Abstract
Biomaterials, traditionally defined as materials used in medical devices, have been used since antiquity, but recently their degree of sophistication has increased significantly. Biomaterials made today are routinely information rich and incorporate biologically active components derived from nature. In the future, biomaterials will assume an even greater role in medicine and will find use in a wide variety of non-medical applications through biologically inspired design and incorporation of dynamic behaviour.
Humankind’s use of materials to augment or repair the body dates to antiquity, when natural materials such as wood were used in an attempt to structurally replace tissues lost to disease or trauma (Fig. 1a). Historically, selection of material was based on availability and the ingenuity of the individual making and applying the prosthetic. In the early part of the twentieth century, naturally derived materials began to be replaced by synthetic polymers, ceramics and metal alloys, which provided better performance, increased functionality and more reproducibility than their naturally derived counterparts. These advances led to a pronounced increase in the range of use and the efficacy of biomaterials, as a result of which millions of lives have been saved or improved by devices such as vascular stents, dental restoratives, artificial hips (Fig. 1b) and contact lenses. On the basis of their application, biomaterials were defined as types of material used in a medical device, and the academic foundation of the field lay in materials science and classical engineering. Materials were desired to perform largely mechanical functions: to prevent biological rejection, which hampered device performance and patient health1, it was preferable that they be ‘inert’ and not interact with the biology of the host organism. Early research and fortuitous accidents linking materials chemistry to biological response provided a rational basis for developing biologically inert substrates and provided a scientific foundation for biomaterials as an intellectually distinct discipline1,2.
Figure 1. History and growth of biomaterials as a field and industry.
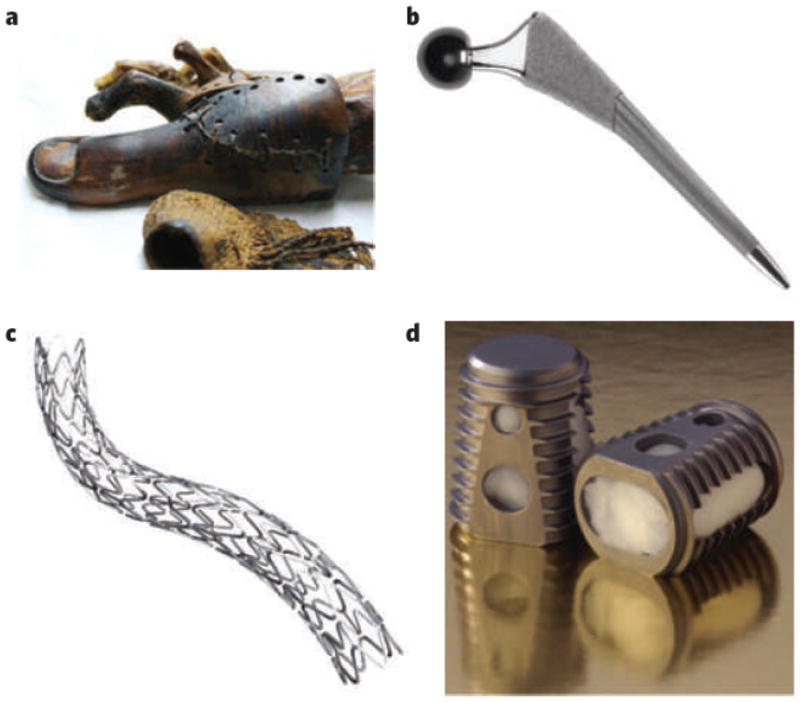
a, Prosthetics fashioned from natural materials: wooden toe, circa 1065–740 bc, used as a prosthetic to replace an amputated toe and identified in an anthropological excavation of the Thebes West tombs, Egypt. (Image courtesy of J. Finch, KNH Centre for Biomedical Egyptology, University of Manchester, UK, and The Egyptian Museum, Cairo.) b, The SYNERGY hip implant is an example of a state-of-the art prosthetic device that uses synthetic materials fabricated and engineered to meet performance demands. (Image courtesy of Smith & Nephew, London.) c, d, Commercially available combination products with both synthetic components and biological activity. c, The TAXUS Express2 Atom Stent, a metal stent from which paclitaxel is eluted into small coronary vessels to prevent restenosis (cell-mediated narrowing of the vessels). (Image courtesy of Boston Scientific Corporation, Massachusetts.) d, The INFUSE Bone Graft device, a combination product that uses both traditional prosthetic components (a steel cage) and a tissue-engineering approach (a bovine type I collagen sponge from which recombinant human bone morphogenetic protein 2 is eluted) to provide stability while spinal tissues are being regenerated. (Image courtesy of G. K. Michelson and Medtronic, Burlington, Massachusetts.)
The molecular biology revolution of the 1970s and advances in genomics and proteomics in the 1990s and 2000s, however, significantly affected the ways in which biomaterials are designed and used. As specific molecules have been implicated in clinically important processes (for example bone morphogenetic protein in osteogenesis), they have been incorporated into materials as bioactive components1. Such combination products, which interface directly with cells and tissues through well-defined molecular pathways to direct biological responses, already represent the state of the art of commercial products such as drug-eluting vascular stents (Fig. 1c). One of these products, Medtronic’s INFUSE Bone Graft device (Fig. 1d), which combines synthetic components with bone morphogenetic protein, accounted for more than US$760 million in sales in 2007 (ref. 3) and is probably near the billion-dollar mark today.
The increasing importance of biomaterials in our society over the past decades can be tracked in a number of ways, including the growth of biomaterials both as an academic discipline and as an important industry. There has been a precipitous increase in scientific publications in the biomaterials field over the past 30 years, and although biomaterials was historically a focus of study in a very small number of schools, the field has expanded markedly in the past 20 years. In the United States alone, there are now more than 75 departments of biomedical engineering (12 existed in 1975), with more than 16,000 enrolled students in 2005 (compared with ~3,000 in 1979). Biomaterials is a major field of study in these programmes and is increasingly being emphasized in other engineering departments. In addition, biomaterials are a critical component of a number of industries, including medical devices, dental restoratives and drug delivery, and are increasingly used in technological applications such as in vitro diagnostics. Together, these applications generate a market of about $200 billion per year in the United States (as of 2007), with a robust annual growth rate of ~9% (refs 4, 5).
