapeutic angiogenesis.
The endothelium, nitric oxide, and angiogenesis
The endothelium is a critical component in the maintenance of normal vascular function and the response to injury. Although many different aspects of endothelial dysfunction exist, in the context of vascular physiology and angiogenesis, it is generally defined as a reduction in the release of nitric oxide in response to a stimulatory agent. This reduction in stimulated NO release can be evaluated by using a number of in vivo or ex vivo experimental methods. Most commonly, ex vivo assessments of arteries or arterioles are performed by using tension or size-based assessment of vascular reactivity.
A strong relation exists between the release of NO and the regulation of blood-vessel growth and development, especially that mediated by the actions of VEGF. Arnal et al. (4) showed that proliferating endothelial cells express about sixfold as much eNOS mRNA as do confluent cells. Substance P and VEGF, which both stimulate the release of NO (93, 134), induce new vessel formation in vivo in addition to increasing the permeability, migration, and proliferation of postcapillary endothelial cells in tissue culture (70). Bouloumie et al. (132–134) demonstrated that VEGF enhances the expression of eNOS in native and cultured endothelial cells, an effect that may be important in the process of VEGF-induced angiogenesis. Inhibitors of NOS suppress angiogenesis, and the proliferative effect of VEGF is decreased in the presence of NOS inhibitors. Uhlmann et al. (112) measured the proliferation and migration of choroidal endothelial cells after VEGF stimulation in the presence or absence of Nώ-nitro-arginine methyl ester (L-NAME), a NO inhibitor, and found that pretreatment with L-NAME attenuated the VEGF-induced angiogenic response, in direct correlation with a reduction in basal NO release. NO may also play a crucial role in the VEGF-mediated angiogenic response of vascular smooth muscle cells (VSMCs). Jozowicz et al. (51) recently examined the effect of exogenous and endogenous NO on the synthesis of VEGF by rat and human VSMCs by exposing cells to exogenous NO donors, or to the genetic augmentation of eNOS or iNOS. NO donors potentiated by twofold the generation of VEGF protein by rat or human VSMCs. Similarly, rat or human VSMCs transiently transfected with plasmid cDNA encoding eNOS or iNOS synthesized up to threefold more VEGF than did those transfected with control plasmid cDNA, an effect that was reversed after treatment with L-NAME, an eNOS inhibitor.
In comparison to VEGF, a lesser number of studies have tied the angiogenic effects of FGF-2 to local NO availability. Still, NO likely acts as an important signal in the angiogenic response to FGF-2 as well, presumably by terminating its proliferative actions and promoting the differentiation of endothelial cells into vascular tubes (6). This role is supported by the work of Muhohara et al. (74), who showed that the inhibition of endothelial NOS by L-NAME attenuated endothelial cell migration but not proliferation in vitro. These authors also demonstrated that endogenous endothelium-derived NO maintains the functional expression of integrin αvβ3, a mediator for endothelial migration, survival, and angiogenesis, suggesting that endothelium-derived NO plays a crucial role in mediating angiogenesis by supporting endothelial cell migration, at least partly via an integrin-dependent mechanism. Recently, Sieber et al. (96) studied the role of NO in the effects of FGF-2 in a rat model of portal hypertension secondary to portal vein ligation. These authors used two Teflon rings, filled with collagen I, that were fixed in the mesenteric cavity, with one supplemented with 100 ng of FGF-2. The role of NO was tested in a subset of animals by adding the NO-formation antagonist Nώ-nitro-l-arginine (NNA) to drinking water. After 16 days, the rings were explanted and embedded, and the vessels were morphometrically counted. FGF-2 significantly stimulated vessel formation per implant in control rats, but not in rats with portal hypertension, suggesting that endothelial dysfunction and diminished NO availability may have played an inhibitory role on the effects of FGF-2. Interestingly, the numbers of ingrown vessels without FGF-2 stimulation were higher in rats that had portal vein ligation compared with controls. NNA substantially inhibited angiogenesis in both groups, and FGF-2 did not reverse angiogenesis prevented by NNA.
It also has been demonstrated that tube development by growing endothelial cells in three-dimensional gels in response to transforming growth factor-β is dependent on NO and inhibited by antagonists of NOS (81). Moreover, the stimulated synthesis and release of endothelium-derived NO by VEGF and FGF-2 has been shown to be largely regulated by tyrosine kinases (93), further implicating the role of NO in blood vessel formation mediated by these two proteins. Interestingly, activity of the tyrosine kinase Src was also found to protect endothelial cells from apoptosis during VEGF-mediated angiogenesis in chick embryos and mice (31).
Indirect evidence also supports for a crucial role of NO in the angiogenic process. In a rat gastric ulcer model, Ma et al. (61) showed that angiogenesis changed in parallel with eNOS expression, and that L-NAME administration significantly reduced both, suggesting that eNOS plays a significant role in gastric ulcer healing. Dewilt et al. (28), in a model of renal subcapsular adenocarcinoma in rats, reported that an additional antitumor effect was demonstrated when L-NAME was added to the synergistic combination of melphalan and tumor necrosis factor (tumor sizes decreased from 70 to 100%), suggesting an antiangiogenic role of L-NAME for the treatment of solid tumors in a systemic or regional setting.
Important in vivo evidence suggesting that endothelial factors play a major role in mediating the angiogenic response is found in the murine studies of Jang (48) and Duan (30), whose apoE- hypercholesterolemic mice exhibited attenuated collateral vessel formation in response to a FGF-2 disk angiogenesis system and hindlimb ischemia, respectively. This inhibition was, in both studies, fully reversed by the oral administration of l-arginine, which is the substrate for endothelial NO production. Overall, the bulk of evidence therefore suggests that NO production and perhaps other yet unidentified endothelial factors play a significant role in mediating the endogenous as well as the exogenous angiogenic responses, and likely accounts for the attenuated effects of angiogenic therapy observed in humans with end-stage, inoperable CAD who display significant endothelial dysfunction (33, 36, 54, 130).
Effects of hypercholesterolemia
Hypercholesterolemia in patients may occur for a variety of reasons, including dietary intake or abnormalities in lipid and cholesterol metabolism. The effects of diet-induced hypercholesterolemia on endothelial function have been repeatedly demonstrated in a number of animal models as well as in clinical studies. Cohen et al. (25) demonstrated that pigs fed a high-cholesterol diet for as little as 9 weeks had attenuated endothelium-dependent relaxation to serotonin in coronary ring segments placed in an organ chamber, despite the absence of intimal proliferative changes on light or electron microscopy. Similarly, Hasdai et al. (40) showed that pigs fed a hypercholesterolemic diet for 10 weeks had reduced vasorelaxation to bradykinin, abnormal responses to the endothelin B–receptor agonist sarafotoxin 6c (41), and impaired arteriolar relaxation to insulin-like growth factor (42). Impairments in smooth muscle function in the setting of hypercholesterolemia have also been demonstrated by Shishido and colleagues (95) in hypercholesterolemic rabbit aortae, in addition to endothelial dysfunction.
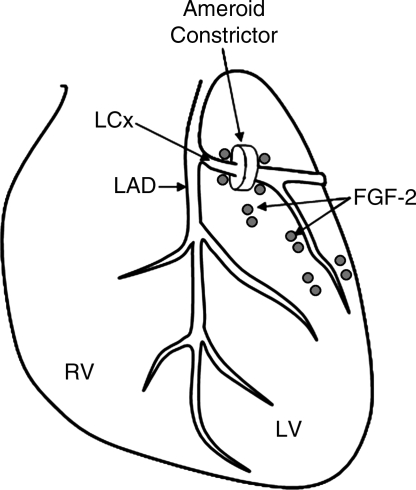
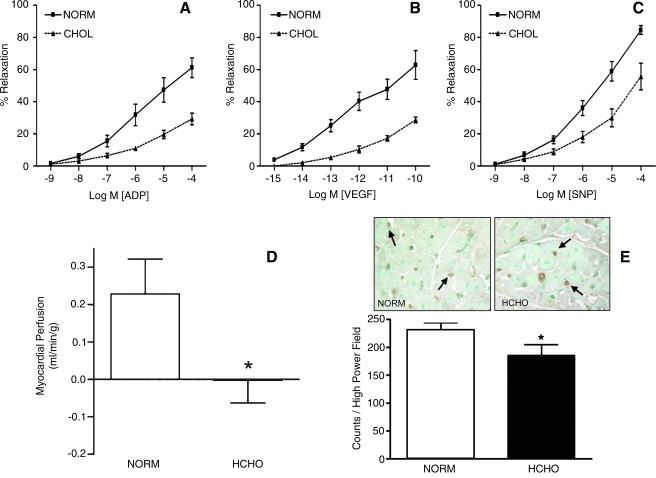
To examine the effects of hypercholesterolemia and endothelial dysfunction on angiogenesis, swine fed a diet rich in fat and cholesterol for 13 weeks were subjected to chronic myocardial ischemia by using a circumflex coronary artery ameroid constrictor (Fig. 1). In this model, the endogenous response to chronic myocardial ischemia as well as the exogenous angiogenic response to sustained-release perivascular administration of VEGF and FGF-2 were evaluated (13, 87, 118). All hypercholesterolemic animals demonstrated impaired coronary microvascular relaxation responses to adenosine diphosphate (ADP) and VEGF, suggesting reduced NO availability and endothelial dysfunction. In addition, hypercholesterolemic animals also exhibited impaired relaxation response to the NO donor, sodium nitroprusside, suggesting abnormalities in smooth muscle relaxation. The endogenous response to myocardial ischemia was impaired in the hypercholesterolemic animals, as evidenced by reduced perfusion of the collateral-dependent territory, as well as reduced endothelial cell density in the ischemic circumflex region (13) (Fig. 2). Furthermore, the response to growth factors, VEGF (118) and FGF-2 (87), was impaired compared with that of normocholesterolemic controls. In addition to the functional effects, a number of abnormalities at the molecular level were observed in this model.
