Abstract
Polyacrylate glue protein analogs of the glue secreted by Phragmatopoma californica, a marine polycheate, were synthesized with phosphate, primary amine, and catechol sidechains with molar ratios similar to the natural glue proteins. Aqueous mixtures of the mimetic polyelectrolytes condensed into liquid complex coacervates around neutral pH. Wet cortical bone specimens bonded with the coacervates, oxidatively crosslinked through catechol sidechains, had bond strengths nearly 40% of the strength of a commercial cyanoacrylate. The unique material properties of complex coacervates may be ideal for development of clinically useful adhesives and other biomaterials.

Keywords: adhesives, biomimetic, complex coacervation, phase behavior, polyelectrolytes
Introduction
Advances in adhesives technology over the last half century or so have lead to the widespread replacement of mechanical fasteners with adhesive bonds in everything from the soles of our shoes to jumbo jet fuselages. Paradoxically, one bonding application that has not benefited much from developments in adhesive technology is medicine. There are few mechanical fasteners in the animal kingdom, yet, torn and fractured tissues are almost always repaired with sutures, staples, screws, pins, and plates. Orthopaedic medicine, in particular, has a striking reliance on metal hardware and power tools for repairing damaged bones. Bonding living tissues is challenging because of the wet environment, aggressive immune surveillance for foreign materials, restricted temperature range, and requirements for non-poisonous reactants, reaction by-products, and degradation products. There are no suitable adhesives in current widespread use in orthopaedic surgery. Modern high-performance adhesives including epoxies, polyurethanes, cyanoacrylates, and polyacrylics have been tested and in some cases are used to repair superficial wounds but have not met the demands of deep tissue bonding or bone fixation.[1–3]
Guided by a natural adhesive produced by a shell-dwelling marine worm, we have taken an alternative approach to developing adhesives for orthopaedic surgery and other medical applications. P. californica lives in the intertidal zone along the coast of California. Like many marine invertebrates, the segmented worm lives in a composite mineral shell for physical protection. Instead of secreting its mineralized shell, P. californica gathers the mineral component adventitiously as sand grains and bits of seashell hash which it bonds together into a tube with small dabs of a proteinaceous glue. The glue sets in less than 30 s under cold seawater[4] and hardens to a tough leathery consistency over several hours. Structurally the glue appears to be an aggregation of 50–80 nm spheres that form a microporous water-filled foam with a steep gradient in porosity, from nearly solid at the outside edges to nearly empty at the center.[4]
The glue is comprised of several highly acidic and basic proteins and a good measure of Mg2+ and Ca2+ ions.[5] The sequences of the published P. californica glue proteins, referred to as Pc1-3, are highly repetitive and relatively simple.[6] Pc1 is comprised mostly of just three residues, glycine (45 mol-%), lysine (14 mol-%), and tyrosine (19 mol-%), which occur as 15 repeats of the decapeptide VGGYGYGGKK. The Pc2 sequence is comprised of several degenerate copies of the dodecapeptide HPAVHKALGGYG. Both proteins are basic with pIs >9. The sequence of Pc3, which exists as two major variants (A,B) and several minor variants, is distinguished by runs of 4–13 serine residues punctuated with single tyrosine residues. Amino acid analysis of secreted glue revealed that 50 mol-% of the total glue amino acid residues are serine or glycine, at least 95% of the serines are phosphorylated,[5] and in total about 1/3 of the tyrosine residues are post-translationally hydroxylated to form 3,4-dihydroxyphenyl-l-alanine (dopa).[6,7] This latter number is likely to be higher because an unknown percentage of dopa residues are lost by oxidation to dopaquinone, subsequent crosslinking with nucleophilic amino acid sidechains, and other oxidation products. Dopa occurs in the adhesive plaque of mussels and has been proposed to play dual roles in interfacial adhesion and cohesive crosslinking.[8] Experiments with dopa-modified AFM tips demonstrated that the catechol form of dopa bonds to wet titanium oxide surfaces with dissociation energies of 22 kcal · mol−1, the strongest non-covalent bond yet measured,[9] providing support for dopa's role in interfacial adhesion.