The ability to engineer biological activity into synthetic materials greatly increases the number of their potential uses and improves their performance in more traditional applications. Moreover, the increasing appreciation of the functionality and complexity of biological systems has caused biomaterials researchers to again consider nature for design inspiration. Unlike most man-made materials, materials used in living systems are frequently multifunctional and dynamic, and are built using ‘bottom-up’ fabrication methods. Both the materials themselves and the biophysical processes involved in their formation are inspiring the design and synthesis of new types of synthetic material that are potentially useful in a wide range of medical and non-medical applications. This widening of the classic view of biomaterials demands an intellectual shift in how these materials are defined. Distinct aspects of this transition in the biomaterials field, and the potential impact on medicine and other industries, are our focus here. We review the current state of the biomaterials field in terms of several major areas of application and design principles, and then we describe emerging and future trends in biomaterials.
Current goals and trajectory of the biomaterials field
The field of biomaterials is in the midst of a revolutionary change in which the life sciences are becoming equal in importance to materials science and engineering as the foundation of the field. Simultaneously, advances in engineering (for example nanotechnology) are greatly increasing the sophistication with which biomaterials are designed and have allowed fabrication of materials with increasingly complex functions. Such sophisticated materials are often designed to mimic a subset of the physicochemical properties of natural materials. Increasingly, nature inspires not only the materials themselves but also the means by which they are made. Whereas synthetic materials are typically engineered on the scale of millimetres or larger and then milled to have micrometrescale or nanometre-scale features, natural materials are constructed on these smaller scales by self-assembly, a bottom-up means of fabrication that facilitates the construction of information-rich, complex structures in a highly reproducible manner with minimal energy input6.
Knowledge gained from fundamental studies is being used in conjunction with fabrication methods such as self-assembly to design biomaterials that interface with the biology of the host. This is typically done by means of binding interactions with cell surface receptors7, to regulate the maintenance, regeneration or even destruction of specific tissues in the body. Key aspects of this line of research include the following: the rich information content of new materials that mimic cellular and extracellular materials, with particular emphasis on presentation of signals in a controlled spatiotemporal manner; provision of non-chemical (for example electrical or mechanical) signals to elicit structural changes in the material or to manipulate cell fate directly; the finding that the physical properties of the materials are probably just as important as their chemistry in terms of the biological response they elicit; and the notion that materials can be designed to regulate host biology at a distance, either by controlling cell trafficking or by trafficking of the material itself in the body.
Biomimetic medical materials and devices
Historically, biological interactions with the host were regulated by the layer of serum proteins adsorbed nonspecifically on surfaces of synthetic materials. A considerable body of research exists on how surface chemistryand topography affect the adsorption of extracellular matrix (ECM) proteins and the presentation of cell-adhesion ligands2. However, it is difficult to engineer the surface of materials to adsorb a precise mixture and arrangement of ECM proteins, and those which initially adsorb may be denatured or displaced. Hence, a current theme in biomaterials design is the combination of synthetics that resist nonspecific protein adsorption and molecular components that regulate host biology in a well-defined manner1.
Inspiration for the design of new biomaterials has been derived from structure–function analysis on various length scales of the extracellular materials that cells use to organize themselves into tissues (Fig. 2a). There has been great progress in elucidating functional domains within large ECM molecules and in using synthetic peptides to mimic key epitopes; perhaps the most common example is the grafting of integrinbinding peptides (for example RGD) onto hydrogel-forming polymers and other non-fouling substrates to facilitate cell adhesion8. Both the epitopes themselves and their spatial organization on micrometre and nanometre scales influence the fate of cells with which they interact9. Epitope spatial organization may be controlled on micrometre and nanometre scales through fabrication of self-assembling materials that present the epitope (Fig. 2b, c) or through direct patterning of the epitopes onto materials that otherwise present an inert background10 (Fig. 2d). This type of patterning can also be performed on a larger length scale to mimic the ability of the natural ECM to create morphogen gradients11 (Fig. 2e).
Figure 2. Information-rich biomimetic materials.
