Abstract
Background
Panax notoginseng saponins (PNS), the main active components of Radix Notoginseng, has been used for treating atherosclerosis, cerebral infarction, and cerebral ischemia. Ginsenosides Rg1, ginsenoside Rb1, and notoginsenoside R1 are the main contributors of biological activities, determination of these three saponins is very important for the in vivo evaluation of PNS. The present study aims to develop a liquid chromatography-tandem mass spectrometry (LC-MS/MS) method for the simultaneous quantification of ginsenosides Rg1, ginsenoside Rb1, and notoginsenoside R1. The use of this method was exemplified in pharmacokinetic study of beagle dog plasma after oral administration of PNS.
Methods
Liquid chromatography-tandem mass spectrometry (LC/MS/MS) method was combined with solid-phase extraction (SPE). This setup was used to determine simultaneously the three major PNS (ginsenoside Rg1, ginsenoside Rb1, and notoginsenoside R1) in beagle dog plasma. Tandem mass spectrometry was performed using electrospray ionization in the positive ion mode.
Results
The lower limits of quantification were 0.5 ng/mL for notoginsenoside R1, 0.82 ng/mL for ginsenoside Rg1, and 1.10 ng/mL for ginsenoside Rb1. The calibration curves for the three saponins were linear over the concentration ranges 2.64-264 ng/mL (r2 = 0.9967, P = 0.003), 3.6-360 ng/mL (r2 = 0.9941, P = 0.004), and 18.7-1870 ng/mL (r2 = 0.9912, P = 0.004) for notoginsenoside R1, ginsenoside Rg1, and ginsenoside Rb1, respectively. Within these concentration ranges, the relative standard deviation (RSD) of intra- and interday assays for the three PNS from beagle dog plasma samples were less than 12%.
Conclusions
This LC/MS/MS method in combination with SPE is useful in the pharmacokinetic study of PNS, such as the simultaneous determination of saponins in beagle dog plasma after oral administration.
Background
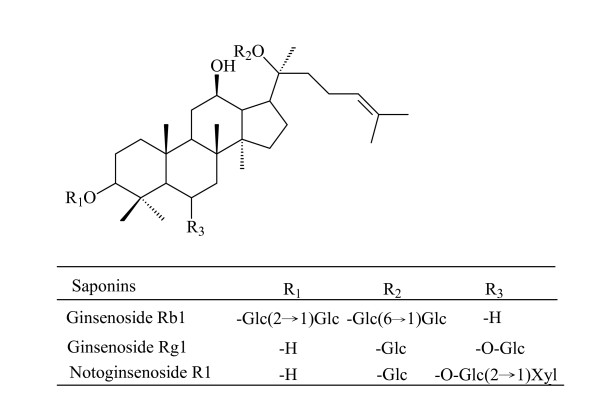
Panax notoginseng saponins (PNS), the main active components of Radix Notoginseng [1], are used for treating atherosclerosis [2], cerebral infarction [3], and cerebral ischemia [4]. The major bioactive saponins of Radix Notoginseng [5] are ginsenoside Rg1, ginsenoside Rb1, and notoginsenoside R1 (Figure 1). In addition, notoginsenoside R1, a saponin unique to Panax notoginseng, has anti-thrombus activity [6]. Thus, determination of these three saponins is very important for the in vivo monitoring and evaluation of PNS.
Figure 1.
Chemical structures of ginsenoside Rb1, ginsenoside Rg1, and notoginsenoside R1
Determination of the limit of quantification (LOQ) of PNS in biological samples is a great challenge for in vivo research. The reported quantitative methods included thin-layer chromatography [7,8], high-performance liquid chromatography (HPLC) with UV detection [9,10], fluorescence detection [11], gas chromatography-mass spectrometry [12], and liquid chromatography-mass spectrometry (LC/MS) with electrospray positive [13] or negative [14] ionization. Although many methods were developed to determine the saponins of PNS in extracts, commercial products, and biological samples, the LOQ values were unsatisfactorily determined in biological samples. For example, the LOQ values of ginsenoside Rg1 and ginsenoside Rb1 were higher than 500 ng/mL in rat biological samples detected by thin-layer chromatography [7,8]; 60 and 5.0 ng/mL, respectively, in rat urine samples by HPLC-UV [15]; and 10.0 ng/mL using a 100- μl plasma sample by LC/MS with negative ionization [10]. There is no report on the determination of notoginsenoside R1 after oral administration of PNS or the simultaneous determination of ginsenosides Rg1 and Rb1 and notoginsenoside R1 in plasma after oral administration of PNS. Thus, it is necessary to develop a sensitive method to determine the saponins in PNS, especially in biological samples.
