Abstract
There is evidence to indicate that the Asp residue in the third transmembrane helix (TMH) of opioid receptors forms a salt bridge with the positively charged nitrogen of endogenous and exogenous opioid ligands. To further examine the role of this electrostatic interaction in receptor binding and activation, we synthesized ‘carba’-analogues of a published fentanyl analogue containing a 3-(guanidinomethyl)-benzyl group in place of the phenyl moiety attached to the ethylamido group (C. Dardonville et al., Bioorg. Med. Chem. 2006, 14, 6570–6580 (1)), in which the piperidine ring nitrogen was replaced with a carbon. As expected, the resulting cis and trans isomers (8a and 8b) showed reduced μ and κ opioid receptor binding affinities as compared to 1, but surprisingly, retained opioid full agonist activity with about half the potency of leucine-enkephalin in the guinea pig ileum assay. In conjunction with performed receptor docking studies, these results indicate that the electrostatic interaction of the protonated nitrogen in the piperidine ring of fentanyl analogues with the Asp residue in the third TMH is not a conditio sine qua non for opioid receptor activation.
Introduction
The positively charged nitrogen in non-peptide opiates and in opioid peptides with agonist or antagonist activity has been thought until recently to be an absolute requirement for binding to opioid receptors, presumably through formation of a salt bridge with the side chain of the Asp residue in the third transmembrane helix (TMH)a of opioid receptors.1 A cyclic beta-casomorphin-derived opioid peptide, CHO-Dmt-c[D-Orn-2-Nal-Pro-Gly], lacking a positively charged nitrogen, was the first example of a neutral opioid compound with significant μ and δ opioid receptor binding affinity and μ and δ antagonist activity.2 Subsequently, it was reported that replacement of the Tyr1 residue in cyclic opioid peptide analogues with 3-(2,6-dimethyl-4- hydroxyphenyl)propanoic acid (Dhp), (2S)-2-methyl-3-(2,6-dimethyl-4- hydroxyphenyl)propanoic acid [(2S)-Mdp] or (3S)-3-methyl-3-(2,6-dimethyl-4- hydroxyphenyl)propanoic acid [(3S)-Mdp] resulted in opioid antagonists lacking a positively charged N-terminal amino group and showing very high opioid receptor binding affinity. 3–5 Recently, a cyclic enkephalin analogue containing Dhp in place of Tyr1 obtained via ring closing metathesis has been described6. This peptide also lacks a positively charged nitrogen and represents the first example of a neutral compound with δ opioid agonist activity.
N-formylnormorphine is an example of a non-peptide opioid compound lacking a positive charge. This compound retained significant μ opioid receptor binding affinity, but was unable to activate the μ opioid receptor, as demonstrated using the [35S]GTPS binding assay.7 More recently, N-formylnormorphine was shown to be a μ and κ opioid antagonist with similar binding affinity for the two receptors (Kiμ = 420 nM; Kiκ = 363 nM) (G. Weltrowska et al. manuscript in preparation).
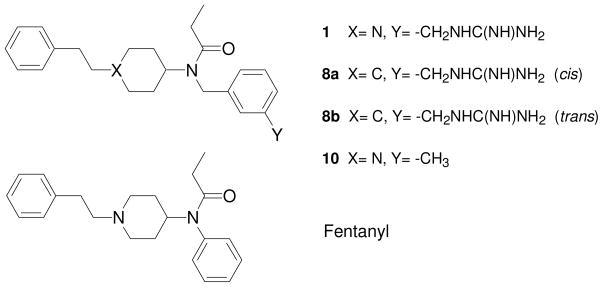
In the μ opioid agonist fentanyl the positively charged nitrogen is contained in the piperidine ring of this compound. In an effort to develop bifunctional μ opioid receptor/I2-imidazoline binding site ligands, a fentanyl analogue containing a 3-(guanidino-methyl)- benzyl group in place of the phenyl moiety attached to the ethylamido group was prepared (Fig. 1, compound 1).8 This compound showed no affinity for the I2-imidazoline binding site but very high μ opioid receptor binding affinity (Kiμ = 0.0098 nM), being about 300-fold more potent than fentanyl under the assay conditions used. This compound was not examined for binding affinity at δ and κ receptors and was not characterized in a functional opioid activity assay. To examine the role of the positively charged nitrogen of fentanyl compounds in the interaction with opioid receptors, we prepared ‘carba’-analogues of compound 1, in which the piperidine nitrogen is replaced with a carbon atom. Both the cis and the trans isomer were obtained and pharmacologically characterized (Fig. 1, compounds 8a and 8b). Compound 1 was chosen as parent structure because the presence of the guanidino group increases water solubility as compared to a carba analogue of fentanyl itself which likely would be poorly soluble in water. To assess the possible contribution of the guanidino group to receptor binding, an analogue of 1 lacking the guanidino group (compound 10) was also synthesized and pharmacologically characterized.
Figure 1.
Structural formulas of fentanyl and fentanyl analogues 1, 8a, 8b and 10.
