Abstract
BCL2 is best known as a multifunctional anti-apoptotic protein. However, little is known about its role in cell adhesive and motility events. Here, we show that BCL2 may play a role in the regulation of cell adhesion, spreading, and motility. When BCL2 was overexpressed in cultured murine and human cell lines, cell spreading, adhesion, and motility were impaired. Consistent with these results, loss of Bcl2 resulted in higher motility observed in Bcl2 null mouse embryonic fibroblast cells compared to its wild type. The mechanism of BCL2 regulation of cell adhesion and motility may involve formation of a complex containing BCL2, actin and gelsolin, which appears to functioally decrease gelsolin-severing activity. We have observed that the lysate from MCF-7 cells and NIH3T3 cells that overexpressed BCL2 enhanced actin polymerization in cell-free in vitro assays. Confocal immunofluorescent localization of BCL2 and F-actin during spreading consistently showed that increased expression of BCL2 resulted in increased F-actin polymerization. Thus, formation of BCL2 and gelsolin complexes (which possibly contains other proteins) appears to play a critical role in regulation of cell adhesion and migration. Given the established correlation of cell motility with cancer metastasis, this result may explain why expression of BCL2 in some tumor cell types reduces the potential for metastasis and shows improved patients prognosis.
Keywords: BCL2, gelsolin, motility, and actin polymerization
Introduction
BCL2 was initially classified as a proto-oncogene because of its anti-apoptotic function 1 and the observation that BCL2 expression resulted in weak tumorigenesis in a transgenic mouse model 2. BCL2 expression in endothelial cells was reported to enhance tumor metastasis 3. Recently, it has been shown that BCL2 expression may, in some cases, be associated with improved prognosis of patients diagnosed with non small cell lung cancer 4, renal cell carcinoma 5, colorectal cancer 6, and melanoma 7. BCL2 expression was not only correlated with an improved prognosis but also with a reduced capacity for distant colonization of breast cancer cells 8. Furthermore, the expression level of BCL2 within differentiated cells changes markedly during tissue development and tumorigenesis 9. However, the physiological role of the BCL2 expression in cell motility has not been well characterized.
Cell adhesion and motility are fundamental functions of both normal cells and metastatic tumor cells involving both transmembrane adhesion receptors and intracellular signaling molecules 10–14. These signaling molecules modulate a wide range of intracellular events such as regulation of actin polymerization, which is necessary for cells to change shape, form lamellipodia, and migrate15–19. The cycle of actin polymerization and depolymerization is regulated by a number of actin-binding proteins, one of which is gelsolin. Normal functioning of these actin binding proteins is required for control of the rate of actin-based cell migration.
The actin capping and severing protein, gelsolin, is thought to be critical to the regulation of focal adhesion, podosome, invadopodium, and other critical functions that govern normal processes, such as wound healing and also disease processes, such as tumor progression 20–22. The critical functions of gelsolin include maintaining G-actin concentrations by its F-actin severing activity and capping the fast growing ends of polymerized actin to regulate the growth and average length of F-actin filaments 20, 23. Membrane ruffling was decreased in fibroblasts without gelsolin expression 24, which demonstrated the importance of gelsolin function in motility. On the other hand, expression of gelsolin enhances cytoskeleton reorganization and cell motility 25, 26.
To understand better the role of BCL2 in the mechanism of cell adhesion and migration, we generated doxycycline controlled expression of murine BCL2 in cultured NIH3T3 cells and a human BCL2 expressed in cultured MCF-7 human mammary adenocarcinoma cells. We found that BCL2 expression inhibited cell adhesion, spreading, and motility. We also find formation of a novel complex that contains BCL2, actin and gelsolin that may play a role in regulation of actin polymerization and de-polymerization. To our knowledge, this is the first report that has shown that BCL2 can function as a regulatory molecule for the actin polymerization in a manner that regulates cell adhesion, spreading and motility.
Results
Expression of BCL2 inhibited cell spreading
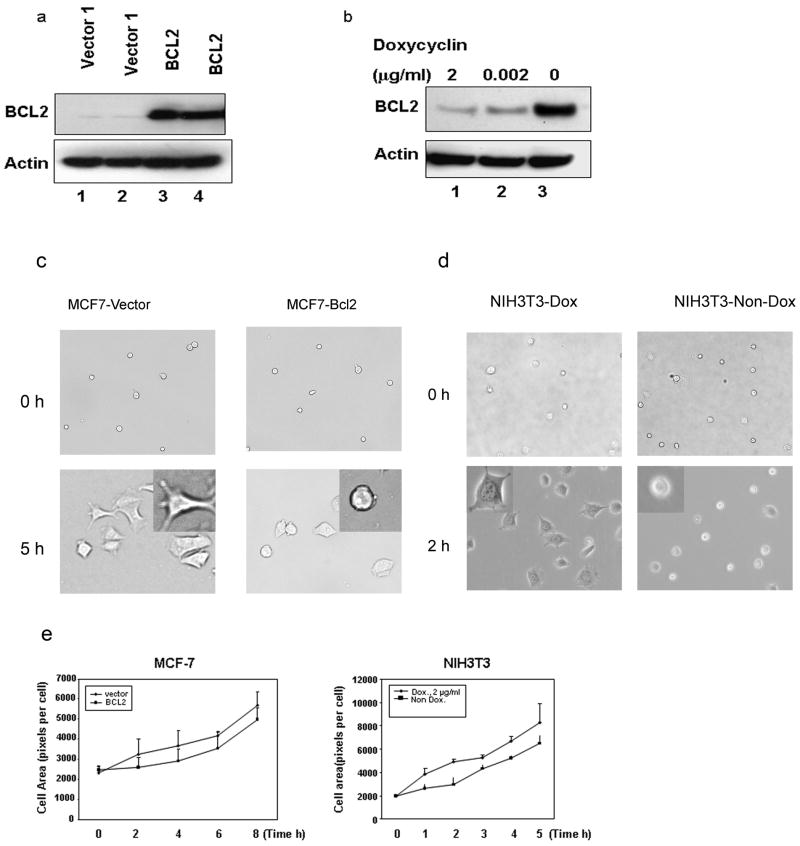
To characterize the consequences of BCL2 expression on cell adhesion, spreading, and motility, human BCL2 expression and empty vector (control) cell lines were established by transfection of pcDNA3-BCL2 or pcDNA3 into cultured MCF-7 cells. The derivative clones that were stably transfected with the BCL2 gene were identified by Western blot analysis (Fig. 1a). Another model used a doxycycline-responsive NIH3T3 cell line transfected with the mouse Bcl2 gene. Different levels of BCL2 expression could be obtained by varying the dosage of doxycyline (Fig. 1b).
Fig. 1. BCL2 expression in human MCF-7 cells and mouse NIH3T3 cells affect cell spreading.
(a). Immunoblot analysis of two independent MCF-7-BCL2 clones (lane 3 and 4) showed higher expression of BCL2 than two MCF-7-Vector clones (lane 1 and 2). (b). Doxycycline inducible NIH3T3-Bcl2 cells expressed varyin g levels of BCL2 depending on concentration of doxycycline. Cells were cultured for three days in the presence of 2 μg/ml (lane 1), 0.002 μg/ml (lane 2) doxycycline or without doxycycline (lane 3). BCL2 expression was analyzed by immunoblot (c, d). Phase microscopy images indicated that BCL2 expression in MCF-7 cells (panel C) and NIH3T3 cells (panel D) inhibited cell spreading. The cells were cultured with or without doxycycline for three days. Cells were harvested and re-plated on collagen-coated plates and the spreading was observed for five to eight hours. In both MCF-7 and NIH3T3 cells, BCL2 expression appeared to result in the presence of more unspread cells. (e). Quantitative analysis of the cell area during spreading confirmed that BCL2 expression significantly (MCF-7 cells, at 0 time point, p = 0.53; at 2, 4, 6, 8 hour time point p < 0.01. NIH3T3 cells, at 0 time point, p = 0.2; at 1, 2, 3, 4, 5 hour time point, p < 0.01) inhibited spreading of both MCF-7 cells (left panel) and NIH3T3 cells (right panel). The results were expressed as averaged pixels per cell.