As LC-MS is useful in drug metabolism and pharmacokinetic studies on active components in Chinese medicines [16-19], we developed a LC-MS method for drug metabolism and pharmacokinetic study of ginsenoside Rg3 and ginsenoside Rh2, and our findings showed that LC-MS and MS/MS methods are sensitive, specific, and convenient for such ginsenoside studies [17-19].
Multiple reactions monitoring (MRM) mass spectrometry has special advantages for quantitative analysis of analyte: it is specific and selective technology that can avoid contaminants in the biological samples. In the present study, we aims to develop a liquid chromatography-tandem mass spectrometry (LC-MS/MS) multiple reactions monitoring method combined with solid-phase extraction preparation of samples for the simultaneous quantification of ginsenosides Rg1, ginsenoside Rb1, and notoginsenoside R1 and to conduct a pharmacokinetic study of beagle dog plasma after oral administration of PNS.
Methods
Chemicals and reagents
Acetonitrile and methanol (HPLC grade) were purchased from Fisher Scientific (NewJersey, USA). Extract-Clean C18 (solid-phase extraction) cartridge columns were purchased from the Alltech Company (Deerfield, USA). Ginsenosides Rg1 and Rb1 and notoginsenoside R1 were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China), and PNS extract was purchased from the Institute of Medicinal Plant Development, Yunnan Branch (Yunnan Province, China). The contents of ginsenosides Rg1 and Rb1 and notoginsenoside R1 in the PNS extract were 37%, 36%, and 10%, respectively, according to HPLC analysis. Glipizide (internal standard) was kindly provided by Professor Jifen Guo at the Academy of Military Medical Sciences (Beijing, China).
Instrumental analysis
The HPLC system consisted of a quaternary LC pump of Agilent 1100 series, from Agilent Technology (Waldbronn, Germany), with a degasser and an autosampler. The mobile phase consisted of 10 mM ammonium acetate solution, 0.1% n-butyl-amine,10% methanol in water (A) and methanol (B). The flow rate was kept at 0.2 mL/min for a total run time of eight minutes. The system was run with a gradient program of 50% B to 90% B in five minutes and 90% B to 50% B in three minutes. The samples were introduced into the electrospray ion source of a triple-stage quadrupole mass spectrometer Finnigan TSQ 7000 with API-2 ion source and performance kit (Thermo Electron, Dreieich, Germany). Electrospray ionization interface parameters were as follows: spray voltage: 4.0 kV; sheath gas (N2): 8 units; capillary heater temperature: 300°C. Multiple reactions monitoring measurements were performed at a multiplier voltage of 1.2 kV.
Animals
Three male beagle dogs, weighing 10-12 kg, were purchased from Tongli Laboratory Animals Center (Beijing, China) for the animal experiments. Experimental animals were maintained in accordance with internationally guidelines for laboratory animal use [20], and the study was approved by the Beijing Animal Care Committee (Beijing China).
Sample preparation
For the assay, a 0.5 mL plasma sample was loaded and drawn through by gravity on a solid-phase extraction cartridge (1 mL, packed with 100 mg of 40 μm octadecyl silica) that was preconditioned by passing through 2 mL of methanol followed by 2 mL of water before loading. Then the solid-phase cartridge was washed with 2 mL of water, 2 mL of 20% (v/v) aqueous methanol solution, and 2 mL of methanol. The final methanol elute was collected and 10 μl of internal standard was added to it. The elute was thereafter evaporated at 40°C to dryness under a stream of nitrogen. The residue was dissolved in 100 μl of 50% (v/v) aqueous methanol solution. A 20 μl sample was injected into the HPLC system for analysis.
Method validation
Calibration curve and quality control sample preparation
Primary mixed stock solutions of notoginsenoside R1, ginsenoside Rg1, and ginsenoside Rb1 were prepared in methanol/water (1:1, v/v). Working standard solutions of the three saponins were prepared by combining the aliquots of each primary solution and diluting with methanol/water (1:1, v/v). The working solution for internal standard (0.1 μg/mL) was prepared using methanol. All stock solutions were stored at 4°C in polypropylene tubes in the dark.