Chemistry
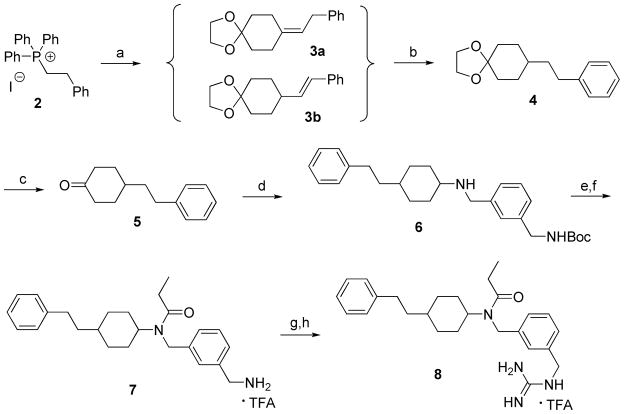
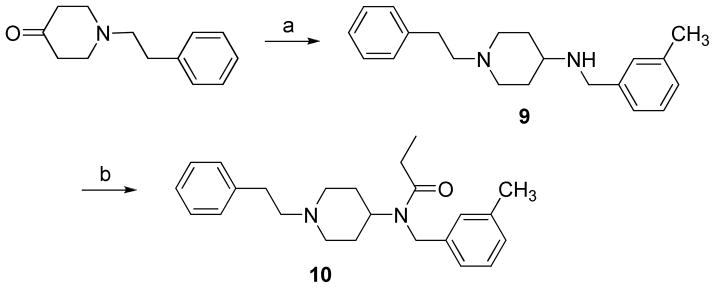
The ‘carba’-analogues 8a and 8b were synthesized according to Scheme 1. 4- phenylethylcyclohexanone 5 was prepared by following a synthetic route described in the literature.9 The Wittig reagent was obtained from phosphonium salt 2 with NaH/DMSO and was condensed with 4,4-(ethylenedioxy)-cyclohexanone to form a mixture of benzylmethylene 3a and phenylmethylene 3b. Catalytic hydrogenation of the mixture of 3a and 3b yielded phenylethyl derivative 4. Removal of the ketal blocking group from 4 with HCl/THF afforded 4-phenylethylcyclohexanone 5. Cyclohexanone 5 was then used to prepare the target compounds 8a and 8b by following synthetic steps described for the preparation of fentanyl analogue 1.8 Reductive amination of 5 with mono-Boc-protected m-xylenediamine provided compound 6. Acylation of 6 with propionic anhydride followed by Boc-deprotection gave amine 7. Reaction of 7 with the Boc-protected thiourea reagent and subsequent Boc-deprotection afforded the target compounds 8a and 8b as the trifluoroacetate salts. Compounds 6, 7 and 8 were obtained as mixtures of cis and trans isomers which could be separated by preparative HPLC. In the case of 8, the stereochemical assignment was made on the basis of the results of homonuclear decoupling NMR experiments. The faster-eluting component was identified as the cis isomer (8a) and the slower-eluting one was the trans isomer (8b). Compound 10 was prepared by reductive amination of 1-phenylethyl-4-piperidone with 3-methylbenzylamine, yielding 9, followed by acylation with propionic anhydride (Scheme 2).
Scheme 1a.
aReagents and conditions: (a) NaH, dry DMSO, 4,4-(ethylenedioxy)-cyclohexanone, rt, 20 h, 70%; (b) Pd/C, H2 (g), EtOAc/AcOH, 60 psi, rt, 2 h, 100%; (c) 2N HCl, THF, rt, 48 h, 82%; (d) tert-Butyl 3-(aminomethyl)benzylcarbamate, NaBH3CN, 3 Å molecular sieves, 1% AcOH/MeOH, rt, 3 h, 71%; (e) (CH3CH2CO)2O, DMAP, pyridine, dry CH2Cl2, rt, 20 h, 95%; (f) 50% TFA/CH2Cl2, rt, 45 min, 63%; (g) N,N′-Boc-thiourea, HgCl2, Et3N, dry CH2Cl2, rt, 3 h, 96%; (h) 50% TFA/CH2Cl2, rt, 1 h, 95%.
Scheme 2a.
aReagents and conditions: (a) 3-methylbenzylamine, NaBH3CN, 3 Å molecular sieves, 1% AcOH/MeOH, rt, 5 h, 50%; (b) (CH3CH2CO)2O, DMAP, pyridine, dry CH2Cl2, rt, 20 h, 73%.
Results and Discussion
In the guinea pig ileum (GPI) assay, the carba analogues 8a and 8b both showed full agonist activity with about half the potency of [Leu5]enkephalin (LENK) (Table 1). Compounds 8a and 8b were about 25-fold less potent agonists than the piperidine nitrogen-containing parent 1, which was characterized as a potent agonist in this assay system (IC50 = 22.4 nM). The agonist effects of 8a, 8b and 1 in the GPI assay were naloxone-reversible with Ke (naloxone) values ranging from 2–13 nM, indicating that they were mediated by opioid receptors. In the δ receptor-representative MVD assay, the carba analogues 8a and 8b displayed about 10-fold lower potency as compared to the potencies determined in the GPI assay, similar to the 5-fold weaker potency seen with compound 1 in this assay system.