The known glue proteins constitute a set of oppositely charged polyelectrolytes at physiological pH, which led us to propose a model based on complex coacervation to explain the foamy structure, fluid character, low interfacial tension, and cohesive properties of the worm's water-borne underwater adhesive.[5] Complex coacervates are an intriguing state of matter.[10] Oppositely charged polyelectrolytes form stoichiometric colloidal complexes in low ionic strength aqueous solutions. When the pH or other solution conditions are adjusted to change the net charge of the complexes, generally described as toward net neutrality,[11] the complexes condense into a liquid phase of concentrated polymers (the coacervate phase) and a dilute equilibrium phase. The coacervate phase is an associative liquid with a dynamic structure in which the individual polymer components diffuse throughout the entire phase.[12] Complex coacervates behave rheologically like a viscous particle dispersion rather than a viscoelastic polymer solution.[13] In the case of P. californica, these properties explain how the worm can secrete its glue under seawater as a watery liquid that readily spreads over the surface of wet mineral substrates, fills gaps, but does not disperse into the ocean. The trigger for the quick set and slower dopa-mediated hardening reaction that occurs after secretion may be the pH jump experienced by the adhesive as it travels through the secretory pathway (pH≈5) to seawater (pH≈8.2).[5]
Complex coacervates have a long history[14] of application in encapsulation of volatile or sensitive compounds, including drugs,[15] because they are formed in aqueous solution at moderate pH from preformed polymers, thereby avoiding non-biocompatible solvents as well as exothermic and promiscuous polymerization chemistries. The physiologically compatible formation process, the unique rheological and material properties, together with the example of P. californica's underwater glue suggested complex coacervates may be well-suited for use as a platform for developing injectable biomedical adhesives, especially for bonding hard mineralized tissues. In this report, we describe a water-borne adhesive created by mimicking the composition and curing mechanisms of the P. californica glue with a composition of synthetic water-soluble 3,4-dihydroxyphenol-containing copolymers and divalent cations.
Experimental Part
Mimetic Copolymer Synthesis and Characterization
Pc3 Analogs
The dopa analog monomer (dopamine methacrylamide, DMA) was prepared by slight modification of a published procedure.[16] Briefly, a borate-dopamine complex was reacted at pH >9 with methacryloyl chloride. After disrupting the borate-catechol bond by acidification, the product was washed with ethyl acetate, recrystallized from hexane, and verified by 1H NMR spectroscopy.
1H NMR (400 MHz, DMSO/TMS): δ = 8.8−8.58[2H, (OH)2–Ar– ], 7.92[1H, –C(=O)–NH– ], 6.64−6.57[2H,C6HH2(OH)2–], 6.42[1H, C6H2H(OH)2–], 5.61[1H, –C(=O)–C(–CH3)=CHH], 5.30[1H, – C(=O)–C(–CH3)=CHH], 3.21[2H, C6H3(OH)2–CH2–CH2(NH)–C(=O)–], 2.55 [2H, C6H3(OH)2–CH2–CH2(NH)–C(=O)–], 1.84 [3H, –C(=O)–C (–CH3)=CH2].
Before polymerization, monoacryloxyethyl phosphate (MAEP, Polysciences) was diluted in MeOH and extracted with hexane to remove dienes.[17] Copolymer 1 was prepared by mixing 90 mol-% MAEP, 8 mol-% DMA, 2 mol-% acrylamide (Aam, Polysciences), and 0.1 mol-% FITC-methacrylamide in MeOH at a final monomer concentration of 5 wt.-%. Free radical polymerization was initiated with 2.2′-azoisobutyronitrile (AIBN) and proceeded 24 h at 60 °C in sealed ampules. Copolymer 1 was recovered by size exclusion chromatography (SEC) in MeOH on a Sephadex LH-20 column (Sigma-Aldrich), concentrated by rotary evaporation, dissolved in DI water, and freeze dried.
The MW and polydispersity index (PDI) of 1 were determined by SEC in DMF on a PLgel column (Polymer Labs) connected to a small angle light scattering detector (Brookhaven BI-MWA) and refractive index monitor (Brookhaven BI-DNDC). The column was calibrated with polystyrene standards. The MW of 1 was 245 kda with a PDI of 1.9. The dopamine sidechain concentration and reactivity was verified by UV-vis spectroscopy (ε280 = 2 600 L · mol−1 · cm−1). The phosphate sidechain concentration was determined by titration with 0.005 m NaOH using an automated titrator (Brinkmann Titrando 808).
Pc1 Analogs
The lysine sidechains of Pc1 were mimicked with N-(3-aminopropyl)methacrylamide hydrochloride (APMA, Polysciences). Copolymer 2 was synthesized by dissolving 10 mol-% APMA and 90 mol-% Aam in DI water, degassing with N2 and initiating polymerization with 2 mol-% ammonium persulfate (Polysciences). Polymerization proceeded at 50 °C for 24 h in sealed ampules. Polymer was recovered by dialysis against water for 3 d, and then freeze dried. The primary amine sidechain content was determined by 1H NMR (400 MHz, DMSO/TMS) from the ratios of δ = (13.45H, –CH3) and δ = [51.04H, RC(=O)CHR2]. The MW and PDI of 2 were determined by SEC in PBS (20 × 10−3 m PO4, 300 × 10−3 m NaCl, pH = 7.2) on a Superose 6 column (Pharmacia). The column was calibrated with poly(2-hydroxypropyl methacrylate) standards. The MW of 2 was 165 kd and PDI was 2.4.
Coacervate Formation and Characterization
A 5 wt.-% aqueous solution of 2 was added dropwise while stirring to a 5 wt.-% aqueous solution of 1 until reaching the target amine/ phosphate ratio. Total copolymer concentration was 50 mg · mL−1. After mixing for 30 min the pH was adjusted with NaOH (6 m). Compositions at pH (<4) conducive to polyelectrolyte complex (PEC) formation were diluted to 1 mg · mL−1 in DI H2O and the zeta potentials and size distribution of PECs were measured on a ZetaSizer 3000HS (Malvern Instruments). At higher pH, coacervated compositions were centrifuged at 2 500 rpm in a microfuge (Eppendorf), at 25 °C for 2 min to collect the coacervate phase. The volume of both phases was measured. The coacervate phases were freeze dried and weighed to determine their mass and concentration.