We monitored cells for both shape and spreading after incubating on collagen type IV substrates. The cells with BCL2 expression tend to spread isotropically, while the vector transfected cells spread anisotropically, as shown in Fig. 1c, d, and e, the cell area increased in a time-dependent manner. However, both the rate and the extent of cell spreading was significantly lower (P< 0.01 is indicated at all time points except the 0 point) in BCL2 overexpressing NIH3T3 cells and human MCF-7 cells. This result suggests that overexpressed BCL2 may inhibit cell spreading.
Expression of BCL2 inhibited cell migration
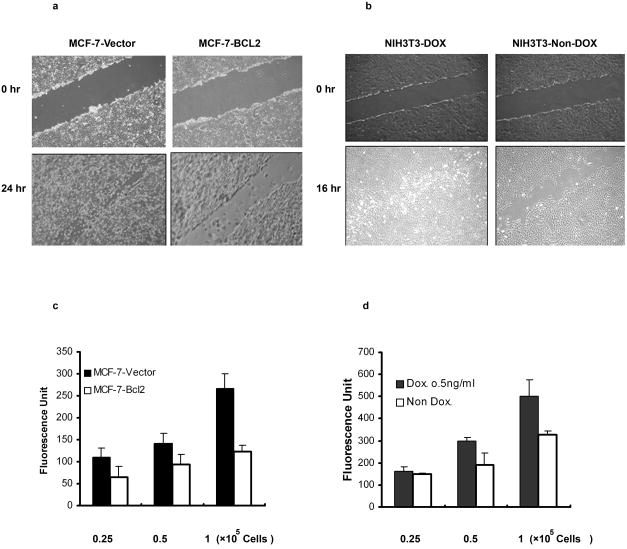
Because of the striking effects of BCL2 expression on cell attachment and spreading, we examined the effects of BCL2 expression on cell migration. Migration of both MCF-7 and NIH3T3 cells were first analyzed using a scratch wound closure assay 27. MCF-7 cells overexpressing human BCL2 demonstrated little migration into the wounded area. In contrast, MCF-7 cells transfected with the empty vector migrated readily across the wound (Fig. 2a). Similarly, NIH3T3 cells cultured without doxycycline, which overexpressed mouse BCL2, appeared to migrate more slowly into the wounded area than did the NIH3T3 cells cultured in the presence of doxycycline, which expressed lower levels of Bcl2 and appeared to migrate faster, completely filling the wound area within 16 hours (Fig. 2b). Similarly, expression of mouse BCL2 in B6 melanoma cells caused decreased cell migration (supplement Fig. 1). Transwell cell migration was also quantified using DMEM containing 10% FBS as the chemoattractant28. Expression of BCL2 in MCF-7 cells inhibited cell motility by over 50% (p< 0.01) (Fig. 2c) and by about 30% in NIH3T3 cells (p <0.05) (Fig. 2d). These results suggest that, in both murine and human cells, lower levels of expression of BCL2 may be optimal for the cell migration, while higher levels of expression of BCL2 inhibited cell motility.
Fig. 2. BCL2 expression inhibited cell motility.
(a). After 24 hours of incubation, there were more cells that moved into and filled the gap by MCF-7-Vector cells (left) compared to the MCF-7-BCL2 cells (right). (Panel b). After 16 hours of incubation, there were more cells that moved into and filled the gap by NIH3T3 cells that do not overexpress BCL2 (Dox., left) compared to NIH3T3 that overexpressed BCL2 (non-dox., right). (c). Quantitative analysis of cell motility by transwell cell migration assay is described in the materials and methods. Approximately 50% fewer cells migrated into the chamber containing10% FBS in MCF-7 cells that overexpressed BCL2 (white) compared to to MCF-7 that expressed normal levels of BCL2 (black), (All groups, p < 0.01). (d). NIH3T3 cells that overexpressed BCL2 migrated significantly less (white bars) compared to those with native BCL2 expression (black bars), (0.25 × 105 cells, p = 0.7; 0.5 × 105 cells, p < 0.05; 1 × 105 cells, p < 0.05). The results were the average value of three experiments.
BCL2 expression enhanced F-actin polymerization during cell spreading
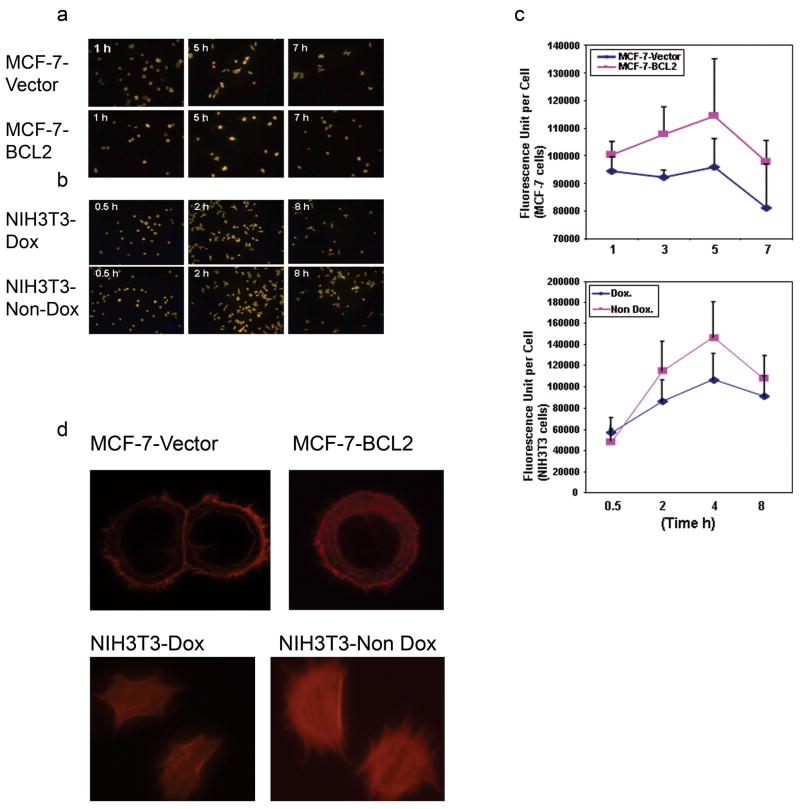
Previously, it has been shown that F-actin polymerization increases for the first several hours during cell spreading and then decreases over 24 hours later 29. We examined the effect of BCL2 expression on the F-actin polymerization in vivo during cell spreading. The cells were fixed and stained with rhodamine phalloidin and the F-actin amount was quantified by measuring the total fluorescence intensity of 500 cells at different time points. As judged by phaolliodin staining, the amount of F-actin in MCF-7-vector cells increased during the first several hours after seeding and peaked after 5 hours (Fig. 3a). BCL2 overexpressing MCF-7 and NIH3T3 cells both had higher fluorescence intensities, suggesting increased F-actin content. (Fig. 3b). Quantitative analysis of fluorescence indicated that the average fluorescence value per cell is significantly higher in MCF-7 cells (p <0.01 in each of the time points) (Figure 3c, upper panel) and in NIH3T3 cells (p <0.01 in each of the time points except the initial time point) (Figure 3c, lower panel) that are overexpressing BCL2. Interestingly, the representative F-actin staining of cells showed that the F-actin exists obviously more in the cytoplasm of the MCF-7-BCL2 cells or NIH3T3-Non Dox cells compared with MCF-7-Vector cells or NIH3T3-Dox cells (Fig. 3d). These results suggested that increasing BCL2 expression results in a concommitant increase of F-actin within cells.