Table 1.
In Vitro Opioid Activity Profiles of Fentanyl Analoguesa
| GPI |
MVD |
Receptor binding |
Ki ratio |
|||
|---|---|---|---|---|---|---|
| Cpd | IC50 (nM) | IC50 (nM) | Kiμ (nM) | Kiδ (nM) | Kiκ (nM) | μ/δ/κ |
| 1 | 22.4 ± 3.7 | 114 ± 9 | 0.448 ± 0.079 | 43.5 ± 4.1 | 0.536 ± 0.043 | 1/97/1.2 |
| 8a | 521 ± 67 | 5820 ± 1040 | 300 ± 8 | 4110 ± 80 | 238 ± 41 | 1/14/0.8 |
| 8b | 590 ± 85 | 4750 ± 790 | 224 ± 13 | 7800 ± 280 | 162 ± 8 | 1/35/0.7 |
| 10 | 647 ± 77 | 610 ± 78 | 96.0 ± 5.9 | 6820 ± 670 | 1850 ± 160 | 1/71/19 |
| Fentanyl | 3.45 ± 0.45b | 9.45 ± 4.05b | 5.9 ± 1.4c | 568 ± 159c | 298 ± 40c | 1/96/51 |
| LENK | 246 ± 39 | 11.4 ± 1.1 | 9.43 ± 2.07 | 2.53 ± 0.35 | 4570 ± 550 | 1/0.3/485 |
In the opioid receptor binding assays, compounds 8a and 8b showed marked binding affinities for μ opioid receptors (Kiμ = 220–300 nM) and κ opioid receptors (160–230 nM) (Table 1). Subnanomolar binding affinities were determined for 1 at both μ and κ receptors. The discrepancy between the μ receptor binding affinity of 1 (Kiμ = 0.448 nM) determined in this study and the Kiμ of 0.0098 nM reported for this compound in the literature8 is likely due to the different μ receptor binding assays used in the two laboratories. The δ opioid receptor binding affinities of 8a, 8b and 1 were more than 10-fold lower than their respective μ and κ receptor binding affinities and these three compounds showed similar μ/δ/κ Ki ratios (Table 1). This result indicates that the carbon replacement of the piperidine nitrogen in 1 did not have much of an effect on the opioid receptor selectivity profile. Furthermore, the opioid receptor binding affinities obtained for 8a, 8b and 1 indicate that the agonist effects produced by these three compounds in the GPI assay are due to activation of both μ and κ receptors which are both present in the ileum. It is also of interest to point out that the configuration at the cyclohexane ring in the carba analogues does not have much of an effect on the in vitro opioid activity profile, as both the cis isomer (8a) and the trans isomer (8b) show very similar agonist potencies and opioid receptor binding affinities, and similar opioid receptor selectivity profiles. Compound 10, an analogue of 1 lacking the guanidino group, showed about 30-fold lower agonist potency than 1 in the GPI assay as well as lower potency in the MVD assay. In the receptor binding assays, compound 10 displayed about 150–200-fold lower μ and δ receptor binding affinities in comparison with 1, and 3500-fold lower κ receptor affinity. These results indicate that the guanidino group of 1 significantly contributes to the receptor binding energy of this compound at all three receptors and to the largest extent at the κ receptor. In comparison with carba analogues 8a and 8b, compound 10 has similar μ and δ receptor binding affinities but about 10-fold lower κ receptor binding affinity. Interestingly, compound 10 has about 15-fold lower μ receptor binding affinity than fentanyl and about 180-fold lower μ agonist potency in the GPI assay (Table 1).
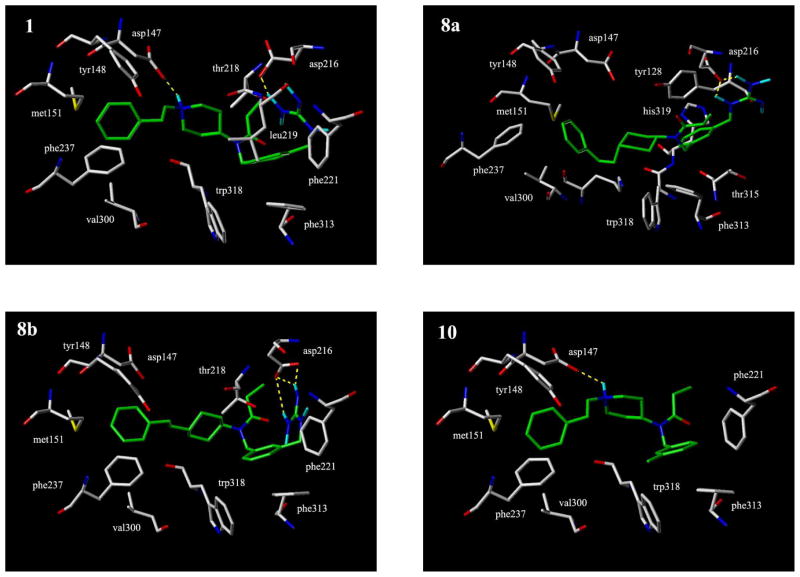
Flexible docking studies of the fentanyl analogues were performed using Mosberg’s model of the μ opioid receptor in the activated state.10 The results indicated that in all cases the phenylethyl group is accommodated in a binding pocket formed by transmembrane helixes (TMHs) 3, 5 and 6, but significant differences in the detailed binding modes among the different ligands are observed (Fig. 2). In the case of the docked fentanyl analogue containing the 3-(guanidinomethyl)-benzyl group (1), the phenylethyl group assumes an almost fully extended conformation with the phenyl ring interacting with the Tyr148, Val300, Phe237 and Met151 side chain residues of the receptor. The piperidine nitrogen forms a salt bridge with Asp147 in the third TMH (nitrogen-oxygen distance = 2.8 Å), as is typically observed with nitrogen-containing opioid receptor ligands. A second salt bridge between the guanidino moiety of the ligand and the side chain of Asp216 in the second extracellular loop close to the interface between the cell membrane and the extracellular domain is also observed. The aromatic ring of the 3- (guanidinomethyl)-benzyl moiety interacts with the side chains of Phe313, Trp318, Thr218 and Leu219.