FIG. 1.
Schematic showing the commonly used model of circumflex coronary artery ameroid constrictor for the creation of chronic myocardial ischemia as well as perivascular implantation of sustained release FGF-2 heparin alginate beads.
FIG. 2.
Summary of functional changes observed in a swine model of hypercholesterolemia and chronic myocardial ischemia. Hypercholesterolemic (HCHO) swine had impaired microvessel relaxation to (A) adenosine diphosphate (ADP), (B) vascular endothelial growth factor (VEGF), and (C) sodium nitroprusside (SNP) compared with normocholesterolemic controls (NORM). HCHO animals had reduced collateral-dependent perfusion (D) and reduced microvessel density (E), indicating impaired angiogenesis. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
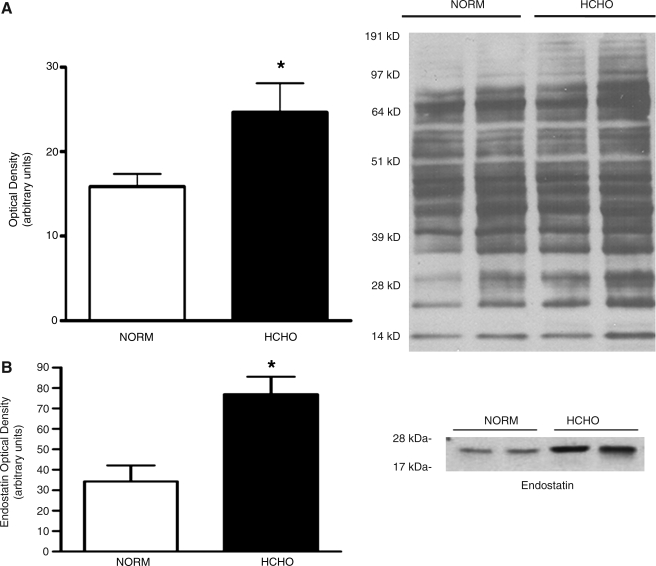
When examined in a prolonged, stable state 7 weeks after ameroid constrictor placement, the animals demonstrated no significant differences in the protein expression of angiogenic growth factors, their receptors, or any of the downstream mediators, including eNOS. An interesting observation, however, was an increase in oxidation levels of structural proteins in hypercholesterolemic animals, the most prominent of those proteins being the structural protein, actin (51 kDa). This suggests increased oxidative burden in hypercholesterolemic animals (Fig. 3). Reactive oxygen species (ROS) can be generated from numerous sources within the cell, including mitochondria, nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) oxidase, xanthine oxidase, and eNOS uncoupling (19). In the context of angiogenesis, they are released in response to angiogenic stimuli (for example, ischemia) and play an important role in angiogenic signaling (66). However, ROS also rapidly combine with NO, forming peroxynitrite, and can, therefore, reduce the amount of bioavailable NO (101). Prolonged, excessive production of ROS can also cause irreversible oxidation of cellular proteins, leading sometimes to altered function (59). The increased burden of oxidative stress in the hypercholesterolemic animal group, as demonstrated by higher levels of oxidized proteins, combined with equivalent levels of eNOS expression in both groups, suggests that the reduced NO bioavailability and resulting endothelial dysfunction in these animals may be due to increased NO degradation, in the presence of ROS, rather than impaired NO synthesis.
FIG. 3.
Molecular findings in the setting of hypercholesterolemia. These included (A) increased protein oxidation, and (B) increased expression of endostatin in hypercholesterolemic (HCHO) versus normocholesterolemic (NORM) animals.
Another interesting observation was that the hypercholesterolemic animals demonstrated a significantly higher expression of the antiangiogenic protein, endostatin. Endostatin was originally identified by O'Reilly et al. (77) from conditioned medium of a hemangioendothelioma cell line as a highly active and endothelial specific angiogenic inhibitor. It is an endogenous 20-kDa protein that is a C-terminal fragment of collagen XVIII produced by proteolytic cleavage, by a variety of matrix metalloproteinases (MMPs). Endostatin not only may inhibit angiogenesis but also may block migration and proliferation of endothelial cells and increase apoptosis (77). Conversely, one of the proposed physiological effects of endostatin is antiatherosclerosis. In 1999, Moulton et al. (71) investigated endostatin as well as TNP-470, another substance known to inhibit the growth of capillaries, in apolipoprotein E–deficient mice fed a high-cholesterol diet for 16 weeks. They found that endostatin significantly reduced intimal neovascularization and plaque growth. More recently, Moulton et al. (72) indicated that loss of collagen XVIII, the source of endostatin, enhanced neovascularization of aorta in the collagen XVIII–knockout mouse. Hence, it is possible that endostatin is an important endogenous protective factor against atherosclerosis in hypercholesterolemia. Nevertheless, endostatin is very likely responsible, at least in part, for the blunted endogenous and exogenous angiogenic response in the setting of hypercholesterolemia.
Last, when the relative efficacies of therapeutic doses of VEGF and FGF-2 were compared in a hypercholesterolemic model, FGF-2 resulted in greater perfusion of the collateral-dependent territory compared with VEGF (17). Interestingly, when evaluated in healthy, normocholesterolemic swine, the effects of intramyocardially delivered VEGF and FGF-2 are not significantly different (47). However, in a model of hypercholesterolemic endothelial dysfunction, FGF-2 appears to be a more effective agent for therapeutic angiogenesis. Possible explanation for this finding may be that VEGF-induced angiogenesis may be more NO dependent and that reduced NO bioavailability, due to increased ROS in this model, makes VEGF less effective. In addition, endostatin has significant inhibitory effects that are specific to VEGF signaling, and its increased expression in this model may be responsible for the reduced angiogenic effect of VEGF.
Diabetes and myocardial angiogenesis
More than 35 million people in the United States are affected by diabetes and glucose intolerance. These individuals carry up to 8 times the risk of cardiovascular events compared with individuals without diabetes, making cardiovascular disease the largest cause of mortality in this population (39). Diabetic patients have accelerated atherosclerosis and also exhibit a diminished angiogenic response to myocardial ischemia, as shown angiographically (3) and in autopsy studies (128). Numerous abnormalities are found at the functional, microvascular, and molecular levels that contribute to this impaired angiogenic response.
Endothelial dysfunction in the presence of diabetes has been demonstrated in various murine (79, 103), rodent (84, 85, 104) and swine models (7) of streptozotocin (STZ)-induced diabetes as well as in coronary and noncoronary vasculature of humans with type I (20, 49, 73) and type II diabetes (68, 80, 91, 116, 121, 126). Although various mechanisms for endothelial dysfunction in diabetes have been proposed, the most commonly invoked mechanism is that of increased oxidative stress. Despite the mildly increased NOS expression and activity demonstrated in in vitro and ex vivo studies of diabetic animals, inactivation of NO by rapid reaction with reactive oxygen species appears to account for decreased NO bioavailability and impaired endothelium-dependent relaxation (19, 52, 84, 85, 102). Rosen et al. (84, 85) demonstrated that this increased oxidative stress in diabetic rat hearts coincides with increased levels of urine nitrites. Endothelial dysfunction seen in these studies was reversed by the administration of antioxidants like superoxide dismutase and tocopherol. Consistent with this hypothesis, increased superoxide production and increased NADPH activity has been demonstrated in coronary arteries of diabetic swine (129). Furthermore, in a diabetic rat model of hindlimb ischemia, Hirata et al. (46) directly correlated a diminished angiogenic response to bone marrow cell implantation to reduced plasma levels of bioavailable NO. The formation of reactive oxygen and nitrogen species, like peroxynitrite, with subsequent increase in nitrotyrosine activates poly(ADP-ribose) polymerase (PARP), a DNA-repair enzyme, which initiates an energy-consuming cycle resulting in cellular dysfunction (79, 103). Activation of PARP also induces transcription factor NF-κB, leading to the activation of a number of pro-inflammatory cytokines such as ICAM-1 and TNF-α, which have also been implicated in diabetic endothelial dysfunction. In addition to the decreased NO bioavailability due to increased oxidative stress, other proposed mechanisms for diabetic endothelial dysfunction include the uncoupling of the homodimeric eNOS (116), leading to reduced NO production, a reduced synthesis of vasodilatory prostacyclin (97), and endothelial cell dysfunction due to the chronic inflammatory state, characterized by elevated levels of inflammatory cytokines.
The dynamic involvement of the extracellular matrix (ECM) in the angiogenic process was previously described (111). Myocardial ischemia, which is a potent stimulus for collateral vessel formation, is associated with an increased breakdown of the various components of the ECM through an increased expression of elastase and matrix metalloproteinases (MMPs) and downregulation of tissue inhibitors of metalloproteinases (TIMPs). In vitro studies have demonstrated a diminished vascular tube formation in response to growth factors in a glycated collagen matrix (56). Nonenzymatic glycation of the ECM proteins also has been shown to reduce the angiogenic response in vivo. Tamarat et al. (105) demonstrated reduced endogenous angiogenesis in a model of hindlimb ischemia in STZ-induced diabetic mice, which was reversed by the administration of aminoguanidine, an inhibitor of advanced glycation end product (AGE) formation. Weirauch et al. (123) studied collateral vessel formation in permanently instrumented dogs under hyperglycemic conditions and demonstrated reduced collateral-dependent perfusion associated with increased MMP-9 activity and increased expression of the antiangiogenic protein, angiostatin.