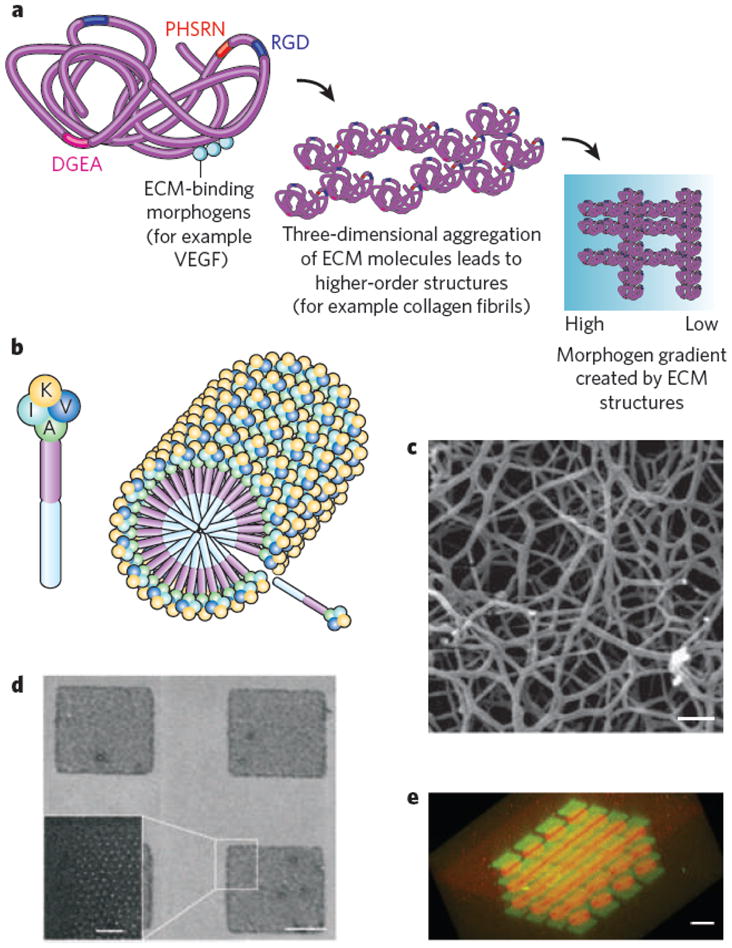
a, Schematic of the natural ECM across different spatial scales. The ECM contains a variety of peptide epitopes (coloured rectangles, labelled with amino-acid sequences of the epitopes) that facilitate integrin-mediated adhesion and other receptor-linked functions. These epitopes are organized in a specific pattern on the nanometre scale within each protein molecule (left) and on the micrometre scale in fibrillar and other structures (centre). The ECM may also regulate the diffusion of soluble proteins, mediating gradients of morphogens between cells on larger length scales (millimetres) (right); the blue colour scale represents one such gradient, with the concentration (from high to low) of morphogen (for example vascular endothelial growth factor (VEGF)) proportional to intensity. b–e, Synthetic mimics of the information-rich natural ECM. b, c, Schematic (b) and scanning electron micrograph (c) analysis of modularly designed peptide amphiphiles that self-assemble into nanofibres presenting a high density of neural-progenitor-binding epitopes (labelled with the amino acids K, V, I and A). Scale bar, 300 nm. (Image reproduced, with permission, from ref. 9). d, Scanning electron micrograph analysis of a surface containing micropatterned islands presenting RGD (adhesive) ligands (white dots) with precisely controlled nanometre-scale spacing. Scale bars, 1 μm (right) and 200 nm (inset, left). (Image reproduced, with permission, from ref. 10.) e, A micrograph of a biomaterial modified to present gradients or other complex spatial patterns of morphogens. The fluorescent dyes Alexa Fluor 488 maleimide (green squares) and Alexa Fluor 546 maleimide (red circles) are placed to demonstrate the spatial precision with which bioactive moieties (for example morphogens) could be patterned. Scale bar, 60 μm. (Image reproduced, with permission, from ref. 11.)
It is possible to use genetic engineering to increase the structural complexity of self-assembling materials and peptides further. Such an approach has been used to generate entirely new proteins that combine modules of different natural ECM molecules to obtain novel functionality, and even to incorporate non-natural amino acids that extend the range of chemical properties (for example to include the ability to undergo photocrosslinking) of cell-synthesized materials12. In some cases, synthetic biomaterials mimic nature not by influencing cells directly through receptor-binding epitopes but indirectly, by regulating the rate of matrix-metalloproteinase-mediated degradation and cellular invasion13 or by initiating and regulating the formation of bioactive inorganic structures (for example mineralized bone or shell14). Both approaches have proved useful in augmenting the formation of mineralized tissues for dental and orthopaedic applications.
The potential utility of information-rich biomaterials that directly manipulate target biological systems is perhaps best exemplified by recent progress in the stem-cell field (see page 433), whereby key cues that regulate stem-cell biology are increasingly being incorporated into sophisticated biomaterials. Fundamental challenges in this field include the ability to expand stem cells ex vivo without using feeder layers, and enhancing the survival of transplanted stem cells and reproducibly regulating their fate in the body. Biomaterials are being used to define precisely the stem-cell microenvironment to meet these challenges15, typically through the provision of high densities of cell-adhesion ligands9, morphogens and other chemical cues16, both to direct cell fate in vitro and to provide a template for the formation of new tissues by transplanted stem cells17. The precisely controlled spatio temporal presentation of morphogens guiding development has inspired the design of biomaterials in which sequences of morphogens18 and spatial gradients of morphogens11,19 can be presented to guide these processes. Notably, soluble morphogens can exhibit enhanced biological activity when they are presented in insoluble form by tethering to biomaterials20, providing another means of using ECM mimics to regulate cell fate.
Regulating biology at a distance
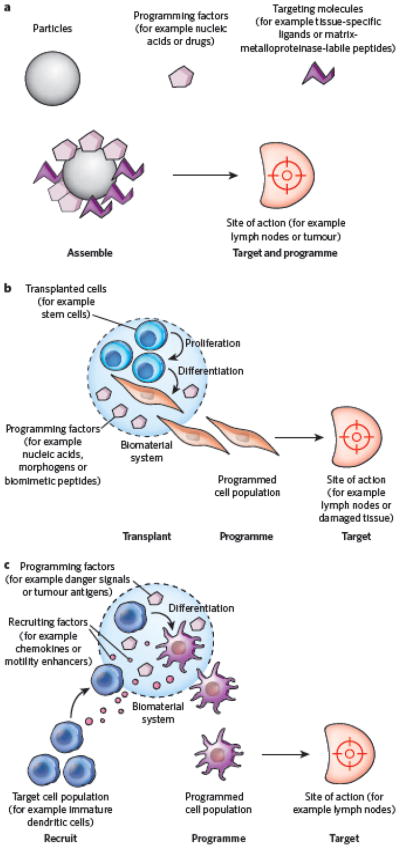
Although biomaterials are typically used to guide the behaviour of cells transplanted with the material or cells in the tissue into which the material is implanted, it has also become apparent that biomaterials can be designed to manipulate specific cell populations that reside in the host at a significant distance from the implant site. This can be done either by targeting the material to specific cells or anatomical locations or by controlling the trafficking of target cell populations (Fig. 3). Recent demonstrations of biomaterials as regulators of the immune system (see page 449) illustrate these two extremes well. Polymeric nano particles can be designed for non-invasive delivery into the body21 and for trafficking through the lymphatic vessels to target T cells in the lymph nodes22. Similarly, nanoparticles are being designed to exploit the chemical and physical differences between normal and tumour-associated vasculature in order to concentrate the particles selectively within or near tumours, allowing subsequent drug-induced cell death23 (Fig. 3a). Materials can also be designed to regulate outward migration of transplanted stem cells, or their differentiated progeny, to populate damaged tissues and promote regeneration efficiently24 (Fig. 3b). Alternatively, biomaterials may program specific cell populations, without transplantation, by recruiting the population of interest (for example by releasing cytokines capable of recruiting immune cells) and subsequently activating these cells once resident in the material. This approach has been used to generate potent, antitumour responses by recruiting and programming immune cells in situ25 (Fig. 3c). Because of their potential to target disease sites that are not yet clinically detectable, materials that regulate host biology at a distance show great promise for treating systemically disseminating diseases such as cancer.