Figure 2.
Receptor docking studies. Fentanyl analogues bound to the μ opoid receptor in the activated state: 1, 8a, 8b, 10.
The lowest-energy conformer of compound 8b, the trans isomer of the ‘carba’-analogue of 1, with the phenylethyl and N-(3-guanidinomethyl)benzylpropanamide moieties equatorial, shows a receptor binding mode which overall is quite similar to that of its parent (1) (Fig. 2). Again, the phenylethyl substituent is in an extended conformation and interacts with the same receptor residues as the phenylethyl group of 1. However, the distance between the carbon replacing the nitrogen in the piperidine ring of 1 and the Asp147 oxygen is larger (5.2 Å as compared to the corresponding distance of 2.8 Å in the case of 1) and the cyclohexane ring is displaced by about 1.2 Å as compared to the piperidine ring in 1. The guanidino group of 8b forms a salt bridge with the side chain of Asp216 but from the opposite side as compared to the salt bridge formation of the guanidino group of 1 with that same residue. The phenyl ring of the 3-(guanidinomethyl)benzyl moiety of 8b interacts with the same receptor moieties as in the case of compound 1 (Phe313, Thr218, Trp318).
In the lowest-energy conformer of compound 8a, the cis isomer of the ‘carba’-analogue 1, the phenylethyl moiety is axial and the N-(3-guanidinomethyl)benzylpropanamide moiety is equatorial. The binding mode of compound 8a differs somewhat from the binding modes of 8b and 1. The phenylethyl group assumes a bent conformation and its aromatic ring is displaced by about 4Å relative to the corresponding aromatic ring of docked 1, interacting with Tyr148, Phe237, Val300 and Lys308. The cyclohexane ring is displaced by 3.6 Å relative to the cyclohexane ring of the trans isomer 8b and the carbon replacing the nitrogen in the piperidine moiety of 1 is located 8.5 Å away from the Asp147 side chain oxygen. The guanidino group of 8a again forms a salt bridge with the side chain of Asp216 and the aromatic ring of its 3- (guanidinomethyl)-benzyl moiety interacts with the side chains of the Tyr128, Thr315 and His319 residues of the receptor.
Compound 10, the analogue of 1 lacking the guanidino group has a binding mode very similar to that of 1, with the phenylethyl moiety in an extended conformation. To compare the μ receptor binding mode of 10 with that of fentanyl, a docking study with fentanyl was also performed (data not shown). Earlier studies on the docking of fentanyl to the μ receptor, using receptor models developed prior to the rhodopsin crystal structure determination have been reviewed.11 In agreement with two of these studies,12,13 the results of the present docking study indicated an interaction of the positively charged piperidine nitrogen with Asp147 in the third TMH and an orientation of the phenylethyl moiety downwards into a cavity formed by TMHs 3, 5 and 6, as described in one of these studies12, whereas in the other study it was located in a cavity formed by TMHs 2, 3 and 7.13 Furthermore, as in the case of one of these studies13, the phenethyl moiety assumed an extended conformation. The aromatic rings of the phenylethyl group and of the 3-methylbenzyl group in 10 interact with the same receptor residues as the corresponding aromatic rings of 1, and the nitrogen in the piperidine ring forms a salt bridge with Asp147. In a comparison of docked fentanyl and docked compound 10, the N-phenylethyl pipiperidine moiety of the two compounds shows the same receptor binding mode. However, the aromatic rings of the N-phenyl group in fentanyl and of the N-3-methylbenzyl group in compound 10 occupy different receptor sites. This is in part due to the presence of the methylene group between the aromatic ring and the amide nitrogen in 10. Furthermore, the torsion angle at the bond between the amide nitrogen and the piperidine ring is quite different for fentanyl (Φ = 172°) and compound 10 (Φ = 11°). Consequently, the propanamide moiety also has a different orientation in the two docked compounds. The different receptor interactions of the N-propanamide substituents may explain the observed decrease in μ opioid agonist potency of 10 as compared to fentanyl (Table 1).14,15