Fig. 3. BCL2 expression enhanced F-actin polymerization during cell spreading in vivo.
Cultured cells were fixed and and then stained with 165 nM Rhodamine phalloidin in PBS for 20 min. Five images were taken and the intensity of fluorescence was analyzed using a Zeiss LSM Imager. At least 500 randomly selected cells per data point were measureded to determine an average fluorescence value. The experiments were repeated twice with similar results. (a). In vivo F-actin staining of MCF-7 cells showed that F-actin increased during cell spreading, peaked at 5 hour and was reduced thereafter. (b). In vivo F-actin staining of NIH3T3 cells shows that F-actin increased during cell spreading, peaked at 4 hour and was reduced thereafter. (c). Quantitative fluorescence analysis showed that the average value per cell was higher in MCF-7 cells that overexpressed BCL2 (upper panel, at 1, 3, 5, 7 hour time points, P<0.01) and NIH3T3 cells (lower panel, at 0.5 hour time point, P = 0.48; at 2, 4, 8 hour time points, P<0.01). These results indicated that BCL2 expression significantly increased F-actin polymerization. (d). Representative F-actin staining of individual cell indicated that MCF-7-BCL2 cells have more extensive F-actin staining in the cytoplasma than do MCF-7-Vector cells (up panels). Representative F-actin staining of individual cells indicated that NIH3T3-Non Dox (right lower panel) have more extensive F-actin staining in the cytoplasm than do NIH3T3-Dox cells (left lower panel)
Loss of Bcl2 results in increased cell migration and decreased actin polymerization rate
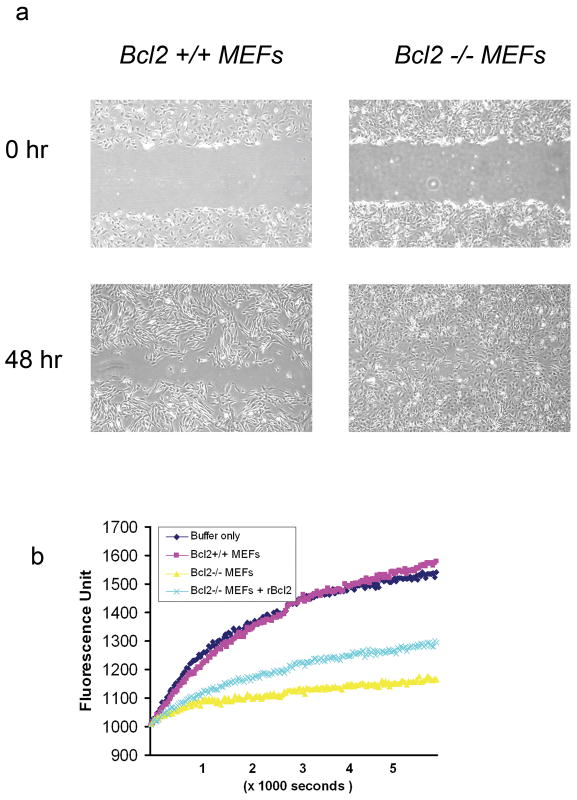
To study further the effect of the level of expression of BCL2 on cell motility, we compared the migration of Bcl2 null MEFs with wild type MEFs. As shown in Fig 4a, Bcl2−/− MEFs had an increased migratory phenotype—these cells completely filled the gap after a 48 hr incubation. In contrast, the Bcl2 +/+ cells did not close the gap after 48 hours. Furthermore, cell lysates from Bcl2 null MEFs significantly inhibit actin polymerization in the in vitro actin polymerization assay. In contrast, cell lysates from Bcl2 +/+ MEFs had little effect on actin polymerization compared by buffer only controls. Addition of 0.5 μg recombinant human BCL2 to the lysate of Bcl2 −/− MEFs partially reversed the inhibition of actin polymerization caused by the Bcl2−/− cell lysates in the in vitro assay (Fig. 4b). Interestingly, addition of anti-mouse BCL2 antibody partially decreased the actin polymerization (supplement Fig. 2), suggesting that the presence of BCL2 itself may be important for its effect on F-actin polymerization. These results are consistent with a mechanism by which expression of BCL2 may enhance actin polymerization and negatively regulate cell motility.
Fig. 4. Loss of Bcl2 results in increased cell migration and lower actin polymerization rate.
(a). Bcl-2−/− MEFs (right) displayed higher motility, completely filling the “wound” after 48 hr. In contrast, Bcl-2 +/+ cells (left) did not completely close the “wound” after 48 hr of incubation. (b). Using an in vitro actin polymerization assay, cell lysates from Bcl-2 null MEFs (yellow) markedly inhibited actin polymerization, while the same amount of cell lysate from Bcl-2 +/+ MEFs had little effect on actin polymerization (red) compared to the control of buffer only (dark blue). Addition of 0.5 μg recombinant human BCL2 to the lysate of Bcl-2 −/− MEFs partially restored the level of actin polymerization in the in vitro assay (light blue).
BCL2 can bind actin and complex with gelsolin
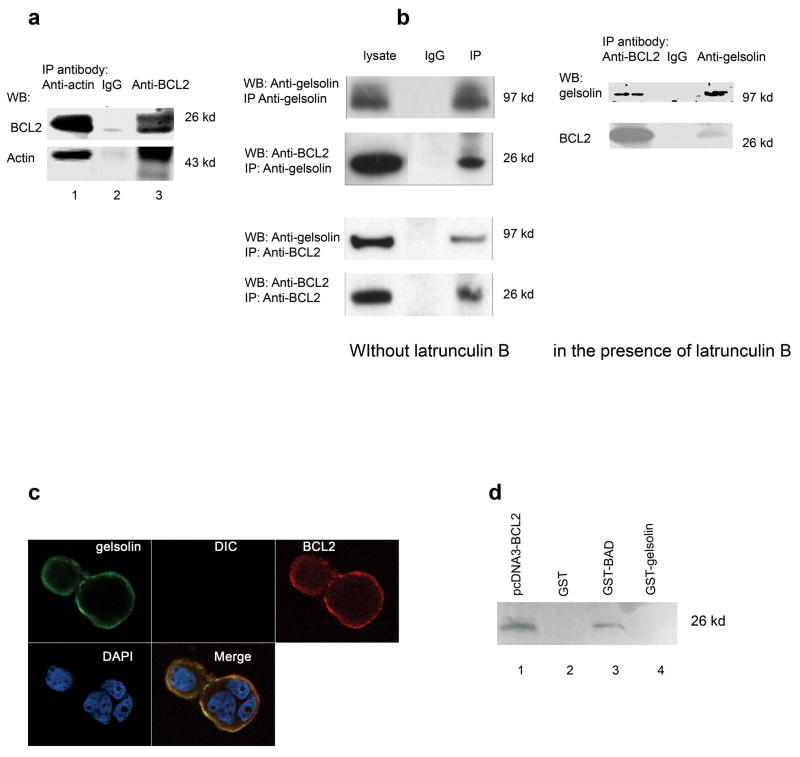
To begin to characterize the mechanism of BCL2 modulation of F-actin polymerization, we used a proteomic approach to identify proteins that could bind BCL2 from NIH3T3 cells that overexpressed BCL2. In addition to actin, a 97 kDa protein was found to complex with BCL2, which was identified as murine gelsolin by mass spectrometry (Table 1). To further investigate whether BCL2 bind actin directly and how BCL2 and gelsolin complex together, we first used recombinant BCL2 and actin protein to perform an immunoprecipitation assay. Human recombinant BCL2 was found to bind with actin directly by immunoprecipitation analysis (Fig. 5a). Then, immunoprecipitation and immunoblotting analysis indicated that both in the presence and absence of latrunculin B, gelsolin was co-precipitated with BCL2 antibody in MCF-7-BCL2 cells. BCL2 can also be precipitated with gelsolin antibody by co- immunoprecipitation and observed in immunoblots(Fig. 5b). Finally, confocal images indicated that BCL2 and gelsolin co-localize in the membrane and cytoplasm of MCF7-BCL2 cells (Fig. 5c). These results suggest that BCL2 and gelsolin can form a complex possibly through interaction with actin in both mouse and human cells.