Figure 3. Regulating biology at a distance: designing materials to target or mimic the niches of specific cell populations.
a, Schematic of microparticles or nanoparticles (grey) whose assembly enables them to target specific anatomical or cellular regions of the body. This targeting occurs on the basis of the particles’ size, shape and presentation of molecules (targeting molecules, purple) that are complementary to specific features of target cell populations. The particles subsequently manipulate cell fate locally through programming factors (pink). b, Schematic of an implantable biomaterial that mimics certain aspects of stem-cell niches in that it activates transplanted progenitor cells to proliferate and programs them to differentiate into cells that migrate into damaged tissues to participate in regeneration. c, Schematic of an implantable biomaterial system that mimics the microenvironment of an infection, allowing the recruitment, programming and subsequent targeting of activated antigen-presenting dendritic cells to the lymph nodes to participate in a potent antitumour response.
Importance of physical variables in biomaterials design
The chemical composition of biomaterials has been the focus of their design for the past few decades, but there is growing appreciation of the importance of other properties, including topological, mechanical and electrical cues, in guiding a biological response. The features of particulate biomaterials on the length scale of individual molecules and cells (tens of nanometres to tens of micrometres) have significant effects on how cells perceive, interact and ingest the material, which affects the efficacy of materials used as drug carriers or vehicles targeting specific cells and tissues in the body21. Regardless of the chemical composition, the cellular response in vitro and in vivo can drastically alter depending on the mechanical properties of biomaterials26. Although the mechanisms responsible for these effects are only beginning to be understood, an underlying hypothesis in this area of research is that mechanosensing is an active cellular process involving dynamic interplay between the ECM and motor proteins coupled to the cytoskeleton. Biomaterials are being used both to study how cell phenotype is regulated by this crosstalk and as fundamental tools to characterize this dynamic interplay27. The ability of cells and natural biopolymers to sense, transmit and respond to mechanical signals is increasingly providing inspiration for new types of sensor, actuator and shape-control material (see page 442).
In addition to mechanical properties and size, external and environmental cues such as temperature and electromagnetic fields are increasingly being used to modulate the performance of biomaterials, often by dynamically altering their structure. Hydrogels, for example, can be designed to change their swelling behaviour and degree of nonspecific protein adsorption in response to temperature28 or binding to specific ligands29. Despite intensive investigation into chemical structure–function relationships in hydrogels, the physics governing macromolecular transport within these materials, and their ability to resist protein adsorption, is still not completely understood and presents opportunities for future tuning of biomaterial performance. Studies have also demonstrated that drug delivery from biomaterials can be manipulated using remotely applied electromagnetic fields30; the same types of field can mediate the in situ assembly of scaffolds for tissue engineering31. Ion flows caused by electromechanical stimulation can probably modulate regeneration32, suggesting that electrochemical signals could be used in the future to alter cell fate directly and, by manipulating bio material structure and presentation of chemical epitopes33, indirectly. In the future, biomaterials may be engineered not only to respond to external fields and forces but also to generate these physical stimuli.
Application of biomaterials beyond medical devices
Biomaterials have crucial roles in the fabrication of devices for biological screening, in basic science studies and in a variety of non-medical fields. Investigations into new diagnostic materials and devices are being driven by several factors: the ever-increasing recognition of the need for early diagnosis and intervention in human disease, particularly at low cost; the need for better in vitro screens for drug efficacy and toxicity; the potential dangers of food and water contamination; and the potential catastrophic results of biological warfare34. These biomaterials are designed on multiple length scales to present and organize arrays of molecules and cells for mechanistic studies and drug screening35.
New approaches to biomaterials fabrication, often incorporating physical as well as chemical fabrication techniques, have paved the way for new approaches to diagnostics. As in the design of biomimetic medical devices, a crucial aspect of this work is the ability to make information-rich materials that assay multiple targets and allow multiple outputs (see page 461). A major feature of these approaches is the ability to capture rare cell populations36. Similarly, materials that change their optical or electrical properties in response to specific biological stimuli have been used to eliminate the need both for traditional probing tools (for example fluorescence) in diagnostics37 and for basic investigations of cell–matrix interactions38.
The increasing appreciation of the roles of insoluble signals from the ECM and physical forces in regulating cell fate has led to the use of biomaterials to construct physiologically relevant in vitro model systems. Perhaps the fastest-growing application of biomaterials for in vitro model systems is in the area of three-dimensional cell culture. Although matrix biologists have appreciated for some time that three-dimensional matrix culture provides more accurate in vitro models of in vivo phenomena (for example angiogenesis) than does two-dimensional cell culture39, it has been difficult to distinguish effects that result from changes in the dimension of the microenvironment from effects that stem purely from the chemical changes required for three-dimensional culture. Biomaterials-based platforms that decouple dimensionality and physical properties from matrix chemistry, and engineering approaches to the analysis of natural ECM, are profoundly altering our understanding of a variety of biological processes, including tumour formation40,41 and early development42, and are also providing more physiologically relevant in vitro model systems for drug screening. In combination with the ability to scale down biological experiments greatly using array-type approaches43, the ability of biomaterials to provide organized, physiologically relevant three-dimensional structures may fundamentally change how mechanistic questions in biology on cellular and tissue scales are approached, much in the same way that screening technologies such as gene arrays are affecting molecular biology. Such changes may be especially important in understanding how chemical inputs are systematically integrated, knowledge that would aid in efforts to develop network-type models of cell signalling for drug development.