The observation that the ‘carba’-analogues of 1, compounds 8a and 8b, behave as full opioid agonists in the GPI assay with about half the potency of leucine-enkephalin represents a novel and important finding. Compounds 8a and 8b have 670- and 500-fold lower μ receptor binding affinity than 1, corresponding to a loss in binding energy (ΔG) of 3.95 and 3.71 kcal/mol respectively. This reduction in receptor binding energy is due to the inability of these ‘carba’-analogues to engage in an electrostatic interaction with Asp147. Such electrostatic interactions typically contribute about 4 kcal/mol to the binding energy in ligand-protein interactions.16 The analogous analysis of the κ receptor binding data of 8a and 8b as compared to 1 indicates a similar loss in binding energy of the two ‘carba’-analogues (3.71 and 3.47 kcal/mol, respectively). This decrease in binding energy is likely due to the elimination of the electrostatic interaction with the Asp residue (Asp138) in the third TMH of the κ receptor which was observed in a study on the docking of 1 to this receptor in the activated state (data not shown). The performed flexible docking studies reveal overall similar μ receptor binding modes for 8a, 8b and 1. In all cases an electrostatic interaction between the guanidino group of these three compounds and the Asp216 side chain of the receptor is observed. The analogue of compound 1 lacking the guanidino group (compound 10) shows a 214-fold decrease in μ receptor binding affinity. This corresponds to a reduction in binding energy of 3.26 kcal/mol, in agreement with the observation that the guanidino group of 1 engages in an electrostatic bond with Asp216.16 The agonist activity of ‘carba’-analogues 8a and 8b can be explained with their overall similar binding modes as compared to 1 and 10, and with their guanidine group interacting with Asp216. The latter electrostatic interaction contributes to binding the active conformation of the receptor and, thereby, compensates to some extent for the loss of the salt bridge with Asp147 in the third TMH.
Conclusions
Compound 1 was confirmed as a ligand with subnanomolar μ receptor binding affinity and, furthermore, it was determined that this fentanyl analogue also has high κ receptor binding affinity as well as high agonist potency in the GPI assay. The most important finding of this study is the novel observation that an electrostatic interaction between the positively charged nitrogen of fentanyl compounds and the Asp147 residue of the μ opioid receptor is not a conditio sine qua non for μ opioid receptor activation. To the best of our knowledge, this has not been demonstrated before for opioid receptor ligands or for other GPCRs having a conserved Asp residue in the third TMH and interacting with endogenous ligands containing an amine moiety. Previously, it has been shown that elimination of the N-terminal amino group or its replacement with a methyl group in opioid peptides containing an N-terminal 2′,6′-dimethyltyrosine (Dmt) residue led to neutral compounds showing high opioid antagonist activity.3–5 Similarly, N-formylnormorphine, which also lacks a positively charged nitrogen, also turned out to be an opioid antagonist (G. Weltrowska et al., manuscript in preparation). Taken together, these results indicate that the elimination of the positively charged nitrogen in opioid compounds may have divergent effects on the efficacy, depending on the receptor binding interactions of other moieties present in the molecule. The nonnitrogenous salvinorin A analogue herkinorin, which also lacks a positive charge, has been reported to be a μ opioid agonist with an EC50 of 500 nM in a functional in vitro assay.17 However, there is evidence to indicate that salvinorin A and its analogues have a receptor binding mode totally different from the binding modes of all classical, nitrogen-containing opioid agonists.18
Experimental Section
General Methods
Reactions were monitored by ascending TLC using pre-coated plates (silica gel 60 F254, 250 μm, Merck Darmstadt, Germany). Flash chromatography was performed with silica gel 60 columns (particle size 230–400 μm). 1H and 13C NMR spectra were recorded on a Varian INOVA 500 MHz spectrometer and referenced with respect to the residual signals of the solvent. The following abbreviations were used in reporting spectra: s = singlet, d = doublet, t = triplet, m = multiplet. Preparative reversed-phase HPLC was performed on a Vydac 218-TP1022 column (22 × 250 mm) with a linear gradient of 50–70% MeOH in 0.1% TFA/H2O over 40 min at a flow rate of 12 mL/min. For analytical reversed-phase HPLC a Vydac 218-TP54 column (5 × 250 mm) was used with a linear gradient of 50–70% MeOH in 0.1% TFA/H2O over 30 min at a flow rate of 1 mL/min. All final compounds were obtained in >95% purity, as established by analytical HPLC. Molecular masses of compounds were determined by electrospray mass spectrometry on a hybrid Q-Tof mass spectrometer interfaced to a Mass Lynx 4.0 data system.
