Abstract
Cardiac gene and cell therapy have both entered clinical trials aimed at ameliorating ventricular dysfunction in patients with chronic congestive heart failure. The transduction of myocardial cells with viral constructs encoding a specific cardiomyocyte Ca2+ pump in the sarcoplasmic reticulum (SR), SRCa2+-ATPase has been shown to correct deficient Ca2+ handling in cardiomyocytes and improvements in contractility in preclinical studies, thus leading to the first clinical trial of gene therapy for heart failure. In cell therapy, it is not clear whether beneficial effects are cell-type specific and how improvements in contractility are brought about. Despite these uncertainties, a number of clinical trials are under way, supported by safety and efficacy data from trials of cell therapy in the setting of myocardial infarction. Safety concerns for gene therapy center on inflammatory and immune responses triggered by viral constructs, and for cell therapy with myoblast cells, the major concern is increased incidence of ventricular arrhythmia after cell transplantation. Principles and mechanisms of action of gene and cell therapy for heart failure are discussed, together with the potential influence of reactive oxygen species on the efficacy of these treatments and the status of myocardial-delivery techniques for viral constructs and cells. Antioxid. Redox Signal. 11, 2025–2042.
Ischemic heart disease and myocardial infarction (MI) remain the dominant causes of death in the industrialized world. Chronic congestive heart failure (CHF) in those who survive an MI is a debilitating disease with a high morbidity, resulting in frequent hospital admissions and considerable pressure on health care resources. Despite significant advances in the pharmacologic treatment of heart failure, the mortality of those with this disease remains high (3). In a significant translational effort by a multitude of groups, gene and cell therapy for heart failure have entered clinical trials. However, the road that has led to this point has been markedly different for gene and cell therapy.
The development of gene therapy has been characterized by extensive experimental work, as well as fits and starts in the area of clinical trials. After promising results in animal models, Baumgartner et al. (12) performed the first open-label study of plasmid DNA encoding VEGF165 to improve blood supply in patients with critical limb ischemia (12), and this study was followed by many other nonrandomized studies, indicating a potential for gene therapy to improve vascularization in ischemic limbs or hearts. However, large randomized studies failed to corroborate these results (26), with the consequence that clinical gene-therapy studies with the goal to improve cardiac vascularization have been abandoned. In the field of chronic CHF, a long trajectory of preclinical experimentation by many groups has resulted in the recent initiation of the first clinical study of viral vector–based gene transfer for CHF (45).
The pathway from bench to bedside for cell therapy has been markedly different and has shown a relatively quick adoption of cell transfer into clinical trials, despite the absence of an understanding of the mechanism through which donor cells improve cardiac function in patients. For example, Asahara et al. (5) published their identification of endothelial progenitor cells in 1997, and a case report describing transfer of bone marrow–derived mononuclear cells in a patient after MI appeared in 2001 (123). In 1998, Taylor et al. (99) published improvement of left ventricular function after skeletal myoblast transfer in a rabbit MI model, and in 2002, the initiation of a clinical study involving myoblast transplantation was announced. Thus, although clinical gene-therapy studies were preceded by numerous preclinical studies, in the field of cell therapy, preclinical and clinical studies progress more along parallel trajectories.
Both approaches have a few things in common, however, beyond the intended clinical syndromes that they aim to ameliorate, which, in the case of this review, is CHF. The therapeutic efficacy of both approaches is affected in patients by the fact that the diseases that resulted in CHF, such as atherosclerosis and diabetes mellitus as well as CHF itself, are characterized by increased intramyocardial oxidative stress, which may reduce the beneficial impact of gene and cell therapy. Considerations regarding delivery methods and efficacy of myocardial delivery show overlap between the two fields. This review discusses principles and mechanisms of action of both approaches, currently available data from clinical trials, the impact of increased oxidative stress on the therapeutic effects in CHF patients, and issues surrounding delivery methods and delivery efficacy.
Gene Therapy
The goal of gene therapy for heart failure of ischemic origin is to achieve therapeutic levels of a protein that will improve cardiac performance through restoration of protein levels essential for normal cardiac function or through knockdown of proteins that impair cardiac function. The DNA that encodes the protein of interest must reach the nucleus of the cardiomyocyte, and this can be achieved by administering it in plasmid form (pDNA), so-called “naked DNA” or by inserting it into the genome of a virus. The viruses that are relevant for cardiac gene therapy are adenovirus, adeno-associated virus (AAV), and lentivirus (41). The viruses differ regarding the size of the insert that they can accommodate, their cardiac tropism, their propensity for integration into the host genome and concomitant cancer risk, the inflammatory response that they initiate, their immunogenicity, and the duration of expression of the therapeutic DNA sequence.
Smith and Helenius (118) wrote an excellent review of the mechanisms through which viruses enter mammalian cells, and the reader is encouraged to refer to their article for further details. Here a few points are highlighted. Viruses use a “Trojan horse” strategy to enter the host cell, in which they extract assistance from the host by using the detailed “insider information” that they have acquired during millions of years of coevolution with their hosts. Many viruses that infect mammalian cells rely on the cell's endocytotic machinery, which has the added advantage that it provides deep entry of the particle into the cell, aided by the natural ability of endocytotic vesicles to traverse barriers imposed by the cytoskeleton, and the highly structured cytoplasm and endocytotic entry helps the particles to escape lysosomal degradation.
Plasmid DNA
Treatment with pDNA constructs has the advantages of being cheap, simple, and safe because these constructs do not initiate inflammation, are not immunogenic, and incur no risk of insertional mutagenesis. All mammalian cell types are capable of internalizing pDNA and bringing it to expression; however, this capability is lost when the cells are cultured in vitro. Thus, it appears that the environment in which the target cell resides is of importance to its capability to internalize pDNA. By using pDNA condensed on the cationic polymer polyethylenimine (PEI) to promote transfection in vitro, Kong et al. (69) demonstrated that the mechanical properties of the substrate to which cells adhere (i.e., its rigidity determined the uptake of PEI-pDNA condensates). On alginate hydrogels in which the elastic modulus (E) could be varied by modifying the extent of cross-linking and with fluorescent resonance energy transfer as a readout, they showed that the transportation of pDNA condensates into cells increased in proportion to E (69).
Gene therapy with pDNA has been hampered by low transfection and expression levels (111), and this is not surprising because it involves cellular uptake of negatively charged molecules (i.e., the phosphate backbone of DNA) through a negatively charged cell membrane. To increase transfection efficacy, pDNA has been complexed with cationic lipids (54), and mechanical approaches such as intramyocardial injection, electroporation, and destruction of targeted ultrasound bubbles that contain the plasmid have also been applied (89), but currently, transfection efficacy remains too low for pDNA-based gene transfer to become a viable strategy for the treatment of heart failure (41, 106). The major advantage of viral constructs over pDNA is their high transduction rate. In rabbits, adenoviral and adeno-associated constructs were 30- to 360-fold more efficient in cardiac transduction than were plasmids (138), and in pigs, up to 75% of cardiomyocytes showed expression of a reporter gene along the needle track after intramyocardial injection of an adenoviral construct (36).
Adenovirus
Named for the adenoid tissue from which they were originally isolated, adenoviruses are double stranded, nonenveloped DNA viruses that are 60–90 nm in diameter and must enter the nucleus for replication. From the family of Adenoviridae, the human subgroup C adenoviruses have been most extensively characterized, and serotypes 2 and 5 are most frequently used as platforms for in vivo gene transfer. Adenoviral constructs can accept inserts of up to 35 kilobases (kb) in size, the largest insert size of all three viral constructs relevant to cardiac gene therapy. Adenoviral constructs exhibit cardiac tropism and generate a very high level of initial gene expression a few days after entry into the target cell, which lasts for ∼2 weeks (106). This duration of expression typically is not sufficient for therapeutic applications in heart failure (136), but it allows proof-of-concept studies in animal models and clinical studies of angiogenesis (122).
In addition to the relatively short-lived expression of the therapeutic DNA sequence, inflammation and immunogenicity are two issues that are inherent to adenoviral gene therapy. Immunogenicity is a significant problem because adenoviral infections are so prevalent in the general population. The immune response triggered by the construct causes significant inflammation and clearance of the infected cells, thus limiting the duration of expression after adenoviral gene transfer (124).
To limit inflammatory and immunogenic responses, the viral genome has been increasingly removed from the constructs, to the point at which the current third-generation adenoviral vectors are called “gutless” because the entire viral genome has been removed, except for the packaging and replication sequence in cis. This means that for growth in vitro, they must be co-infected with a helper virus that provides all the necessary viral proteins in trans (34). Helper-dependent vectors accept insert sizes up to 35 kb (89). In a comparison of first- and third-generation adenoviral constructs for cardiac gene transfer, however, no differences were found in duration of reporter gene expression, despite a lower number of monocytes/macrophages, total T cells, CD4+ and CD8+ T cells, and less inflammatory cytokine production in the animals that received the third-generation construct (34). The authors of this article postulated that because adenovirus can infect dendritic cells (59), which then can initiate a cytotoxic immune response, even gutless constructs can still have abrogated duration of expression due to host immune responses.
Adenoassociated virus
In 1965, Atchison et al. (7) described a viral particle that was associated with a simian adenovirus. The particles were antigenically different from the adenovirus but were incapable of replication in the absence of the adenovirus. Thus, they appeared to be a defective satellite virus that was dependent on co-infection with a helper virus. Later it was established that AAV belongs to the dependovirus genus of the parvoviridiae. Nine human subtypes and AAV2 are known; the most common subtype was first described in 1968 as a co-infecting agent during an adenovirus outbreak in a children's nursery (16), but no known human pathology has been attributed to AAV2. The defective replication and the absence of any human pathology make this virus an attractive candidate for gene-transfer applications.
AAV is a 20-nm icosahedral single-strand DNA virus that can accommodate an insert size of 4.5 kb. When used in gene-transfer studies, AAV transgene expression results primarily from extrachromosomal viral genomes that persist as double-stranded circular or linear episome (91). In cardiac gene-transfer studies, AAV was shown to be as efficient as adenovirus in transducing cardiac cells, and stable reporter-gene expression was observed for periods up to 1 year (134). After a single intravenous injection, skeletal and cardiac muscle were efficiently transduced, whereas the reporter gene could not be identified in brain, lung, liver, spleen, intestine, kidney, or testes. Even though the transgene was not expressed in these tissues, the rAAV6 genome was detectable in homogenates of all organs, which is in keeping with the broad tissue tropism of AAV (19). To increase specificity of rAAV for the target cell, constructs can be generated that contain cell type–specific gene-regulatory elements (137), and others have proposed to integrate cell type–specific ligands in the viral capsid (19).
Lentivirus
Lentiviruses are a genus within the family of Retroviridae. Retroviruses are RNA viruses that rely on conversion of their RNA sequences to DNA and integration in the host genome for replication after infection of the host cell; therefore, their replication depends on cell division. Lentiviruses have a more-complex structure than retroviruses and can multiply in nondividing cells. Gene-transfer constructs based on lentiviruses do not induce inflammatory and immune responses (143); they have the same transfection efficiency in the myocardium as adenoviruses, but the duration of transgene expression is longer, and their capability to accept inserts up to 18 kb puts them at an advantage over AAVs (71). Lentivirus encoding SERCA2 was used successfully to ameliorate heart failure in a rat model of myocardial infarction (92).
Lentiviral vectors are derived from the human immunodeficiency type 1 (HIV-1) virus; thus, the tropism of the wild-type HIV-1 virus for CD4+ T cells must be overcome the construct to become suitable for gene-therapy applications. In addition, lentivirus-based gene transfer is hampered by impaired nuclear translocation of the vectors (35). To improve tropism for other cells types than T cells, a pseudotype lentiviral vector was generated in which the lentiviral envelope glycoprotein was replaced by the G glycoprotein of vesicular stomatitis virus (105). To address the problem of poor nuclear translocation, Follenzi et al. (35) generated a self-inactivating lentiviral construct in which a 118-bp sequence of HIV-1 polymerase (pol) encompassing the central polypurine tract (cPPT) and termination sequences was inserted upstream of an expression cassette for enhanced green fluorescent protein. They showed that the cPPT sequence increased transgene expression by promoting a rate-limiting step of infection that occurs after reverse transcription of viral RNA and concurrent with nuclear translocation. Once in the nucleus, vector transcripts with or without the cPPT sequence were similarly processed. However, safety concerns regarding clinical application of lentivirus, centering on the construct's derivation from wild-type HIV-1, thus far have prevented the application of lentivirus-based gene transfer for heart failure in patients.
Therapeutic Targets
In chronic heart failure, numerous changes in the homeostasis of the remaining viable myocytes occur, and especially the changes in Ca2+ handling offer opportunities for therapeutic intervention (64).
Excitation–contraction coupling denotes the series of events that lead from the myocyte action potential to contraction of myofilaments in the cell, and this process was described in detail by Bers (15). In summary, on depolarization, a small amount of Ca2+ enters the myocyte through voltage-dependent L-type Ca2+ channels. These Ca2+ ions activate the ryanodine receptors in the sarcoplasmic reticulum (SR). The ryanodine receptors are located adjacent to the L-type Ca2+ channels in the cell membrane and release a large amount of Ca2+ into the cytosol, where the ions bind troponin C on the myofilaments of the sarcomere, triggering contraction. Sarcomere relaxation on return of Ca2+ from the cytosol into the sarcoplasmic reticulum requires energy and is the result of a specific Ca2+ pump, SRCa2+-ATPase (SERCA). The principal isoform in cardiomyocytes is SERCA2a. The activity of SERCA2a is regulated by a closely associated protein in the sarcoplasmic reticulum, phospholamban, which regulates SERCA2a, dependent on its phosphorylation state. Dephosphorylated phospholamban inhibits SERCA2a. In a final step, Ca2+ is removed from the myocyte by the Na+/Ca2+ exchange.