Table 1.
Proteins precipitated by anti-BCL2 antibody
| Protein | Accession | #MW |
|---|---|---|
| Ribosome protein S19 | gi12963511 | 16076 |
| Ribosome protein S10 | gi127170 | 19883 |
| Ribosome protein 3A | gi3914914 | 29481 |
| tropomyosin | gi1351289 | 32675 |
| actin | gi1703121 | 41809 |
| translation elongation factor 1 alpha 1 | gi15277711 | 42997 |
| vimentin | gi2078001 | 51533 |
| tubulin alpha 1 | gi6755901 | 50104 |
| protein disulfide-isomerase precursor | gi68462 | 57023 |
| glucose-regulated protein precursor (GRP 78) | gi121570 | 72334 |
| nucleolin | gi7106377 | 76677 |
| gelsolin | gi6754078 | 80827 |
| procollagen, type I, alpha 2 | gi6680980 | 129524 |
| collagen alpha 1 | gi2506350 | 137859 |
| myosin | gi6981236 | 226197 |
Fig. 5. BCL2 binds actin and complexes with gelsolin.
(a). Recombinant human BCL2 was mixed with actin and immunoprecipitation was carried out as described in the Materials and Methods. Both proteins were precipitated by either rabbit anti-BCL2 (human) or goat anti-Actin (human) antibody as shown in lane 1 and 3. The antibodies used in the western blot were either mouse anti-human BCL2 or rabbit anti-Actin respectively. The rabbit IgG alone control in lane 2 shows that nothing is immunoprecipitated in the absence of specific antibodies. (b). Gelsolin and BCL2 were co-immunoprecipitated from lysates of MCF-7 that overexpress BCL2 (MCF-7-BCL2 cells) with either goat anti-gelsolin or mouse anti-human BCL2 (BD Biosciences). The antibodies used in western blot were either mouse anti-gelsolin or mouse anit- human BCL2 (BD Biosciences) respectively. The experiment in the left pannel was performed in the absence of latrunculin B. The complex containing both Bcl2 and gelsolin was precipitated using either anti-gelsolin (top) or anti-BCL2 (bottom) antibody. In contrast, mouse IgG can precipitate neither gelsolin nor BCL2 (middle lanes of both top and bottom). The experiment in the right panel was performed in the presence of 0.5 μg/ml latrunculin B with similar result. (c). Confocal microscopy showed the co-localization of gelsolin with BCL2 in MCF-7-BCL2 cells. The merged image indicated both gelsolin and BCL2 were located in cell membrane and cytoplasm. These experiments were repeated twice with similar results. (d). Using the GST pull-down assays, BCL2 was found to be bound to BAD (lane 3), but did not bind directly to gelsolin in vitro (lane 4). Lane 1 showed the in vitro translated human BCL2 protein. Lane 2 showed a GST negative control. The pull-down proteins were separated by electrophoresis and visualized with Typhoon 8600 Variable Mode Imager.
We also examined whether purified human BCL2 could bind with human gelsolin directly in vitro. A GST pull-down assay showed that BCL2 could bind with BAD but not with the GST-gelsolin fusion protein (Fig. 5d). This result suggests that BCL2 may not bind directly to gelsolin in vivo.
BCL2 expression inhibited the actin severing activity of gelsolin while enhancing actin polymerization in vitro
Inhibition of gelsolin severing activity has been shown to result in decreased cell migration; conversely, increasing intracellular gelsolin resulted in increased cell migration 26. We therefore tested the hypothesis that BCL2 inhibition of cell motility could be mediated by gelsolin. Total gelsolin expression was appeared to be unaffected by expression of BCL2 in both the NIH3T3 and MCF-7 cells, although gelsolin expression in NIH3T3 cells was lower than that of MCF-7 cells (Fig. 6a). When tested in an in vitro, fluorescence-based assay for F-actin severing activity 30, the fluorescence intensity of the reaction containing lysates from MCF-7 cells transfected with only empty vector decreased within minutes. In contrast, no fluorescence decay was observed in the reaction with lysates from cells that overexpress BCL2 containing the same amount of total protein (Fig. 6b). This result suggests that lysates from cells that overexpress BCL2 has decreased gelsolin severing activity compared to cell lysates from MCF7-vector cells containing normal levels of expression of BCL2.
Fig. 6. BCL2 expression down-regulated gelsolin severing activity and enhanced actin polymerization in vitro.
(a). Gelsolin expression level was not changed by BCL2 expression in either NIH3T3 cells (left panel) or MCF-7 cells (right panel). (b). Rhodamine phalloidin labeled F-actin was incubated with dialyzed protein lysate from vector transfected (red) or BCL2 overexpressing (blue) MCF-7 cells in the presence of calcium. Fluorescence recording indicated that the gelsolin severing activity was inhibited in cell lysates from MCF-7 cells that over expressed BCL2. The fluorescence decayed within minutes in control MCF-7-Vector cells (blue) indicating a normal severing activity causing the loss of F-actin. These experiments were repeated 3× and yielded similar results each time. (c). The actin polymerization assay was peformed with controls of buffer only, buffer without ATP, buffer in the presence of either 1μM Jaspakinolide, an actin polymerization agent, or 0.5 μg/ml latrunculin B, an actin depolymerization agent. Pyrene actin (2.3 μM) was incubated with 10 μg of dialyzed cell lysates in polymerization buffer. Actin polymerization was dramatically inhibited by latrunculin (light blue). The actin polymerization process was normal or increased when incubated with buffer (dark blue) or Jaspkinolide (yellow). Lysates from MCF-7-Vector cells consisting of 10 μg total protein inhibited actin polymerization (brown). Lysates (10 μg total protein) from MCF-7 cells that overexpressed BCL2 enhanced actin polymerization (purple). (d). Compared to buffer-only control conditions (dark blue), lysates containing 10 μg total protein from NIH3T3 cells (doxycycline added) inhibited actin polymerization (brown), while 10 μg lysates from NIH3T3 cells (without doxycycline) that overexpressed Bcl2 enhanced actin polymerization (purple). Latrunculin B completely inhibited F-actin polymerization (light blue) down to the level of negative control conditions from which ATP was deleted (magenta). Jaspkinolide also activated F-actin polymerization (yellow). This experiment was repeated three times with each of the above cell lines and yielded similar results.
To address further the role of BCL2 expression in the process of G-actin polymerization to F-actin, we assayed the polymerization of purified G-actin in the presence of cell lysates from control cells and those overexpressing BCL2. As shown in Fig. 6c, lysate from control MCF-7-vector cells caused a slight decrease in fluorescence, suggesting the presence of actin severing activity, while lysate from MCF-7 cells overexpressing BCL2 restored the F-actin polymerization rate to the level of that observed with the normal buffer alone. Similarly, we observed that lysate from NIH3T3 cells overexpressing BCL2 had an activating effect on F-actin polymerization (Fig. 6d). While the actin polymerization agent, jaspakinolide increased the actin polymerization rate, the actin depolymerization agent, latrunculin B significantly decrease the actin polymerization rate. These results conform the findings that higher level of BCL2 enhances F-actin polymerization.