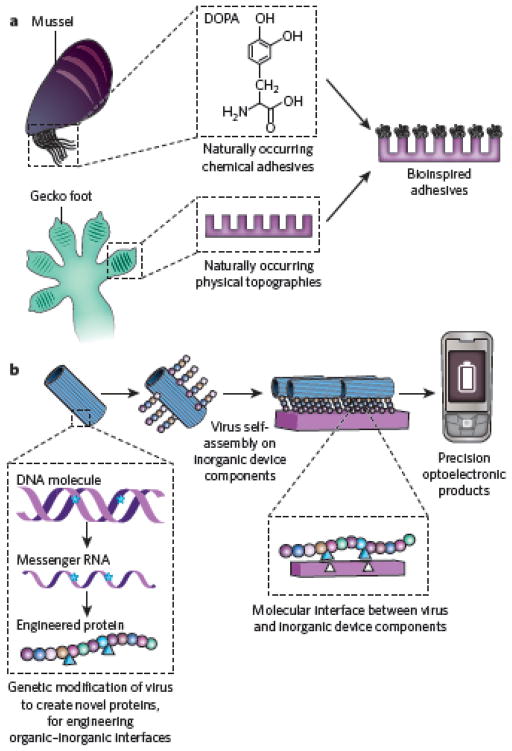
Although bioinspired materials have had an increasing role in diagnostic devices and basic science, the fastest area of growth may be the application of such materials to fields outside medicine and biology. For example, just as fragments of the ECM facilitate mammalian cell adhesion to synthetic materials, functional chemical epitopes and physically patterned topographies used to facilitate surface adhesion in organisms such as mussels and geckos may improve the performance of synthetic adhesives in both medical and industrial applications44 (Fig. 4a). Likewise, the materials used in nature for sensing are inspiring the development of biomimetic sensors; for example, salient features of the compound eyes of insects have been replicated in completely artificial materials designed to recapitulate the function of these natural sensors45. Although self-assembly is useful in constructing nanoscale devices, the assembly of synthetic polymers alone may be insufficient to provide the hierarchical organization such devices require. However, this organization can be accomplished by the molecular templating of whole microorganisms that have been genetically engineered to facilitate functionalities these organisms lack in nature (for example adhesion to metallic surfaces, for making devices such as batteries46) (Fig. 4b).
Figure 4. Using components of biological organisms and materials in novel applications.
a, The chemical and physical properties of materials used by organisms to facilitate surface adhesion can be mimicked, allowing the generation of synthetic coatings that modify surface chemistry or prevent biofouling. For example, 3,4(OH)2 phenylalanine (DOPA), a naturally occurring chemical adhesive used to facilitate the adhesion of mussels to surfaces in wet environments, has been combined with the physically patterned nanopillar topography found in the toes of geckos, which facilitates strong adhesion in dry environments, to produce novel adhesives that work in both wet and dry environments. b, The molecular templating of whole viruses allows high-precision, multiscale patterning of electronic devices. Genetic modification of the organism (left) is used to engender bimolecular organic–inorganic interactions that lead to the coating of viruses with desired inorganic materials and their macromolecular assembly (centre). Low-cost, high-precision energy-storage systems (right) are one potential application of this concept46.
Biomaterials of the future
Advances in biomaterials will include the development of more functional medical materials and the expanded use of biomaterials into new fields of application. However, the future may also present an opportunity for practitioners in the field to rethink fundamentally the way in which inspiration is drawn from biology. Understanding the way in which complex dynamic behaviours are accomplished in nature may lead to the design of novel materials that mimic nature not through presenting active motifs replicated exactly from biological molecules but rather through reproducing the functional behaviour of these biological materials to obtain properties that are currently unavailable (Fig. 5). The application of the molecular templating of viruses to optoelectronic device fabrication is one early example of such an approach46.
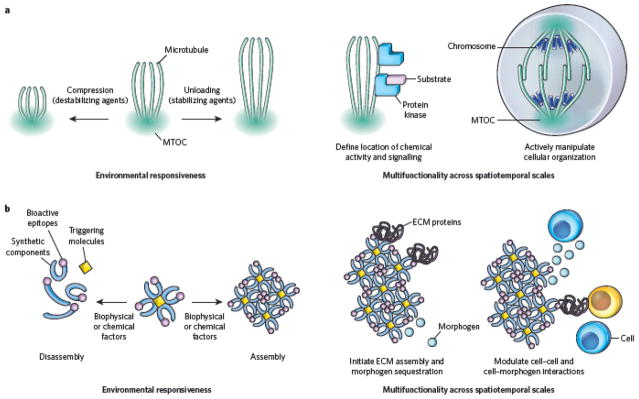
Figure 5. The future: rethinking how inspiration is drawn from biology, and applying biological design principles to new areas.
a, Microtubules are an example of a multifunctional natural material with a dynamic structure, responsiveness to multiple environmental cues (for example chemical stimuli and mechanical forces) and the ability to regulate a variety of events at the molecular and cellular levels. The dynamic nature of microtubules allows these polymers, which are assembled from relatively simple monomeric components (tubulins) at microtubule-organizing centres (MTOCs), to operate with a high degree of functional complexity in cells. Their dynamic assembly and disassembly in response to both chemical and mechanical stimuli from the environment is shown (left). Alterations in microtubule assembly can regulate a variety of cellular events (right), ranging from protein-mediated signalling (for example by regulating the localization of a protein kinase), which occurs on the nanometre scale, to mechanical control over cellular structure and organization during mitosis, which occurs on the micrometre scale. b, Potential biomedical devices inspired by microtubules. Structurally simple synthetic polymers can be designed to self-assemble in response to triggering molecules (for example ECM proteins or ions) and further assemble or disassemble in response to other environmental cues (for example pH, matrix metalloproteinases and physical forces) (left). Assembled polymers can subsequently regulate signalling between and within target cell populations to bring about biologically complex changes in their local environment. For example, they may bind to ECM proteins, initiating assembly or restructuring of the ECM and thereby manipulating cells locally (right). At the same time, they may sequester morphogens, generating gradients that alter cell behaviour over longer distances. As is the case for the natural materials that inspired their design, these synthetics undergo reorganization in response to changes in the local environment, subsequently altering the ways in which they interact with cells. This allows relatively simple materials to carry out the complex functions of both integrating multiple inputs from the environment and providing multiple outputs (cell-interactive stimuli) to regulate local biological events.