Altered expression of angiogenic growth factors and related mediators also is known to occur with diabetes. Diminished activation of HIF-1α, a transcription factor that triggers the angiogenic response, in an acute ischemia model (62, 78, 106), as well as alterations in the expression of angiopoietins and the tie-2 receptor, have been shown in diabetic rats (78). Sasso et al. (88) studied the expression of VEGF and its downstream mediators in myocardial biopsies of patients with or without type II diabetes and found that whereas VEGF expression was increased, VEGF-receptor activation and downstream signaling were reduced.
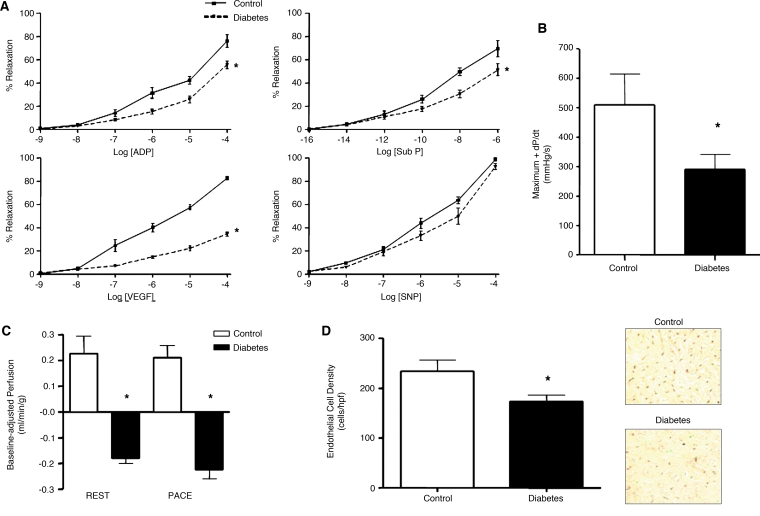
The effects of diabetes on the angiogenic response was studied in a large-animal model of a 15-week period of alloxan-induced diabetes in Yucatan miniswine with subsequent creation of chronic myocardial ischemia by using a circumflex coronary artery ameroid constrictor (16). In this model, diabetic animals successfully achieved blood glucose levels between 250 and 400 mg/dl and demonstrated significant impairments in coronary microvascular relaxation to ADP and substance P. Furthermore, microvascular relaxation in response to VEGF was even further reduced, suggesting an impairment in VEGF signaling beyond the reduction in bioavailable NO. Collateral-dependent perfusion in the circumflex territory was profoundly reduced and was also associated with reduced left ventricular function. Consistent with the finding of reduced perfusion and function, diabetic animals had reduced vascular density compared with normoglycemic controls in the ischemic territory (Fig. 4). Together, these findings demonstrate an impaired endogenous angiogenic response to chronic myocardial ischemia.
FIG. 4.
Summary of functional changes in a swine model of alloxan-induced diabetes and chronic myocardial ischemia. Diabetic animals (DM) exhibited (A) endothelial dysfunction, (B) impaired left ventricular systolic function, (C) reduced collateral-dependent perfusion during rest and pacing, and (D) reduced microvessel density in the ischemic territory compared with nondiabetic controls. ADP, adenosine diphosphate; Sub P, substance P; VEGF, vascular endothelial growth factor; SNP, sodium nitroprusside. *p < 0.01. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
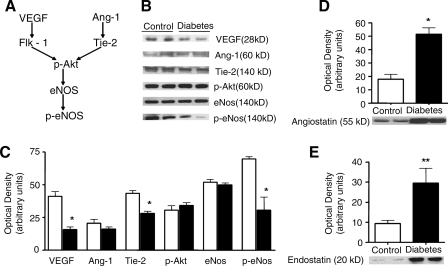
At the molecular level, the myocardium of these animals exhibited reduced expression of VEGF and of the angiopoietin receptor, Tie-2, as well as reduced phosphorylation and activation of eNOS. Expression of antiangiogenic proteins, endostatin (about a threefold increase) and particularly, angiostatin (about a 4.5-fold increase) was also significantly elevated in diabetic animals versus controls (Fig. 5). Overall, this large-animal model successfully characterized the functional, microvascular, and molecular abnormalities that have been observed in human diabetes and offers an opportunity to evaluate novel therapeutic strategies to enhance the angiogenic response in the setting of diabetes.
FIG. 5.
(A) Schematic of proangiogenic signaling pathway, and (B) expression, and (C) quantification of proangiogenic mediators. Diabetic swine demonstrated reduced VEGF, Tie-2, and phospho-eNOS expression. Expression of antiangiogenic proteins, angiostatin (D), and endostatin (E) was significantly elevated in diabetic swine. VEGF, vascular endothelial growth factor; Ang-1, angiopoietin-1; eNOS, endothelial nitric oxide synthase.
Role of Substrate Modification
In response to limited therapeutic efficacy of growth factor–based angiogenic therapy in clinical trials, researchers have investigated a number of strategies to enhance the angiogenic response. These include the search for a more-potent angiogenic agent or combination of agents, improved and sustained delivery strategies that may involve sustained-release devices or gene-based or cell-based delivery, as well as more-targeted delivery strategies (i.e., intramyocardial and perivascular delivery compared with intravenous or intracoronary routes). In addition, some investigators explored substances that can modify the substrate to improve the response to the therapeutic angiogenic agent. Substrate-modifying strategies are briefly discussed below.
l-Arginine
Numerous studies have clearly demonstrated that the presence of endothelial dysfunction is associated with impaired endogenous and exogenous angiogenic responses. This endothelial dysfunction is secondary to a reduction in bioavailable NO, which may occur because of reduced NO production or increased consumption. l-Arginine, the substrate for endothelial nitric oxide synthase, can increase the amount of bioavailable NO by increasing its production. Evidence for its pro-endothelial effects comes from animal studies as well as demonstration of improved coronary vasorelaxation in response to acetylcholine in patients with coronary disease (83). In the context of angiogenesis, studies by Jang (48) and Duan (30) in a hypercholesterolemic mouse model of hindlimb ischemia were the first to demonstrate amelioration of a blunted angiogenic response to FGF-2 administration in vivo through dietary supplementation with l-arginine. These concepts have since been validated in a large-animal model of hypercholesterolemic swine. In these animals, oral supplementation with l-arginine reversed endothelial dysfunction and improved collateral-dependent perfusion in response to chronic myocardial ischemia (75), as well as with VEGF (117) and FGF-2 (119) supplementation. These findings have been further translated to the clinical setting in a trial in which patients undergoing coronary artery bypass surgery with at least one non-revascularizable territory were randomly assign to receive placebo, VEGF alone, l-arginine alone, or VEGF and l-arginine in a factorial trial design (86). Although small in size, this study demonstrated trends toward smaller perfusion defects and trends toward improved angina scores in the combination-therapy group. Contrary to these findings, the VINTAGE MI clinical trial (90), which randomized patients to l-arginine versus placebo after acute myocardial infarction, had increased mortality in the l-arginine group. It is important to recognize in this context that patients with acute myocardial infarction are a very different patient population compared with patients with chronic stable coronary disease and that l-arginine supplementation may still hold promise as an adjunctive therapy for therapeutic angiogenesis. Finally, it should be noted that l-arginine is also a substrate for arginase and leads to the formation of polyamines, and the role of this pathway in the setting of angiogenesis has not been well elucidated.
Statins
3-Hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) are commonly used in patients with coronary disease. In addition to inhibiting the rate-limiting step in cholesterol biosynthesis, statins appear to have a number of off-target or pleiotropic effects. Statins have been shown to improve peripheral and coronary endothelial dysfunction in coronary artery disease patients (8). They can increase NO bioavailability by activating Akt, which subsequently leads to endothelial nitric oxide synthase (eNOS) activation (94), as well as through its antioxidant effects (63). Interestingly, in vitro and murine studies have suggested a biphasic and dose-dependent effect of statins on angiogenesis (114, 124). In a swine model of hypercholesterolemia, oral supplementation with high-dose atorvastatin reversed the hypercholesterolemia-induced endothelial dysfunction, as demonstrated by normalization of microvessel relaxation to ADP and VEGF. However, improvements in endothelial function did not lead to improved endogenous angiogenic response to chronic myocardial ischemia (14) or to exogenous VEGF protein administration (12). In this model, atorvastatin-treated animals had significantly and persistently elevated levels of Akt phosphorylation, a known effect of statins, as well as increased expression of the antiangiogenic protein, endostatin. These abnormalities were also replicated in normocholesterolemic swine (11). These studies communicate that, in addition to endothelial function, other determinants of the angiogenic response can affect the response to substrate-modifying therapies.