In heart failure, peak systolic Ca2+ concentration is reduced, and diastolic concentration is increased. Concomitantly, SERCA2a mRNA and protein levels (48), as well as enzymatic activity (30), are reduced, and phospholamban has been shown to be hypophosphorylated because of desensitization of the β-adrenergic signaling pathway (48, 86); this, in all likelihood, further reduces SERCA2a activity.
Gene-transfer approaches to restore SERCA2a activity have shown improvement of ventricular function in preclinical studies. For example, in a large-animal model of LV volume overload, Kawase et al. (63) ruptured the chordae tendinae of 16 Yorkshire-Landrace pigs and infused AAV encoding SERCA2a or saline into the coronary arteries 2 months later. At 2 months after treatment, the animals that had received AAV had increased LV inotropy by LV pressure–volume measurements compared with the saline-treated group, and LV dimensions had decreased (63). These encouraging preclinical results led to the initiation of a clinical study using an AAV construct encoding SERCA2a in heart-failure patients (45).
Other gene-transfer approaches aimed at restoring Ca2+ handling by the cardiomyocyte with viral vectors have targeted phospholamban. A competitive approach was studied by Hoshijima et al. (57), by using AAV encoding a pseudophosphorylated mutant of human phospholamban to treat heart failure in BIO14.6 cardiomyopathic hamsters. The mutation consisted of the substitution of the Ser16 amino acid residue with a glutamate, which resulted in a conformational change that mimics the effect of phosphorylation of phospholamban at position 16. Sustained improvement of cardiac function was measured by echocardiography and pressure–volume in the animals treated with AAV expressing mutant phospholamban up to 30 weeks after gene transfer. Protein phosphatase 1 (PP1) is the major Ser/Thr phosphatase in cardiomyocytes, and it dephosphorylates phospholamban at Ser 16. Yamada et al. (140) hypothesized that myocardial gene transfer of inhibitor-2 (INH-2), an endogenous PP1 inhibitor, would increase phospholamban phosphorylation and ameliorate cardiac function in cardiomyopathic UMX7.1 hamsters. Two treatment groups were studied; one group received recombinant adenovirus (AdV) encoding INH-2 and was followed up for 1 week, whereas the other group received AAV-INH-2 and was followed up for 3 months. After INH-2 gene transfer, a significant decrease in microsomal PP1 activity was noted, concomitant with increased phospholamban phosphorylation at Ser 16. At 7 days after AdV-INH2 transfer, a significant reduction in LV size by echocardiography occurred, whereas control animals showed further increase of LV dimensions and deterioration of LV function. In the AAV-INH2 group, 3-month survival was significantly higher than that in the control group.
Finally, another avenue through which phospholamban could be affected in the failing heart is through adenylyl cyclase (AC) gene transfer. Adenylyl cyclase is an effector enzyme activated by a G protein coupled to a transmembrane receptor. In the heart, a typical example of such a G protein–coupled receptor is the β1-adrenergic receptor. Stimulation of β1-adrenergic receptors leads to activation of AC, which synthesizes the second-messenger cyclic AMP, which in turn activates protein kinase A to phosphorylate phospholamban and the ryanodine receptors (75).
However, other proteins relevant to cardiac Ca2+ cycling are also phosphorylated by protein kinase A, and the molecular mechanism responsible for functional improvement after AC gene transfer remains to be elucidated (46). Nine isoforms of AC are expressed in mammals, but AC5 and AC6 are the predominant isoforms in the heart. In a porcine model of pacing-induced heart failure, Lai et al. (72) documented a significant reduction in LV dimensions and improvement in LV function by echocardiography and LV pressure/volume measurements after intracoronary infusion of AdV-AC6. Thus far, no studies of cardiac AC5 gene transfer have been reported, and reports on the effect of AC5 in heart failure are difficult to reconcile. When transgenic mice with hypertrophic heart failure due to overexpression of the α-subunit of the G protein–coupled receptor were crossed with transgenic mice that overexpressed AC5, the double-transgenic mice showed a significant improvement in cardiac function (126). Conversely, in a pressure-overload model involving aortic banding in AC5-knockout mice, LV function remained unchanged compared with baseline measurements (95). In addition, a discrepancy between gene-transfer studies aimed at increasing phosphorylated phospholamban was summarized earlier in heart failure studies using genetic models. In a mouse model of hypertrophic heart failure, silencing of phospholamban expression led to a restoration of cardiac function (112). Minamisawa et al. (86) proposed that in heart failure, the inhibitory effects of dephosphorylated phospholamban predominate because phosphorylation is reduced because of blunted β-adrenergic receptor signaling, and consequently, a reduction in protein kinase A–dependent phosphorylation of phospholamban and knockout of phospholamban would prevent the accumulation of dephosphorylated phospholamban.
Safety and Side Effects
Adenoviral vectors are widely used and studied, and the insights into their pathogenicity continue to evolve. It has become clear that even gutless or helper-dependent adenoviral constructs are capable of initiating inflammatory and immune responses. A review of recent progress in this field has been published, and highlights of findings in vivo are summarized later (47). The problem of preexisting immunity was mentioned earlier, and it was shown that adenoviral vectors may be more toxic in the presence of antiadenoviral antibodies (133). The reasons for this increased toxicity are unknown. Adenoviruses are strong inducers of interferons in the cells that they infect, and this may contribute to their toxicity in vivo. Interferons are a class of small soluble proteins that induce non-infected cells to produce an antiviral protein that inhibits viral multiplication. After liver transduction with adenoviral vectors, an upregulation was observed in interferon-related genes, including MCP-1, IP-10, RANTES, and MIP-2 with a peak at 6–12 h after injection, and no difference was seen between first-generation constructs and helper-dependent vectors (47). A second response was seen at 5–7 days after injection that was elicited by vector-derived gene transcription.
Conversely, the changes in cytokine levels are typically transient, and in cardiac gene-transfer studies adenoviral vectors have shown an excellent safety profile (52), with the most frequently reported side effect being a transient and mild fever (106). However, the severity of these effects may be dose related, as suggested by the death of a patient in an adenoviral gene-transfer trial for ornithine transcarbamylase deficiency (an X-linked inborn error of urea synthesis in the liver) who received a very high dose of 6 × 1011 viral particles/kg (103). The doses used in clinical cardiac gene-transfer trials have ranged between 1 × 1010 and 4 × 1010 viral particles (42, 52, 122). Because adenoviral vectors do not integrate into the host genome, the risk of malignant transformation of transduced cells is anticipated to be low. Indeed, this is corroborated by clinical studies with adenoviral vectors, as no increased incidence of neoplasia was observed in these studies (60).
The inflammatory and immune responses to AAV and lentiviral vectors have not been studied as extensively as those for adenovirus, but in baboons, myocarditis has been reported after intramyocardial injection of an adeno-associated construct encoding human TNF receptor II (81). This myocarditis was attributed to a cellular immune response with circulating anti-AAV antibodies.
AAVs and lentiviral vectors have not been used in clinical heart-failure studies in part because of their unattractive safety profile, whereas the first clinical gene-transfer study with AAVs has been initiated (45). Although AAVs and lentiviral vectors remain relevant to the development of new strategies for the treatment of heart failure, because they allow mechanistic and proof-of-principle studies in animals, the defective replication and lack of any human pathology that can be ascribed to AAVs are attractive features of this vector in clinical studies. Moreover, for gene-transfer approaches to be effective in heart failure, expression of the target gene at high levels for many months is likely to be necessary, and as mentioned earlier, AAV has been shown to meet this requirement at least in animal studies (134).
In conjunction with long-term expression, control of expression is an important safety requirement, and the detrimental effect of unregulated gene expression was clearly illustrated in a study by Lee et al. (73). This study assessed the effect of myoblast implantation in mice after these cells had been transduced ex vivo with a retrovirus encoding VEGF, and the authors observed that heart failure and hemangiomas developed in the animals. The most widely used tool to regulate gene expression is the tetracycline (Tet) regulatory system based on the repressor/operator elements of an Escherichia coli tetracycline-resistance operon (39). The Tet-OFF system was developed first, and it operates by coordinated interaction of two components, the Tet-repressor protein (TetR) and the tetracycline response element (TRE). In bacteria, TetR inhibits gene transcription, but when fused to a herpes simplex viral protein (VP) domain, VP 16, it becomes a transcriptional activator called transactivator. The transactivator/TRE complex can be fused to cell type–specific promoters (e.g., the CMV promoter), and when Tet binds the complex, transcription is abrogated. Randomly induced mutations of the Tet-OFF transactivator led to the development of the Tet-ON system in which the construct is similar to that of the Tet-OFF system, except for alterations in four amino acids in the transactivator that result in activation of transcription on binding of tetracycline (40). For applications in heart failure, the Tet-ON system would appear to be more appropriate because tetracycline would need to be taken by the patient only for the desired duration of gene expression.
Another system that offers conditional expression of genes encoded by viral vectors relies on hypoxia. Hypoxia-inducible factor-1α (HIF-1α) is a master-switch transcriptional activator that induces expression of numerous genes under conditions of hypoxia. Under normoxic conditions, HIF-1α interacts with the von Hippel–Landau protein that recruits prolyl hydroxylases. On hydroxylation of HIF-1α, it is ubiquitinated and subsequently destroyed by the S26 proteasome. This hydroxylation is oxygen dependent and absent or reduced under hypoxic conditions. Fusion of DNA-binding and dimerization domains of HIF-1α with the VP16 transcriptional activator generates a powerful, oxygen-dependent inducer of HIF-1α–dependent gene expression. One of the genes that is activated by HIF-1α is brain natriuretic peptide (BNP), which has a cardioprotective effect. Luo et al. (76) showed that BNP was upregulated in human cardiomyocytes transfected with HIF-1α/VP16 only after exposure to hypoxia. Thus, the HIF-1α/VP16 complex may be viewed as a “vigilant system” that lies dormant until the heart is exposed to hypoxia and that will shut down again on restoration of normoxia. The efficacy and safety of this concept remain to be studied in clinical studies, however, and more generally, none of the systems for controlled expression of vector-based genes has reached the phase of clinical trials.
Cell Therapy
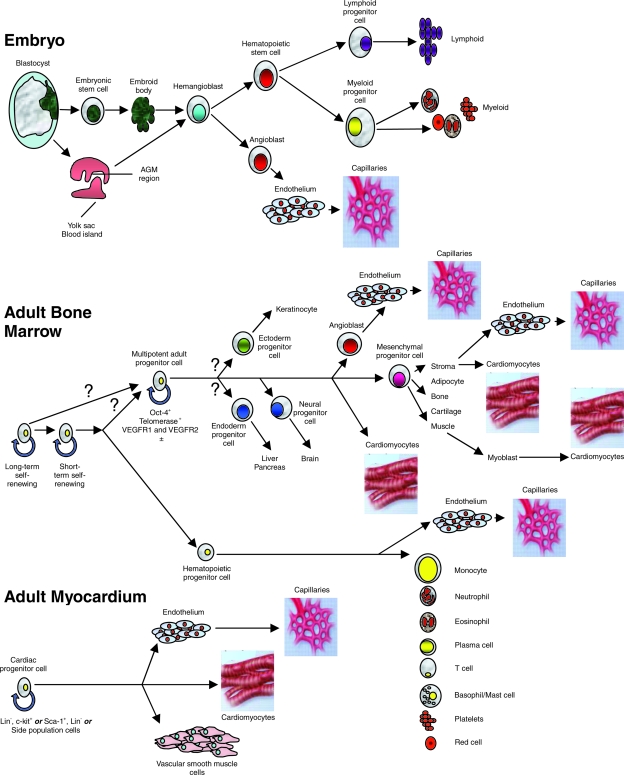
The expanding literature describing the use of multipotent cells to improve cardiac function has made it necessary to define clearly the cell types that are being used for these types of treatment. Different cells in adult organs have different degrees of regenerative capacity. Some progenitor cells are capable of producing only one type of differentiated cell; for example, spermatogonial cells are unipotent and differentiate into spermatozoa. Other cells have a broader differentiation repertory. One bone marrow–precursor cell can reconstitute all lineages found in the bone marrow, and thus, these cells can be described as being multipotent (Fig. 1) (50). Likewise, tripotent cardiac progenitor cells have been demonstrated in the adult heart to have a capability to differentiate into cardiomyocytes, endothelial cells, and vascular smooth muscle cells (13). These multipotent progenitor cells must be distinguished from “true” stem cells, which are clonogenic, and have the capability of unlimited self-renewal by symmetric division while maintaining a stable diploid karyotype, but conversely can give rise to all differentiated cell types through asymmetric division, with one daughter cell retaining the stem cell phenotype and the other giving rise to differentiated cell types (117). Stem cells that meet these criteria have not been identified in adult organs.
FIG. 1.