Mechanical Bond Testing
Bone test specimens, ≈1 cm3, were cut with a band saw from bovine femur cortical bone, obtained from a local grocery store, sanded with 320 grit sandpaper, and stored at −20 °C. NaIO4 at a 1:2 molar ratio to dopa sidechains was evenly applied to one face each of two wet bone specimens. Forty μL, a volume sufficient to completely fill the space between 1 cm2 bone interfaces, of the test coacervate solution was applied with a pipette, the bone specimens were pressed together squeezing out a small excess of adhesive, clamped, and immediately wrapped in PBS (20 × 10−3 m PO4, 150 × 10−3 m NaCl, pH = 7.4) soaked gauze. The applied coacervate contained ascorbate at a 1:5 molar ratio to dopa to prevent premature crosslinking. The bonded specimens were incubated at 37 °C for at least 24 h in a sealed container containing soaked sponges to maintain 100% humidity. Reference specimens were bonded with 40 μL Loctite 401 superglue in exactly the same manner. A commercial non-medical grade cyanoacrylate was used because there are no hard tissue medical adhesives available for comparison. Mechanical tests were performed on a custom built material testing system using a 1 kg load cell. The instrument was controlled and data aquired using LabView (National Instruments). One bone of a bonded pair was clamped laterally 1 mm from the bond interface. The second bone was pressed with a cross-head speed of 0.02 mm · s−1 against a dull blade positioned 1 mm lateral to the bond interface. Bond strength tests were performed at room temperature immediately after unwrapping the wet specimens to prevent drying. After testing, the bonds were examined for failure mode. The bonded area was measured by tracing an outline of the bone contact surface on paper, cutting out the trace, and determining its area from the weight of the paper cut-out. At least 6 specimens were tested for each condition.
Statistical Analysis
Errors are reported as the standard error of the mean.
Results and Discussion
Mimetic Copolymers
We reasoned based on the simple repetitive sequences and probable lack of higher order protein structure that the key and essential features of the glue proteins giving rise to the structure and bonding mechanism of the P. californica glue are their polyelectrolytic and oppositely charged nature, the density of the charged sidechains, the overall ratio of positive to negative charges, the chemical nature and pKa of the charged sidechains, the interactions of divalent cations with the polyphosphate protein, and the molar ratio and distribution of the redox active dopa sidechains. These features can be readily copied with acrylic copolymers. The polyamide backbone, chiral amino acid subunits, exact sidechain sequence, and precise molecular weight, on the other hand, may not be essential for an effective adhesive but rather fixed, non-optional features of biological protein synthesis. To test this hypothesis copolymers with glue protein mimetic sidechains were synthesized.
Analogs of the acidic Pc3 proteins 1 were synthesized by free radical copolymerization of monoacryloxyethyl phosphate (MAEP), dopamine methacrylate (DMA), and acrylamide (Aam) (Figure 1a). A trace of fluoroscein isothiocyanate (FITC) modified methacrylate was included for visualization of the copolymer. The UV-vis spectrum of 1 contained a single absorption peak at 280 nm characteristic of the catechol form of dopamine (Figure 1b). Addition of a 1:1 molar ratio of NaIO4 to 1 at pH = 5.0 oxidized the dopa catechol to dopaquinone with an absorption peak near 395 nm as expected. The dopaquinone peak was stable for several hours at pH < 5.
Figure 1.
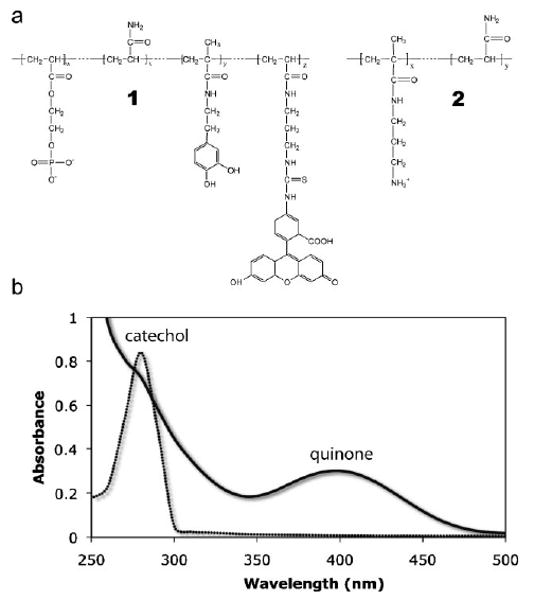
Structure and UV-vis characterization of mimetic copolymers: a) The Pc3 analog, 1, contained 88.4 mol-% phosphate, 9.7 mol-% dopamide, and 0.1 mol-% FITC sidechains. The Pc1 analog, 2, contained 8.1 mol-% amine sidechains. The balance was acrylamide subunits in both cases. b) A single peak at 280 nm characteristic of the catechol form of 3,4-dihydroxyphenol was present in the spectrum of 1. Following oxidation with NaIO4 a peak at 395 nm corresponding to the quinone form appeared confirming the expected redox behavior of the 3,4-dihydroxyphenol-containing polymer.