One focus of research on the new generation of bioinspired materials will probably be the development of ‘smart’, multifunctional nanoparticles or implants for use in our bodies. These complex materials would integrate multiple inputs from chemical and physical stimuli to determine their behaviour (Fig. 5b). Such materials could target desired anatomical regions, monitor health, and report on and actively intervene in biological crises. Biological systems have already inspired the development of cell-programming matrices based on our abstract understanding of dynamic biological processes such as infection, and these matrices accomplish their task with a small subset of key molecular stimuli27. New ex vivo biosensors capable of predicting disease are also likely to result from our understanding of living materials, as are new energy-storage devices, optical materials and other devices. Materials that selectively interact with specific cell populations, for use in diagnostic or therapeutic applications, may even be created by understanding and ultimately harnessing the dynamic cues provided by specific cell types (for example stem cells) to modify in situ, or assemble in situ, complex devices or materials from simple input templates.
A critical intellectual step in biomaterials design is the recognition both that biological polymers and organisms can be used as models of, or templates for, multifunctional, dynamic devices and that components of natural systems can be used for purposes other than that which they serve in nature46. This requires an abstract understanding of the biophysical properties imparted by certain molecular structures. This understanding is being applied in the context of self-assembling natural materials such as DNA, which originally was considered solely as an information-storage system but recently has inspired the development of new types of nanomaterial with precisely defined structures6, as well as self-assembling synthetic polymers (inspired by the highly regulated base-pairing of DNA). Some of the best-characterized self-assembling molecules in cell biology are the filamentous polymers that form the cytoskeleton. These information-rich polymers provide structural support that changes in response to environmental cues, and they also form a nexus for chemical signalling, by defining the location for synthetic activity, and regulate the movement of materials within the cell (Fig. 5a). What is perhaps most striking about these polymers from a materials standpoint, however, is that they can exhibit such a high degree of functional complexity while being relatively simple in composition. For example, microtubules consist of monomers that self-assemble in response to both chemical cues and mechanical loading47 and rapidly disassemble and reassemble, providing distinct functions (Fig. 5a). Multifunctional synthetic materials with a subset of these functions are currently being developed. Nature is also inspiring micrometre-sized and nanometresized robots powered by stimulus-responsive soft actuators to augment bottom-up fabrication technologies48. Likewise, DNA may inspire the construction of actuators that mimic this biopolymer’s dynamic assembly and responsiveness to environmental cues49.
Beyond the devices and materials themselves, biological inspiration may revolutionize the methods used to produce and transform raw materials in the chemical and materials industries. For example, living plants can process, in huge quantities, a much greater variety of liquids and materials than are produced by humankind commercially, but they do so without the energy cost or waste streams typical of our chemical industry (Fig. 6). Applying lessons from nature may not only allow the synthesis of new chemicals but also significantly reduce the costs and environmental impacts associated with the manufacturing of current chemicals and drugs.
Figure 6. The future: drawing inspiration from nature to rethink how materials and pharmaceuticals are manufactured.
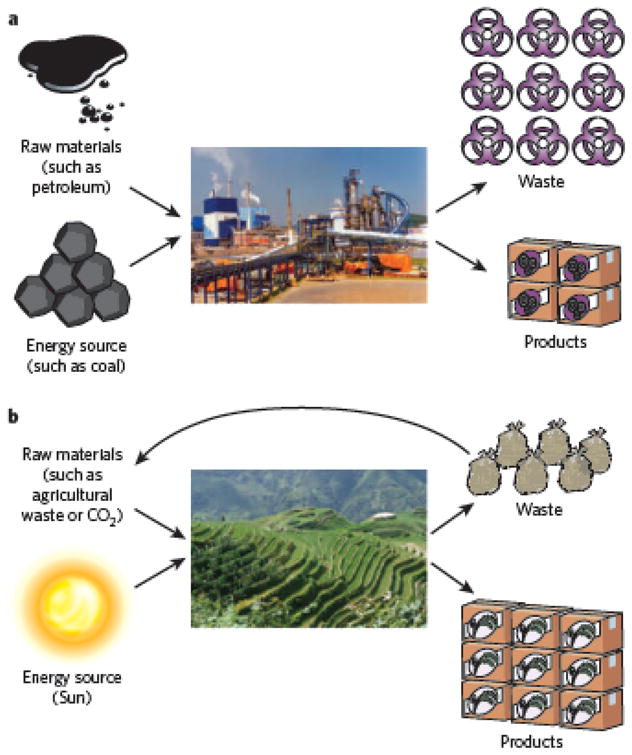
Schematic of a typical factory used for materials manufacturing, with associated inputs of raw materials and energy, and output waste streams (a). Schematic of a rice terrace, with its associated inputs and outputs (b). The relative sizes of manufactured products and the associated wastes shown represent the scale of waste streams and input materials in the respective schemes. The ability of natural systems to use renewable energy sources (for example solar energy) effectively and to recycle waste streams when generating products is inspiring novel approaches to manufacturing.