Hemangioblasts can be derived from blastocyst-derived embryonic stem cells, and in vivo, they arise from the yolk sac or AGM region of the early embryo. With appropriate stimulation in vitro, hemangioblasts develop into hematopoietic progenitor cells that can give rise to all cell lines of the lymphoid and hematopoietic system, or they can develop into angioblasts that form endothelial cells and capillaries. In adult bone marrow, self-renewing cells differentiate into hemangioblasts that generate all cells of hematopoietic lineage, but cells of monocyte lineage can also differentiate into endothelial cells. The origin of the multipotent adult progenitor cell is unknown, but its progeny forms all mesenchymal tissues, and it can differentiate into cardiomyocytes and endothelial cells. Skeletal muscle is of mesenchymal lineage and can dedifferentiate into a myoblast phenotype, and then can differentiate into a cell with cardiomyocyte features. In adult myocardium, several multipotent cells have been identified by immunohistochemistry and fluorescence-activated cell sorting, and by sorting for side population cells. These cells have been shown to differentiate into endothelial cells and capillaries, cardiomyocytes, and vascular smooth muscle cells. (Reproduced with permission of Nature Publishing Group from ref. 27. © 2006.)
In 1993 Koh et al. (68) reported the feasibility of cell transplantation to the myocardium. The group used atrial cardiomyocytes from transgenic mice in which cardiomyocyte proliferation had been induced by targeted expression of simian virus 40 large T antigen and documented myocyte survival up to 4 months after transplantation of these cells into hearts of syngeneic mice. However, no evidence of electromechanical coupling was found between the transplanted and host cardiomyocytes, and this prompted the group to study fetal cardiomyocytes from transgenic mice carrying a fusion construct encoding α-cardiac myosin heavy chain promoter and β-galactosidase (120). On electron microscopy after implantation of these cells into hearts of syngeneic mice, the transplanted cells were readily identifiable because the X-gal product is an electron-dense precipitate, and the authors observed numerous intercalated disks between transplanted and host cardiomyocytes. Later, resident cardiac progenitor cells were identified in mice by a number of groups by using various markers, and the reader is referred to an excellent review on this subject by Wu et al. (139). For some of these cells, a capability was demonstrated to differentiate into endothelial cells, vascular smooth muscle cells, and cardiomyocytes (61), whereas others demonstrated improvement in LV function in murine MI models after intravenous injection of cardiac progenitor cells (24). The demonstration of cardiac progenitor cells in mice initiated studies of cells obtained from adult human hearts [for example, tissue samples from patients who underwent surgery for aortic stenosis (131) or heart transplantation (101)] yielded cardiac progenitor cells that were positive for the cardiac progenitor cell marker c-Kit. When these c-Kit+ cells were isolated by flow cytometry, they exhibited a tripotent differentiation capability in vitro, giving rise to cardiomyocytes, vascular smooth muscle cells, and endothelial cells (13). A relation appears to exist between the molecular signature of cardiac progenitor cells and their capability to differentiate in a uni-, bi-, or tripotent manner, and this relation is currently being investigated by a number of groups (139). In a parallel development, Asahara et al. (5) discovered that a cell type can be isolated from human peripheral blood on the basis of cell surface–antigen expression that is capable of differentiating into endothelial cells in vitro and that contributes to neovascularization in vivo (5).
Because of these discoveries, many different cell types have been shown to exhibit multipotent differentiation capacities. The cells that have been applied to stimulate post-MI wound healing are of a greater variety in their origin than are the cells that have been transplanted into the chronically failing heart. Functional improvement after cell transplantation after MI has been demonstrated in preclinical and clinical studies for homogenous and heterogenous autologous cell populations from peripheral blood and bone marrow (33, 97, 113), cells of mesenchymal origin such as bone marrow stromal cells (28), adipose stromal cells (129), epicardium-derived cells (130), and mesenchymal progenitor cells (49), as well as cardiac progenitor cells (94) and stem cells (56). It is unclear whether these different cell types achieve functional improvement through a final common pathway or whether the improvement is the result of different mechanisms attributable to different progenitor cell types. Moreover, direct comparisons of different cell types in MI models yield conflicting results, with some studies showing a difference in functional improvement between cell types (62), and others showing equivalency (2).
For the treatment of chronic CHF, the cell types have been restricted primarily to skeletal myoblasts and bone marrow–derived cells (Table 1). Because these cells are injected into nonviable vascular scars (82), the primary rationale for cell therapy in CHF has been to increase the number of contractile units, as opposed to promoting neovascularization and reducing cardiomyocyte death (67).
Table 1.
Clinical Studies of Cellular Cardiomyoplasty for Chronic Congestive Heart Failure
| Indication + year of publication | Pts. (no.) | E.F, pre − post tx (%) | Follow-up | Death: arrhythmia | Cell type | Cell (no.) | Delivery method | Results | |
|---|---|---|---|---|---|---|---|---|---|
| MI >10 d to <3 mo before tx, ischemic CMP, 2003 NR |
6 | 36.2 ± 10.6 48.8 ± 5.6 |
3–10 mo | None | BMC | 1.5 × 106 | CABG + epicardial injection | Improved LVEF and improved perfusion by scintigraphy | (121) |
| Old MI + ischemic CMP, 2003 NR |
12 | 35.5 ± 2.3 53.5 ± 4.98 |
3 mo | None | Skeletal myoblasts | 1.75–3 × 108 | CABG + epicardial injection | Improved LVEF and improved viability by 18F-FDG-PET | (53) |
| Old MI, 2003 NR |
5 | 36 ± 11 41 ± 9 |
6 mo | 1 pt. requiring ICD for NSVT | Skeletal myoblasts | 2.96 × 108 | Catheter-based endomyocardial injection | Improved LVEF and regional thickening (MRI) in cell tx arm | (119) |
| Old MI + ischemic CMP, 2003 NR |
10 | 24 ± 1 32 ± 1 |
10.9 mo | 4 pts. VT requiring ICD | Skeletal myoblasts | 8.71 × 108 | CABG + epicardial injection | Improved LVEF and regional thickening (echo) in cell tx arm | (84) |
| CHF pre HT, 2003 NR |
5 | n.a. | 68–191 d | None | Skeletal myoblasts | 2.8 × 108 | LVAD + epicardial injection | Myoblasts identified at heart recovery | (96) |
| Old MI + ischemic CMP, 2003 NR |
14 | 20 ± 9 29 ± 13 |
4 mo | 1 pt. SCD | BMC | 3 ± 0.4 × 107 | Catheter-based endomyocardial injection | Improved LVEF and mechanical improvement by EMM | (98) |
| Old MI + ischemic CMP, 2004 NR |
10 | 35.2 (25–40) 42 (29–47) |
12 mo | 1 death, 2 pts. VT requiring amiodarone | Skeletal myoblasts | 2.45 ± 2.42 × 107 | CABG + epicardial injection | Improved LVEF | (116) |
| Old MI + ischemic CMP, 2005 NR |
30 | 28 ± 9 36 ± 11 at 24 mo |
33–45 mo | 4 deaths, 5 pts. VT 2 requiring ICD, 1 pt with VF on LVAD | Skeletal myoblasts | 1 × 107–3 × 108 | CABG (n = 24) or LAVD (n = 6) + epicardial injection | Improved LVEF and improved viability by 18F-FDG-PET | (29) |
| Old MI, 2006 R |
75 | BMC: 41 ± 11 → 43 ± 10 CPC: 39 ± 10 → 39 ± 10 No cells: 43 ± 13 → 42 ± 13 |
3 mo | 1 death | BMC (n = 28) CPC (n = 24) No cells (n = 23) |
BMC: 2.05 ± 1.1 × 108 CPC: 2.2 ± 1.1 × 107 |
IC infusion | Improved LVEF in BMC group | (6) |
| Old ant. MI, 2007 NR |
12 | 27.2 ± 6.8 29.7 ± 7.3 |
11.3 ± 3 mo | None | BMC | 2.79 ± 0.89 × 107 | IC infusion | Improved LVEF and improved perfusion by scintigraphy | (78) |
| Old MI, 2007 NR |
10 | 35.2 ± 4.6 42.3 ± 5.1 |
12 mo | 1 pt. VT requiring ICD | BMC | 8.6 ± 3 × 107 | Catheter-based endomyocardial injection | Improved LVEF | (25) |
| Old MI, 2007 NR |
15 | 23 ± 8 27 ± 9 |
3 mo | 1 death | BMC | 9.4 ± 1.4 × 107 | Catheter-based endomyocardial injection | Improved LVEF | (14) |
| Old MI + ischemic CMP, 2008 NR |
14 | 35 ± 10 37 ± 9 |
4 yr | 3 deaths | Skeletal myoblasts | 2.06 (1.5–2.94) × 107 | Catheter-based endomyocardial injection | No improvement in LVEF and tissue Doppler indices | (135) |
| Old MI + ischemic CMP, 2007 NR |
11 | 19 ± 1 19 ± 2 |
4 mo | 1 death, 1 pt VT requiring ICD | BMC | 2.5 × 107 | IC infusion | No improvement in LVEF and WMSI | (107) |
| Old MI + ischemic CMP, 2008 R |
97 | High dose: 29.6 → 35.1 Low dose: 25.2 → 32.3 Placebo: 28.7 → 32.5 |
6 mo | 5 deaths, VT all pts. prophylactic CD | Skeletal myoblasts: 30 high dose 33 low dose 34 placebo |
High dose: 8 × 108 Low dose: 4 × 108 |
CABG + epicardial injection | No difference in LVEF between groups, more arrhythmia in myoblast groups | (83) |
Ant, anterior; BMC, bone marrow–derived mononuclear cell; CABG, coronary artery bypass surgery; CPC, circulating progenitor cell; CMP, cardiomyopathy; d, day; Dec, decreased; DSE, dobutamine stress echocardiography; Echo, echocardiography; EF, ejection fraction; EMM, electromechanical mapping; FDG-PET, fluorodeoxyglucose positron emission tomography; HT, heart transplantation; IC, intracoronary; ICD, internal cardiac defibrillator; LV, left ventricular; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MRI, magnetic resonance imaging; mo, month; n.a., not applicable; NR, nonrandomized; NSVT, nonsustained ventricular tachycardia; Pts, patients; R, randomized; SAE, serious adverse event; SCD, sudden cardiac death; tx, therapy; vs., versus; VT, ventricular tachycardia; WMSI, wall-motion score index; yr, year.
Myoblast cells
Skeletal myoblasts are cells that reside in a quiescent state underneath the basal lamina of skeletal muscle fibers; they are mobilized quickly in response to muscle injury and contribute to muscle healing. Since the identification of myoblasts, a cell has been discovered in the same location that can completely regenerate skeletal muscle fibers, and these cells are called satellite cells (22). Satellite cells, like all “organ-specific stem cells,” such as hematopoietic “stem” cells and cardiac progenitor cells, are rare cells that are capable of self-renewal and regeneration of the organ in which they reside. Collins et al. (22) demonstrated in mice that a single muscle fiber associated with seven satellite cells was capable of originating 100 new myofibers containing thousands of nuclei. It is unclear how many satellite cells were included in the myoblast populations that have been used for cardiac myoblast transplantation studies. It seems unlikely that they contained satellite cells, because virtually all protocols for isolating satellite cells have resulted in their activation and differentiation toward a myocyte phenotype (102). Thus, it would seem that procedures to isolate and culture myoblasts would also result in satellite cell activation and myogenic differentiation.
As explained by the pioneers of myoblast transplantation, Menasché (83) and Taylor (141), myoblasts are attractive candidates for cardiac cell transplantation for a number of reasons. Under the correct culture conditions, hundreds of millions of cells can be obtained from a small muscle biopsy. The cells are well into the myogenic differentiation pathway, which makes it very unlikely that they will undergo malignant transformation after transplantation, and they are resistant to ischemia, which is important because they are implanted in an avascular myocardial scar. Since the first experiment by Taylor et al. (125), the beneficial effect of myoblasts on cardiac function has been demonstrated in a number of ways in animal models. By using sonomicrometry, He et al. (51) showed an increase in end-systolic pressure and a decrease in end-systolic volume in a dog MI model. In a study using magnetic resonance imaging (MRI), van den Bos et al. (132) showed improved wall thickening and a reduction in left ventricular remodeling after MI in rabbits, and in a rabbit model of ventricular aneurysm, myoblast transplantation showed improved wall motion at rest and under dobutamine stress by sonomicrometry (31).
Other cells
Although bone marrow cells are less resistant to ischemia than are myoblasts, they too have been applied in approaches to improve cardiac function in CHF. The majority of studies with bone marrow–derived cells have been performed in the subacute period of post-MI wound healing, in which the MI wound still is in transition (hemorrhagic “mushy” infarct) and is better vascularized than in the end stage of healing, which is characterized by the presence of a scar. Nevertheless, when bone marrow cells were injected in the periphery and the center of a myocardial scar in a rabbit MI model, the animals showed functional improvement (128). Cells of mesenchymal origin have also been shown to improve cardiac function when injected after the myocardial scar has formed. In rats, Mazo et al. (80) injected cells derived from adipose stroma 1 month after MI; these showed functional improvement by echocardiography and increased tissue viability by 18F-FDG microPET.
Clinical Studies
Whereas currently only one clinical study of viral vector–based gene transfer for heart failure has been initiated (45), multiple clinical studies of cell transplantation for this syndrome have been completed, and the results have been mixed, with some studies showing improvement, and one randomized study showing no benefit over placebo injections (Table 1). From the limited experience with long-term clinical follow-up, it appears, however, that any functional improvement is not sustained after 4 years (135).