Beagle dog plasma calibration standards of the three saponins were prepared by spiking standard solutions with various concentrations of drug-free beagle dog plasma. Standard solutions of six different concentrations of the three saponins were obtained: 2.64, 5.28, 13.2, 26.4, 52.8, and 264 ng/mL for notoginsenoside R1; 3.60, 7.20, 18.0, 36.0, 72.0, and 360 ng/mL for ginsenoside Rg1; and 18.7, 37.4, 93.5, 187, 374, and 1870 ng/mL for ginsenoside Rb1. Three calibration curves were established by determining the peak area ratio (analyte/internal standard) versus the saponin concentrations in samples processed as described in Sample preparation.
Quality control samples were prepared in bulk by adding 50 μl of the appropriate working standard solutions to blank beagle dog plasma. The quality control samples at 2.8, 14, and 140 ng/mL for notoginsenoside R1; 3.8, 19, and 190 ng/mL for ginsenoside Rg1; and 18, 180, and 900 ng/mL for ginsenoside Rb1 were stored at -20°C until analysis.
Accuracy and precision
Batches of quality control samples were analyzed on four different days to validate the method. In each batch, quality control samples were assayed in sets of six replicates to evaluate the accuracy and intra- and interday precision. The accuracy and relative standard deviation (RSD) were used to evaluate the method.
Recovery and sensitivity
The relative recovery of this method was determined by comparing the concentration calculated from the calibration curve to the known concentration. Recovery was evaluated with four replicates, and samples were prepared as the quality control samples.
The LOQ values were measured using a series of diluted standard plasma samples. For the concentration to be accepted as the LOQ, the signal-to-noise ratio had to be greater than or equal to five and the percent deviation for the analyte concentration and the relative standard deviation had to be within 20%.
Pharmacokinetic study
PNS extract was administered orally at a dose of 90 mg/kg to beagle dogsthat had been fasting but had free access to water for 18 hours prior to the experiment. Each blood sample (3 mL) was transferred to a heparinized glass tube at 0 hr, 0.5 hr, 1 hr, 2 hr, 3 hr, 4 hr, 6 hr, 8 hr, 10 hr, 12 hr, 16 hr, 24 hr, 36 hr, 48 hr and 72 hr after administration and immediately centrifuged at 1500 × g for 15 minutes at 8-10°C. The plasma was then transferred to another glass tube and stored at -20°C until analysis.
Results and discussion
Sample preparation
Sugar moieties are extracted incompletely into organic solvents. Therefore, solid-phase extraction was used to remove excess ingredients in plasma and achieve good selectivity. When the concentration of methanol was below 20%, the three saponins were strongly absorbed by the C18 stationary phase, while the interfering compounds in plasma could be eluted under this condition. The excellent selectivity of solid-phase extraction helped to improve the LC-MS/MS analysis.
Performance and validation of the analytical method
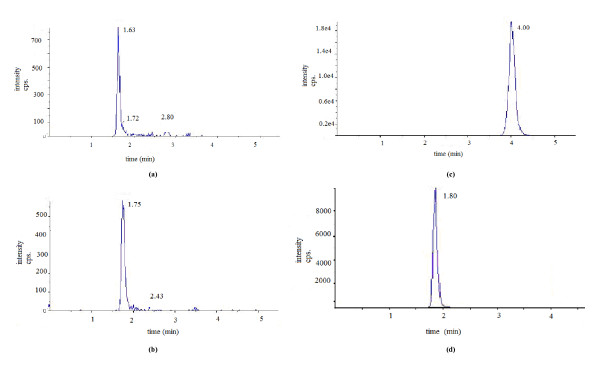
The three saponins and internal standard in plasma were completely separated within six minutes without significant interference (Figure 2), demonstrating the specificity of this method. The retention times were as follows: 1.56, 1.67, 3.87, and 1.80 minutes for notoginsenoside R1, ginsenoside Rg1, ginsenoside Rb1, and internal standard, respectively.
Figure 2.
HPLC chromatograms of (a) notoginsenoside R1; (b) ginsenoside Rg1; (c) ginsenoside Rb1; and (d) internal standard (glipizide).