The acidic residues in the natural glue are predominantly phosphoserine. The basic residues, on the other hand, are represented by roughly equal parts of lysine, histidine, and arginine.[6,7] For this initial report the focus was narrowed to creating analogs of the lysine-rich glue proteins, Pc1 for example. Analogs 2 of the lysine-rich basic proteins were created by copolymerizing various ratios of the primary amine containing monomer, N-(3-aminopropyl)methacrylamide hydrochloride (APMA), with Aam as a neutral filler (Figure 1a). Although Pc1 contains about 10 mol-% dopa,[7] the dopamide monomer was not included in 2 to allow better control of the spontaneous oxidative crosslinking of dopamide-containing copolymers at elevated pH.
Phase Behavior of Mixed Polyelectrolytes
The phase behavior of 1 and 2 mixed at a 1:1 molar ratio of phosphate to amine sidechains (50 mg · mL−1 combined concentration) over the pH range 3–10 is shown in Figure 2a. The calculated net copolymer charge normalized to the total ionizable sidechain concentration is shown in Figure 2b. Ascorbate, a reductant, was added at a 1:5 molar ratio to dopa to retard oxidation of dopa by O2 and subsequent crosslinking at elevated pH. At low pH, the polyelectrolytes formed a stable milky solution of colloidal polyelectrolyte complexes (PECs). The mean diameter of the PECs at pH = 2.1, determined by dynamic light scattering, was 360 nm with a narrow dispersity and increased to 1080 nm at pH = 4.0 (Figure 2c). The crossover of the zeta potential from positive to negative at pH = 3.6 fit well with the calculated pH dependent net charge of the complexes (Figure 2b). The particle size could not be measured accurately above pH = 4 because the complexes flocculated. As the net charge increased due to the deprotonation of the phosphate sidechains, the copolymers condensed into a dense second phase. At pH = 5.1 the separated phase had the character of a loose low density precipitate. At pH = 7.2 and 8.3 the dense phase had the character of a cohesive liquid complex coacervate (Figure 3, Supporting Information, video 1). The copolymers were concentrated about three-fold to 148 and 153 mg · mL−1, respectively, in the coacervated phases. At pH = 9.5 the polyelectrolyte mixture formed a dense non-liquid ionic gel. At pH = 10 the copolymers went into solution and spontaneously crosslinked through the dopaquinone and amine sidechains into a clear hydrogel.
Figure 2.
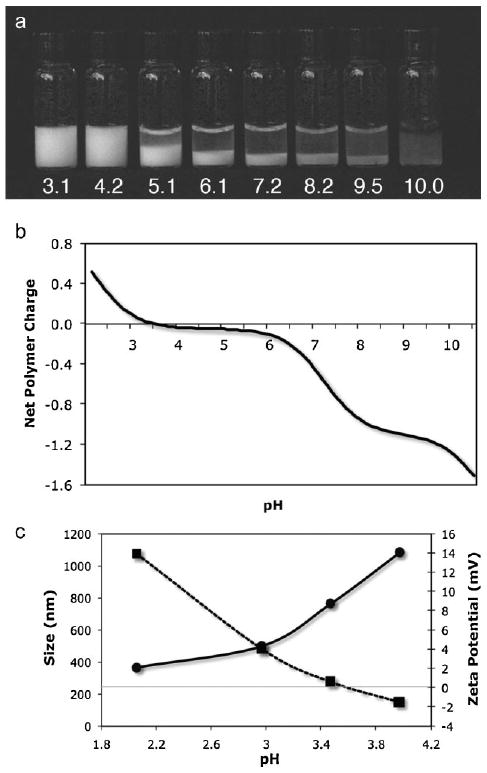
pH-dependent complex coacervation of mixed polyelectrolytes. a) At low pH, a 50 mg · mL−1 mixture of 1 and 2 having equal quantities of amine and phosphate sidechains formed stable colloidal PECs. As the pH increased the polymers condensed into a dense liquid complex coacervate phase. At pH = 10 the copolymers went into solution and oxidatively crosslinked into a clear hydrogel. b) The net charge of the copolymer sidechains as a function of pH calculated from the copolymer sidechain densities. c) The diameter of the PECs (circles) increased nearly three-fold over the pH range 2–4. Above pH = 4 the complexes flocculate and their size could not be measured. The zeta potential (squares) was zero near pH = 3.6 in agreement with the calculated net charge.
Figure 3.