One explanation for the difference between clinical and experimental data could be that most experimental studies have been performed in young otherwise healthy animals, and evidence suggests that a young systemic environment restores the regenerative capacity of progenitor cells. In a parabiosis experiment with young and old mice, which exposed the old mice to factors present in the young serum, Conboy et al. (23) showed that old satellite cells regained their regenerative capacity by enhanced expression of the Notch ligand (Delta), increased Notch activation, and increased proliferation in vitro. Thus, the exposure of transplanted cells to serum factors in young animal models of MI might favorably affect their regenerative capacity compared with transplantation in old patients. In this context, a study by Mangi et al. (77) is of interest; bone marrow–derived mesenchymal progenitor cells were transfected ex vivo with a retroviral construct encoding Akt because this procedure significantly improved the viability of these cells after transplantation.
Although skeletal myoblasts have been shown to improve LV function in preclinical studies and, as described earlier, have a number of characteristics that make them attractive for cell-transfer approaches to improve cardiac function, the largest clinical randomized study to date did not show any improvement in LV function after myoblast transplantation (83). Moreover, an increase in early ventricular arrhythmia was found in the myoblast group, and therefore, the lack of functional improvement in combination with an increased risk of ventricular arrhythmia makes it doubtful that this particular cell type will acquire a place in clinical heart-failure therapy within the near future.
Mechanism of Benefit
Although no doubt exists that the mechanism of action of gene transfer relies on expression of the gene of interest by the host cell, it is unclear how transplanted cells improve cardiac function after MI. Considerable controversy occurs over the hypothesis that cells transdifferentiate into cardiac myocytes. Evidence in support of this hypothesis comes from a study in a child with a mutation in the gene for cardiac troponin T (TnT) (110). After heart transplantation for heart failure, peripheral blood was collected, and circulating progenitor cells were isolated and cocultured with neonatal rat cardiomyocytes. After 6 days, the cardiac marker protein, α-sarcomeric actin, could be detected in the progenitor cells. The fact that these cells originated from the patient's circulation was confirmed by the presence of the mutated TnT gene. Conversely, proof for transdifferentiation in vivo has been more difficult to obtain. By using bone marrow cells from transgenic mice expressing enhanced green fluorescent protein and injected into the periinfarct zone of wild-type mice, Scherschel et al. (114) were unable to show that these cells acquired intracellular Ca2+ transients in response to membrane depolarization in situ by monitoring the Ca2+-sensitive fluorophore rod-2 in the perfused, isolated hearts by two-photon laser scanning fluorescence microscopy. Others have also provided evidence that hematopoietic cells do not transdifferentiate into cardiomyocytes after transplantation to the heart (90). The reasons for the discrepancies between these studies are manifold and can be attributed both to a poor understanding of the biologic effect of cell transfer in vivo and to deficiencies in the readouts that are used to study the effects of cell transfer in animal models. For example, the use of fluorescent labels to track and locate cells after transfer into the infarcted heart is prone to error because the macrophages, contractile proteins, and collagen in the infarct area render the tissue highly autofluorescent, making the identification of fluorescently labeled cells prone to error. The use of reporter genes encoding β-galactosidase in combination with the Cre/lox system or Y chromosome in situ hybridization allows easier recognition of transplanted cells.
Little evidence exists that skeletal myoblasts undergo transdifferentiation, and it has been postulated that the mechanical effects of cell transfer in terms of modification of the scar by a new inert tissue mass may in itself favorably alter ventricular geometry and cardiac output (27). Another plausible mechanism of benefit from cell transfer, which has been suggested primarily for skeletal myoblasts, is cell integration or electromechanical coupling (44). The presence of connexin-43 between myoblasts and cardiomyocytes has been demonstrated with immunohistochemistry in rat hearts (21), and others found action potential–induced Ca2+ transients in synchrony with host cardiomyocytes in a small number of transplanted myoblasts, but the duration of these [Ca2+]i transients was heterogeneous compared with that of the neighboring cardiomyoblasts (108). Interestingly, in the same study, evidence was noted for fusion between myoblasts and resident cardiomyocytes, based on the identification of cells that expressed both a donor- and a host-derived reporter gene, and these fused cells were in the same anatomic location as the cells that showed the heterogeneous [Ca2+]i transients; thus, the authors concluded that these transients are likely to be result of cell fusion and not an expression of bona fide electromechanical coupling. Cell fusion is a not-infrequent event observed after cell transfer to the heart, not only after myoblast transfer, but also after transplantation of bone marrow–derived cells (93). Another manner in which contact and exchange of molecules between host and donor cells can be established has been proposed by Koyanagi et al. (70), who found evidence that contact between transplanted and resident cells can be established by naturally occurring nanotubules that facilitate intercellular transport of macromolecules, which then could be responsible for the phenotype switches that have been observed by multiple groups. In summary, no conclusive evidence exists that electromechanical coupling occurs after cell transfer in vivo (104). The electrical heterogeneity introduced by the nonsynchronous electrical activity of transplanted myoblasts may have caused the increase in ventricular arrhythmias observed in clinical myoblast studies (83).
The most recent hypothesis is that the transplanted cells exert their beneficial effect via a paracrine mechanism (66) in which molecules from transplanted cells affect changes in resident cells. For example, Gnecchi et al. (38) demonstrated a significant reduction of MI size and a reduction of apoptosis in rat hearts after injection of mesenchymal progenitor cells that overexpressed the survival gene Akt1 (Akt-MPC) (77), as well as after injection of media with a high concentration of Akt1 obtained from cultures of Akt-MPC (38). Later, members of the same group reported that the most prominently expressed and secreted protein by Akt-MPC was the secreted frizzled-related protein 2 (Sfrp2), a known modulator of Wnt signaling, and they showed that Sfrp2 promotes survival of cardiomyocytes after MI and that these effects were significantly reduced after knockdown of Sfrp2 with siRNA (87). Wnt proteins form a family of highly conserved secreted signaling molecules that regulate cell-to-cell interactions during embryogenesis and are expressed at low levels in adult organs under normal conditions. After MI, however, Wnt levels are increased, and overexpression of an antagonist of Wnt signaling in mice has been shown significantly to reduce MI size and improve cardiomyocyte survival (11). The paracrine hypothesis is in better alignment with the low retention of transplanted cells after delivery (see later) and the high mortality within the first 24 h after transplantation (88). It could explain the observed functional improvement in the face of this extensive cell loss early after cell transfer.
Safety and Side Effects
The aggregate of clinical studies of cell transplantation in the subacute MI-healing phase and in chronic CHF has shown that the procedure is safe (1). Notably, no increased incidence of malignancies was found in these studies. The risk of potentially fatal ventricular arrhythmia after skeletal myoblast transplantation in patients with chronic CHF (83), together with the lack of demonstrable efficacy in the first clinical randomized trial, is a major impediment to further development of this cell type for clinical heart-failure therapy.
Oxidative Stress
In addition to the influence of recipient age mentioned earlier, the therapeutic effect of gene and cell transfer is also likely to be influenced by oxidative stress in the recipient myocardium. Oxidative stress can be described as an imbalance in the generation of reactive oxygen species (ROS) and opposing antioxidant molecules in favor of the former. In the overwhelming majority of cases, atherosclerosis is the cause of ischemic CHF, and this state of vascular inflammation, together with its precipitating factors (hyperlipidemia, diabetes mellitus, hypertension, metabolic syndrome, or smoking), is characterized by increased levels of ROS (20). In addition to the pathologic states leading to CHF, the syndrome itself is characterized by increased myocardial ROS levels generated by endothelial cells, vascular smooth muscle cells, and cardiomyocytes (4, 58). It has been suggested that increased levels of ROS in patients compared with animal models may explain some of the discrepancies that have been observed between preclinical and clinical gene- and cell-transfer studies (18).
Within cells, ROS are generated by a number of processes that include the electron-transport chain, the xanthine oxidase/dehydrogenase system, uncoupling of eNOS, and a number of enzymes, such as the cytochrome P450 family and NADPH oxidases, with NADPH oxidases being the most important source of ROS (4). Importantly, mitochondria of cardiomyocytes are a main source of ROS (4), and Terentyev et al. (127) showed that the diastolic “leak” of Ca2+ from the SR, which is a characteristic of failing cardiomyocytes and is responsible for the lack of excitation–contraction coupling in these cells, can be attributed to modification of the ryanodine receptor on the SR by ROS. Thus, it is conceivable that transfer of AAV encoding SERCA2a, as proposed by Hajjar et al. (45), may not be sufficient to restore excitation–contraction coupling in the presence of continued ryanodine receptor modification by ROS in the failing cardiomyocyte. Strategies that alter the redox state of the failing heart may have to accompany this gene-therapy approach. Fortunately, many pharmacotherapeutics used in heart-failure patients have been shown to reduce the prevalence of ROS [for example, ACE inhibitors and angiotensin-receptor blockers have been shown to reduce cardiovascular oxidative stress (32,109)]. Evidence indicates that statins reduce NADPH oxidase activity and ROS generation (115), and tight glycemic control in diabetic patients with CHF is likely to reduce oxidative stress in the failing heart (142). It remains to be seen whether these medications reduce oxidative stress in the failing heart to the extent at which the effect of viral SERCA2a gene transfer is not nullified by the effect of ROS on other determinants of intracellular Ca2+ flow (for example, the ryanodine receptor).
As with gene transfer, the effects of cell transfer may be altered by the redox state of the patient heart. For example, the production of ROS by mitochondria is regulated to a large extent by the adapter protein p66Shc (85). This protein has been identified as a master regulator of ROS production and is encoded by the mammalian protooncogene locus SHC, which also encodes two similar adapter proteins, p52Shc and p46Shc (85). The unique role of p66Shc to amplify basal and stimulus-dependent ROS generation in mitochondria is conveyed by an N-terminal extension that contains an S36 phosphorylation site that is critical to this capability (43). Recently Guo et al. (43) reported evidence for the existence of a redox-sensitive pathway initiated by α1- adrenergic receptor (α1-AR) activation, which leads to p66Shc phosphorylation with subsequent increases in Akt phosphorylation, inactivation of the forkhead transcription factor FOXO3a, and decreases in expression of the antioxidant enzyme manganese superoxide dismutase (MnSOD). Thus, p66Shc phosphorylation decreases ROS detoxification by activating the Akt-FOXO3a pathway that regulates MnSOD. This finding may have implications for cell-transfer strategies that rely on ex vivo transfection of cells with constructs that encode Akt to promote their survival in the host myocardium (77). In the redox environment of the patient heart, the Akt protein synthesized by these cells may be diverted away from an Akt-mediated antiapoptotic pathway, although the progenitor cell may not have α1-AR because the donor cell may be exposed to phosphorylated p66Shc from the host cell through fusion or connection via nanotubules mentioned earlier, or both. As with viral gene transfer, it is as yet uncertain whether the effects of cell transfer will be modified by the redox-sensitive mechanism summarized earlier and whether the standard medical regimen of CHF patient will be sufficient to alter the redox state of the recipient heart or whether additional antioxidant strategies will have to be used for cell transfer to reach its full therapeutic potential.
Delivery Methods
The optimal delivery strategy for viral vectors and cells remains to be established, and compared with the number of preclinical and clinical studies aimed at understanding mechanisms of action and clinical efficacy, work comparing and optimizing delivery methods has been lagging. Both viral gene vectors and multipotent cells are typically transferred into the myocardium by mechanical techniques, with intracoronary infusion (6) and intramyocardial injection being the most frequently applied. Intramyocardial injection can be into the endocardium by using injection catheters (10, 25, 119) and into the epicardium during open-chest coronary artery bypass surgery (83). Other techniques include retroinfusion into coronary veins (10, 17), delivery of vectors via extracorporeal circulation (100), or a percutaneous closed-loop recirculatory system (65).