Discussion
We have shown that increased expression of BCL2, a multifunctional protein, inhibited cell adhesion, spreading and migration. The mechanistic basis for these effects appears to involve enhanced actin polymerization and reduced gelsolin severing activity which is associated with the formation of a complex that contains both gelsolin and BCL2. These results suggest that BCL2 may function as a noval cytoplasmic factor that regulates cell motility.
The area of the cell adhering to the substrate during spreading is thought to be related with the total magnitude of force exerted by the cell 29, 31. Force is again generated when actin monomers polymerize into actin filaments in the plasma membrane. The reduction of the spreading of cells that overexpress BCL2 suggests that there may be abnormal actin polymerization close to the the plasma membrane. We show here that actin polymerization and depolymerization are directly modulated by the level of BCL2 expression and are linked to both cell spreading and cell motility. Consistent with this observation, BCL2 over-expression in MCF-7 cells inhibits the cell-cell adhesion caused by 12G10 antibody, resembling the effects of actin polymerization agent (supplement Fig. 3). Previously published studies showed that expression of BCL2 results in an increase of simple attachment of osteoblasts to Primaria™ substrates 32 and knocking out BCL2 results in decreased attachment of ureteric bud cells to vironectin and fibronectin 33. These studies use a different endpoint (simple cell attachment) than does our study (cell spreading), which could possible explain the different conclusions.
Cell migration involves a multistep process that requires formation of new adhesions at the leading edge while breaking adhesions at the trailing edge 16, 34–36, a process that is mediated by its integrin receptors on the cell surface 10, 37–42. Cell migration also requires cytoskeltal remodeling, a process that is mediated by a number of cytoplalsmic proteins one of which is gelsolin 34, 43, 44. It is thought that it is the amount of free gelsolin level relative to its severing activity that is critical, rather than simply the total amount of gelsolin 26. Our results are consistent with these findings and further suggest that gelsolin can form a complex with other proteins that may reduce its severing activity but enhance the nucleation function. The nucleating activity of gelsolin at the plasma membrane predominates in lamellipodia, membrane ruffles, and adhesion foci 45. Gelsolin associates with membrane through polyphosphoinositides and non-polyphosphoinositides lipids 46,47. The binding of these molecules can dissociate gelsolin from the capped actin and increase the amount of F-actin 48. Our finding that cytosol from MCF-7 cells that overexpress BCL2 has decreased gelsolin severing activity and higher actin polymerization rates, as judged by in vitro assays, suggests that the rate and amount of actin monomers in the region behind lamellipodium recycled to the cell front (treadmilling) to extend the lamelliopodium protrusion may reduce. Interpretation of the results of the gelsolin severing assay require careful consideration since other severing proteins might affect the actin depolymerization. We propose that gelsolin may be functionally more important in mediating this BCL2 function. Finally, our results are consistent with those from previously published studies showing that BCL2-null cells have a greater motility 33 49. We hypothesize that rapid F-actin assembly and disassembly, which is required to maintain normal cell elasticity, cell adhesion and cell motility, may be crippled in cells overexpressing BCL2 possibly because of the down-regulation of gelsolin severing activity.
We have also described a novel gelsolin-BCL2 containing complex in cells that overexpress BCL2 suggesting that signaling complexes may exist to directly inhibit actin depolymerization and thus affect cytoskeleton remodeling. We did not observe a striking change of Rac-GTP expression induced by BCL2 expression (data not shown), indicating that there may be an alternate, Rac-GTP-independent, pathway by which BCL2 inhibits gelsolin function and actin depolymerization. It is likely both BCL2 and gelsolin bind actin and therefore complex together. The scaffolding adaptor protein, paxillin was also detected in BCL2 antibody precipitated complex (data not shown), confirming the findings by others that paxillin can bind both BCL2 and gelsolin 50, 51 to form a complex that can regulate physiological processes that require cell migration 50, 52. The mechanism by which BCL2 complexes with gelsolin has not been fully investigated but will be important to determine. We propose the hypothesis that BCL2 complexes with actin, paxillin, gelsolin, and other integrin associated proteins, and negatively regulate the motility related pathways.
The finding that BCL2 expression can inhibit motility of certain cell lines suggests that there may be a reduced potential of these cells to metastasize and may help explain why there is a higher survival rate in patients with certain tumor types that overexpress BCL2 53. This conclusion is, however, inconsistent with other reports that BCL2 may enhance tumor growth and metastasis 54, 55. The critical parameter may, in fact, be the overall balance between the anti-apoptotic and the anti-migratory activities of BCL2 that determines whether BCL2 epxression is associated with increased or decreased survival in cancer patients.
The BCL2–gelsolin regulation of F-acting depolymerization may have a broader significance for cell division and apoptosis since both processes require cytoskeleton reorganization. We propose that down-regulation of the gelsolin severing activity and decreased degradation of F-actin induced by BCL2 expression may be a novel mechanism by which apoptosis can be inhibited. For example, BCL2 can attenuate cadmium induced apoptosis and necrosis 56, 57. Cadmium also causes association of gelsolin with actins and subsequent loss of F-actin during the process of apoptosis 30. Therefore, we speculate that BCL2 may inhibit apoptosis also by affecting the gelsolin severing activity.
In conclusion, we have demonstrated that expression of BCL2 in two distinct cell lines inhibited cell adhesion and migration via a mechanism involving inhibited gelsolin severing activity and enhanced actin polymerization. The finding that BCL2 may regulate cell motility and adhesion should also be very useful for the investigation of the role of BCL2 in tumor metastasis, tissue and organ development, and homing of stem cells to damaged and regenerating tissues.
Materials and Methods
Plasmids and cell culture
The pcDNA3-BCL2 plasmid was obtained by inserting a Not I cut fragment from pCMV-XL5-hBCL2 containing human BCL2 cDNA (Accession: NM__00063, OriGene Technologies) into the Not I site of pcDNA3. The pcDNA3-BCL2 or pcDNA3 empty vector was transfected separately into the MCF-7 cells (ATCC, Masassas, VA) with lipofectamine (Invitrogen). The cells were cultured in 10% DMEM containing 10% Fetal Bovine Serum (FBS, Clontech) for 24 hours after transfection, then replated into 150 mm culture dishes in culture media supplemented with G418 (750 μg/ml) and cultured for 14 days of growth. Visible clones were isolated and transferred to individual dishes. The derivative clones with high expression of BCL2 protein were obtained and amplified for assays. Fragments of NheI and XbaI (blunt) of murine Bcl2 from pUSEamp-Bcl2 (Upstate) were sub-cloned into NheI and Not I (blunt) sites of pTRE2hyg (Clontech, Mountain View, CA). The resulting plasmid was transfected into the NIH3T3 Tet-off cell line (Clontech). Doxycycline responsive cell clones were selected by culturing the transfected cells in the presence of hygromycin (300 μg/ml) and identified by western blot analysis. The cells were maintained in DMEM containing 10% FBS plus hygromycin (200 μg/ml) and with or without Doxycycline (DOX., 2 μg/ml). The Bcl-2 wild type mouse embryonic fibroblast (MEFs) and Bcl-2 null MEFs were generous gifts from Dr. Christine M. Sorenson, University of Wisconsin, Madison. The MEF cells were grown in DMEM containing 10% Fetal Calf Serum (FCS) plus 5.3 ng/ml of gamma interferon (R&D Inc.), 10−5 M β-mercaptoethanol.