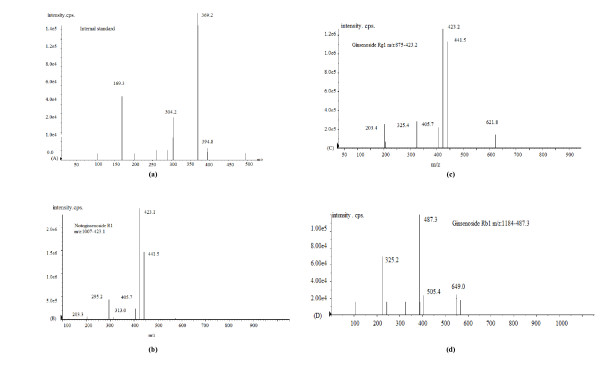
MS/MS transitions monitored in the positive ion mode were m/z 1007 → m/z 423 for notoginsenoside R1 (MW 932), m/z 875 → m/z 423 for ginsenoside Rg1 (MW 800), m/z 1183 → m/z 487 for ginsenoside Rb1 (MW 1108), and m/z 494 → m/z 369.1 for internal standard. MS/MS spectra (daughter ion scans) of the three saponins and internal standard are shown in Figure 3.
Figure 3.
Tandem mass spectra (daughter ion scans) of (a) internal standard (glipizide); (b) notoginsenoside R1 parent ion; (c) ginsenoside Rg1; and (d) ginsenoside Rb1.
In this method, standard curves are linear over the range of 2.64-264 ng/mL for notoginsenoside R1, 3.60-360 ng/mL for ginsenoside Rg1, and 18.7-1870 ng/mL for ginsenoside Rb1, with all correlation coefficients larger than 0.99. The LOQ values are 0.5 mL for notoginsenoside R1, 0.82 ng/mL for ginsenoside Rg1, and 1.10 ng/mL for ginsenoside Rb1. The results of the analysis are shown in Table 1.
Table 1.
Calibration curves of the three Panax notoginseng saponins in beagle dog plasma
| Analyte | Standard curves (linear) | P | r2 | Test range (ng/mL) | Limit of quantification (ng/mL) |
|---|---|---|---|---|---|
| NotoginsenosideR1 | Y = 0.0011X+0.0004 | 0.003 | 0.9967 | 2.64--264 | 0.50 |
| Ginsenoside Rg1 | Y = 0.0009X+0.0007 | 0.004 | 0.9941 | 3.60--360 | 0.82 |
| Ginsenoside Rb1 | Y = 0.0016X+ 0.0283 | 0.004 | 0.9912 | 18.7--1870 | 1.10 |
Note: Y, peak area ratio (analyte/internal standard); X, concentration of analyte in plasma (ng/mL)
The results of the assay of precision and accuracy are shown in Table 2. The relative standard deviation of the method was less than 12.0%. Recovery test results are shown in Table 3.
Table 2.
Intra- and interday variability for the assay of the three Panax notoginseng saponins in beagle dog plasma (n = 6)
|
Spiked conc. (ng/mL) |
Intraday | Interday | ||||
|---|---|---|---|---|---|---|
| Measured conc. (ng/mL) | RSD (%)a | Accuracy (%)b | Measured conc. (ng/mL) | RSD (%) | Accuracy (%) | |
| Notoginsenoside R1 | ||||||
| 2.8 | 2.8 | 1.27 | 100.0 | 2.68 | 1.49 | 95.71 |
| 14 | 13.9 | 0.76 | 99.29 | 12.89 | 9.62 | 92.1 |
| 140 | 148 | 8.22 | 105.7 | 131.67 | 10.11 | 105.7 |
| Ginsenoside Rg1 | ||||||
| 3.8 | 3.6 | 5.13 | 94.7 | 3.96 | 11.96 | 104.2 |
| 19 | 17.6 | 3.65 | 92.6 | 18.3 | 8.95 | 96.3 |
| 190 | 197.3 | 6.59 | 103.8 | 193.7 | 7.66 | 101.7 |
| Ginsenoside Rb1 | ||||||
| 18 | 18.1 | 3.07 | 100.6 | 17.6 | 5.36 | 96.11 |
| 180 | 165.3 | 2.73 | 91.83 | 179.7 | 5.05 | 99.50 |
| 900 | 912.67 | 3.90 | 101.3 | 871.3 | 2.38 | 96.87 |
Note: aRelative standard deviation; bAccuracy (%) = [1--(nominal concentration -- mean of measured concentration)/nominal concentration] × 100
Table 3.