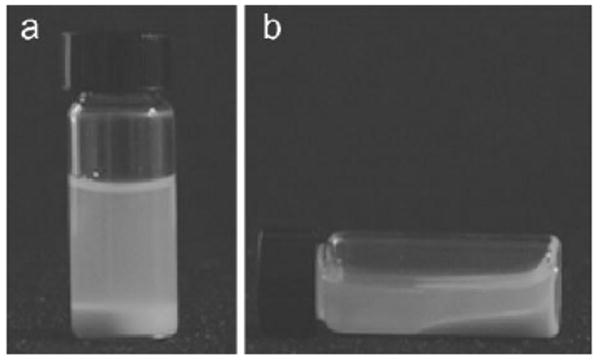
Liquid character of complex coacervate. The solution of 1 and 2 contained equal quantities of amine and phosphate sidechains, pH = 7.4.
The divalent cations Mg2+ and Ca2+ play a crucial but not well understood role in the structure, strength, and adhesiveness of the natural glue. Extraction of divalent cations with the chelator EDTA resulted in a 50% decrease in compressive strength of P. californica tubes, a ten-fold decrease in adhesiveness, and collapse of the glues porous structure.[18] The effect of divalent cations on the phase behavior of the mimetic polyelectrolytes was investigated by mixing 1 and 2 at amine to phosphate sidechain ratios ranging from 1:1 to 0:1 with divalent cation to phosphate sidechain ratios ranging from 0:1 to 1:1 to create a coacervate phase diagram (Figure 4). The pH was fixed at 8.2, the pH of seawater, and divalent cations were added as a 4:1 mixture of Mg2+ and Ca2+, the approximate Mg2+/ Ca2+ ratio in the natural glue determined by elemental analysis. The highest mass of coacervate (dark gray squares) occurred in mixtures with higher amine to phosphate sidechain ratios and lower divalent cation to phosphate sidechain ratios. Mixtures with lower polyamine ratios were clear (clear squares) even at higher divalent cation/phosphate sidechain ratios. At higher amine/phosphate and divalent cation/phosphate ratios the solutions were turbid (light gray squares) with slight precipitates but much less turbid than solutions containing PECs (medium gray squares).
Figure 4.
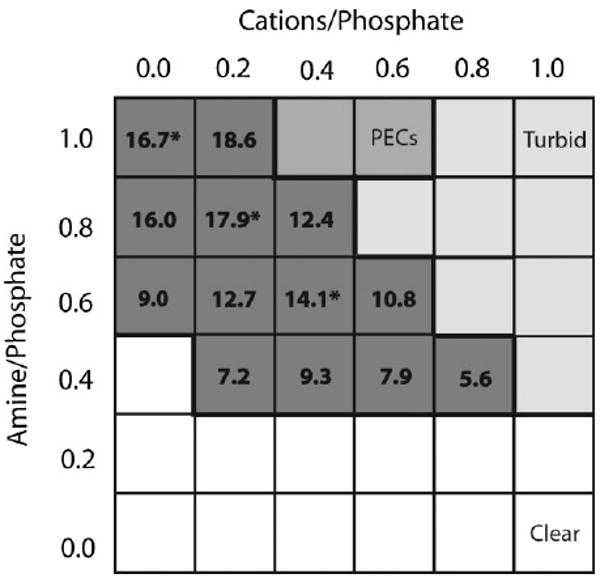
Phase diagram of polyelectrolytes and divalent cations. The amine to phosphate sidechain and phosphate sidechain to divalent cation ratios were varied at a fixed pH = 8.2. The state of the solutions represented in a gray scale. The mass (mg) of the coacervate phase is indicated in the dark grey squares. The compositions indicated with an asterisk were used to test bond strength.
Coacervated Bone Adhesive Strength
The shear modulus and strength at failure were measured with bovine cortical bone specimens bonded while wet with the three coacervating compositions marked with an asterisk in Figure 4. The coacervate density in the three compositions increased with increasing divalent cation ratios (to 120, 125, and 130 mg · mL−1, respectively). Both the modulus and bond strength of the fully hydrated specimens increased with increasing divalent cation concentration, reaching 37% of the strength of wet bones bonded with a commercial cyanoacrylate adhesive (Figure 5a). The cyanoacrylate adhesive was used as a reference point because there are no bone adhesives in clinical use for comparison. The strength of the mimetic adhesive is also about 1/3 the strength of natural P. californica glue estimated to be 350 kPa[18] and mussel byssal glue estimated to range from 320 to 750 kPa dependent on the season.[19] In almost all cases the bonds failed cohesively leaving adhesive on both bone interfaces, which suggested the compositions formed strong interfacial bonds with hydroxyapatite. The bonds were dimensionally stable, neither shrinking nor swelling appreciably after complete submersion in PBS pH = 7.2 for several months (Figure 5b). Dimensional stability during cure and long term exposure to water is an important requirement for a useful bone adhesive.
Figure 5.