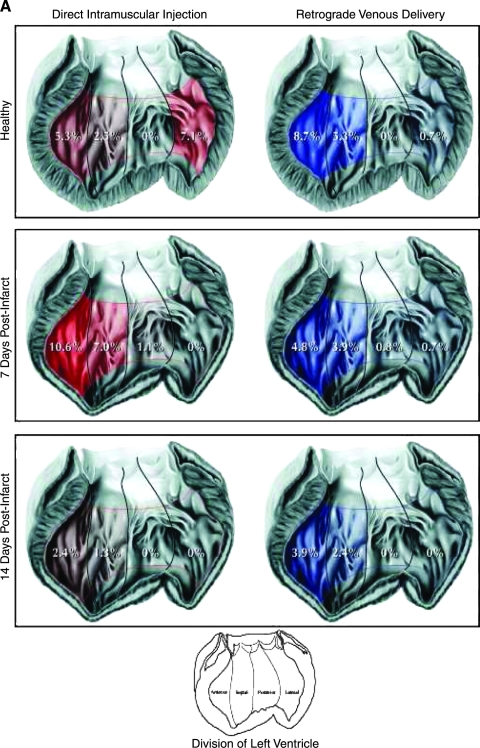
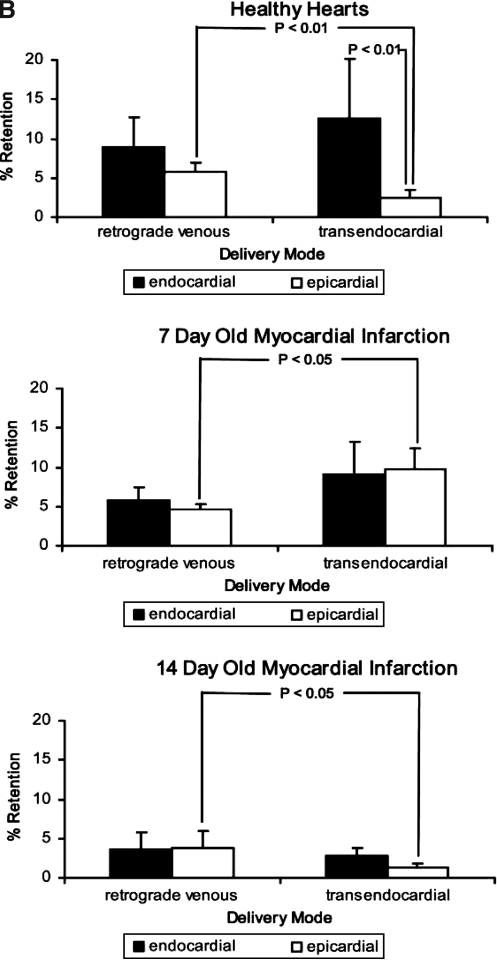
Few studies quantify the retention of the agent that was delivered, and likewise, few data compare the efficacy of different delivery systems. This is surprising because delivery efficacy is likely to affect the therapeutic effect. In a porcine MI model, we compared catheter-based intramyocardial injection with retrograde intravenous infusion with radiolabeled microspheres to quantify the amount of injectate that was retained in the myocardium; we found that overall retention was comparable between the two methods, but that it was low and decreased with the age of the myocardial infarction (Fig. 2) (10). A difference was found between the two methods regarding myocardial distribution, because the catheter was navigated to the infarct zone, and the majority of spheres were distributed in that area, whereas after retrograde venous infusion, the spheres were retained mostly in the periinfarct area in midventricular segments. A novel strategy that allows intravenous injection of agents and thus has the advantage of being less invasive is the use of ultrasound bubbles that are targeted to the cells of interest by incorporation of antibodies or peptide-recognition sequences in the bubble shell and that carry a payload of vectors (79). On arrival in the target area, these bubbles are destroyed by a high-energy ultrasound pulse that makes the vector available in the target tissue. No studies investigated the effect of different delivery modes on functional recovery of the heart after gene or cell transfer.
FIG. 2.
Microsphere retention and distribution in normal hearts and in hearts with 1- and 2-week-old MI. The retention is expressed as percentage of the total microspheres injected. Values <0.5% are shown as 0. (A) Moving from left to right in these hearts, the anterior wall is shown on the left side, followed by the septum, posterior wall, and lateral wall. (Reproduced with permission of John Wiley and Sons, Inc. from ref. 10. © 2006.) (B) When comparing endo- and epicardial distribution, in healthy hearts, significantly more retention of microspheres is found in the epicardium after retrograde infusion; this relation is reversed in 7-day-old MI, but in 2-week-old MI characterized by a transmural scar, retrograde infusion deposits more spheres in the epicardium than does transendocardial injection (Reproduced with permission of John Wiley and Sons, Inc. from ref. 10. © 2006.)
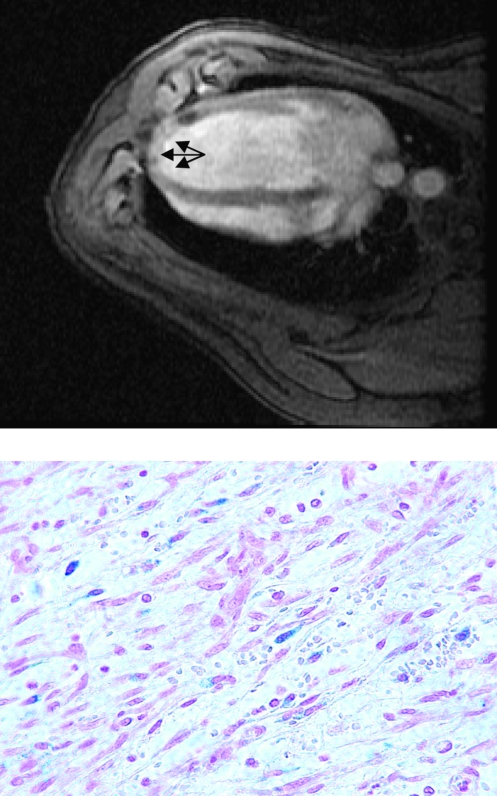
Methods to track cells after injection include radioactive labeling, but cells can also be labeled with iron particles ex vivo, and we (9) and others (37, 55) have shown the feasibility of locating transplanted cells in the infarcted heart by MRI (Fig. 3). The advantage of iron labeling and MRI is that, in conjunction with localizing the cells, cardiac function can measured, and thus, the effect of cells on cardiac recovery can be assessed during the same imaging session. Because the disruption of the magnetic field extends to a much larger area than the labeled cells, conventional T2*-weighted MRI of iron-labeled cells creates a “shadowing” effect that obscures the immediate surroundings of the cells and that impedes quantification of the signal. Recently, a novel positive-contrast technique was developed that addresses this problem (74). Finally, when iron labels are used, it is impossible to distinguish between viable cells and cells that have died after transplantation because in the latter situation, even when the cell has lysed the iron particle may still be retained in the myocardium for a time. However, when imaged within hours after injection, the majority of transplanted cells appear to be viable (Fig. 3), and therefore, MRI of iron-labeled cells may prove useful to study early distribution of injected cells throughout the myocardium. The capability of MRI to identify scar tissue would be of benefit especially to determine the relation between transplanted cells and myocardial scar (8). The lack of a contrast agent that distinguishes between viable and nonviable cells currently limits MRI and other cell-tracking techniques to distribution studies early after injection. In the future, imaging particles that are sensitive to differences between the intra- and extracellular milieus may become available that would solve this problem.
FIG. 3.
Vascular smooth muscle cells labeled ex vivo with iron particles (Feridex i.v.; Bayer Healthcare, Leverkusen, Germany) and injected retrogradely into the anterior interventricular vein in a porcine model of myocardial infarction, 1 week after induction of myocardial infarction by a 45-min balloon occlusion in the midsegment of the left anterior descending coronary artery. T2*-weighted magnetic resonance imaging shows a dark shadow indicating accumulation of the cells at the border of the infarct zone (arrow). After Prussian blue staining, iron-labeled cells are visible on 4-μm sections from the myocardium, and the fact that the iron label is retained within the cytoplasm of the labeled cells can be interpreted as indicating that these cells are still intact and viable (lower panel). (Reproduced with permission of John Wiley and Sons, Inc. from ref. 9. © 2004.)
Discussion
Gene and cell therapy for heart failure are exciting and rapidly evolving fields that require a multidisciplinary approach to succeed. Molecular biologists, chemists, imaging specialists, clinicians, and those of us who develop cardiac devices all must come together to take advantage of the promises that these approaches hold. The first clinical study of viral vector–based gene therapy for chronic heart failure is in progress, and for cell therapy, the clinical studies to date show that the treatment is safe and produces a modest but significant improvement of left ventricular function above and beyond conventional therapy when applied in the subacute phase of MI healing (1). Further studies must show whether this benefit is sustained during long-term follow-up and whether it translates into a reduction of morbidity and mortality after MI.
The clinical studies of cell therapy for heart failure present a more mixed picture, with the first randomized comparison of myoblast transfer showing no benefit over placebo treatment and an increased incidence of ventricular arrhythmia in the myoblast group. The loss of functional benefit 4 years after myoblast transplantation is disappointing. Gene therapy and cell therapy may come together to address this lack of efficacy and sustained benefit in studies in which cells are transduced with viral vectors encoding sequences that improve their survival and therapeutic potency. Within this context, it is important to realize that the effects of ROS on myocardial gene and cell transfer remain largely unaccounted for and that a strategy that combines gene and cell transfer may be especially relevant to reducing the detrimental effects of ROS on this type of therapeutic approach.
Finally, different delivery methods must be compared regarding their effect on therapeutic efficacy of the vectors and cells, and more-sophisticated methods must be developed to understand the effects of gene and cell therapy on myocardial neovascularization, perfusion, oxygenation, and cardiac function.
Abbreviations
α1-AR, α1-adrenergic receptor; AAV, adenoassociated virus; AC, adenylyl cyclase; ACE, angiotensin-converting enzyme; AdV, adenovirus; cPPT, central polypurine tract; eNOS, endothelial nitric oxide synthase; HIF-1α, hypoxia-inducible factor-1α; HIV-1, human immunodeficiency virus-1; INH-2, inhibitor-2; LTR, long terminal repeat; LV, left ventricle; MI, myocardial infarction; MnSOD, manganese superoxide dismutase; MPC, mesenchymal progenitor cell; MRI, magnetic resonance imaging; NADPH, nicotinamide dinucleotide phosphate; pDNA, plasmid DNA; PEI, polyethylenimine; PET, positron emission tomography; pol, polymerase gene; PP1, protein phosphatase 1; ROS, reactive oxygen species; SERCA, sarcoplasmic reticulum Ca2+-ATPase; Sfrp2, secreted frizzled-related protein 2; siRNA, small interfering ribonucleic acid; SR, sarcoplasmic reticulum; Tet, tetracycline; VEGF165, 165-amino acid isoform of vascular endothelial growth factor; VP, viral protein.
References
- 1.Abdel-Latif A. Bolli R. Tleyjeh IM. Montori VM. Perin EC. Hornung CA. Zuba-Surma EK. Al-Mallah M. Dawn B. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 2.Agbulut O. Vandervelde S. Al Attar N. Larghero J. Ghostine S. Leobon B. Robidel E. Borsani P. Le Lorc'h M. Bissery A. Chomienne C. Bruneval P. Marolleau JP. Vilquin JT. Hagège A. Samuel JL. Menasché P. Comparison of human skeletal myoblasts and bone marrow-derived CD133+ progenitors for the repair of infarcted myocardium. J Am Coll Cardiol. 2004;44:458–463. doi: 10.1016/j.jacc.2004.03.083. [DOI] [PubMed] [Google Scholar]
- 3.American Heart Association. Heart disease and stroke statistics update. Dallas, TX: American Heart Association; 2008. [Google Scholar]
- 4.Anilkumar N. Sirker A. Shah AM. Redox sensitive signaling pathways in cardiac remodeling, hypertrophy and failure. Front Biosci. 2009;14:3168–3187. doi: 10.2741/3443. [DOI] [PubMed] [Google Scholar]
- 5.Asahara T. Murohara T. Sullivan A. Silver M. van der Zee R. Li T. Witzenbichler B. Schatteman G. Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 6.Assmus B. Honold J. Schächinger V. Britten MB. Fischer-Rasokat U. Lehmann R. Teupe C. Pistorius K. Martin H. Abolmaali ND. Tonn T. Dimmeler S. Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 7.Atchison RW. Casto BC. Hammon WM. Adenovirus-associated defective virus particles. Science. 1965;149:754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- 8.Baklanov DV. Demuinck ED. Thompson CA. Pearlman JD. Novel double contrast MRI technique for intramyocardial detection of percutaneously transplanted autologous cells. Magn Reson Med. 2004;52:1438–1442. doi: 10.1002/mrm.20292. [DOI] [PubMed] [Google Scholar]
- 9.Baklanov DV. de Muinck ED. Thompson CA. Pearlman JD. Novel double contrast MRI technique for intramyocardial detection of percutaneously transplanted autologous cells. Magn Reson Med. 2004;52:1438–1442. doi: 10.1002/mrm.20292. [DOI] [PubMed] [Google Scholar]
- 10.Baklanov DV. Moodie KM. McCarthy FE. Mandrusov E. Chiu J. Aswonge G. Cheng J. Chow M. Simons M. de Muinck ED. Comparison of transendocardial and retrograde coronary venous intramyocardial catheter delivery systems in healthy and infarcted pigs. Catheter Cardiovasc Interv. 2006;68:416–423. doi: 10.1002/ccd.20841. [DOI] [PubMed] [Google Scholar]
- 11.Barandon L. Couffinhal T. Ezan J. Dufourcq P. Costet P. Alzieu P. Leroux L. Moreau C. Dare D. Duplaa C. Reduction of infarct size and prevention of cardiac rupture in transgenic mice overexpressing FrzA. Circulation. 2003;108:2282–2289. doi: 10.1161/01.CIR.0000093186.22847.4C. [DOI] [PubMed] [Google Scholar]
- 12.Baumgartner I. Pieczek A. Manor O. Blair R. Kearney M. Walsh K. Isner JM. Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation. 1998;97:1114–1123. doi: 10.1161/01.cir.97.12.1114. [DOI] [PubMed] [Google Scholar]
- 13.Bearzi C. Rota M. Hosoda T. Tillmanns J. Nascimbene A. De Angelis A. Yasuzawa-Amano S. Trofimova I. Siggins RW. Lecapitaine N. Cascapera S. Beltrami AP. D'Alessandro DA. Zias E. Quaini F. Urbanek K. Michler RE. Bolli R. Kajstura J. Leri A. Anversa P. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beeres SL. Bax JJ. Dibbets-Schneider P. Stokkel MP. Fibbe WE. van der Wall EE. Schalij MJ. Atsma DE. Intramyocardial injection of autologous bone marrow mononuclear cells in patients with chronic myocardial infarction and severe left ventricular dysfunction. Am J Cardiol. 2007;100:1094–1098. doi: 10.1016/j.amjcard.2007.04.056. [DOI] [PubMed] [Google Scholar]