Recovery of the three Panax notoginseng saponins from beagle dog plasma (n = 4)
| Analyte | Spiked conc. (ng/mL) | Recovery (%)a, b | RSD (%)c |
|---|---|---|---|
| Notoginsenoside R1 | 2.8 | 96.0 ± 6.72 | 6.99 |
| 14 | 104.0 ± 7.71 | 7.41 | |
| 140 | 102.3 ± 7.82 | 7.82 | |
| Ginsenoside Rg1 | 3.8 | 99.9 ± 6.89 | 6.90 |
| 19 | 102.4 ± 6.73 | 6.57 | |
| 190 | 98.96 ± 8.06 | 8.14 | |
| Ginsenoside Rb1 | 18 | 102.6 ± 9.85 | 9.60 |
| 180 | 101.1 ± 10.26 | 9.14 | |
| 900 | 105.0 ± 8.32 | 7.92 |
Note: aRecovery (%) = [1 -- (spiked concentration -- measured concentration)/spiked concentration] × 100; bValues are the mean ± SD (n = 4); c Relative standard deviation
Pharmacokinetics study of the three saponins
The pharmacokinetic parameters are listed in Table 4. In this study, notoginsenoside R1 and ginsenoside Rg1 could hardly be detected after 24 hours, whereas ginsenoside Rb1 could still be detected at four days. Thus, the half-life of ginsenoside Rb1 is much longer than those of ginsenoside Rg1 and notoginsenoside R1.
Table 4.
Pharmacokinetic parameters of the three saponins
| Parameters | Notoginsenoside R1 | Ginsenoside Rg1 | Ginsenoside Rb1 |
|---|---|---|---|
| Cmax (ng/mL) | 37.57 ± 24.55 | 95.57 ± 69.30 | 1080.00 ± 186.82 |
| Tmax (h) | 2.67 ± 1.15 | 2.63 ± 1.15 | 8.67 ± 6.43 |
| T1/2 (h) | 3.35 ± 0.55 | 4.55. ± 1.22 | 18.274 ± 2.55 |
| AUC | 107.02 ± 17.39 | 233.42 ± 87.69 | 35178.68 ± 12236.26 |
Conclusions
This LC-MS/MS assay combined with solid-phase extraction was simple, rapid, highly sensitive, and precise for the determination of notoginsenoside R1, ginsenoside Rg1, and ginsenoside Rb1 in beagle dog plasma samples after oral administration of PNS at 90 mg/kg. Although thin-layer chromatography and HPLC-UV methods had been used to study the pharmacokinetics of saponins in PNS [5,6], the specific and more sensitive LC-MS/MS method allows for the simultaneous determination of the three saponins and the quantification of PNS in biological samples, making this as a promising method for the in vivo study of PNS.
Abbreviations
PNS: Panax notoginseng saponins; LC-MS/MS: liquid chromatography-tandem mass spectrometry; HPLC: high-performance liquid chromatography; SPE: solid-phase extraction; MRM: Multiple reaction monitoring; IS: internal standard; QC: quality control; LOQ: limit of quantification; RSD: Relative standard deviation.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
WC designed the study, conducted the animal experiments and performed the statistical analysis. YJD drafted the manuscript. CYZ conceived the study and participated in its design. All authors read and approved the final manuscript.
Contributor Information
Wei Chen, Email: woidyj520@163.com.
Yunjie Dang, Email: dangyunjie@yeal.net.
Chunyan Zhu, Email: cyzhu@implad.ac.cn.
Acknowledgements
The project was supported by the National Natural Science Foundation of China (30672673) and National S&T Major Special Project on Major New Drug Innovation (2009ZX09301-003). We also thank Professors Ji-Fen Guo and Shan-Yi Qiao at Institute of Pharmacology and Toxicology, Academy of Military Medical Sciences (Beijing, China)for their help in this study.