Cell spreading
For the cell spreading assays, cells were plated onto collagen type-IV coated wells at 5500 cells per cm2 in DMEM. Five images were taken from random locations at 1.5–2 hour intervals using phase microscopy (Olympus, IX70, and Tokyo, Japan). The cell surface area was determined and calculated automatically by image analysis using a FluoChemTM IS-8900 (Alpha Innotech). Each data point is the average of five images ± SEM.
Scratch wound assay and transwell cell migration assay
The migration of cells was assessed using a scratch wound assay 58. Approximately 5 –10 × 105 cells were grown on 35 mm collagen coated plates for 24–48 hours to form confluent monolayer of cells. Gaps were created in confluent cell layers using micropipette tips, rinsed with DMEM media. Cells were then cultured with DMEM media with 1% FBS (or 2% FCS for MEFS) to minimize the effect of cell proliferation. The wound closure was monitored by phase contrast microscopy and photographed over 16 hours for murine or 24 hours for human cells.
Transwell migration assays with serum as the chemoattractant were performed using the QCM™ 24-Well Fluorimetric Cell Migration Assay kit (Chemicon). Briefly, 500 μl DMEM containing 10% FBS were added to the lower chambers. Cells suspended in 300 μl DMEM without FBS were added to the upper compartment of the Transwell unit with 8-μm pore size polycarbonate inserts. Cells were allowed to migrate for 16 hours (NIH3T3 cells) or 24 hours (MCF7 cells) at 37°C in a humidified atmosphere containing 5% CO2. The medium with cells from the top side of the inserts was carefully removed by aspiration. The cells from the underside of the inserts were completely dislodged, lysed, and stained by Cyquant GR Dye (Molecular Probes, Inc). 200 μl of cell lysate were then transferred to 96-well plate. The plates were read using Spectra MAX Gemini XS (Molecular Devices, Sunnyvale, CA) with a 485/538 nm filter set.
Actin severing and polymerization assays
The gelsolin severing activity was measured as described previously 59. Briefly, 1 mg/ml purified rabbit muscle actin (Cytoskeleton, Denver, CO) was diluted to 30 μg/ml and resuspended in polymerization buffer (50 mM KCl, 2 mM MgCl2, 0.5 mM ATP, 2 mM Tris, pH 8.0), incubated one hour and sedimented by centrifugation at 100,000 g for 1 hour to remove the unpolymerized actin. Rhodamine phalloidin (1 μM; Molecular Probes, Eugene, OR) was added to actin filaments at a final concentration of 0.4 μM. The rate of florescence loss at 590 nm (530 nm excitation wavelength) was measured by fluorometry (FL600, Microplate Fluorescence Reader, BioTEK). Cell lysates from vector only control cells or MCF-7 cells overexpressing BCL2 was prepared with proteinase inhibitors in lysis buffer containing 50 mM KCl, 2 mM MgCl2, 0.5 mM ATP, 2 mM Tris, pH 8.0, 1 mM EGTA, and 1 % Triton X-100. The lysates were dialyzed (Slide-A-Lyzer 10K, Pierce) at 4 °C with three changes of buffer containing 50 mM KCl, 2 mM MgCl2, 2 mM Tris, 1 mM EGTA, 0.5 mM β–mercaptoethanol. The volume of cell lysate was adjusted to 400 μl using dialysis buffer. The final actin filament concentration in polymerization buffer was 0.4 μM. The severing assay was started by adding 2 mM CaCl2, and 1 mM EGTA.
Actin polymerization in the presence of cell lysates was measured in a standard pyrene-actin fluorescence assay 30. The cell lysates were dialyzed as described above. Lysate (10 μl) with equal amount of total protein was added to the final reaction volume (200 μl). The pyrene-actin was diluted to 2.3 μM in general actin buffer (5 mM Tris-HCl PH 8.0, 0.2 mM CaCl2) containing 0.2 mM ATP and 0.5 mM DTT and stored on ice for 60 minutes to depolymerize actin oligomers. The tubes were centrifuged at 14,000 g force for 30 minutes at 4 °C. The supernants containing pyrene-actin were mixed with the lysate, added into 96-well plate and held for three min. In the control wells, we added either 1μM jaspakinolide, an actin polymerization agent, or 0.5 μg/ml latrunculin B, an actin depolymerization agent. After the addition of 1/10 volume of 10 × of polymerization buffer (10 × contains 500mM KCl, 10 mM MgCl2), the kinetics of pyrene fluorescence upon initiation of actin polymerization was monitored for 2 hours at 405 nm with an excitation wavelength of 355 nm with the same Spectra Max Gemini XS.
Confocal immunofluorescence microscopy of protein localization
Approximately 104 cells were cultured on 35 mm glass bottom micro dishes (Matek Corp., Ashlan, MA, USA) for 2 days. Cells were fixed with 10% formalin in PBS for 10 minutes followed by permeabilization for 5 minutes with ice cold methanol and 5 minutes of ice-cold acetone. Cells were washed three times with PBS and then blocked in 1% BSA. The fixed and permeabilized cells were incubated with 20 μg/ml goat anti-human BCL2 (R&D) and 2.5 μg/ml mouse anti-gelsolin (BD Biosciences) at 22–25 °C for 1 hour. The cells were then washed three times with PBS followed by a 40 minute incubation with a 1:100 dilution of FITC labeled anti-mouse IgG and Alexa 647 conjugated chicken anti-goat IgG (1:200 dilution). After washing four times with PBS, the cells were mounted on slides with Mountshield containing DAPI and imaged using a Zeiss LSM 510 NLO confocal/multiphoton laser-scanning attachment (Carl Zeiss, Inc., Thornwood, NY) on the Zeiss Axiovert 100M microscope. Three image acquisitions occurred sequentially with a C-Apo 40x/1.2 W objective lens and optimized dichroics. For DAPI localization, two-photon excitation was used at 750 nm, using a BP 435–485 emission filter. For FITC, the excitation wavelength was 488 nm and the BP 500–550 filter was used for emission. For Alexa Fluor 647, a 633nm laser was used for excitation and an LP 650 filter for emission. For the two confocal channels, the pinhole was set to ~1 Airy Unit so that z-resolution would match that of the two-photon excitation. The DIC image was acquired simultaneously with the FITC image. XY alignment of the DAPI image was performed post-acquisition using the DIC image as reference, and all channels were contrast-stretched, using Zeiss LSM Image Examiner software.
The cells for F-actin staining were treated with 0.1% Triton X-100 in PBS for 5 minutes after fixation described above. Rhodamine phalloidin (5 μl) from 6.6 M stock solution was diluted into 200 μl PBS and placed onto the coverslip for 20 minutes at 22–25 °C. Cells were then washed four times with PBS. Images and the intensity of fluorescence were obtained with an Olympus IX 70 fluorescence microscope (Olympus, Japan) and a DXC-S500 (Sony, Japan) color digital camera. Images were analyzed with Zeiss LSM Image examiner. Quantitative analysis of the images was performed with Zeiss LSM Image Examiner software. A background value was determined from the pixel intensity histogram of each image, taken as the statistical mode above zero. This value was subtracted from the mean pixel value for all pixels above a threshold determined by visual exclusion of background pixels. This threshold was used subsequently for all images. The background-subtracted mean pixel value was then multiplied by the pixel area to get total fluorescence intensity, and this number was divided by the number of cells to get the average fluorescence per cell, all in arbitrary fluorescence units.
Supplementary Material
Acknowledgments
The authors thank Dr. Jau-shyong Hong, Dr. Bob Petrovich, Pamela D. Arora, and Dr. Yanhong Liao for materials, Dr. Jeff Chou for statistics and Dr. James Williams for Mass Spectra analysis. We thank Dr. Azad Boni for providing plasmid expressing GST-bad. The Division of Intramural Research of the NIEHS, NIH supported this research through projects Z01-ES02302511 and Z01-ES2120714.