- 15.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 16.Blacklow NR. Hoggan MD. Kapikian AZ. Austin JB. Rowe WP. Epidemiology of adenovirus-associated virus infection in a nursery population. Am J Epidemiol. 1968;88:368–378. doi: 10.1093/oxfordjournals.aje.a120897. [DOI] [PubMed] [Google Scholar]
- 17.Boekstegers P. Kupatt C. Current concepts and applications of coronary venous retroinfusion. Basic Res Cardiol. 2004;99:373–381. doi: 10.1007/s00395-004-0486-3. [DOI] [PubMed] [Google Scholar]
- 18.Boodhwani M. Sellke FW. Therapeutic angiogenesis in diabetes and hypercholesterolemia: influence of oxidative stress. Antioxid Redox Signal. 2009 doi: 10.1089/ars.2009.2439. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buning H. Ried MU. Perabo L. Gerner FM. Huttner NA. Enssle J. Hallek M. Receptor targeting of adeno-associated virus vectors. Gene Ther. 2003;10:1142–1151. doi: 10.1038/sj.gt.3301976. [DOI] [PubMed] [Google Scholar]
- 20.Cai H. Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 21.Chedrawy EG. Wang JS. Nguyen DM. Shum-Tim D. Chiu RC. Incorporation and integration of implanted myogenic and stem cells into native myocardial fibers: anatomic basis for functional improvements. J Thorac Cardiovasc Surg. 2002;124:584–590. doi: 10.1067/mtc.2002.122544. [DOI] [PubMed] [Google Scholar]
- 22.Collins CA. Olsen I. Zammit PS. Heslop L. Petrie A. Partridge TA. Morgan JE. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Conboy IM. Conboy MJ. Wagers AJ. Girma ER. Weissman IL. Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 24.Dawn B. Stein AB. Urbanek K. Rota M. Whang B. Rastaldo R. Torella D. Tang XL. Rezazadeh A. Kajstura J. Leri A. Hunt G. Varma J. Prabhu SD. Anversa P. Bolli R. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc Natl Acad Sci U S A. 2005;102:3766–3771. doi: 10.1073/pnas.0405957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de la Fuente LM. Stertzer SH. Argentieri J. Penaloza E. Miano J. Koziner B. Bilos C. Altman PA. Transendocardial autologous bone marrow in chronic myocardial infarction using a helical needle catheter: 1-year follow-up in an open-label, nonrandomized, single-center pilot study (the TABMMI study) Am Heart J. 2007;154:e1–e7. doi: 10.1016/j.ahj.2007.04.051. [DOI] [PubMed] [Google Scholar]
- 26.de Muinck ED. Simons M. Re-evaluating therapeutic neovascularization. J Mol Cell Cardiol. 2004;36:25–32. doi: 10.1016/j.yjmcc.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 27.de Muinck ED. Thompson C. Simons M. Progress and prospects: cell based regenerative therapy for cardiovascular disease. Gene Ther. 2006;13:659–671. doi: 10.1038/sj.gt.3302680. [DOI] [PubMed] [Google Scholar]
- 28.Dezawa M. Ishikawa H. Itokazu Y. Yoshihara T. Hoshino M. Takeda S. Ide C. Nabeshima Y. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science. 2005;309:314–217. doi: 10.1126/science.1110364. [DOI] [PubMed] [Google Scholar]
- 29.Dib N. Michler RE. Pagani FD. Wright S. Kereiakes DJ. Lengerich R. Binkley P. Buchele D. Anand I. Swingen C. Di Carli MF. Thomas JD. Jaber WA. Opie SR. Campbell A. McCarthy P. Yeager M. Dilsizian V. Griffith BP. Korn R. Kreuger SK. Ghazoul M. MacLellan WR. Fonarow G. Eisen HJ. Dinsmore J. Diethrich E. Safety and feasibility of autologous myoblast transplantation in patients with ischemic cardiomyopathy: four-year follow-up. Circulation. 2005;112:1748–1755. doi: 10.1161/CIRCULATIONAHA.105.547810. [DOI] [PubMed] [Google Scholar]
- 30.DiPaola NR. Sweet WE. Stull LB. Francis GS. Schomisch Moravec C. Beta-adrenergic receptors and calcium cycling proteins in non-failing, hypertrophied and failing human hearts: transition from hypertrophy to failure. J Mol Cell Cardiol. 2001;33:1283–1295. doi: 10.1006/jmcc.2001.1390. [DOI] [PubMed] [Google Scholar]
- 31.Emani SM. Ellis MJ. Dibernardo LR. Colgrove S. Glower DD. Taylor DA. Systolic contraction within aneurysmal rabbit myocardium following transplantation of autologous skeletal myoblasts. J Surg Res. 2006;135:202–208. doi: 10.1016/j.jss.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 32.Fiordaliso F. Cuccovillo I. Bianchi R. Bai A. Doni M. Salio M. De Angelis N. Ghezzi P. Latini R. Masson S. Cardiovascular oxidative stress is reduced by an ACE inhibitor in a rat model of streptozotocin-induced diabetes. Life Sci. 2006;79:1211–1219. doi: 10.1016/j.lfs.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 33.Flaherty MP. Abdel-Latif A. Li Q. Hunt G. Ranjan S. Ou Q. Tang XL. Johnson RK. Bolli R. Dawn B. Noncanonical Wnt11 signaling is sufficient to induce cardiomyogenic differentiation in unfractionated bone marrow mononuclear cells. Circulation. 2008;117:2241–2252. doi: 10.1161/CIRCULATIONAHA.107.741066. [DOI] [PubMed] [Google Scholar]
- 34.Fleury S. Driscoll R. Simeoni E. Dudler J. von Segesser LK. Kappenberger L. Vassalli G. Helper-dependent adenovirus vectors devoid of all viral genes cause less myocardial inflammation compared with first-generation adenovirus vectors. Basic Res Cardiol. 2004;99:247–256. doi: 10.1007/s00395-004-0471-x. [DOI] [PubMed] [Google Scholar]
- 35.Follenzi A. Ailles LE. Bakovic S. Geuna M. Naldini L. Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nat Genet. 2000;25:217–222. doi: 10.1038/76095. [DOI] [PubMed] [Google Scholar]
- 36.French BA. Mazur W. Geske RS. Bolli R. Direct in vivo gene transfer into porcine myocardium using replication-deficient adenoviral vectors. Circulation. 1994;90:2414–2424. doi: 10.1161/01.cir.90.5.2414. [DOI] [PubMed] [Google Scholar]
- 37.Garot J. Unterseeh T. Teiger E. Champagne S. Chazaud B. Gherardi R. Hittinger L. Gueret P. Rahmouni A. Magnetic resonance imaging of targeted catheter-based implantation of myogenic precursor cells into infarcted left ventricular myocardium. J Am Coll Cardiol. 2003;41:1841–1846. doi: 10.1016/s0735-1097(03)00414-5. [DOI] [PubMed] [Google Scholar]
- 38.Gnecchi M. He H. Liang OD. Melo LG. Morello F. Mu H. Noiseux N. Zhang L. Pratt RE. Ingwall JS. Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 39.Gossen M. Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gossen M. Freundlieb S. Bender G. Muller G. Hillen W. Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- 41.Gray SJ. Samulski RJ. Optimizing gene delivery vectors for the treatment of heart disease. Expert Opin Biol Ther. 2008;8:911–922. doi: 10.1517/14712598.8.7.911. [DOI] [PubMed] [Google Scholar]
- 42.Grines CL. Watkins MW. Mahmarian JJ. Iskandrian AE. Rade JJ. Marrott P. Pratt C. Kleiman N. Angiogene GTSG: a randomized, double-blind, placebo-controlled trial of Ad5FGF-4 gene therapy and its effect on myocardial perfusion in patients with stable angina. J Am Coll Cardiol. 2003;42:1339–1347. doi: 10.1016/s0735-1097(03)00988-4. [DOI] [PubMed] [Google Scholar]
- 43.Guo J. Gertsberg Z. Ozgen N. Steinberg SF. p66Shc links alpha1-adrenergic receptors to a reactive oxygen species-dependent AKT-FOXO3A phosphorylation pathway in cardiomyocytes. Circ Res. 2009;104:660–669. doi: 10.1161/CIRCRESAHA.108.186288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hagege AA. Carrion C. Menasche P. Vilquin JT. Duboc D. Marolleau JP. Desnos M. Bruneval P. Viability and differentiation of autologous skeletal myoblast grafts in ischaemic cardiomyopathy. Lancet. 2003;361:491–492. doi: 10.1016/S0140-6736(03)12458-0. [DOI] [PubMed] [Google Scholar]
- 45.Hajjar RJ. Zsebo K. Deckelbaum L. Thompson C. Rudy J. Yaroshinsky A. Ly H. Kawase Y. Wagner K. Borow K. Jaski B. London B. Greenberg B. Pauly DF. Patten R. Starling R. Mancini D. Jessup M. Design of a phase 1/2 trial of intracoronary administration of AAV1/SERCA2a in patients with heart failure. J Card Fail. 2008;14:355–367. doi: 10.1016/j.cardfail.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Hammond HK. Adenylyl cyclase gene transfer in heart failure. Ann N Y Acad Sci. 2006;1080:426–436. doi: 10.1196/annals.1380.032. [DOI] [PubMed] [Google Scholar]
- 47.Hartman ZC. Appledorn DM. Amalfitano A. Adenovirus vector induced innate immune responses: impact upon efficacy and toxicity in gene therapy and vaccine applications. Virus Res. 2008;132:1–14. doi: 10.1016/j.virusres.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hasenfuss G. Pieske B. Calcium cycling in congestive heart failure. J Mol Cell Cardiol. 2002;34:951–969. doi: 10.1006/jmcc.2002.2037. [DOI] [PubMed] [Google Scholar]
- 49.Hashemi SM. Ghods S. Kolodgie FD. Parcham-Azad K. Keane M. Hamamdzic D. Young R. Rippy MK. Virmani R. Litt H. Wilensky RL. A placebo controlled, dose-ranging, safety study of allogenic mesenchymal stem cells injected by endomyocardial delivery after an acute myocardial infarction. Eur Heart J. 2008;29:251–259. doi: 10.1093/eurheartj/ehm559. [DOI] [PubMed] [Google Scholar]
- 50.Hawley RG. Ramezani A. Hawley TS. Hematopoietic stem cells. Methods Enzymol. 2006;419:149–179. doi: 10.1016/S0076-6879(06)19007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He KL. Yi GH. Sherman W. Zhou H. Zhang GP. Gu A. Kao R. Haimes HB. Harvey J. Roos E. White D. Taylor DA. Wang J. Burkhoff D. Autologous skeletal myoblast transplantation improved hemodynamics and left ventricular function in chronic heart failure dogs. J Heart Lung Transplant. 2005;24:1940–1949. doi: 10.1016/j.healun.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 52.Hedman M. Hartikainen J. Syvanne M. Stjernvall J. Hedman A. Kivela A. Vanninen E. Mussalo H. Kauppila E. Simula S. Narvänen O. Rantala A. Peuhkurinen K. Nieminen MS. Laakso M. Yla-Herttuala S. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: phase II results of the Kuopio Angiogenesis Trial (KAT) Circulation. 2003;107:2677–2683. doi: 10.1161/01.CIR.0000070540.80780.92. [DOI] [PubMed] [Google Scholar]
- 53.Herreros J. Prosper F. Perez A. Gavira JJ. Garcia-Velloso MJ. Barba J. Sanchez PL. Canizo C. Rabago G. Marti-Climent JM. Hernández M. López-Holgado N. González-Santos JM. Martin-Luengo C. Alegria E. Autologous intramyocardial injection of cultured skeletal muscle-derived stem cells in patients with non-acute myocardial infarction. Eur Heart J. 2003;24:2012–2020. doi: 10.1016/j.ehj.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 54.Herweijer H. Wolff JA. Progress and prospects: naked DNA gene transfer and therapy. Gene Ther. 2003;10:453–458. doi: 10.1038/sj.gt.3301983. [DOI] [PubMed] [Google Scholar]
- 55.Hill JM. Dick AJ. Raman VK. Thompson RB. Yu ZX. Hinds KA. Pessanha BS. Guttman MA. Varney TR. Martin BJ. Dunbar CE. McVeigh ER. Lederman RJ. Serial cardiac magnetic resonance imaging of injected mesenchymal stem cells. Circulation. 2003;108:1009–1014. doi: 10.1161/01.CIR.0000084537.66419.7A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hodgson DM. Behfar A. Zingman LV. Kane GC. Perez-Terzic C. Alekseev AE. Puceat M. Terzic A. Stable benefit of embryonic stem cell therapy in myocardial infarction. Am J Physiol Heart Circ Physiol. 2004;287:H471–H9. doi: 10.1152/ajpheart.01247.2003. [DOI] [PubMed] [Google Scholar]
- 57.Hoshijima M. Ikeda Y. Iwanaga Y. Minamisawa S. Date MO. Gu Y. Iwatate M. Li M. Wang L. Wilson JM. Wang Y. Ross Jr J. Chien KR. Chronic suppression of heart-failure progression by a pseudophosphorylated mutant of phospholamban via in vivo cardiac rAAV gene delivery. Nat Med. 2002;8:864–871. doi: 10.1038/nm739. [DOI] [PubMed] [Google Scholar]