Insulin
Glycemic control remains the mainstay of treatment in diabetes and has been shown to improve both macrovascular and microvascular complications of diabetes (1, 2). Insulin treatment, with or without oral hypoglycemic agents, is the most common method used clinically to achieve glycemic control. Insulin has multifaceted effects on the myocardium, which mainly involve the regulation of fuel consumption, glucose transport, glycogen synthesis, and glycolysis (18). More relevant to vascular function, however, is the demonstrated ability of insulin to increase endothelial nitric oxide (NO) availability in the vasculature (125). Furthermore, the insulin receptor, which is present in the myocardium, is a tyrosine kinase receptor that shares many of the downstream mediators common to angiogenic growth factors and their receptors [e.g., PI3 kinase and MAP kinases (125)]. In addition to its direct effects on the myocardium and coronary vasculature, insulin exerts indirect effects through the reduction in systemic blood glucose levels. By reducing blood glucose levels, insulin can avoid the adverse effects of chronic hyperglycemia, which include increased oxidative stress, chronic inflammation, and the nonenzymatic glycation of proteins, particularly in the extracellular matrix (122, 129). In the large-animal model of alloxan-induced diabetes and chronic circumflex territory myocardial ischemia described earlier, parenteral insulin treatment was successfully used to achieve glycemic control (fasting blood glucose, <150 mg/dl) (15). Insulin treatment resulted in significant improvements in, but not normalization of, diabetic endothelial dysfunction. Significant improvements also were observed in collateral-dependent perfusion, as well as systolic and diastolic left ventricular function and capillary density in the ischemic territory. These functional findings of an improved endogenous angiogenic response were accompanied by increased expression of proangiogenic growth factors VEGF and angiopoietin-1, as well as its receptor, Tie-2. Last, the expression of the antiangiogenic proteins, angiostatin and endostatin, which were significantly elevated in the setting of diabetes, was reduced with insulin therapy. Thus, insulin treatment holds significant potential as a substrate-modulating agent for therapeutic angiogenesis in the setting of diabetes.
Other agents
A number of agents hold some potential as substrate-modifying agents for therapeutic angiogenesis because of either their pro-endothelial or antioxidant properties. Ascorbic acid and α-tocopherol (vitamins C and E) have demonstrated antioxidant properties that may be useful in improving endothelial function in certain disease states. Preliminary evidence from rodent models of hindlimb ischemia suggests that supplementation with vitamins C and E, along with l-arginine, may enhance the angiogenic effect of bone marrow cell infusion (76). Resveratrol, an ingredient found in red wine, has been shown to have antioxidant and cardioprotective effects in a variety of disease states and may have a beneficial effect on angiogenesis (26, 107). Other agents that have pro-endothelial properties and may play a role in enhancing therapeutic angiogenesis include angiotensin-converting enzyme (ACE) inhibitors, PPAR-γ agonists, and other oral hypoglycemic agents. These substances must be studied in large-animal models, to better elucidate their effects on the coronary vasculature and the angiogenic response.
Conclusions
For more than a decade, cardiovascular researchers and clinicians have explored therapeutic angiogenesis by using growth factors or cell-based therapies as treatment options for patients with advanced coronary artery disease. A number of therapeutic agents have undergone extensive preclinical evaluation followed by phase I, II, and III clinical trials. Despite encouraging results in animal models, clinical trials have showed minimal measurable benefits in patients. These investigations, however, have given us a new level of insight into the complexity of the angiogenic process and determinants of therapeutic success. For angiogenic therapy to become a clinically viable therapeutic option, it will have to involve modulation of multiple potent growth factors and be delivered in a targeted manner to the desired territory, with a sustained effect and without major adverse effects. Additionally, the therapeutic strategy will have to address the antiangiogenic influences present in the host tissue. Improved understanding of factors influencing the substrate with approaches for substrate modification will likely be an important part of this therapeutic strategy.
Acknowledgments
We are grateful for financial support from grant R01 HL69024 from the National Institutes of Health (Dr. Sellke).
Abbreviations
ACE, Angiotensin-converting enzyme; ADP, adenosine diphosphate; AGE, advanced glycation end product; CAD, coronary artery disease; CABG, coronary artery bypass graft; DNA, deoxyribonucleic acid; ECM, extracellular matrix; FGF, fibroblast growth factor; HMG-CoA, 3-hydroxy-3-methylglutaryl coenzyme A; ICAM, intracellular adhesion molecule; L-NAME, Nώ-nitro-l-arginine methyl ester; MMP, matrix metalloproteinase; mRNA, messenger ribonucleic acid; NADPH, nicotinamide adenine dinucleotide phosphate hydrogen; NO, nitric oxide; NOS, nitric oxide synthase; NNA, Nώ-nitro-l-arginine; PARP, poly(ADP-ribose) polymerase; PCI, percutaneous coronary intervention; PDGF, platelet-derived growth factor; PIGF, placental growth factor; ROS, reactive oxygen species; STZ, streptozotocin; TIMP, tissue inhibitors of metalloproteinases; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor; VSMC, vascular smooth muscle cell.
References
- 1.American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2005;28:S4–S36. [PubMed] [Google Scholar]
- 2.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus: the Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.Abaci A. Oguzhan A. Kahraman S. Eryol NK. Unal S. Arinc H. Ergin A. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation. 1999;99:2239–2242. doi: 10.1161/01.cir.99.17.2239. [DOI] [PubMed] [Google Scholar]
- 4.Arnal JF. Yamin J. Dockery S. Harrison DG. Regulation of endothelial nitric oxide synthase mRNA, protein, and activity during cell growth. Am J Physiol. 1994;267:C1381–C1388. doi: 10.1152/ajpcell.1994.267.5.C1381. [DOI] [PubMed] [Google Scholar]
- 5.Asahara T. Takahashi T. Masuda H. Kalka C. Chen D. Iwaguro H. Inai Y. Silver M. Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Babaei S. Teichert-Kuliszewska K. Monge JC. Mohamed F. Bendeck MP. Stewart DJ. Role of nitric oxide in the angiogenic response in vitro to basic fibroblast growth factor. Circ Res. 1998;82:1007–1015. doi: 10.1161/01.res.82.9.1007. [DOI] [PubMed] [Google Scholar]
- 7.Bagwell CA. Brophy C. Enhanced arterial contractile responses in diabetic hypercholesterolemic pig carotid arteries. Int J Surg Invest. 2000;1:477–481. [PubMed] [Google Scholar]
- 8.Balk EM. Karas RH. Jordan HS. Kupelnick B. Chew P. Lau J. Effects of statins on vascular structure and function: a systematic review. Am J Med. 2004;117:775–790. doi: 10.1016/j.amjmed.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 9.Bernotat-Danielowski S. Sharma HS. Schott RJ. Schaper W. Generation and localisation of monoclonal antibodies against fibroblast growth factors in ischaemic collateralised porcine myocardium. Cardiovasc Res. 1993;27:1220–1228. doi: 10.1093/cvr/27.7.1220. [DOI] [PubMed] [Google Scholar]
- 10.Bonow RO. Smaha LA. Smith SC., Jr. Mensah GA. Lenfant C. World Heart Day 2002: the international burden of cardiovascular disease: responding to the emerging global epidemic. Circulation. 2002;106:1602–1605. doi: 10.1161/01.cir.0000035036.22612.2b. [DOI] [PubMed] [Google Scholar]
- 11.Boodhwani M. Mieno S. Feng J. Sodha NR. Clements RT. Xu SH. Sellke FW. Atorvastatin impairs the myocardial angiogenic response to chronic ischemia in normocholesterolemic swine. J Thorac Cardiovasc Surg. 2008;135:117–122. doi: 10.1016/j.jtcvs.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 12.Boodhwani M. Mieno S. Voisine P. Feng J. Sodha N. Li J. Sellke FW. High-dose atorvastatin is associated with impaired myocardial angiogenesis in response to vascular endothelial growth factor in hypercholesterolemic swine. J Thorac Cardiovasc Surg. 2006;132:1299–1306. doi: 10.1016/j.jtcvs.2006.05.060. [DOI] [PubMed] [Google Scholar]