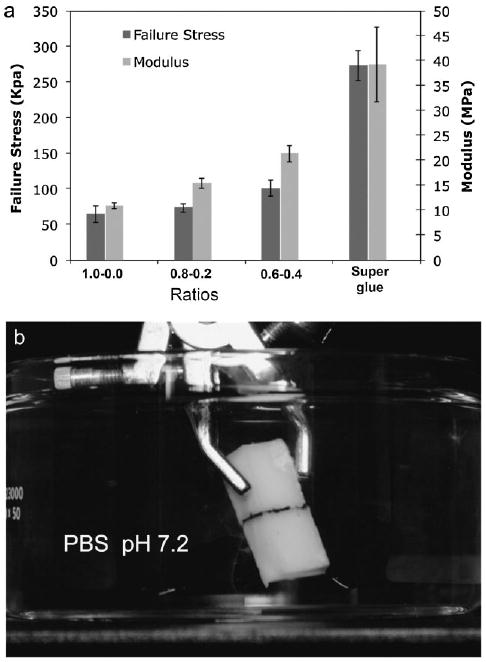
Bond strength, shear modulus, and dimensional stability of coacervate bonded bones. a) Bond strength at failure increased ≈50% and the stiffness doubled as the divalent cation ratio went from 0 to 0.4 relative to phosphate sidechains. Specimens wet bonded with a commercial cyanoacrylate adhesive were used as a reference (n = 6 for all conditions). b) Bonds of adhered bone specimens fully submerged in PBS for four months (pH = 7.2) did not swell appreciably.
A conceptual model of the phase behavior and bonding mechanism of the mimetic coacervated adhesive is presented in Figure 6. At low pH the oppositely charged polyelectrolytes associate electrostatically into nanocomplexes with a net positive surface charge that stabilizes the suspension (Figure 6a). With increasing pH the net charge of the complexes changes from positive to negative but remains near net neutrality. The complexes form loose precipitates (Figure 3a) over the pH range where the phosphate sidechains have a single negative charge. As the phosphate sidechains undergo a second ionization with rising pH the polyphosphates become more extended and rigid due to the increasing double negative charge density. The excess negative charges (Figure 6b) are shielded only by monovalent ions in the low ionic strength solution. Eventually the densely charged polyphosphates extend to form a cohesive liquid network with dynamic ionic junctions formed by the more compact polyamines and divalent cations (Figure 6b). The liquid coacervate has low initial viscosity, specific gravity greater than one, and being mostly water by weight, low interfacial tension in an aqueous environment, all of which contribute to its ability to wet the bone surface, a prerequisite for effective underwater adhesion.
Figure 6.
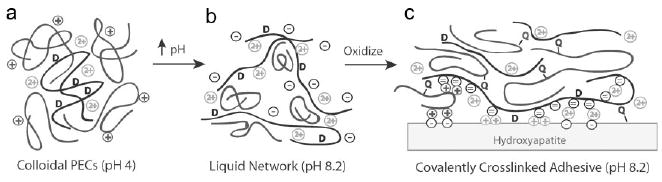
Model of pH dependent coacervate structure and adhesive mechanisms. a) The polyphosphate (black) with low charge density paired with the polyamine (dark grey) form nm-scale complexes. The complexes have a net positive charge. b) Extended high charge density polyphosphates form a network connected by more compact lower charge density polyamines and when present divalent cations (light grey symbols). The net charge on the copolymers is negative. c) Oxidation of 3,4-dihydroxyphenol (D) by O2 or an added oxidant initiates crosslinking between the quinone (Q) and primary amine sidechains. The coacervate can adhere to the hydroxyapatite surface through electrostatic interactions, 3,4-dihydroxyphenol sidechains, and quinone-mediated covalent coupling to matrix proteins.
The coacervate adheres to bone (and other minerals) through several mechanisms (Figure 6c). The surface of the bone's hydroxyapatite mineral phase [Ca5(PO4)3(OH)] is an array of both positive and negative charges.[20] The negative polyphosphate can interact directly with the positive surface charges or it can be bridged to the negative surface charges through the positive polyamine and/or divalent cations. Likewise, direct interaction of the polyamine with the negative surface charges would contribute to adhesion. Molecules containing catechol moieties have been shown to have strong absorptive properties and to readily wet hydroxyapatite.[21] Further contributions to strong interfacial adhesion may therefore come from direct bonding of unoxidized dopamide sidechains to hydroxyapatite. Sidechains oxidized to dopaquinone could covalently couple to nucleophilic sidechains of bone matrix proteins as another factor in strong adhesion. Cohesive strength develops through intermolecular covalent coupling between the dopaquinone sidechains on the polyphosphate and the primary amine sidechains of the polyamine (Figure 6c).
The increase in bond strength with increasing divalent cation concentrations requires additional experimentation to fully understand its mechanistic basis. One contributing factor is likely the increase in crosslink density as singly charged amine sidechains were substituted with doubly charged cations. The increased density of the cation containing coacervate phases may be due to more compact junctions between polyphosphates and divalent cations compared to polyamine junctions. Divalent cation bridging between the polyphosphate and hydroxyapatite surface may be more effective than polyamine bridging or direct polyphosphate binding contributing to stronger adhesion. The divalent cations may also affect polyphosphate conformation, the number of effective intermolecular crosslinks, as well as polyphosphate hydration and solubility.