- 58.Irani K. Oxidant signaling in vascular cell growth, death, and survival: a review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circ Res. 2000;87:179–183. doi: 10.1161/01.res.87.3.179. [DOI] [PubMed] [Google Scholar]
- 59.Jooss K. Yang Y. Fisher KJ. Wilson JM. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. J Virol. 1998;72:4212–4223. doi: 10.1128/jvi.72.5.4212-4223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jozkowicz A. Dulak J. Helper-dependent adenoviral vectors in experimental gene therapy. Acta Biochim Pol. 2005;52:589–599. [PMC free article] [PubMed] [Google Scholar]
- 61.Kattman SJ. Huber TL. Keller GM. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11:723–732. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 62.Kawamoto A. Iwasaki H. Kusano K. Murayama T. Oyamada A. Silver M. Hulbert C. Gavin M. Hanley A. Ma H. Kearney M. Zak V. Asahara T. Losordo DW. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114:2163–2169. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- 63.Kawase Y. Ly HQ. Prunier F. Lebeche D. Shi Y. Jin H. Hadri L. Yoneyama R. Hoshino K. Takewa Y. Sakata S. Peluso R. Zsebo K. Gwathmey JK. Tardif J-C. Tanguay J-F. Hajjar RJ. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J Am Coll Cardiol. 2008;51:1112–1119. doi: 10.1016/j.jacc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 64.Kaye DM. Hoshijima M. Chien KR. Reversing advanced heart failure by targeting Ca2+ cycling. Annu Rev Med. 2008;59:13–28. doi: 10.1146/annurev.med.59.052407.103237. [DOI] [PubMed] [Google Scholar]
- 65.Kaye DM. Preovolos A. Marshall T. Byrne M. Hoshijima M. Hajjar R. Mariani JA. Pepe S. Chien KR. Power JM. Percutaneous cardiac recirculation-mediated gene transfer of an inhibitory phospholamban peptide reverses advanced heart failure in large animals. J Am Coll Cardiol. 2007;50:253–260. doi: 10.1016/j.jacc.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 66.Kinnaird T. Stabile E. Burnett MS. Shou M. Lee CW. Barr S. Fuchs S. Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 67.Kocher AA. Schuster MD. Szabolcs MJ. Takuma S. Burkhoff D. Wang J. Homma S. Edwards NM. Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 68.Koh GY. Soonpaa MH. Klug MG. Field LJ. Long-term survival of AT-1 cardiomyocyte grafts in syngeneic myocardium. Am J Physiol. 1993;264:H1727–H1733. doi: 10.1152/ajpheart.1993.264.5.H1727. [DOI] [PubMed] [Google Scholar]
- 69.Kong HJ. Liu J. Riddle K. Matsumoto T. Leach K. Mooney DJ. Non-viral gene delivery regulated by stiffness of cell adhesion substrates. Nat Mater. 2005;4:460–464. doi: 10.1038/nmat1392. [DOI] [PubMed] [Google Scholar]
- 70.Koyanagi M. Brandes RP. Haendeler J. Zeiher AM. Dimmeler S. Cell-to-cell connection of endothelial progenitor cells with cardiac myocytes by nanotubes: a novel mechanism for cell fate changes? Circ Res. 2005;96:1039–1041. doi: 10.1161/01.RES.0000168650.23479.0c. [DOI] [PubMed] [Google Scholar]
- 71.Kumar M. Keller B. Makalou N. Sutton RE. Systematic determination of the packaging limit of lentiviral vectors. Hum Gene Ther. 2001;12:1893–1905. doi: 10.1089/104303401753153947. [DOI] [PubMed] [Google Scholar]
- 72.Lai NC. Roth DM. Gao MH. Tang T. Dalton N. Lai YY. Spellman M. Clopton P. Hammond HK. Intracoronary adenovirus encoding adenylyl cyclase VI increases left ventricular function in heart failure. Circulation. 2004;110:330–336. doi: 10.1161/01.CIR.0000136033.21777.4D. [DOI] [PubMed] [Google Scholar]
- 73.Lee RJ. Springer ML. Blanco-Bose WE. Shaw R. Ursell PC. Blau HM. VEGF gene delivery to myocardium: deleterious effects of unregulated expression. Circulation. 2000;102:898–901. doi: 10.1161/01.cir.102.8.898. [DOI] [PubMed] [Google Scholar]
- 74.Liu W. Dahnke H. Jordan EK. Schaeffter T. Frank JA. In vivo MRI using positive-contrast techniques in detection of cells labeled with superparamagnetic iron oxide nanoparticles. NMR Biomed. 2008;21:242–250. doi: 10.1002/nbm.1187. [DOI] [PubMed] [Google Scholar]
- 75.Lohse MJ. Engelhardt S. Eschenhagen T. What is the role of beta-adrenergic signaling in heart failure? Circ Res. 2003;93:896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- 76.Luo Y. Jiang C. Belanger AJ. Akita GY. Wadsworth SC. Gregory RJ. Vincent KA. A constitutively active hypoxia-inducible factor-1alpha/VP16 hybrid factor activates expression of the human B-type natriuretic peptide gene. Mol Pharmacol. 2006;69:1953–1962. doi: 10.1124/mol.105.017905. [DOI] [PubMed] [Google Scholar]
- 77.Mangi AA. Noiseux N. Kong D. He H. Rezvani M. Ingwall JS. Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 78.Manginas A. Goussetis E. Koutelou M. Karatasakis G. Peristeri I. Theodorakos A. Leontiadis E. Plessas N. Theodosaki M. Graphakos S. Cokkinos DV. Pilot study to evaluate the safety and feasibility of intracoronary CD133(+) and CD133(−) CD34(+) cell therapy in patients with nonviable anterior myocardial infarction. Catheter Cardiovasc Interv. 2007;69:773–781. doi: 10.1002/ccd.21023. [DOI] [PubMed] [Google Scholar]
- 79.Mayer CR. Bekeredjian R. Ultrasonic gene and drug delivery to the cardiovascular system. Adv Drug Deliv Rev. 2008;60:1177–1192. doi: 10.1016/j.addr.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 80.Mazo M. Planat-Benard V. Abizanda G. Pelacho B. Leobon B. Gavira JJ. Penuelas I. Cemborain A. Penicaud L. Laharrague P. Joffre C. Boisson M. Ecay M. Collantes M. Barba J. Casteilla L. Prósper F. Transplantation of adipose derived stromal cells is associated with functional improvement in a rat model of chronic myocardial infarction. Eur J Heart Fail. 2008;10:454–462. doi: 10.1016/j.ejheart.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 81.McTiernan CF. Mathier MA. Zhu X. Xiao X. Klein E. Swan CH. Mehdi H. Gibson G. Trichel AM. Glorioso JC. Feldman AM. McLurry KR. London B. Myocarditis following adeno-associated viral gene expression of human soluble TNF receptor (TNFRII-Fc) in baboon hearts. Gene Ther. 2007;14:1613–1622. doi: 10.1038/sj.gt.3303020. [DOI] [PubMed] [Google Scholar]
- 82.Menasche P. Skeletal myoblasts as a therapeutic agent. Prog Cardiovasc Dis. 2007;50:7–17. doi: 10.1016/j.pcad.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 83.Menasche P. Alfieri O. Janssens S. McKenna W. Reichenspurner H. Trinquart L. Vilquin JT. Marolleau JP. Seymour B. Larghero J. Lake S. Chatellier G. Solomon S. Desnos M. Hagege AA. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189–1200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 84.Menasche P. Hagege AA. Vilquin JT. Desnos M. Abergel E. Pouzet B. Bel A. Sarateanu S. Scorsin M. Schwartz K. Bruneval P. Benbunan M. Marolleau J-P. Duboc D. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol. 2003;41:1078–1083. doi: 10.1016/s0735-1097(03)00092-5. [DOI] [PubMed] [Google Scholar]
- 85.Migliaccio E. Giorgio M. Mele S. Pelicci G. Reboldi P. Pandolfi PP. Lanfrancone L. Pelicci PG. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 86.Minamisawa S. Hoshijima M. Chu G. Ward CA. Frank K. Gu Y. Martone ME. Wang Y. Ross J Jr. Kranias EG. Giles WR. Chien KR. Chronic phospholamban-sarcoplasmic reticulum calcium ATPase interaction is the critical calcium cycling defect in dilated cardiomyopathy. Cell. 1999;99:313–322. doi: 10.1016/s0092-8674(00)81662-1. [DOI] [PubMed] [Google Scholar]
- 87.Mirotsou M. Zhang Z. Deb A. Zhang L. Gnecchi M. Noiseux N. Mu H. Pachori A. Dzau V. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007;104:1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Muller-Ehmsen J. Whittaker P. Kloner RA. Dow JS. Sakoda T. Long TI. Laird PW. Kedes L. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J Mol Cell Cardiol. 2002;34:107–116. doi: 10.1006/jmcc.2001.1491. [DOI] [PubMed] [Google Scholar]
- 89.Muller OJ. Katus HA. Bekeredjian R. Targeting the heart with gene therapy-optimized gene delivery methods. Cardiovasc Res. 2007;73:453–462. doi: 10.1016/j.cardiores.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 90.Murry CE. Soonpaa MH. Reinecke H. Nakajima H. Nakajima HO. Rubart M. Pasumarthi KB. Virag JI. Bartelmez SH. Poppa V. Bradford G. Dowell JD. Williams DA. Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 91.Nakai H. Storm TA. Kay MA. Recruitment of single-stranded recombinant adeno-associated virus vector genomes and intermolecular recombination are responsible for stable transduction of liver in vivo. J Virol. 2000;74:9451–9463. doi: 10.1128/jvi.74.20.9451-9463.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Niwano K. Arai M. Koitabashi N. Watanabe A. Ikeda Y. Miyoshi H. Kurabayashi M. Lentiviral vector-mediated SERCA2 gene transfer protects against heart failure and left ventricular remodeling after myocardial infarction in rats. Mol Ther. 2008;16:1026–1032. doi: 10.1038/mt.2008.61. [DOI] [PubMed] [Google Scholar]
- 93.Nygren JM. Jovinge S. Breitbach M. Sawen P. Roll W. Hescheler J. Taneera J. Fleischmann BK. Jacobsen SE. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10:494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- 94.Oh H. Bradfute SB. Gallardo TD. Nakamura T. Gaussin V. Mishina Y. Pocius J. Michael LH. Behringer RR. Garry DJ. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Okumura S. Takagi G. Kawabe J. Yang G. Lee MC. Hong C. Liu J. Vatner DE. Sadoshima J. Vatner SF. Ishikawa Y. Disruption of type 5 adenylyl cyclase gene preserves cardiac function against pressure overload. Proc Natl Acad Sci U S A. 2003;100:9986–9990. doi: 10.1073/pnas.1733772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pagani FD. DerSimonian H. Zawadzka A. Wetzel K. Edge AS. Jacoby DB. Dinsmore JH. Wright S. Aretz TH. Eisen HJ. Aaronson KD. Autologous skeletal myoblasts transplanted to ischemia-damaged myocardium in humans: histological analysis of cell survival and differentiation. J Am Coll Cardiol. 2003;41:879–88. doi: 10.1016/s0735-1097(03)00081-0. [DOI] [PubMed] [Google Scholar]
- 97.Peichev M. Naiyer AJ. Pereira D. Zhu Z. Lane WJ. Williams M. Oz MC. Hicklin DJ. Witte L. Moore MA. Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 98.Perin EC. Dohmann HF. Borojevic R. Silva SA. Sousa AL. Mesquita CT. Rossi MI. Carvalho AC. Dutra HS. Dohmann HJ. Silva GV. Belém L. Vivacqua R. Rangel FOD. Esporcatte R. Geng YJ. Vaughn WK. Assad JAR. Mesquita ET. Willerson JT. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation. 2003;107:2294–2302. doi: 10.1161/01.CIR.0000070596.30552.8B. [DOI] [PubMed] [Google Scholar]
- 99.Pouzet B. Vilquin JT. Menasche P. [Myocardial implantation of muscle cells] Presse Med. 2002;31:1569–1576. [PubMed] [Google Scholar]
- 100.Preovolos AC. Mennen MT. Bilney A. Mariani J. Kaye DM. Power JM. Development of a novel perfusion technique to allow targeted delivery of gene therapy: the V-Focus system. J Extra Corpor Technol. 2006;38:51–52. [PMC free article] [PubMed] [Google Scholar]
- 101.Quaini F. Urbanek K. Beltrami AP. Finato N. Beltrami CA. Nadal-Ginard B. Kajstura J. Leri A. Anversa P. Chimerism of the transplanted heart. N Engl J Med. 2002;346:5–15. doi: 10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- 102.Rando TA. The adult muscle stem cell comes of age. Nat Med. 2005;11:829–831. doi: 10.1038/nm0805-829. [DOI] [PubMed] [Google Scholar]