Syntheses of compounds 8a, 8b and 10
Phenylethyltriphenylphosphonium iodide (2)
A solution of (2-iodoethyl)benzene (10.4 mL, 72 mmol, 1.2 equiv.) and 15.64 g (60 mmol) triphenylphosphine in 80 mL xylene was heated at 90°C under argon for 24h. After cooling in an ice bath, the formed precipitate was collected, washed with Et2O and dried in vacuo. Crystallization from acetone-ethylether yielded 2 as white crystals (25.0 g, 83%); mp 130–132° C. 1H NMR (500 MHz, DMSO-d6) δ 7.77–7.95 (m, 15H), 7.24–7.35 (m, 5H), 3.92–3.98 (m, 2H), 2.86–2.91 (m, 2H). HRMS (EI) m/e calcd for C26H24P [M]+ 367.1610, obsd 367.1611.
8-(2-phenylethylidene)-1,4-dioxaspiro[4.5]decane (3)
1.2 g (30 mmol) NaH (60% oil dispersion) was stirred in 75 mL DMSO (dry) at 60° C under argon for 1h. After cooling of the solution to room temperature, 12.8 g (26 mmol) of phosphonium salt 2 was added under stirring. 4,4-(ethylenedioxy)-cyclohexanone (4.06 g, 26 mmol) was then added and the reaction mixture was stirred for 20 h. The mixture was then poured over ice and extracted with petroleum ether. The organic layers were combined, dried over MgSO4 and evaporated. Flash chromatography on silica gel with hexane-EtOAc (6:1, v/v) afforded 3 as a mixture of 8-(2-phenylethylidene)-1,4-dioxaspiro[4.5]decane (3a) and 8-styryl-1,4-dioxaspiro[4.5]decane (3b) in the form of a colorless oil (4.5 g, 70%). The mixture of 3a and 3b was used without separation in the subsequent hydrogenation step.
8-phenylethyl-1,4-dioxaspiro[4.5]decane (4)
An argon-purged reaction vessel was charged with 3a and 3b (4.5 g, 18 mmol), 10% Pd/C (0.5 g) in 75 mL EtOAc and AcOH (10 μL). The reaction vessel was then pressurized to 60 psi with hydrogen and the mixture was stirred for 2h at room temperature. After filtration the solvent was evaporated to yield 4 as a colorless oil (4.5 g, 100%). 1H NMR (500 MHz, CDCl3) δ 7.18–7.35 (m, 5H), 3.95 (s, 4H), 2.63–2.66 (t, 2H, J = 8.0 Hz), 1.28–1.82 (m, 11H). 13C NMR (125 MHz, CDCl3) δ 142.7, 128.5, 125.7, 108.5, 64.4, 38.2, 36.5, 33.1, 29.5, 28.9 HRMS (EI) m/e calcd for C16H23O2 [M+H]+ 247.1698, obsd 247.1691.
4-phenylethylcyclohexanone (5)
4.5 g (18 mmol) of 4 in a mixture of 50 mL THF and 25 mL 2N HCl was stirred for 48 h at room temperature. After evaporation of the solvent, the product was partitioned between EtOAc and H2O. Evaporation of the organic layer afforded 5 (3.0 g, 82%) as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 7.18–7.38 (m, 5H), 2.68–2.71 (t, 2H, J = 8.0 Hz) 1.42–2.45 (m, 11H). 13C NMR (125 MHz, CDCl3) δ 211.8, 142.7, 128.5, 128.2, 125.7, 41.0, 38.3, 36.5, 31.2, 28.1. HRMS (EI) m/e calcd for C14H19O [M+H]+ 203.1436, obsd 203.1431.
tert-Butyl-3((4-phenylethylcyclohexylamino)methyl) benzylcarbamate (6)
NaBH3CN (942 mg, 15 mmol) was added to a solution of mono-Boc-protected m-xylenediamine8 (2.8 g, 12 mmol), 4-phenylethylcyclohexanone (5) (2.02 g, 10 mmol) and 3Å molecular sieves in 1% AcOH/MeOH dry, and the mixture was stirred for 3h at room temperature. After filtration on a pad of Celite the organic solvent was evaporated and 6 was obtained as a 50%:50% mixture of the cis and trans isomers, as determined by integration of the isomeric proton peaks at 2.94 and 3.08 ppm in the 1H NMR spectrum. Purification by flash chromatography on silica gel with CH2Cl2/7N NH3 in MeOH (97:3) afforded 6 (3.080 g, 71% [3g of cis/trans mixture, 40 mg of isomer 1 and 40 mg of isomer 2]). Isomer 1: 1H NMR (500 MHz, CDCl3) δ 8.45 (s, 1H), 7.08–7.25 (m, 9H), 5.12 (s, 1H), 4.12 (s, 2H), 4.08 (s, 2H), 3.08 (br s, 1H). 2.60 (t, 2H, J = 8.0 Hz), 1.42–1.9 (m, 11H), 1.4 (s, 9H). 13C NMR (125 MHz, CDCl3) δ 156.2, 142.6, 137.4, 136.2, 128.5, 128.3, 128.1, 127.7, 125.9, 80.0, 56.3, 52.5, 45.4, 38.3, 36.7, 32.1, 28.4. HRMS (EI) m/e calcd for C27H39N2O2 [M+H]+ 423.3012 obsd 423.3013. Isomer 2: 1H NMR (500 MHz, CDCl3) δ 8.45 (s, 1H), 7.08–7.22 (m, 9H), 5.12 (br s, 1H), 4.12 (s, 1H), 4.05 (br s, 1H), 2.94 (br s, 1H), 2.60 (t, 2H, J = 8.0 Hz), 2.10 (br, 2H), 1.98 (br, 2H), 1.25–1.4 (br m, 14H), 0.81 (br m, 2H). 13C NMR (125 MHz, CDCl3) δ 156.2, 142.6, 137.4, 136.2, 128.5, 128.3, 128.1, 127.7, 125.9, 80.0, 56.3, 52.5, 45.5, 38.3, 36.7, 32.1, 28.4. HRMS (EI) m/e calcd for C27H39N2O2 [M+H]+ 423.3012 obsd 423.3018.