Nomenclature
- BCL2
human gene
- Bcl2
mouse gene
- BCL2
human and mouse protein
References
- 1.Reed JC. Double identity for proteins of the Bcl-2 family. Nature. 1997;387(6635):773–776. doi: 10.1038/42867. [DOI] [PubMed] [Google Scholar]
- 2.Strasser A, Harris AW, Cory S. E mu-bcl-2 transgene facilitates spontaneous transformation of early pre-B and immunoglobulin-secreting cells but not T cells. Oncogene. 1993;8(1):1–9. [PubMed] [Google Scholar]
- 3.Kumar P, Ning Y, Polverini PJ. Endothelial cells expressing Bcl-2 promotes tumor metastasis by enhancing tumor angiogenesis, blood vessel leakiness and tumor invasion. Lab Invest. 2008;88(7):740–749. doi: 10.1038/labinvest.2008.46. [DOI] [PubMed] [Google Scholar]
- 4.Yilmaz A, Savas I, Dizbay Sak S, et al. Distribution of Bcl-2 gene expression and its prognostic value in non-small cell lung cancer. Tuberk Toraks. 2005;53(4):323–329. [PubMed] [Google Scholar]
- 5.Itoi T, Yamana K, Bilim V, Takahashi K, Tomita F. Impact of frequent Bcl-2 expression on better prognosis in renal cell carcinoma patients. Br J Cancer. 2004;90(1):200–205. doi: 10.1038/sj.bjc.6601454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leahy DT, Mulcahy HE, O’Donoghue DP, Parfrey NA. bcl-2 protein expression is associated with better prognosis in colorectal cancer. Histopathology. 1999;35(4):360–367. doi: 10.1046/j.1365-2559.1999.00743.x. [DOI] [PubMed] [Google Scholar]
- 7.Divito KA, Berger AJ, Camp RL, et al. Automated quantitative analysis of tissue microarrays reveals an association between high Bcl-2 expression and improved outcome in melanoma. Cancer Res. 2004;64(23):8773–8777. doi: 10.1158/0008-5472.CAN-04-1387. [DOI] [PubMed] [Google Scholar]
- 8.Neri A, Marrelli D, Roviello F, et al. Bcl-2 expression correlates with lymphovascular invasion and long-term prognosis in breast cancer. Breast Cancer Res Treat. 2006;99(1):77–83. doi: 10.1007/s10549-006-9183-2. [DOI] [PubMed] [Google Scholar]
- 9.Rathmell JC, Thompson CB. Pathways of apoptosis in lymphocyte development, homeostasis, and disease. Cell. 2002;109(Suppl):S97–107. doi: 10.1016/s0092-8674(02)00704-3. [DOI] [PubMed] [Google Scholar]
- 10.Akiyama SK, Olden K, Yamada KM. Fibronectin and integrins in invasion and metastasis. Cancer Metastasis Rev. 1995;14(3):173–189. doi: 10.1007/BF00690290. [DOI] [PubMed] [Google Scholar]
- 11.Bokel C, Brown NH. Integrins in development: moving on, responding to, and sticking to the extracellular matrix. Dev Cell. 2002;3(3):311–321. doi: 10.1016/s1534-5807(02)00265-4. [DOI] [PubMed] [Google Scholar]
- 12.Kedrin D, van Rheenen J, Hernandez L, Condeelis J, Segall JE. Cell motility and cytoskeletal regulation in invasion and metastasis. J Mammary Gland Biol Neoplasia. 2007;12(2–3):143–152. doi: 10.1007/s10911-007-9046-4. [DOI] [PubMed] [Google Scholar]
- 13.Ridley AJ, Schwartz MA, Burridge K, et al. Cell migration: integrating signals from front to back. Science. 2003;302(5651):1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 14.Webb DJ, Brown CM, Horwitz AF. Illuminating adhesion complexes in migrating cells: moving toward a bright future. Curr Opin Cell Biol. 2003;15(5):614–620. doi: 10.1016/s0955-0674(03)00105-4. [DOI] [PubMed] [Google Scholar]
- 15.Disanza A, Steffen A, Hertzog M, et al. Actin polymerization machinery: the finish line of signaling networks, the starting point of cellular movement. Cell Mol Life Sci. 2005;62(9):955–970. doi: 10.1007/s00018-004-4472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kassis J, Lauffenburger DA, Turner T, Wells A. Tumor invasion as dysregulated cell motility. Semin Cancer Biol. 2001;11(2):105–117. doi: 10.1006/scbi.2000.0362. [DOI] [PubMed] [Google Scholar]
- 17.Pantaloni D, Le Clainche C, Carlier MF. Mechanism of actin-based motility. Science. 2001;292(5521):1502–1506. doi: 10.1126/science.1059975. [DOI] [PubMed] [Google Scholar]
- 18.Pollard TD. The cytoskeleton, cellular motility and the reductionist agenda. Nature. 2003;422(6933):741–745. doi: 10.1038/nature01598. [DOI] [PubMed] [Google Scholar]
- 19.Small JV, Stradal T, Vignal E, Rottner K. The lamellipodium: where motility begins. Trends Cell Biol. 2002;12(3):112–120. doi: 10.1016/s0962-8924(01)02237-1. [DOI] [PubMed] [Google Scholar]
- 20.Silacci P, Mazzolai L, Gauci C, et al. Gelsolin superfamily proteins: key regulators of cellular functions. Cell Mol Life Sci. 2004;61(19–20):2614–2623. doi: 10.1007/s00018-004-4225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linder S, Aepfelbacher M. Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol. 2003;13(7):376–385. doi: 10.1016/s0962-8924(03)00128-4. [DOI] [PubMed] [Google Scholar]
- 22.De Corte V, Bruyneel E, Boucherie C, et al. Gelsolin-induced epithelial cell invasion is dependent on Ras-Rac signaling. Embo J. 2002;21(24):6781–6790. doi: 10.1093/emboj/cdf680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larson L, Arnaudeau S, Gibson B, et al. Gelsolin mediates calcium-dependent disassembly of Listeria actin tails. Proc Natl Acad Sci U S A. 2005;102(6):1921–1926. doi: 10.1073/pnas.0409062102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azuma T, Witke W, Stossel TP, Hartwig JH, Kwiatkowski DJ. Gelsolin is a downstream effector of rac for fibroblast motility. Embo J. 1998;17(5):1362–1370. doi: 10.1093/emboj/17.5.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cunningham CC, Stossel TP, Kwiatkowski DJ. Enhanced motility in NIH 3T3 fibroblasts that overexpress gelsolin. Science. 1991;251(4998):1233–1236. doi: 10.1126/science.1848726. [DOI] [PubMed] [Google Scholar]
- 26.Arora PD, McCulloch CA. Dependence of fibroblast migration on actin severing activity of gelsolin. J Biol Chem. 1996;271(34):20516–20523. doi: 10.1074/jbc.271.34.20516. [DOI] [PubMed] [Google Scholar]
- 27.Akiyama SK. Functional analysis of cell adhesion: quantitation of cell-matrix attachment. Methods Cell Biol. 2002;69:281–296. doi: 10.1016/s0091-679x(02)69018-1. [DOI] [PubMed] [Google Scholar]
- 28.Gildea JJ, Harding MA, Gulding KM, Theodorescu D. Transmembrane motility assay of transiently transfected cells by fluorescent cell counting and luciferase measurement. Biotechniques. 2000;29(1):81–86. doi: 10.2144/00291st02. [DOI] [PubMed] [Google Scholar]
- 29.Reinhart-King CA, Dembo M, Hammer DA. The dynamics and mechanics of endothelial cell spreading. Biophys J. 2005;89(1):676–689. doi: 10.1529/biophysj.104.054320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Apostolova MD, Christova T, Templeton DM. Involvement of gelsolin in cadmium-induced disruption of the mesangial cell cytoskeleton. Toxicol Sci. 2006;89(2):465–474. doi: 10.1093/toxsci/kfj035. [DOI] [PubMed] [Google Scholar]