- 13.Boodhwani M. Nakai Y. Mieno S. Voisine P. Bianchi C. Araujo EG. Feng J. Michael K. Li J. Sellke FW. Hypercholesterolemia impairs the myocardial angiogenic response in a swine model of chronic ischemia: role of endostatin and oxidative stress. Ann Thorac Surg. 2006;81:634–641. doi: 10.1016/j.athoracsur.2005.07.090. [DOI] [PubMed] [Google Scholar]
- 14.Boodhwani M. Nakai Y. Voisine P. Feng J. Li J. Mieno S. Ramlawi B. Bianchi C. Laham R. Sellke FW. High-dose atorvastatin improves hypercholesterolemic coronary endothelial dysfunction without improving the angiogenic response. Circulation. 2006;114:I402–1408. doi: 10.1161/CIRCULATIONAHA.105.000356. [DOI] [PubMed] [Google Scholar]
- 15.Boodhwani M. Sodha NR. Mieno S. Ramlawi B. Xu SH. Feng J. Clements RT. Ruel M. Sellke FW. Insulin treatment enhances the myocardial angiogenic response in diabetes. J Thorac Cardiovasc Surg. 2007;134:1453–1460. doi: 10.1016/j.jtcvs.2007.08.025. Epub November 5, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boodhwani M. Sodha NR. Mieno S. Xu SH. Feng J. Ramlawi B. Clements RT. Sellke FW. Functional, cellular, and molecular characterization of the angiogenic response to chronic myocardial ischemia in diabetes. Circulation. 2007;116(Suppl 11):131–137. doi: 10.1161/CIRCULATIONAHA.106.680157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boodhwani M. Voisine P. Ruel M. Sodha NR. Feng J. Xu SH. Bianchi C. Sellke FW. Comparison of vascular endothelial growth factor and fibroblast growth factor-2 in a swine model of endothelial dysfunction. Eur J Cardiothorac Surg. 2008;33:645–650. doi: 10.1016/j.ejcts.2007.12.016. discussion 251–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brownsey RW. Boone AN. Allard MF. Actions of insulin on the mammalian heart: metabolism, pathology and biochemical mechanisms. Cardiovasc Res. 1997;34:3–24. doi: 10.1016/s0008-6363(97)00051-5. [DOI] [PubMed] [Google Scholar]
- 19.Cai H. Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 20.Calver A. Collier J. Vallance P. Inhibition and stimulation of nitric oxide synthesis in the human forearm arterial bed of patients with insulin-dependent diabetes. J Clin Invest. 1992;90:2548–2554. doi: 10.1172/JCI116149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 22.Carmeliet P. Manipulating angiogenesis in medicine. J Intern Med. 2004;255:538–561. doi: 10.1111/j.1365-2796.2003.01297.x. [DOI] [PubMed] [Google Scholar]
- 23.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 24.Casscells W. Speir E. Sasse J. Klagsbrun M. Allen P. Lee M. Calvo B. Chiba M. Haggroth L. Folkman J, et al. Isolation, characterization, and localization of heparin-binding growth factors in the heart. J Clin Invest. 1990;85:433–441. doi: 10.1172/JCI114456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen RA. Zitnay KM. Haudenschild CC. Cunningham LD. Loss of selective endothelial cell vasoactive functions caused by hypercholesterolemia in pig coronary arteries. Circ Res. 1988;63:903–910. doi: 10.1161/01.res.63.5.903. [DOI] [PubMed] [Google Scholar]
- 26.Csiszar A. Labinskyy N. Podlutsky A. Kaminski PM. Wolin MS. Zhang C. Mukhopadhyay P. Pacher P. Hu F. de Cabo R. Ballabh P. Ungvari Z. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol. 2008;294:H2721–H2735. doi: 10.1152/ajpheart.00235.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cuevas P. Carceller F. Ortega S. Zazo M. Nieto I. Gimenez-Gallego G. Hypotensive activity of fibroblast growth factor. Science. 1991;254:1208–1210. doi: 10.1126/science.1957172. [DOI] [PubMed] [Google Scholar]
- 28.de Wilt JH. Manusama ER. van Etten B. van Tiel ST. Jorna AS. Seynhaeve AL. ten Hagen TL. Eggermont AM. Nitric oxide synthase inhibition results in synergistic anti-tumour activity with melphalan and tumour necrosis factor alpha-based isolated limb perfusions. Br J Cancer. 2000;83:1176–1182. doi: 10.1054/bjoc.2000.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Detillieux KA. Sheikh F. Kardami E. Cattini PA. Biological activities of fibroblast growth factor-2 in the adult myocardium. Cardiovasc Res. 2003;57:8–19. doi: 10.1016/s0008-6363(02)00708-3. [DOI] [PubMed] [Google Scholar]
- 30.Duan J. Murohara T. Ikeda H. Katoh A. Shintani S. Sasaki K. Kawata H. Yamamoto N. Imaizumi T. Hypercholesterolemia inhibits angiogenesis in response to hindlimb ischemia: nitric oxide-dependent mechanism. Circulation. 2000;102:III370–III376. doi: 10.1161/01.cir.102.suppl_3.iii-370. [DOI] [PubMed] [Google Scholar]
- 31.Eliceiri BP. Paul R. Schwartzberg PL. Hood JD. Leng J. Cheresh DA. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell. 1999;4:915–924. doi: 10.1016/s1097-2765(00)80221-x. [DOI] [PubMed] [Google Scholar]
- 32.Ellis SG. Chew D. Chan A. Whitlow PL. Schneider JP. Topol EJ. Death following creatine kinase-MB elevation after coronary intervention: identification of an early risk period: importance of creatine kinase-MB level, completeness of revascularization, ventricular function, and probable benefit of statin therapy. Circulation. 2002;106:1205–1210. doi: 10.1161/01.cir.0000028146.71416.2e. [DOI] [PubMed] [Google Scholar]
- 33.Esper RJ. Vilarino J. Cacharron JL. Machado R. Ingino CA. Garcia Guinazu CA. Bereziuk E. Bolano AL. Suarez DH. Kura M. Impaired endothelial function in patients with rapidly stabilized unstable angina: assessment by noninvasive brachial artery ultrasonography. Clin Cardiol. 1999;22:699–703. doi: 10.1002/clc.4960221104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faham S. Hileman RE. Fromm JR. Linhardt RJ. Rees DC. Heparin structure and interactions with basic fibroblast growth factor. Science. 1996;271:1116–1120. doi: 10.1126/science.271.5252.1116. [DOI] [PubMed] [Google Scholar]
- 35.Folkman J. Angiogenic therapy of the human heart. Circulation. 1998;97:628–629. doi: 10.1161/01.cir.97.7.628. [DOI] [PubMed] [Google Scholar]
- 36.Fukuchi M. Giaid A. Endothelial expression of endothelial nitric oxide synthase and endothelin-1 in human coronary artery disease: specific reference to underlying lesion. Lab Invest. 1999;79:659–670. [PubMed] [Google Scholar]
- 37.Grines CL. Watkins MW. Helmer G. Penny W. Brinker J. Marmur JD. West A. Rade JJ. Marrott P. Hammond HK. Engler RL. Angiogenic Gene Therapy (AGENT) trial in patients with stable angina pectoris. Circulation. 2002;105:1291–1297. doi: 10.1161/hc1102.105595. [DOI] [PubMed] [Google Scholar]
- 38.Grines CL. Watkins MW. Mahmarian JJ. Iskandrian AE. Rade JJ. Marrott P. Pratt C. Kleiman N. A randomized, double-blind, placebo-controlled trial of Ad5FGF-4 gene therapy and its effect on myocardial perfusion in patients with stable angina. J Am Coll Cardiol. 2003;42:1339–1347. doi: 10.1016/s0735-1097(03)00988-4. [DOI] [PubMed] [Google Scholar]
- 39.Grundy SM. Garber A. Goldberg R. Havas S. Holman R. Lamendola C. Howard WJ. Savage P. Sowers J. Vega GL. Prevention Conference VI: Diabetes and Cardiovascular Disease: writing group IV: lifestyle and medical management of risk factors. Circulation. 2002;105:e153–e158. doi: 10.1161/01.cir.0000014022.85836.96. [DOI] [PubMed] [Google Scholar]
- 40.Hasdai D. Mathew V. Schwartz RS. Holmes DR., Jr Lerman A. The effect of basic fibroblast growth factor on coronary vascular tone in experimental hypercholesterolemia in vivo and in vitro. Coron Artery Dis. 1997;8:299–304. doi: 10.1097/00019501-199705000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Hasdai D. Mathew V. Schwartz RS. Smith LA. Holmes DR., Jr Katusic ZS. Lerman A. Enhanced endothelin-B-receptor-mediated vasoconstriction of small porcine coronary arteries in diet-induced hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1997;17:2737–2743. doi: 10.1161/01.atv.17.11.2737. [DOI] [PubMed] [Google Scholar]
- 42.Hasdai D. Nielsen MF. Rizza RA. Holmes DR., Jr Richardson DM. Cohen P. Lerman A. Attenuated in vitro coronary arteriolar vasorelaxation to insulin-like growth factor I in experimental hypercholesterolemia. Hypertension. 1999;34:89–95. doi: 10.1161/01.hyp.34.1.89. [DOI] [PubMed] [Google Scholar]
- 43.Hedman M. Hartikainen J. Syvanne M. Stjernvall J. Hedman A. Kivela A. Vanninen E. Mussalo H. Kauppila E. Simula S. Narvanen O. Rantala A. Peuhkurinen K. Nieminen MS. Laakso M. Yla-Herttuala S. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: phase II results of the Kuopio Angiogenesis Trial (KAT) Circulation. 2003;107:2677–2683. doi: 10.1161/01.CIR.0000070540.80780.92. [DOI] [PubMed] [Google Scholar]