- 103.Raper SE. Chirmule N. Lee FS. Wivel NA. Bagg A. Gao GP. Wilson JM. Batshaw ML. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80:148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 104.Reinecke H. MacDonald GH. Hauschka SD. Murry CE. Electromechanical coupling between skeletal and cardiac muscle: implications for infarct repair. J Cell Biol. 2000;149:731–740. doi: 10.1083/jcb.149.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ricks DM. Kutner R. Zhang XY. Welsh DA. Reiser J. Optimized lentiviral transduction of mouse bone marrow-derived mesenchymal stem cells. Stem Cells Dev. 2008;17:441–450. doi: 10.1089/scd.2007.0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rissanen TT. Yla-Herttuala S. Current status of cardiovascular gene therapy. Mol Ther. 2007;15:1233–1247. doi: 10.1038/sj.mt.6300175. [DOI] [PubMed] [Google Scholar]
- 107.Rouy D. Lebrun F. Berchem G. Delagardelle C. Beissel J. Wagner DR. Cell therapy for severe chronic heart failure: the Luxembourg experience. Biomed Mater Eng. 2008;18:S27–S31. [PubMed] [Google Scholar]
- 108.Rubart M. Soonpaa MH. Nakajima H. Field LJ. Spontaneous and evoked intracellular calcium transients in donor-derived myocytes following intracardiac myoblast transplantation. J Clin Invest. 2004;114:775–783. doi: 10.1172/JCI21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rueckschloss U. Quinn MT. Holtz J. Morawietz H. Dose-dependent regulation of NAD(P)H oxidase expression by angiotensin II in human endothelial cells: protective effect of angiotensin II type 1 receptor blockade in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2002;22:1845–1851. doi: 10.1161/01.atv.0000035392.38687.65. [DOI] [PubMed] [Google Scholar]
- 110.Rupp S. Koyanagi M. Iwasaki M. Diehl F. Bushoven P. Schranz D. Zeiher AM. Dimmeler S. Genetic proof-of-concept for cardiac gene expression in human circulating blood-derived progenitor cells. J Am Coll Cardiol. 2008;51:2289–2290. doi: 10.1016/j.jacc.2008.01.062. [DOI] [PubMed] [Google Scholar]
- 111.Rutanen J. Rissanen TT. Markkanen JE. Gruchala M. Silvennoinen P. Kivela A. Hedman A. Hedman M. Heikura T. Orden MR. Stacker SA. Achen MG. Hartikainen J. Yla-Herttuala S. Adenoviral catheter-mediated intramyocardial gene transfer using the mature form of vascular endothelial growth factor-D induces transmural angiogenesis in porcine heart. Circulation. 2004;109:1029–1035. doi: 10.1161/01.CIR.0000115519.03688.A2. [DOI] [PubMed] [Google Scholar]
- 112.Sato Y. Kiriazis H. Yatani A. Schmidt AG. Hahn H. Ferguson DG. Sako H. Mitarai S. Honda R. Mesnard-Rouiller L. Frank KF. Beyermann B. Wu G. Fujimori K. Dorn II GW. Kranias EG. Rescue of contractile parameters and myocyte hypertrophy in calsequestrin overexpressing myocardium by phospholamban ablation. J Biol Chem. 2001;276:9392–9399. doi: 10.1074/jbc.M006889200. [DOI] [PubMed] [Google Scholar]
- 113.Schachinger V. Erbs S. Elsasser A. Haberbosch W. Hambrecht R. Holschermann H. Yu J. Corti R. Mathey DG. Hamm CW. Süselbeck T. Assmus B. Tonn T. Dimmeler S. Zeiher AM. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 114.Scherschel JA. Soonpaa MH. Srour EF. Field LJ. Rubart M. Adult bone marrow-derived cells do not acquire functional attributes of cardiomyocytes when transplanted into peri-infarct myocardium. Mol Ther. 2008;16:1129–1137. doi: 10.1038/mt.2008.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shang F. Zhao L. Zheng Q. Wang J. Xu Z. Liang W. Liu H. Liu S. Zhang L. Simvastatin inhibits lipopolysaccharide-induced tumor necrosis factor-alpha expression in neonatal rat cardiomyocytes: the role of reactive oxygen species. Biochem Biophys Res Commun. 2006;351:947–952. doi: 10.1016/j.bbrc.2006.10.134. [DOI] [PubMed] [Google Scholar]
- 116.Siminiak T. Kalawski R. Fiszer D. Jerzykowska O. Rzezniczak J. Rozwadowska N. Kurpisz M. Autologous skeletal myoblast transplantation for the treatment of postinfarction myocardial injury: phase I clinical study with 12 months of follow-up. Am Heart J. 2004;148:531–537. doi: 10.1016/j.ahj.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 117.Smith A. A glossary for stem cell biology. Nature. 2006;441:1060. [Google Scholar]
- 118.Smith AE. Helenius A. How viruses enter animal cells. Science. 2004;304:237–242. doi: 10.1126/science.1094823. [DOI] [PubMed] [Google Scholar]
- 119.Smits PC. van Geuns RJ. Poldermans D. Bountioukos M. Onderwater EE. Lee CH. Maat AP. Serruys PW. Catheter-based intramyocardial injection of autologous skeletal myoblasts as a primary treatment of ischemic heart failure: clinical experience with six-month follow-up. J Am Coll Cardiol. 2003;42:2063–2069. doi: 10.1016/j.jacc.2003.06.017. [DOI] [PubMed] [Google Scholar]
- 120.Soonpaa MH. Koh GY. Klug MG. Field LJ. Formation of nascent intercalated disks between grafted fetal cardiomyocytes and host myocardium. Science. 1994;264:98–101. doi: 10.1126/science.8140423. [DOI] [PubMed] [Google Scholar]
- 121.Stamm C. Westphal B. Kleine HD. Petzsch M. Kittner C. Klinge H. Schumichen C. Nienaber CA. Freund M. Steinhoff G. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet. 2003;361:45–46. doi: 10.1016/S0140-6736(03)12110-1. [DOI] [PubMed] [Google Scholar]
- 122.Stewart DJ. Hilton JD. Arnold JM. Gregoire J. Rivard A. Archer SL. Charbonneau F. Cohen E. Curtis M. Buller CE. Mendelsohn FO. Dib N. Page P. Ducas J. Plante S. Sullivan J. Macko J. Rasmussen C. Kessler PD. Rasmussen HS. Angiogenic gene therapy in patients with nonrevascularizable ischemic heart disease: a phase 2 randomized, controlled trial of AdVEGF(121) (AdVEGF121) versus maximum medical treatment. Gene Ther. 2006;13:1503–1511. doi: 10.1038/sj.gt.3302802. [DOI] [PubMed] [Google Scholar]
- 123.Strauer BE. Brehm M. Zeus T. Gattermann N. Hernandez A. Sorg RV. Kogler G. Wernet P. [Intracoronary, human autologous stem cell transplantation for myocardial regeneration following myocardial infarction] Dtsch Med Wochenschr. 2001;126:932–938. doi: 10.1055/s-2001-16579-2. [DOI] [PubMed] [Google Scholar]
- 124.Szelid Z. Sinnaeve P. Vermeersch P. Gillijns H. Pellens M. Laurysens V. Van Pelt N. Flameng W. Sergeant P. Herijgers P. Pokreisz R. van Zonneveld A-J. Verbeken E. Collen D. Janssens S. Preexisting antiadenoviral immunity and regional myocardial gene transfer: modulation by nitric oxide. Hum Gene Ther. 2002;13:2185–2195. doi: 10.1089/104303402320987879. [DOI] [PubMed] [Google Scholar]
- 125.Taylor DA. Atkins BZ. Hungspreugs P. Jones TR. Reedy MC. Hutcheson KA. Glower DD. Kraus WE. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med. 1998;4:929–933. doi: 10.1038/nm0898-929. [DOI] [PubMed] [Google Scholar]
- 126.Tepe NM. Liggett SB. Transgenic replacement of type V adenylyl cyclase identifies a critical mechanism of beta-adrenergic receptor dysfunction in the G alpha q overexpressing mouse. FEBS Lett. 1999;458:236–240. doi: 10.1016/s0014-5793(99)01147-3. [DOI] [PubMed] [Google Scholar]
- 127.Terentyev D. Gyorke I. Belevych AE. Terentyeva R. Sridhar A. Nishijima Y. de Blanco EC. Khanna S. Sen CK. Cardounel AJ. Carnes CA. Györke S. Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ Res. 2008;103:1466–1472. doi: 10.1161/CIRCRESAHA.108.184457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Thompson RB. Emani SM. Davis BH. van den Bos EJ. Morimoto Y. Craig D. Glower D. Taylor DA. Comparison of intracardiac cell transplantation: autologous skeletal myoblasts versus bone marrow cells. Circulation. 2003;108(suppl 1):II264–II271. doi: 10.1161/01.cir.0000087657.29184.9b. [DOI] [PubMed] [Google Scholar]
- 129.Traktuev DO. Merfeld-Clauss S. Li J. Kolonin M. Arap W. Pasqualini R. Johnstone BH. March KL. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 130.Urayama K. Guilini C. Turkeri G. Takir S. Kurose H. Messaddeq N. Dierich A. Nebigil CG. Prokineticin receptor-1 induces neovascularization and epicardial-derived progenitor cell differentiation. Arterioscler Thromb Vasc Biol. 2008;28:841–849. doi: 10.1161/ATVBAHA.108.162404. [DOI] [PubMed] [Google Scholar]
- 131.Urbanek K. Quaini F. Tasca G. Torella D. Castaldo C. Nadal-Ginard B. Leri A. Kajstura J. Quaini E. Anversa P. Intense myocyte formation from cardiac stem cells in human cardiac hypertrophy. Proc Natl Acad Sci U S A. 2003;100:10440–10445. doi: 10.1073/pnas.1832855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.van den Bos EJ. Thompson RB. Wagner A. Mahrholdt H. Morimoto Y. Thomson LE. Wang LH. Duncker DJ. Judd RM. Taylor DA. Functional assessment of myoblast transplantation for cardiac repair with magnetic resonance imaging. Eur J Heart Fail. 2005;7:435–443. doi: 10.1016/j.ejheart.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 133.Varnavski AN. Calcedo R. Bove M. Gao G. Wilson JM. Evaluation of toxicity from high-dose systemic administration of recombinant adenovirus vector in vector-naive and pre-immunized mice. Gene Ther. 2005;12:427–436. doi: 10.1038/sj.gt.3302347. [DOI] [PubMed] [Google Scholar]
- 134.Vassalli G. Bueler H. Dudler J. von Segesser LK. Kappenberger L. Adeno-associated virus (AAV) vectors achieve prolonged transgene expression in mouse myocardium and arteries in vivo: a comparative study with adenovirus vectors. Int J Cardiol. 2003;90:229–238. doi: 10.1016/s0167-5273(02)00554-5. [DOI] [PubMed] [Google Scholar]
- 135.Veltman CE. Soliman OI. Geleijnse ML. Vletter WB. Smits PC. ten Cate FJ. Jordaens LJ. Balk AH. Serruys PW. Boersma E. van Domburg RT. van der Giessen WJ. Four-year follow-up of treatment with intramyocardial skeletal myoblasts injection in patients with ischaemic cardiomyopathy. Eur Heart J. 2008;29:1386–1396. doi: 10.1093/eurheartj/ehn171. [DOI] [PubMed] [Google Scholar]
- 136.Vinge LE. Raake PW. Koch WJ. Gene therapy in heart failure. Circ Res. 2008;102:1458–1470. doi: 10.1161/CIRCRESAHA.108.173195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Walther W. Stein U. Cell type specific and inducible promoters for vectors in gene therapy as an approach for cell targeting. J Mol Med. 1996;74:379–392. doi: 10.1007/BF00210632. [DOI] [PubMed] [Google Scholar]
- 138.Wright MJ. Wightman LM. Lilley C. de Alwis M. Hart SL. Miller A. Coffin RS. Thrasher A. Latchman DS. Marber MS. In vivo myocardial gene transfer: optimization, evaluation and direct comparison of gene transfer vectors. Basic Res Cardiol. 2001;96:227–236. doi: 10.1007/s003950170053. [DOI] [PubMed] [Google Scholar]
- 139.Wu SM. Chien KR. Mummery C. Origins and fates of cardiovascular progenitor cells. Cell. 2008;132:537–543. doi: 10.1016/j.cell.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yamada M. Ikeda Y. Yano M. Yoshimura K. Nishino S. Aoyama H. Wang L. Aoki H. Matsuzaki M. Inhibition of protein phosphatase 1 by inhibitor-2 gene delivery ameliorates heart failure progression in genetic cardiomyopathy. FASEB J. 2006;20:1197–1199. doi: 10.1096/fj.05-5299fje. [DOI] [PubMed] [Google Scholar]
- 141.Zenovich AG. Davis BH. Taylor DA. Comparison of intracardiac cell transplantation: autologous skeletal myoblasts versus bone marrow cells. Handb Exp Pharmacol. 2007:117–165. doi: 10.1007/978-3-540-68976-8_6. [DOI] [PubMed] [Google Scholar]
- 142.Zhang L. Zalewski A. Liu Y. Mazurek T. Cowan S. Martin JL. Hofmann SM. Vlassara H. Shi Y. Diabetes-induced oxidative stress and low-grade inflammation in porcine coronary arteries. Circulation. 2003;108:472–478. doi: 10.1161/01.CIR.0000080378.96063.23. [DOI] [PubMed] [Google Scholar]
- 143.Zhao J. Pettigrew GJ. Thomas J. Vandenberg JI. Delriviere L. Bolton EM. Carmichael A. Martin JL. Marber MS. Lever AM. Lentiviral vectors for delivery of genes into neonatal and adult ventricular cardiac myocytes in vitro and in vivo. Basic Res Cardiol. 2002;97:348–358. doi: 10.1007/s00395-002-0360-0. [DOI] [PubMed] [Google Scholar]