The minimum bond strength required for repairing fractured bones with an adhesive in a clinical setting is difficult to specify because of the broad range of bone and fracture types. Even current metal fixators are not used to recreate weight bearing joints but rather function as internal splints to stabilize the fracture during healing. Weight is kept off of the joint until bony healing has occurred. The bond strengths of the first generation adhesives, 40% of the bond strength of a cyanoacrylate adhesive, may be already sufficient for some applications since the clinical goal will not be to reconstruct a permanent weight bearing joint. One clinical target of the mimetic adhesive is the reconstruction of highly fragmented articular surfaces for which maintaining precise alignment is critical for restoring joint function. It is especially difficult to maintain alignment of small bone fragments by drilling them with screws and wires. An adjunctive adhesive could reduce the number or volume of metal fixators while helping maintain accurate alignment of small bone fragments to improve clinical outcomes for this orthopaedic population. Additional orthopaedic applications for the mimetic adhesive may include craniofacial reconstructions and securing engineered tissue scaffolds[22] to native bony tissues, both of which are non-weight bearing applications.
In addition to sufficient bond strength, deliverability, and appropriate curing kinetics, the coacervate-based adhesives will have to meet several more specifications for use in the clinic. They must be non-toxic, must not elicit foreign body responses, and should be biodegradability to allow natural healing in the bonded areas. Regarding cytotoxicity, the coacervates are self-organized in aqueous solution from pre-polymerized components so tissues are not exposed to toxic reactants or reaction by-products, nor to heat generated by exothermic in situ polymerization of monomers. Literature precedent also suggests the coacervate adhesives will have low cytotoxicity. Nuttelman et. al.[23] created PEG hydrogels with pendant phosphate groups using ethylene glycol methacrylate phosphate, the methacrylic version of the phosphate monomer used to mimic Pc3. The viability of human mesenchymal stem cells improved dramatically and a bone-like mineral phase formed in the phosphate functionalized hydrogels. Regarding biocompatibility, important questions remain to be addressed, but in general water-soluble methacrylates are not expected to elicit immune responses.[24] Ultimately, the adhesive should be resorbed over a period of several months to allow natural bone healing. The phosphoester linkages of 1 are susceptible to hydrolysis,[25] which may provide suitable rates of adhesive degradation. If necessary, additional ester bonds will be incorporated into later generation adhesives to increase the resorption rate.
The idea of using principles or materials derived from natural adhesives produced by sessile marine organisms in medicine is not new.[26] The most studied of these marine adhesives are produced by mussels who meet the challenge of living in turbulent wave-swept ocean environments by gluing collagenous byssal threads to solid surfaces with a collection of proteins assembled into an adhesive plaque. The adhesive plaque proteins are spatially distributed in adhesive plaque consistent with distinct chemical roles in byssal thread bonding.[27] It will be difficult to reconstruct all of the critical features of the highly organized mussel adhesive into effective mimetic adhesives. The P. californica adhesive, in contrast, has been adapted for the comparatively simple task of gluing two similar and external mineral substrates together. Correspondingly, its glue appears to be simpler in composition, to be less structurally organized, likely requires less sophisticated biological processing, and may therefore be an excellent model for clinically practical adhesives.
The first generation mimetic adhesive described here qualitatively reproduced several observed or predicted features of the natural glue: the nanoparticulate structure, pH dependent phase behavior, ability to bond wet mineral substrates, and oxidative dopa-mediated curing. As the composition, application methods, curing trigger and kinetics are optimized it seems reasonable to expect significant improvements in the adhesive bond strength to hydroxyapatite. In an iterative cycle of discovery and implementation, additional features of the P. californica adhesive, both already known and yet to be appreciated, will be built into the mimetic adhesive system for the dual purposes of testing hypotheses about natural glue structure and bonding mechanisms and to further improve the synthetic adhesive.
Conclusion
Complex coacervates are a state of matter with ideal but so far unexploited material and rheological properties for injectable medical adhesives. The known utility of complex coacervates for encapsulation of bioactives can be exploited in future incarnations to create mimetic adhesives that simultaneously fix bone fractures and deliver antibiotics, pain relievers, and/or growth factors to provide greater patient comfort and accelerate bone healing.
Supplementary Material
Acknowledgments
We thank Rick Vance and Andrew Guss for early mechanical testing and bone sample preparation. RJS thanks Thomas Higgins and Charles Saltzmann for helpful orthopaedic surgery discussions and Herb Waite for suggesting P. californica as a sabbatical research project in his lab several years ago.
Footnotes
Supporting information for this article is available at the bottom of the article's abstract page, which can be accessed from the journal's homepage at http://www.mbs-journal.de, or from the author.
Supporting Information: Video of phase behavior and properties of complex coacervates.
Contributor Information
Hui Shao, Department of Bioengineering, University of Utah, Salt Lake City, UT 84112.
Kent N. Bachus, Department of Orthopaedics, University of Utah, Salt Lake City, UT 84112
Russell J. Stewart, Email: rstewart@eng.utah.edu, Department of Bioengineering, University of Utah, Salt Lake City, UT 84112.