- 44.Henry TD. Abraham JA. Review of preclinical and clinical results with vascular endothelial growth factors for therapeutic angiogenesis. Curr Intervent Cardiol Rep. 2000;2:228–241. [PubMed] [Google Scholar]
- 45.Henry TD. Annex BH. McKendall GR. Azrin MA. Lopez JJ. Giordano FJ. Shah PK. Willerson JT. Benza RL. Berman DS. Gibson CM. Bajamonde A. Rundle AC. Fine J. McCluskey ER. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation. 2003;107:1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 46.Hirata K. Li TS. Nishida M. Ito H. Matsuzaki M. Kasaoka S. Hamano K. Autologous bone marrow cell implantation as therapeutic angiogenesis for ischemic hindlimb in diabetic rat model. Am J Physiol Heart Circ Physiol. 2003;284:H66–H70. doi: 10.1152/ajpheart.00547.2002. [DOI] [PubMed] [Google Scholar]
- 47.Hughes GC. Biswas SS. Yin B. Coleman RE. DeGrado TR. Landolfo CK. Lowe JE. Annex BH. Landolfo KP. Therapeutic angiogenesis in chronically ischemic porcine myocardium: comparative effects of bFGF and VEGF. Ann Thorac Surg. 2004;77:812–818. doi: 10.1016/j.athoracsur.2003.09.060. [DOI] [PubMed] [Google Scholar]
- 48.Jang JJ. Ho HK. Kwan HH. Fajardo LF. Cooke JP. Angiogenesis is impaired by hypercholesterolemia: role of asymmetric dimethylarginine. Circulation. 2000;102:1414–1419. doi: 10.1161/01.cir.102.12.1414. [DOI] [PubMed] [Google Scholar]
- 49.Johnstone MT. Creager SJ. Scales KM. Cusco JA. Lee BK. Creager MA. Impaired endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. Circulation. 1993;88:2510–2516. doi: 10.1161/01.cir.88.6.2510. [DOI] [PubMed] [Google Scholar]
- 50.Jones EL. Craver JM. Guyton RA. Bone DK. Hatcher CR., Jr Riechwald N. Importance of complete revascularization in performance of the coronary bypass operation. Am J Cardiol. 1983;51:7–12. doi: 10.1016/s0002-9149(83)80003-4. [DOI] [PubMed] [Google Scholar]
- 51.Jozkowicz A. Cooke JP. Guevara I. Huk I. Funovics P. Pachinger O. Weidinger F. Dulak J. Genetic augmentation of nitric oxide synthase increases the vascular generation of VEGF. Cardiovasc Res. 2001;51:773–783. doi: 10.1016/s0008-6363(01)00344-3. [DOI] [PubMed] [Google Scholar]
- 52.Karasu C. Time course of changes in endothelium-dependent and -independent relaxation of chronically diabetic aorta: role of reactive oxygen species. Eur J Pharmacol. 2000;392:163–173. doi: 10.1016/s0014-2999(00)00140-0. [DOI] [PubMed] [Google Scholar]
- 53.Kastrup J. Jorgensen E. Ruck A. Tagil K. Glogar D. Ruzyllo W. Botker HE. Dudek D. Drvota V. Hesse B. Thuesen L. Blomberg P. Gyongyosi M. Sylven C. Direct intramyocardial plasmid vascular endothelial growth factor-A165 gene therapy in patients with stable severe angina pectoris: a randomized double-blind placebo-controlled study: the Euroinject One trial. J Am Coll Cardiol. 2005;45:982–988. doi: 10.1016/j.jacc.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 54.Kaufmann PA. Gnecchi-Ruscone T. Schafers KP. Luscher TF. Camici PG. Low density lipoprotein cholesterol and coronary microvascular dysfunction in hypercholesterolemia. J Am Coll Cardiol. 2000;36:103–109. doi: 10.1016/s0735-1097(00)00697-5. [DOI] [PubMed] [Google Scholar]
- 55.Kleisli T. Cheng W. Jacobs MJ. Mirocha J. Derobertis MA. Kass RM. Blanche C. Fontana GP. Raissi SS. Magliato KE. Trento A. In the current era, complete revascularization improves survival after coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2005;129:1283–1291. doi: 10.1016/j.jtcvs.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 56.Kuzuya M. Satake S. Ai S. Asai T. Kanda S. Ramos MA. Miura H. Ueda M. Iguchi A. Inhibition of angiogenesis on glycated collagen lattices. Diabetologia. 1998;41:491–499. doi: 10.1007/s001250050937. [DOI] [PubMed] [Google Scholar]
- 57.Laham R. Simons M. Growth factor therapy in ischemic heart disease. In: Rubanyi G, editor. Angiogenesis in health and disease. New York: Marcel Decker; 2000. pp. 451–475. [Google Scholar]
- 58.Laham RJ. Sellke FW. Edelman ER. Pearlman JD. Ware JA. Brown DL. Gold JP. Simons M. Local perivascular delivery of basic fibroblast growth factor in patients undergoing coronary bypass surgery: results of a phase I randomized, double-blind, placebo-controlled trial. Circulation. 1999;100:1865–1871. doi: 10.1161/01.cir.100.18.1865. [DOI] [PubMed] [Google Scholar]
- 59.Li JM. Shah AM. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1014–R1030. doi: 10.1152/ajpregu.00124.2004. [DOI] [PubMed] [Google Scholar]
- 60.Losordo DW. Vale PR. Hendel RC. Milliken CE. Fortuin FD. Cummings N. Schatz RA. Asahara T. Isner JM. Kuntz RE. Phase 1/2 placebo-controlled, double-blind, dose-escalating trial of myocardial vascular endothelial growth factor 2 gene transfer by catheter delivery in patients with chronic myocardial ischemia. Circulation. 2002;105:2012–2018. doi: 10.1161/01.cir.0000015982.70785.b7. [DOI] [PubMed] [Google Scholar]
- 61.Ma L. Wallace JL. Endothelial nitric oxide synthase modulates gastric ulcer healing in rats. Am J Physiol Gastrointest Liver Physiol. 2000;279:G341–G346. doi: 10.1152/ajpgi.2000.279.2.G341. [DOI] [PubMed] [Google Scholar]
- 62.Marfella R. D'Amico M. Di Filippo C. Piegari E. Nappo F. Esposito K. Berrino L. Rossi F. Giugliano D. Myocardial infarction in diabetic rats: role of hyperglycaemia on infarct size and early expression of hypoxia-inducible factor 1. Diabetologia. 2002;45:1172–1181. doi: 10.1007/s00125-002-0882-x. [DOI] [PubMed] [Google Scholar]
- 63.Mason RP. Walter MF. Jacob RF. Effects of HMG-CoA reductase inhibitors on endothelial function: role of microdomains and oxidative stress. Circulation. 2004;109:II34–II41. doi: 10.1161/01.CIR.0000129503.62747.03. [DOI] [PubMed] [Google Scholar]
- 64.Maulik N. Reactive oxygen species drives myocardial angiogenesis? Antioxid Redox Signal. 2006;8:2161–2168. doi: 10.1089/ars.2006.8.2161. [DOI] [PubMed] [Google Scholar]
- 65.Maulik N. Redox signaling of angiogenesis. Antioxid Redox Signal. 2002;4:805–815. doi: 10.1089/152308602760598963. [DOI] [PubMed] [Google Scholar]
- 66.Maulik N. Das DK. Redox signaling in vascular angiogenesis. Free Radic Biol Med. 2002;33:1047–1060. doi: 10.1016/s0891-5849(02)01005-5. [DOI] [PubMed] [Google Scholar]
- 67.McNeer JF. Conley MJ. Starmer CF. Behar VS. Kong Y. Peter RH. Bartel AG. Oldham HN., Jr Young WG., Jr Sabiston DC., Jr Rosati RA. Complete and incomplete revascularization at aortocoronary bypass surgery: experience with 392 consecutive patients. Am Heart J. 1974;88:176–182. doi: 10.1016/0002-8703(74)90007-6. [DOI] [PubMed] [Google Scholar]
- 68.Momose M. Abletshauser C. Neverve J. Nekolla SG. Schnell O. Standl E. Schwaiger M. Bengel FM. Dysregulation of coronary microvascular reactivity in asymptomatic patients with type 2 diabetes mellitus. Eur J Nucl Med Mol Imaging. 2002;29:1675–1679. doi: 10.1007/s00259-002-0977-0. [DOI] [PubMed] [Google Scholar]
- 69.Moon MR. Sundt TM., 3rd Pasque MK. Barner HB. Gay WA., Jr Damiano RJ., Jr. Influence of internal mammary artery grafting and completeness of revascularization on long-term outcome in octogenarians. Ann Thorac Surg. 2001;72:2003–2007. doi: 10.1016/s0003-4975(01)03144-7. [DOI] [PubMed] [Google Scholar]
- 70.Morbidelli L. Chang CH. Douglas JG. Granger HJ. Ledda F. Ziche M. Nitric oxide mediates mitogenic effect of VEGF on coronary venular endothelium. Am J Physiol. 1996;270:H411–H415. doi: 10.1152/ajpheart.1996.270.1.H411. [DOI] [PubMed] [Google Scholar]
- 71.Moulton KS. Heller E. Konerding MA. Flynn E. Palinski W. Folkman J. Angiogenesis inhibitors endostatin or TNP-470 reduce intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Circulation. 1999;99:1726–1732. doi: 10.1161/01.cir.99.13.1726. [DOI] [PubMed] [Google Scholar]
- 72.Moulton KS. Olsen BR. Sonn S. Fukai N. Zurakowski D. Zeng X. Loss of collagen XVIII enhances neovascularization and vascular permeability in atherosclerosis. Circulation. 2004;110:1330–1336. doi: 10.1161/01.CIR.0000140720.79015.3C. [DOI] [PubMed] [Google Scholar]
- 73.Mullen MJ. Clarkson P. Donald AE. Thomson H. Thorne SA. Powe AJ. Furuno T. Bull T. Deanfield JE. Effect of enalapril on endothelial function in young insulin-dependent diabetic patients: a randomized, double-blind study. J Am Coll Cardiol. 1998;31:1330–1335. doi: 10.1016/s0735-1097(98)00099-0. [DOI] [PubMed] [Google Scholar]