N-(3-(aminomethyl)benzyl)-N-(4-phenylethylcyclohexyl)propionamide (7)
Propionic anhydride (1 mL, 7.8 mmol) was added at room temperature to a solution of amine 6 (mixture of cis/trans isomers), DMAP (0.1 equiv.) and pyridine (2.11 mL, 26 mmol) in dry CH2Cl2, and the mixture was stirred for 20 h at room temperature. After evaporation of the organic solvent, the oily residue was dissolved in EtOAc, extracted with a saturated NH4Cl aqueous solution, brine and water, and dried under MgSO4 and concentrated. The crude product (2.5 g, 95%) was then taken up in 50% TFA/CH2Cl2 and the solution was stirred for 45 min at room temperature. The volatiles were evaporated and the resulting crude oil was triturated with dry Et2O. Purification of the oily residue by flash chromatography on silica gel with CH2Cl2/7N NH3 in MeOH (97:3) afforded 7 as a mixture of cis and trans isomers (1.2 g, 63%). The mixture was used in the next synthetic step without separation of the isomers. For the analytical characterization the isomers were separated by preparative HPLC of a 100 mg sample of the mixture. Isomer 1: 1H NMR (500 MHz, DMSO-d6) δ 8.26 (s, 2H), 7.25–7.49 (m, 9H), 4.58 (d, 2H, J = 23 Hz), 4.36 (br t, 0.5H), 4.05–4.11 (dd, 2H, J1 = 5 Hz, J2 = 17 Hz) 3.82 (br t, 0.5H), 3.16 (m, 0.5H), 2.82 (d, 0.5H, J = 5 Hz), 2.52 (m, 1H), 2.21 (m, 1H), 1.44–1.71 (m, 11H), 1.12 (t, 1.5H, J = 7 Hz), 1.0 (t, 1.5H, J = 7 Hz). 13C NMR (125 MHz, DMSO-d6) δ 171.7, 142.4, 141.3, 136.2, 128.5, 128.3, 128.1, 127.7, 125.9, 125.2, 46.7, 46.2, 38.3, 36.7, 29.4, 28.8, 26.6, 10.3. HRMS (EI) m/e calcd for C25H35N2O [M+H]+ 379.2749, obsd 379.2735. Isomer 2: 1H NMR (500 MHz, DMSO-d6) δ 8.22 (br s, 2H), 7.15–7.41 (m, 9H), 4.46 (d, 2H, J = 24 Hz), 4.31 (t, 0.5H, J = 12 Hz), 3.99 (m, 2H), 3.72 (t, 0.5H, J = 12 Hz), 3.05 (m, 0.5H), 2.75 (d + m, 1.5H, J = 5Hz), 2.47 (m, 3H), 2.13 (m, 1H), 1.75 (d, 2H, J= 12 Hz), 1.56 (t, 2H, J = 18 Hz), 0.92–1.48 (m, 8H). 13C NMR (125 MHz, DMSO-d6) δ 171.7, 142.4, 141.3, 136.2, 128.5, 128.3, 128.1, 127.7, 125.4, 125.2, 46.7, 46.2, 38.3, 36.7, 29.4, 28.8, 26.6, 10.3. HRMS (EI) m/e calcd for C25H35N2O [M+H]+ 379.2749, obsd 379.2738.