References
- Gan FY, Zheng GZ. Review of study on chemical constituent of Panax notoginseng. Chin Pharm J. 1992;2:138–142. [Google Scholar]
- Wang N, Wan JB, Li MY, Wang YT. Advances in studies on Panax notoginseng against atherosclerosis. Chin Tradit Herb Drugs. 2008;5:787–791. [Google Scholar]
- Yao XH, Li XJ. Panax notoginseng saporins injection in treatment of cerebral infarction with a multicenter study. Chin J New Drugs Clin Rem. 2001;20:257–260. [Google Scholar]
- Yao XH, Li XJ. Protective effects and its mechanism of panaxatriol saponins isolated from Panax notoginseng on cerebral ischemia. Zhongguo Zhong Yao Za Zhi. 2002;27(5):371–373. [PubMed] [Google Scholar]
- Ma WG, Mizutani M, Malterud KE, Lu SL, Ducrey B, Tahara S. Saponins from the roots of Panax notoginseng. Phytochemistry. 1999;52(6):1133–1139. doi: 10.1016/S0031-9422(99)00364-7. [DOI] [Google Scholar]
- Sheng-wu HEN, an WANGY, i WANGY, i- juan WANGL, Zhong-me i HE, Ben-x iang WANG. Study on Anti-Tumor Activity of Ginsenoside Rg1 and Rh1. Journal of Jilin university (Medicine Edition) 2003;29(1):25–28. [Google Scholar]
- Odani T, Tanizawa H, Takino Y. Studies on the absorption, distribution, excretion and metabolism of ginseng saponins. II. The absorption, distribution and excretion of ginsenoside Rg1 in the rat. Chem Pharm Bull. 1983;31(1):292–298. doi: 10.1248/cpb.31.292. [DOI] [PubMed] [Google Scholar]
- Odani T, Tanizawa H, Takino Y. Studies on the absorption, distribution, excretion and metabolism of ginseng saponins. β. The absorption, distribution and excretion of ginsenoside Rg1 in the rat. Chem Pharm Bull. 1983;31(3):1059–1066. doi: 10.1248/cpb.31.1059. [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Sung JH, Matsumiya S, Uchiyama M. Main ginseng saponin metabolites formed by intestinal bacteria. Planta Med. 1996;62(5):453–457. doi: 10.1055/s-2006-957938. [DOI] [PubMed] [Google Scholar]
- Xu QF, Fang XL, Chen DF. Pharmacokinetics and bioavailability of ginsenoside Rb1 and Rg1 from Panax notoginseng in rats. J Ethnopharmacol. 2003;84(2-3):187–192. doi: 10.1016/S0378-8741(02)00317-3. [DOI] [PubMed] [Google Scholar]
- Shangguan D, Han H, Zhao R, Zhao Y, Xiong S, Liu G. New method for high-performance liquid chromatographic separation and fluorescence detection of ginsenosides. J Chromatogr A. 2001;910(2):367–372. doi: 10.1016/S0021-9673(00)01208-5. [DOI] [PubMed] [Google Scholar]
- Cui JF, Björkhem I, Eneroth P. Gas chromatographic-mass spectrometric determination of 20(S)-protopanaxadiol and 20(S)-protopanaxatriol for study on human urinary excretion of ginsenosides after ingestion of ginseng preparations. J Chromatogr B. 1997;689(2):349–355. doi: 10.1016/S0378-4347(96)00304-0. [DOI] [PubMed] [Google Scholar]
- Tawab MA, Bahr U, Karas M, Wurglics M, Schubert-Zsilavecz M. Degradation of ginsenosides in human after oral administration. Drug Metab Dispos. 2003;31(8):1065–1071. doi: 10.1124/dmd.31.8.1065. [DOI] [PubMed] [Google Scholar]
- Ji HY, Lee HW, Kim HK, Kim HH, Chang SG, Sohn DH, Kim J, Lee HS. Simultaneous determination of ginsenoside Rb1 and Rg1 in human plasma by liquid chromatography-mass spectrometry. J Pharm Biomed Anal. 2004;35(1):207–212. doi: 10.1016/j.jpba.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Li L, Zhang JL, Sheng YX, Ye G, Guo HZ, Guo DA. Liquid chromatographic method for determination of four active saponins from Panax notoginseng in rat urine using solid-phase extraction. J Chromatogr B. 2004;808(2):177–183. doi: 10.1016/j.jchromb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Mauri P, Pietta P. Electrospray characterization of selected medicinal plant extracts. J Pharm Biomed Anal. 2000;23(1):61–68. doi: 10.1016/S0731-7085(00)00264-8. [DOI] [PubMed] [Google Scholar]
- Chan TWD, But PPH, Cheng SW, Kwok IMY, Lau FW, Xu HX. Differentiation and authentication of Panax ginseng, Panax quinquefolius, and ginseng products by using HPLC/MS. Anal Chem. 2000;72(6):1281–1287. doi: 10.1021/ac990819z. [DOI] [PubMed] [Google Scholar]
- Qian T, Jiang ZH, Cai Z. High performance liquid chromatography coupled with tandem mass spectrometry applied for metabolic study of ginsenoside Rb1 on rat. Anal Biochem. 2006;352(1):87–96. doi: 10.1016/j.ab.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Qian T, Cai Z, Wong RNS, Jiang ZH. Liquid chromatography/mass spectrometric analysis of rat samples for in vivo metabolism and pharmacokinetic studies of ginsenoside Rh2. Rapid Commun Mass Spectrom. 2005;19(23):3549–3554. doi: 10.1002/rcm.2232. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal (U.S.) Guide for the care and use of laboratory animals. Washington, D.C. National Academy Press; 1996. [Google Scholar]