- 74.Murohara T. Witzenbichler B. Spyridopoulos I. Asahara T. Ding B. Sullivan A. Losordo DW. Isner JM. Role of endothelial nitric oxide synthase in endothelial cell migration. Arterioscler Thromb Vasc Biol. 1999;19:1156–1161. doi: 10.1161/01.atv.19.5.1156. [DOI] [PubMed] [Google Scholar]
- 75.Nakai Y. Voisine P. Bianchi C. Xu SH. Feng J. Malik T. Rosinberg A. Sellke FW. Effects of L-arginine on the endogenous angiogenic response in a model of hypercholesterolemia. Surgery. 2005;138:291–298. doi: 10.1016/j.surg.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 76.Napoli C. Williams-Ignarro S. de Nigris F. de Rosa G. Lerman LO. Farzati B. Matarazzo A. Sica G. Botti C. Fiore A. Byrns RE. Sumi D. Sica V. Ignarro LJ. Beneficial effects of concurrent autologous bone marrow cell therapy and metabolic intervention in ischemia-induced angiogenesis in the mouse hindlimb. Proc Natl Acad Sci U S A. 2005;102:17202–17206. doi: 10.1073/pnas.0508534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O'Reilly MS. Boehm T. Shing Y. Fukai N. Vasios G. Lane WS. Flynn E. Birkhead JR. Olsen BR. Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 78.Ohashi H. Takagi H. Koyama S. Oh H. Watanabe D. Antonetti DA. Matsubara T. Nagai K. Arai H. Kita T. Honda Y. Alterations in expression of angiopoietins and the Tie-2 receptor in the retina of streptozotocin induced diabetic rats. Mol Vis. 2004;10:608–617. [PubMed] [Google Scholar]
- 79.Pacher P. Liaudet L. Soriano FG. Mabley JG. Szabo E. Szabo C. The role of poly(ADP-ribose) polymerase activation in the development of myocardial and endothelial dysfunction in diabetes. Diabetes. 2002;51:514–521. doi: 10.2337/diabetes.51.2.514. [DOI] [PubMed] [Google Scholar]
- 80.Papaioannou GI. Seip RL. Grey NJ. Katten D. Taylor A. Inzucchi SE. Young LH. Chyun DA. Davey JA. Wackers FJ. Iskandrian AE. Ratner RE. Robinson EC. Carolan S. Engel S. Heller GV. Brachial artery reactivity in asymptomatic patients with type 2 diabetes mellitus and microalbuminuria (from the Detection of Ischemia in Asymptomatic Diabetics: Brachial Artery Reactivity Study) Am J Cardiol. 2004;94:294–299. doi: 10.1016/j.amjcard.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 81.Papapetropoulos A. Desai KM. Rudic RD. Mayer B. Zhang R. Ruiz-Torres MP. Garcia-Cardena G. Madri JA. Sessa WC. Nitric oxide synthase inhibitors attenuate transforming-growth-factor-beta 1-stimulated capillary organization in vitro. Am J Pathol. 1997;150:1835–1844. [PMC free article] [PubMed] [Google Scholar]
- 82.Pecher P. Schumacher BA. Angiogenesis in ischemic human myocardium: clinical results after 3 years. Ann Thorac Surg. 2000;69:1414–1419. doi: 10.1016/s0003-4975(00)01162-0. [DOI] [PubMed] [Google Scholar]
- 83.Quyyumi AA. Dakak N. Diodati JG. Gilligan DM. Panza JA. Cannon RO., 3rd Effect of L-arginine on human coronary endothelium-dependent and physiologic vasodilation. J Am Coll Cardiol. 1997;30:1220–1227. doi: 10.1016/s0735-1097(97)00279-9. [DOI] [PubMed] [Google Scholar]
- 84.Rosen P. Ballhausen T. Bloch W. Addicks K. Endothelial relaxation is disturbed by oxidative stress in the diabetic rat heart: influence of tocopherol as antioxidant. Diabetologia. 1995;38:1157–1168. doi: 10.1007/BF00422364. [DOI] [PubMed] [Google Scholar]
- 85.Rosen P. Ballhausen T. Stockklauser K. Impairment of endothelium dependent relaxation in the diabetic rat heart: mechanisms and implications. Diabetes Res Clin Pract. 1996;31(suppl):S143–S155. doi: 10.1016/0168-8227(96)01242-9. [DOI] [PubMed] [Google Scholar]
- 86.Ruel M. Beanlands RS. Lortie M. Chan V. Camack N. deKemp RA. Suuronen EJ. Rubens FD. DaSilva JN. Sellke FW. Stewart DJ. Mesana TG. Concomitant treatment with oral L-arginine improves the efficacy of surgical angiogenesis in patients with severe diffuse coronary artery disease: the Endothelial Modulation in Angiogenic Therapy randomized controlled trial. J Thorac Cardiovasc Surg. 135:762–770. doi: 10.1016/j.jtcvs.2007.09.073. 770 e1, 2008. [DOI] [PubMed] [Google Scholar]
- 87.Ruel M. Wu GF. Khan TA. Voisine P. Bianchi C. Li J. Laham RJ. Sellke FW. Inhibition of the cardiac angiogenic response to surgical FGF-2 therapy in a swine endothelial dysfunction model. Circulation. 2003;108(suppl 1):II335–II340. doi: 10.1161/01.cir.0000087903.75204.ad. [DOI] [PubMed] [Google Scholar]
- 88.Sasso FC. Torella D. Carbonara O. Ellison GM. Torella M. Scardone M. Marra C. Nasti R. Marfella R. Cozzolino D. Indolfi C. Cotrufo M. Torella R. Salvatore T. Increased vascular endothelial growth factor expression but impaired vascular endothelial growth factor receptor signaling in the myocardium of type 2 diabetic patients with chronic coronary heart disease. J Am Coll Cardiol. 2005;46:827–834. doi: 10.1016/j.jacc.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 89.Schneider H. Huse K. Arterial gene therapy. Lancet. 1996;348:1380–1381. doi: 10.1016/S0140-6736(05)65442-6. author reply 1381–1382. [DOI] [PubMed] [Google Scholar]
- 90.Schulman SP. Becker LC. Kass DA. Champion HC. Terrin ML. Forman S. Ernst KV. Kelemen MD. Townsend SN. Capriotti A. Hare JM. Gerstenblith G. L-arginine therapy in acute myocardial infarction: the Vascular Interaction With Age in Myocardial Infarction (VINTAGE MI) randomized clinical trial. JAMA. 2006;295:58–64. doi: 10.1001/jama.295.1.58. [DOI] [PubMed] [Google Scholar]
- 91.Sekiya M. Suzuki J. Watanabe K. Funada J. Otani T. Akutsu H. Beneficial effect of troglitazone, an insulin-sensitizing antidiabetic agent, on coronary circulation in patients with non-insulin-dependent diabetes mellitus. Jpn Circ J. 2001;65:487–490. doi: 10.1253/jcj.65.487. [DOI] [PubMed] [Google Scholar]
- 92.Sellke FW. Laham RJ. Edelman ER. Pearlman JD. Simons M. Therapeutic angiogenesis with basic fibroblast growth factor: technique and early results. Ann Thorac Surg. 1998;65:1540–1544. doi: 10.1016/s0003-4975(98)00340-3. [DOI] [PubMed] [Google Scholar]
- 93.Sellke FW. Wang SY. Stamler A. Lopez JJ. Li J. Simons M. Enhanced microvascular relaxations to VEGF and bFGF in chronically ischemic porcine myocardium. Am J Physiol. 1996;271:H713–H7120. doi: 10.1152/ajpheart.1996.271.2.H713. [DOI] [PubMed] [Google Scholar]
- 94.Shiojima I. Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res. 2002;90:1243–1250. doi: 10.1161/01.res.0000022200.71892.9f. [DOI] [PubMed] [Google Scholar]
- 95.Shishido T. Tasaki K. Takeishi Y. Takasaki S. Miyamoto T. Itoh M. Takahashi H. Kubota I. Ito T. Katano Y. Wakabayashi I. Tomoike H. Chronic hypertriglyceridemia in young watanabe heritable hyperlipidemic rabbits impairs endothelial and medial smooth muscle function. Life Sci. 2004;74:1487–501. doi: 10.1016/j.lfs.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 96.Sieber CC. Sumanovski LT. Stumm M. van der Kooij M. Battegay E. In vivo angiogenesis in normal and portal hypertensive rats: role of basic fibroblast growth factor and nitric oxide. J Hepatol. 2001;34:644–650. doi: 10.1016/s0168-8278(00)00064-7. [DOI] [PubMed] [Google Scholar]
- 97.Silberbauer K. Clopath P. Sinzinger H. Schernthaner G. Effect of experimentally induced diabetes on swine vascular prostacyclin (PGI2) synthesis. Artery. 1980;8:30–36. [PubMed] [Google Scholar]
- 98.Simons M. Annex BH. Laham RJ. Kleiman N. Henry T. Dauerman H. Udelson JE. Gervino EV. Pike M. Whitehouse MJ. Moon T. Chronos NA. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: double-blind, randomized, controlled clinical trial. Circulation. 2002;105:788–793. doi: 10.1161/hc0802.104407. [DOI] [PubMed] [Google Scholar]
- 99.Simons M. Bonow RO. Chronos NA. Cohen DJ. Giordano FJ. Hammond HK. Laham RJ. Li W. Pike M. Sellke FW. Stegmann TJ. Udelson JE. Rosengart TK. Clinical trials in coronary angiogenesis: issues, problems, consensus: an expert panel summary. Circulation. 2000;102:E73–E86. doi: 10.1161/01.cir.102.11.e73. [DOI] [PubMed] [Google Scholar]
- 100.Slavin J. Fibroblast growth factors: at the heart of angiogenesis. Cell Biol Int. 1995;19:431–444. doi: 10.1006/cbir.1995.1087. [DOI] [PubMed] [Google Scholar]
- 101.Stocker R. Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 102.Stockklauser-Farber K. Ballhausen T. Laufer A. Rosen P. Influence of diabetes on cardiac nitric oxide synthase expression and activity. Biochim Biophys Acta. 2000;1535:10–20. doi: 10.1016/s0925-4439(00)00078-8. [DOI] [PubMed] [Google Scholar]
- 103.Szabo C. PARP as a drug target for the therapy of diabetic cardiovascular dysfunction. Drug News Perspect. 2002;15:197–205. doi: 10.1358/dnp.2002.15.4.840052. [DOI] [PubMed] [Google Scholar]