Accomplishing this transformation in the biomaterials field will require an improved understanding of how cells receive information from materials and how key signalling pathways process this information to dictate biological responses50; it will not suffice simply to make materials and empirically test for their effects on cell or host responses. In addition, gaining an abstract understanding of how the basic building blocks of biological systems are coordinated and integrated, in a manner analogous to the unit operations approach that revolutionized the chemical industry in the twentieth century, is likely to be an important step. This will require the development and application of new tools from biology, engineering and the physical sciences. Likewise, biophysical models of the materials themselves and their interaction with cells will also be necessary. The biomaterials field, both as an academic pursuit and as an industry, is quickly becoming unrecognizable in terms of its current definition. The field will need to be redefined to encompass materials that direct biology and those whose design and functions are inspired by natural materials; future generations of biomaterials are likely to be critical components in many facets of modern society.
Acknowledgments
We thank D. Ingber for discussions. We acknowledge funding from the US National Institutes of Health (National Institute of Dental and Craniofacial Research). N.H. is supported by a National Science Foundation Graduate Research Fellowship.
Footnotes
Author Information Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
References
- 1.Ratner BD, Bryant SJ. Biomaterials: where we have been and where we are going. Annu Rev Biomed Eng. 2004;6:41–75. doi: 10.1146/annurev.bioeng.6.040803.140027. This is an excellent, comprehensive review of the history of the biomaterials field. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mroz T, Yamashita T, Lieberman I. The on- and off-label use of rhBMP-2 (INFUSE) in Medicare and non-Medicare patients. Spine J. 2008;8:41S–42S. [Google Scholar]
- 4.Shahani S. Advanced Drug Delivery Systems: New Developments, New Technologies Report No. PHM006F. Business Communications Company; 2006. [Google Scholar]
- 5.King RG, Donohue GF. Estimates of medical device spending in the United States. AMSA; 2007. < http://www.amsa.org/AMSA/libraries/committee_docs/king_paper_medical_device_spending.sflb.ashx>. [Google Scholar]
- 6.Shih WM, Quispe JD, Joyce GF. A 1.7-kilobase single-stranded DNA that folds into a nanoscale octahedron. Nature. 2004;427:618–621. doi: 10.1038/nature02307. [DOI] [PubMed] [Google Scholar]
- 7.Shin H, Zygourakis K, Farach-Carson MC, Yaszemski MJ, Mikos AG. Attachment, proliferation, and migration of marrow stromal osteoblasts cultured on biomimetic hydrogels modified with an osteopontin-derived peptide. Biomaterials. 2004;25:895–906. doi: 10.1016/s0142-9612(03)00602-1. [DOI] [PubMed] [Google Scholar]
- 8.Massia SP, Hubbell JA. Covalently attached GRGD on polymer surfaces promotes biospecific adhesion of mammalian cells. Ann NY Acad Sci. 1990;589:261–270. doi: 10.1111/j.1749-6632.1990.tb24251.x. [DOI] [PubMed] [Google Scholar]
- 9.Silva GA, et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303:1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 10.Arnold M, et al. Activation of integrin function by nanopatterned adhesive surfaces. ChemPhysChem. 2004;5:383–388. doi: 10.1002/cphc.200301014. [DOI] [PubMed] [Google Scholar]
- 11.Wosnick JH, Shoichet MS. Three-dimensional chemical patterning of transparent hydrogels. Chem Mater. 2008;20:55–60. [Google Scholar]
- 12.Carrico IS, et al. Lithographic patterning of photoreactive cell-adhesive proteins. J Am Chem Soc. 2007;129:4874–4875. doi: 10.1021/ja070200b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lutolf MP, et al. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nature Biotechnol. 2003;21:513–518. doi: 10.1038/nbt818. This paper describes how materials can be designed to mimic key aspects of natural ECM (for example enzyme-mediated degradation) and function as templates for tissue regeneration. [DOI] [PubMed] [Google Scholar]
- 14.Shin K, Jayasuriya AC, Kohn DH. Effect of ionic activity products on the structure and composition of mineral self assembled on three-dimensional poly(lactide-co-glycolide) scaffolds. J Biomed Mater Res A. 2007;83:1076–1086. doi: 10.1002/jbm.a.31437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li YJ, Chung EH, Rodriguez RT, Firpo MT, Healy KE. Hydrogels as artificial matrices for human embryonic stem cell self-renewal. J Biomed Mater Res A. 2006;79:1–5. doi: 10.1002/jbm.a.30732. [DOI] [PubMed] [Google Scholar]
- 16.Benoit DS, Schwartz MP, Durney AR, Anseth KS. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nature Mater. 2008;7:816–823. doi: 10.1038/nmat2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin’oka T, et al. Midterm clinical results of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg. 2005;129:1330–1338. doi: 10.1016/j.jtcvs.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 18.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric systems for dual growth factor delivery. Nature Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 19.Phillips JE, Burns KL, Le Doux JM, Guldberg RE, Garcia AJ. Engineering graded tissue interfaces. Proc Natl Acad Sci USA. 2008;105:12170–12175. doi: 10.1073/pnas.0801988105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan VH, et al. Tethered epidermal growth factor provides a survival advantage to mesenchymal stem cells. Stem Cells. 2007;25:1241–1251. doi: 10.1634/stemcells.2006-0320. [DOI] [PubMed] [Google Scholar]