References
- 1.Leonard F. Hemostatic applications of alpha-cyanoacrylates: bonding mechanism and physiological degradation of bonds. In: Manly RS, editor. Adhesion in Biological Systems. Academic Press; New York: 1970. p. 185. [Google Scholar]
- 2.Donkerwolcke M, Burny F, Muster D. Biomaterials. 1998;19:1461. doi: 10.1016/s0142-9612(98)00059-3. [DOI] [PubMed] [Google Scholar]
- 3.Heiss C, Kraus R, Schluckebier D, Stiller AC, Wenisch S, Schnettler R. Eur J Trauma. 2006;32:141. [Google Scholar]
- 4.Stevens MJ, Steren RE, Hlady V, Stewart RJ. Langmuir. 2007;23:5045. doi: 10.1021/la063765e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart RJ, Weaver JC, Morse DE, Waite JH. J Exp Biol. 2004;207:4727. doi: 10.1242/jeb.01330. [DOI] [PubMed] [Google Scholar]
- 6.Zhao H, Sun C, Stewart RJ, Waite JH. J Biol Chem. 2005;280:42938. doi: 10.1074/jbc.M508457200. [DOI] [PubMed] [Google Scholar]
- 7.Waite JH, Jensen RA, Morse DE. Biochemistry. 1992;31:5733. doi: 10.1021/bi00140a007. [DOI] [PubMed] [Google Scholar]
- 8.Waite JH. Ann N Y Acad Sci. 1999;875:301. doi: 10.1111/j.1749-6632.1999.tb08513.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee H, Scherer NF, Messersmith PB. Proc Natl Acad Sci USA. 2006;103:12999. doi: 10.1073/pnas.0605552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bungenberg de Jong HG. Morphology of coacervates. In: Kruyt HR, editor. Colloid Science. Elsevier Publishing Company; Amsterdam: 1949. p. 431. [Google Scholar]
- 11.Cornelus G, de Kruif CG. Curr Opin Colloid Interface Sci. 2004;9:340. [Google Scholar]
- 12.Weinbreck F, Rollema HS, Tromp RH, de Kruif CG. Langmuir. 2004;20:6389. doi: 10.1021/la049908j. [DOI] [PubMed] [Google Scholar]
- 13.Weinbreck F, Wientjes RHW, Nieuwenhuijse H, Robijn GW, de Kruif CG. J Rheol. 2004;48:1215. [Google Scholar]
- 14.US 2 730 456. National Cash Register Company, invs.: B. K. Green, L. Schleicher 1956
- 15.Burgess DJ. Complex coacervation: microcapsule formation. In: Dubin PL, editor. Macromolecular complexes in chemistry and biology. Springer Verlag; Berlin: 1994. p. 285. [Google Scholar]
- 16.Lee BP, Huang K, Nunalee FN, Shull KR, Messersmith PB. J Biomater Sci Polym Ed. 2004;15:449. doi: 10.1163/156856204323005307. [DOI] [PubMed] [Google Scholar]
- 17.Nakamae K, Nizuka T, Miyata T, Furukawa M, Nishino T, Kato K. J Biomater Sci Polym Ed. 1997;9:43. doi: 10.1163/156856297x00254. [DOI] [PubMed] [Google Scholar]
- 18.Sharma B, Williams CG, Kim TK, Sun D, Malik A, Khan M. Tissue Eng. 2007;13:405. doi: 10.1089/ten.2006.0068. [DOI] [PubMed] [Google Scholar]
- 19.Young GA, Crisp DJ. Marine animals and adhesion. In: Allen KW, editor. Adesion 6. Aplied Science Publishers; London: 1982. p. 19. [Google Scholar]
- 20.Long JR, Shaw WJ, Stayton PS, Drobny GP. Biochemistry. 2001;40:15451. doi: 10.1021/bi010864c. [DOI] [PubMed] [Google Scholar]
- 21.Chirdon WM, O'Brien WJ, Robertson RE. J Biomed Mater Res B Appl Biomater. 2003;66:532. doi: 10.1002/jbm.b.10041. [DOI] [PubMed] [Google Scholar]
- 22.Khan K, Yaszemski MJ, Mikos AG, Laurencin CT. J Bone Joint Surgery (American) 2008;90:36. doi: 10.2106/JBJS.G.01260. [DOI] [PubMed] [Google Scholar]
- 23.Nuttelman CR, Benoit DS, Tripodi MC, Anseth KS. Biomaterials. 2006;7:1377. doi: 10.1016/j.biomaterials.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 24.Ratner BD, Hoffman AS, Schoen FJ, Lemons JE. Biomaterials Science. 2nd. Academic Press; New York: 1996. [Google Scholar]
- 25.Wang DA, Williams CG, Li Q, Sharma B, Elisseeff JH. Biomaterials. 2003;24:3969. doi: 10.1016/s0142-9612(03)00280-1. [DOI] [PubMed] [Google Scholar]
- 26.Benedict CV, Picciano T. Adhesives from marine mussels. In: Hemingway W, Conner AH, Branham SJ, editors. Adhesives from renewable resources. American Chemical Society; Washington, DC: 1989. p. 465. [Google Scholar]
- 27.Waite JH, Andersen NH, Jewhurst S, Sun C. J Adhesion. 2005;81:297. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