- 104.Tada H. Muramatsu I. Nakai T. Kigoshi S. Miyabo S. Effects of chronic diabetes on the responsiveness to endothelin-1 and other agents of rat atria and thoracic aorta. Gen Pharmacol. 1994;25:1221–1228. doi: 10.1016/0306-3623(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 105.Tamarat R. Silvestre JS. Huijberts M. Benessiano J. Ebrahimian TG. Duriez M. Wautier MP. Wautier JL. Levy BI. Blockade of advanced glycation end-product formation restores ischemia-induced angiogenesis in diabetic mice. Proc Natl Acad Sci U S A. 2003;100:8555–8560. doi: 10.1073/pnas.1236929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Taniyama Y. Morishita R. Hiraoka K. Aoki M. Nakagami H. Yamasaki K. Matsumoto K. Nakamura T. Kaneda Y. Ogihara T. Therapeutic angiogenesis induced by human hepatocyte growth factor gene in rat diabetic hind limb ischemia model: molecular mechanisms of delayed angiogenesis in diabetes. Circulation. 2001;104:2344–2350. doi: 10.1161/hc4401.098470. [DOI] [PubMed] [Google Scholar]
- 107.Thirunavukkarasu M. Penumathsa SV. Koneru S. Juhasz B. Zhan L. Otani H. Bagchi D. Das DK. Maulik N. Resveratrol alleviates cardiac dysfunction in streptozotocin-induced diabetes: role of nitric oxide, thioredoxin, and heme oxygenase. Free Radic Biol Med. 2007;43:720–729. doi: 10.1016/j.freeradbiomed.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tio RA. Tan ES. Jessurun GA. Veeger N. Jager PL. Slart RH. de Jong RM. Pruim J. Hospers GA. Willemsen AT. de Jongste MJ. van Boven AJ. van Veldhuisen DJ. Zijlstra F. PET for evaluation of differential myocardial perfusion dynamics after VEGF gene therapy and laser therapy in end-stage coronary artery disease. J Nucl Med. 2004;45:1437–1443. [PubMed] [Google Scholar]
- 109.Tofukuji M. Metais C. Li J. Franklin A. Simons M. Sellke FW. Myocardial VEGF expression after cardiopulmonary bypass and cardioplegia. Circulation. 98:II242–II246. discussion II247–II248, 1998. [PubMed] [Google Scholar]
- 110.Toyota E. Matsunaga T. Chilian WM. Myocardial angiogenesis. Mol Cell Biochem. 2004;264:35–44. doi: 10.1023/b:mcbi.0000044372.65864.18. [DOI] [PubMed] [Google Scholar]
- 111.Tyagi SC. Vasculogenesis and angiogenesis: extracellular matrix remodeling in coronary collateral arteries and the ischemic heart. J Cell Biochem. 1997;65:388–394. [PubMed] [Google Scholar]
- 112.Uhlmann S. Friedrichs U. Eichler W. Hoffmann S. Wiedemann P. Direct measurement of VEGF-induced nitric oxide production by choroidal endothelial cells. Microvasc Res. 2001;62:179–189. doi: 10.1006/mvre.2001.2334. [DOI] [PubMed] [Google Scholar]
- 113.Unger EF. Goncalves L. Epstein SE. Chew EY. Trapnell CB. Cannon RO., 3rd Quyyumi AA. Effects of a single intracoronary injection of basic fibroblast growth factor in stable angina pectoris. Am J Cardiol. 2000;85:1414–1419. doi: 10.1016/s0002-9149(00)00787-6. [DOI] [PubMed] [Google Scholar]
- 114.Urbich C. Dernbach E. Zeiher AM. Dimmeler S. Double-edged role of statins in angiogenesis signaling. Circ Res. 2002;90:737–744. doi: 10.1161/01.res.0000014081.30867.f8. [DOI] [PubMed] [Google Scholar]
- 115.Vale PR. Losordo DW. Milliken CE. McDonald MC. Gravelin LM. Curry CM. Esakof DD. Maysky M. Symes JF. Isner JM. Randomized, single-blind, placebo-controlled pilot study of catheter-based myocardial gene transfer for therapeutic angiogenesis using left ventricular electromechanical mapping in patients with chronic myocardial ischemia. Circulation. 2001;103:2138–2143. doi: 10.1161/01.cir.103.17.2138. [DOI] [PubMed] [Google Scholar]
- 116.van Etten RW. de Koning EJ. Verhaar MC. Gaillard CA. Rabelink TJ. Impaired NO-dependent vasodilation in patients with type II (non-insulin-dependent) diabetes mellitus is restored by acute administration of folate. Diabetologia. 2002;45:1004–1010. doi: 10.1007/s00125-002-0862-1. [DOI] [PubMed] [Google Scholar]
- 117.Voisine P. Bianchi C. Khan TA. Ruel M. Xu SH. Feng J. Li J. Malik T. Rosinberg A. Sellke FW. Normalization of coronary microvascular reactivity and improvement in myocardial perfusion by surgical vascular endothelial growth factor therapy combined with oral supplementation of l-arginine in a porcine model of endothelial dysfunction. J Thorac Cardiovasc Surg. 2005;129:1414–1420. doi: 10.1016/j.jtcvs.2004.12.046. [DOI] [PubMed] [Google Scholar]
- 118.Voisine P. Bianchi C. Ruel M. Malik T. Rosinberg A. Feng J. Khan TA. Xu SH. Sandmeyer J. Laham RJ. Sellke FW. Inhibition of the cardiac angiogenic response to exogenous vascular endothelial growth factor. Surgery. 2004;136:407–415. doi: 10.1016/j.surg.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 119.Voisine P. Li J. Bianchi C. Khan TA. Ruel M. Xu SH. Feng J. Rosinberg A. Malik T. Nakai Y. Sellke FW. Effects of L-arginine on fibroblast growth factor 2-induced angiogenesis in a model of endothelial dysfunction. Circulation. 2005;112:I202–1207. doi: 10.1161/CIRCULATIONAHA.104.526350. [DOI] [PubMed] [Google Scholar]
- 120.Wang SY. Friedman M. Johnson RG. Weintraub RM. Sellke FW. Adrenergic regulation of coronary microcirculation after extracorporeal circulation and crystalloid cardioplegia. Am J Physiol. 1994;267:H2462–H2470. doi: 10.1152/ajpheart.1994.267.6.H2462. [DOI] [PubMed] [Google Scholar]
- 121.Watts GF. O'Brien SF. Silvester W. Millar JA. Impaired endothelium-dependent and independent dilatation of forearm resistance arteries in men with diet-treated non-insulin-dependent diabetes: role of dyslipidaemia. Clin Sci (Lond) 1996;91:567–573. doi: 10.1042/cs0910567. [DOI] [PubMed] [Google Scholar]
- 122.Wautier JL. Schmidt AM. Protein glycation: a firm link to endothelial cell dysfunction. Circ Res. 2004;95:233–238. doi: 10.1161/01.RES.0000137876.28454.64. [DOI] [PubMed] [Google Scholar]
- 123.Weihrauch D. Lohr NL. Mraovic B. Ludwig LM. Chilian WM. Pagel PS. Warltier DC. Kersten JR. Chronic hyperglycemia attenuates coronary collateral development and impairs proliferative properties of myocardial interstitial fluid by production of angiostatin. Circulation. 2004;109:2343–2348. doi: 10.1161/01.CIR.0000129225.67353.1F. [DOI] [PubMed] [Google Scholar]
- 124.Weis M. Heeschen C. Glassford AJ. Cooke JP. Statins have biphasic effects on angiogenesis. Circulation. 2002;105:739–745. doi: 10.1161/hc0602.103393. [DOI] [PubMed] [Google Scholar]
- 125.White MF. Insulin signaling in health and disease. Science. 2003;302:1710–1711. doi: 10.1126/science.1092952. [DOI] [PubMed] [Google Scholar]
- 126.Williams SB. Cusco JA. Roddy MA. Johnstone MT. Creager MA. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1996;27:567–574. doi: 10.1016/0735-1097(95)00522-6. [DOI] [PubMed] [Google Scholar]
- 127.Yancopoulos GD. Davis S. Gale NW. Rudge JS. Wiegand SJ. Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 128.Yarom R. Zirkin H. Stammler G. Rose AG. Human coronary microvessels in diabetes and ischaemia: morphometric study of autopsy material. J Pathol. 1992;166:265–270. doi: 10.1002/path.1711660308. [DOI] [PubMed] [Google Scholar]
- 129.Zhang L. Zalewski A. Liu Y. Mazurek T. Cowan S. Martin JL. Hofmann SM. Vlassara H. Shi Y. Diabetes-induced oxidative stress and low-grade inflammation in porcine coronary arteries. Circulation. 2003;108:472–478. doi: 10.1161/01.CIR.0000080378.96063.23. [DOI] [PubMed] [Google Scholar]
- 130.Zhang X. Zhao SP. Li XP. Gao M. Zhou QC. Endothelium-dependent and -independent functions are impaired in patients with coronary heart disease. Atherosclerosis. 2000;149:19–24. doi: 10.1016/s0021-9150(99)00288-9. [DOI] [PubMed] [Google Scholar]
- 131.Zhang Y. Li J. Partovian C. Sellke FW. Simons M. Syndecan-4 modulates basic fibroblast growth factor 2 signaling in vivo. Am J Physiol Heart Circ Physiol. 2003;284:H2078–H2082. doi: 10.1152/ajpheart.00942.2001. [DOI] [PubMed] [Google Scholar]
- 132.Ziche M. Role of nitric oxide in the angiogenesis of avascular tissue. Osteoarthritis Cartilage. 1999;7:403–405. doi: 10.1053/joca.1998.0225. [DOI] [PubMed] [Google Scholar]
- 133.Ziche M. Morbidelli L. Choudhuri R. Zhang HT. Donnini S. Granger HJ. Bicknell R. Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Invest. 1997;99:2625–2634. doi: 10.1172/JCI119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ziche M. Morbidelli L. Masini E. Amerini S. Granger HJ. Maggi CA. Geppetti P. Ledda F. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest. 1994;94:2036–2044. doi: 10.1172/JCI117557. [DOI] [PMC free article] [PubMed] [Google Scholar]