- 21.Tsapis N, Bennett D, Jackson B, Weitz DA, Edwards DA. Trojan particles: large porous carriers of nanoparticles for drug delivery. Proc Natl Acad Sci USA. 2002;99:12001–12005. doi: 10.1073/pnas.182233999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reddy ST, et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nature Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 23.Park JH, et al. Systematic surface engineering of magnetic nanoworms for in vivo tumor targeting. Small. 2009;5:694–700. doi: 10.1002/smll.200801789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silva EA, Kim ES, Kong HJ, Mooney DJ. Material-based deployment enhances the efficacy of endothelial progenitor cells. Proc Natl Acad Sci USA. 2008;105:14347–14352. doi: 10.1073/pnas.0803873105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ali OA, Huebsch N, Cao L, Dranoff G, Mooney DJ. Infection-mimicking materials to program dendritic cells in situ. Nature Mater. 2009;8:151–158. doi: 10.1038/nmat2357. This paper describes how biomaterials can be designed to regulate host biology at a distance by recruiting, locally programming and subsequently dispersing target cell populations to produce potent biological responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. This paper demonstrates the importance of physical properties of biomaterials in controlling cellular response. [DOI] [PubMed] [Google Scholar]
- 27.Tan JL, et al. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc Natl Acad Sci USA. 2003;100:1484–1489. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park TG, Hoffman AS. Synthesis and characterization of pH- and or temperaturesensitive hydrogels. J Appl Polym Sci. 1992;46:659–671. [Google Scholar]
- 29.Podual K, Doyle FJ, Peppas NA. Glucose-sensitivity of glucose oxidase-containing cationic copolymer hydrogels having poly(ethylene glycol) grafts. J Control Release. 2000;67:9–17. doi: 10.1016/s0168-3659(00)00195-4. [DOI] [PubMed] [Google Scholar]
- 30.Edelman ER, Brown L, Taylor J, Langer R. In vitro and in vivo kinetics of regulated drug release from polymer matrices by oscillating magnetic fields. J Biomed Mater Res. 1987;21:339–353. doi: 10.1002/jbm.820210307. [DOI] [PubMed] [Google Scholar]
- 31.Alsberg E, Feinstein E, Joy MP, Prentiss M, Ingber DE. Magnetically-guided selfassembly of fibrin matrices with ordered nano-scale structure for tissue engineering. Tissue Eng. 2006;12:3247–3256. doi: 10.1089/ten.2006.12.3247. [DOI] [PubMed] [Google Scholar]
- 32.Adams DS, Masi A, Levin M. H+ pump-dependent changes in membrane voltage are an early mechanism necessary and sufficient to induce tail regeneration. Development. 2007;134:1323–1335. doi: 10.1242/dev.02812. [DOI] [PubMed] [Google Scholar]
- 33.Lahann J, et al. A reversibly switching surface. Science. 2003;299:371–374. doi: 10.1126/science.1078933. [DOI] [PubMed] [Google Scholar]
- 34.Martinez AW, Phillips ST, Whitesides GM. Three-dimensional microfluidic devices fabricated in layered paper and tape. Proc Natl Acad Sci USA. 2008;105:19606–19611. doi: 10.1073/pnas.0810903105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khetani SR, Bhatia SN. Microscale culture of human liver cells for drug development. Nature Biotechnol. 2008;26:120–126. doi: 10.1038/nbt1361. [DOI] [PubMed] [Google Scholar]
- 36.Nagrath S, et al. Isolation of rare circulating tumor cells in cancer patients by microchip technology. Nature. 2007;450:1235–1239. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stern E, et al. Label-free immunodetection with CMOS-compatible semiconducting nanowires. Nature. 2007;445:519–522. doi: 10.1038/nature05498. [DOI] [PubMed] [Google Scholar]
- 38.Gupta VK, Dubrovsky TB, Abbott NL. Optical amplification of ligand–receptor binding using liquid crystals. Science. 1998;279:2077–2080. doi: 10.1126/science.279.5359.2077. [DOI] [PubMed] [Google Scholar]
- 39.Madri JA, Pratt BM, Tucker AM. Phenotypic modulation of endothelial cells by transforming growth factor-β depends upon the composition and organization of the extracellular matrix. J Cell Biol. 1988;106:1375–1384. doi: 10.1083/jcb.106.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fischbach C, et al. Cancer cell angiogenic capability is regulated by 3D culture and integrin engagement. Proc Natl Acad Sci USA. 2009;106:399–404. doi: 10.1073/pnas.0808932106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghajar CM, et al. The effect of matrix density on the regulation of 3-D capillary morphogenesis. Biophys J. 2008;94:1930–1941. doi: 10.1529/biophysj.107.120774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu M, et al. Encapsulated three-dimensional culture supports the development of nonhuman primate secondary follicles. Biol Reprod. 2009;81:587–593. doi: 10.1095/biolreprod.108.074732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci USA. 2006;103:2480–2487. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee H, Scherer NF, Messersmith PB. A reversible wet/dry adhesive inspired by mussels and geckos. Nature. 2007;448:338–341. doi: 10.1038/nature05968. [DOI] [PubMed] [Google Scholar]
- 45.Jeong KH, Kim J, Lee LP. Biologically inspired artificial compound eyes. Science. 2006;312:557–561. doi: 10.1126/science.1123053. [DOI] [PubMed] [Google Scholar]
- 46.Nam KT, et al. Virus-enabled synthesis and assembly of nanowires for lithium ion battery electrodes. Science. 2006;312:885–888. doi: 10.1126/science.1122716. This paper discusses the engineering of non-medical materials through the templating of viruses. The precisely tuned patterns of spatial features of the natural organism promise distinct performance advantages. [DOI] [PubMed] [Google Scholar]
- 47.Needleman DJ, et al. Higher-order assembly of microtubules by counterions: from hexagonal bundles to living necklaces. Proc Natl Acad Sci USA. 2004;101:16099–16103. doi: 10.1073/pnas.0406076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sidorenko A, Krupenkin T, Taylor A, Fratzl P, Aizenberg J. Reversible switching of hydrogel-actuated nanostructures into complex micropatterns. Science. 2007;315:487–490. doi: 10.1126/science.1135516. [DOI] [PubMed] [Google Scholar]
- 49.Omabegho T, Sha R, Seeman NC. A bipedal DNA Brownian motor with coordinated legs. Science. 2009;324:67–71. doi: 10.1126/science.1170336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kyriakides TR, et al. The CC chemokine ligand, CCL2/MCP1, participates in macrophage fusion and foreign body giant cell formation. Am J Pathol. 2004;165:2157–2166. doi: 10.1016/S0002-9440(10)63265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]