- 31.Li S, Guan JL, Chien S. Biochemistry and biomechanics of cell motility. Annu Rev Biomed Eng. 2005;7:105–150. doi: 10.1146/annurev.bioeng.7.060804.100340. [DOI] [PubMed] [Google Scholar]
- 32.Zhang W, Pantschenko AG, McCarthy MB, Gronowicz G. Bone-targeted overexpression of Bcl-2 increases osteoblast adhesion and differentiation and inhibits mineralization in vitro. Calcif Tissue Int. 2007;80(2):111–122. doi: 10.1007/s00223-006-0168-2. [DOI] [PubMed] [Google Scholar]
- 33.Sheibani N, Scheef EA, Dimaio TA, et al. Bcl-2 expression modulates cell adhesion and migration promoting branching of ureteric bud cells. J Cell Physiol. 2007;210(3):616–625. doi: 10.1002/jcp.20858. [DOI] [PubMed] [Google Scholar]
- 34.Lambrechts A, Van Troys M, Ampe C. The actin cytoskeleton in normal and pathological cell motility. Int J Biochem Cell Biol. 2004;36(10):1890–1909. doi: 10.1016/j.biocel.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 35.Moissoglu K, Schwartz MA. Integrin signalling in directed cell migration. Biol Cell. 2006;98(9):547–555. doi: 10.1042/BC20060025. [DOI] [PubMed] [Google Scholar]
- 36.Suetsugu S, Takenawa T. Regulation of cortical actin networks in cell migration. Int Rev Cytol. 2003;2229:245–286. doi: 10.1016/s0074-7696(03)29006-9. [DOI] [PubMed] [Google Scholar]
- 37.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84(3):359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 38.Howe A, Aplin AE, Alahari SK, Juliano RL. Integrin signaling and cell growth control. Curr Opin Cell Biol. 1998;10(2):220–231. doi: 10.1016/s0955-0674(98)80144-0. [DOI] [PubMed] [Google Scholar]
- 39.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 40.Huttenlocher A, Sandborg RR, Horwitz AF. Adhesion in cell migration. Curr Opin Cell Biol. 1995;7(5):697–706. doi: 10.1016/0955-0674(95)80112-x. [DOI] [PubMed] [Google Scholar]
- 41.Ruoslahti E. Fibronectin and its integrin receptors in cancer. Adv Cancer Res. 1999;76:1–20. doi: 10.1016/s0065-230x(08)60772-1. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 43.Feldner JC, Brandt BH. Cancer cell motility--on the road from c-erbB-2 receptor steered signaling to actin reorganization. Exp Cell Res. 2002;272(2):93–108. doi: 10.1006/excr.2001.5385. [DOI] [PubMed] [Google Scholar]
- 44.Zigmond SH. Beginning and ending an actin filament: control at the barbed end. Curr Top Dev Biol. 2004;63:145–188. doi: 10.1016/S0070-2153(04)63005-5. [DOI] [PubMed] [Google Scholar]
- 45.Chou J, Stolz DB, Burke NA, Watkins SC, Wells A. Distribution of gelsolin and phosphoinositol 4,5-bisphosphate in lamellipodia during EGF-induced motility. Int J Biochem Cell Biol. 2002;34(7):776–790. doi: 10.1016/s1357-2725(01)00177-7. [DOI] [PubMed] [Google Scholar]
- 46.Janmey PA, Stossel TP. Gelsolin-polyphosphoinositide interaction. Full expression of gelsolin-inhibiting function by polyphosphoinositides in vesicular form and inactivation by dilution, aggregation, or masking of the inositol head group. J Biol Chem. 1989;264(9):4825–4831. [PubMed] [Google Scholar]
- 47.Mere J, Chahinian A, Maciver SK, et al. Gelsolin binds to polyphosphoinositide-free lipid vesicles and simultaneously to actin microfilaments. Biochem J. 2005;386(Pt 1):47–56. doi: 10.1042/BJ20041054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chellaiah M, Hruska K. Osteopontin stimulates gelsolin-associated phosphoinositide levels and phosphatidylinositol triphosphate-hydroxyl kinase. Mol Biol Cell. 1996;7(5):743–753. doi: 10.1091/mbc.7.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ziehr J, Sheibani N, Sorenson CM. Alterations in cell-adhesive and migratory properties of proximal tubule and collecting duct cells from bcl-2 −/− mice. Am J Physiol Renal Physiol. 2004;287(6):F1154–1163. doi: 10.1152/ajprenal.00129.2004. [DOI] [PubMed] [Google Scholar]
- 50.Sorenson CM. Interaction of bcl-2 with Paxillin through its BH4 domain is important during ureteric bud branching. J Biol Chem. 2004;279(12):11368–11374. doi: 10.1074/jbc.M310079200. [DOI] [PubMed] [Google Scholar]
- 51.Chellaiah MA, Biswas RS, Yuen D, Alvarez UM, Hruska KA. Phosphatidylinositol 3,4,5-trisphosphate directs association of Src homology 2-containing signaling proteins with gelsolin. J Biol Chem. 2001;276(50):47434–47444. doi: 10.1074/jbc.M107494200. [DOI] [PubMed] [Google Scholar]
- 52.Sorenson CM, Sheibani N. Focal adhesion kinase, paxillin, and bcl-2: analysis of expression, phosphorylation, and association during morphogenesis. Dev Dyn. 1999;215(4):371–382. doi: 10.1002/(SICI)1097-0177(199908)215:4<371::AID-AJA8>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 53.Callagy GM, Webber MJ, Pharoah PD, Caldas C. Meta-analysis confirms BCL2 is an independent prognostic marker in breast cancer. BMC Cancer. 2008;8:153. doi: 10.1186/1471-2407-8-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Monni O, Joensuu H, Franssila K, et al. BCL2 overexpression associated with chromosomal amplification in diffuse large B-cell lymphoma. Blood. 1997;90(3):1168–1174. [PubMed] [Google Scholar]
- 55.Pinkas J, Martin SS, Leder P. Bcl-2-mediated cell survival promotes metastasis of EpH4 betaMEKDD mammary epithelial cells. Mol Cancer Res. 2004;2(10):551–556. [PubMed] [Google Scholar]
- 56.Ishido M, Ohtsubo R, Adachi T, Kunimoto M. Attenuation of both apoptotic and necrotic actions of cadmium by Bcl-2. Environ Health Perspect. 2002;110(1):37–42. doi: 10.1289/ehp.0211037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martin SS, Leder P. Human MCF10A mammary epithelial cells undergo apoptosis following actin depolymerization that is independent of attachment and rescued by Bcl-2. Mol Cell Biol. 2001;21(19):6529–6536. doi: 10.1128/MCB.21.19.6529-6536.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lipton A, Klinger I, Paul D, Holley RW. Migration of mouse 3T3 fibroblasts in response to a serum factor. Proc Natl Acad Sci U S A. 1971;68(11):2799–2801. doi: 10.1073/pnas.68.11.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Allen PG, Janmey PA. Gelsolin displaces phalloidin from actin filaments. A new fluorescence method shows that both Ca2+ and Mg2+ affect the rate at which gelsolin severs F-actin. J Biol Chem. 1994;269(52):32916–32923. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.