N-(3-(guanidinomethyl)benzyl)-N-(4-phenylethylcyclohexyl)propionamide TFA salt (8)
To a solution of 7 (500 mg, 1 mmol), N,N′-di-(tert-butoxycarbonyl)-thiourea (327 mg, 1.2 mmol) and TEA (0.68 mL) in dry CH2Cl2 (20 mL), HgCl2 (325 mg, 1.2 mmol) was added. After stirring under argon for 3h at room temperature, the reaction mixture was filtered and the organic phase was washed with water, dried over MgSO4 and concentrated. The resulting crude oil (600 mg, 96%) was dissolved in 50% TFA/CH2Cl2 and the solution was stirred for 1h at room temperature. The volatiles were then evaporated and the crude oily residue was triturated with dry Et2O. After evaporation of Et2O, the TFA salt of 8 (mixture of cis and trans isomers) was obtained as a very hygroscopic solid (400 mg, 95%). The cis and trans isomers were separated by preparative HPLC and were identified by homonuclear decoupling NMR experiments (see “Supporting Information”). Isomer 1 (cis, 8a): 1H NMR (500 MHz, DMSO-d6) δ 7.94 (d, 1H, J = 27 Hz), 7.09–7.37 (m, 9H), 4.50 (d, 2H, J = 23 Hz), 4.32 (dd, 2H, J = 6Hz), 4.26 (br t, 0.5H), 3.73 (br 0.5H), 2.75 (d, 1H, J = 5 Hz), 2.46 (m, 3H), 2.12 (m, 1H), 1.17–1.63 (m, 12H), 1.04 (t, 1.5H, J = 7 Hz), 0.92 (t, 1.5H, J = 7 Hz). 13C NMR (125 MHz, DMSO-d6) δ 171.7, 158.0, 142.6, 137.4, 128.5, 128.1, 127.7, 125.9, 53.3, 46.7, 44.9, 38.3, 36.7, 29.4, 29.1, 28.8, 26.6, 10.2. HRMS (EI) m/e calcd for C26H37N4O [M+H]+ 421.2967, obsd 421.2955. Isomer 2 (trans, 8b): 1H NMR (500 MHz, DMSO-d6) δ 7.94 (d, 1H, J = 27 Hz), 7.08–7.37 (m, 9H), 4.46 (d, 2H, J = 26 Hz), 4.33–4.37 (dd, 2.5H, J = 6 Hz), 3.73 (br t, 0.5H), 2.74 (d, 1H, J = 5 Hz), 2.53 (t, 2H, J = 8 Hz), 2.40 (m, 1H), 2.12 (m, 1H), 1.75 (d, 2H, J = 12 Hz), 1.54 (m, 2H), 0.98–1.46 (m, 12H). 13C NMR (125 MHz, DMSO-d6) δ 171.7, 158.0, 142.6, 137.4, 128.5, 128.1, 127.7, 125.0, 53.3, 46.7, 44.9, 38.3, 36.7, 29.4, 29.1, 28.8, 26.6, 10.2. HRMS (EI) m/e calcd for C26H37N4O [M+H]+ 421.2967, obsd 421.2955.
N-(3-methylbenzyl)-1-phenylethylpiperidine-4-amine (9)
NaBH3CN (0.55 g, 8.9 mmol) was added to a solution of 3-methylbenzylamine (0.88 mL, 7 mmol), 1-phenylethyl-4-piperidinone (1.2 g, 5.9 mmol) and 3Å sieves in 1% AcOH/MeOH (dry). The mixture was stirred at room temperature for 5 h, filtered over Celite and washed with dry MeOH. The combined organic filtrates were evaporated and the crude product was purified by flash chromatography on silica gel (CHCl3/MeOH, 9:1), affording 9 as a colorless oil (0.9 g, 50%). 1H NMR (500 MHz, CDCl3) δ 7.08–7.32 (m, 9H), 3.81 (s, 2H), 3.0 (d, 2H, J = 14 Hz), 2.83 (m, 2H), 2.58 (m, 4H), 2.37 (s, 3H), 2.15 (t, 2H, J = 11 Hz), 1.97 (d, 2H, J = 12 Hz), 1.50 (dd, 2H, J = 4 Hz and 11 Hz). 13C NMR (125 MHz, CDCl3) δ 140.5, 140.4, 129.1, 129.0, 128.9, 128.6, 128.5, 127.9, 126.3, 125.3, 60.7, 54.0, 51.0, 45.0, 38.9, 33.8, 32.5, 24.0, 21.6. HRMS (EI) m/e calcd for C21H29N2 [M+H]+ 309.2325, obsd 309.2319.
N-(3-methylbenzyl)-N-(1-phenylethylpiperidine-4-yl)propionamide (10)
Propionic anhydride (0.55 mL, 4.35 mmol) was added at room temperature to a solution of amine 9 (0.9 g, 2.9 mmol), DMAP (30 mg, 0.1 equiv.) and pyridine (1.17 mL, 14.5 mmol) in dry CH2Cl2. After stirring the reaction mixture for 20 h, the organic solvent was evaporated and the crude product was dissolved in AcOEt. The solution was washed with sat. NH4Cl aqueous solution, brine and water, dried over MgSO4 and concentrated. The resulting crude oil was purified by flash chromatography on silica gel (CH2Cl2/MeOH/TEA, 95:5:0.2), affording 10 as a colorless oil (0.8 g, 73%). 1H NMR (500 MHz, CDCl3) δ 6.93–7.34 (m, 9H), 4.88 (m, 1H), 4.52 (s, 2H), 3.66 (d, 2H, J = 12 Hz), 3.16 (br, 2H), 3.00 (m, 2H), 2.77 (br, 2H), 2.37 (d, 2H, J = 8 Hz), 2.35 (s, 3H), 2.11 (dd, 2H, J = 11 Hz and 36 Hz), 1.82 (d, 2H, J = 12 Hz), 1.14 (t, 3H, J = 8 Hz). 13C NMR (125 MHz, CDCl3) δ 175.8, 139.1, 137.6, 135.8, 129.3, 128.8, 128.5, 127.7, 126.4, 122.7, 58.6, 52.8, 49.0, 46.5, 45.0, 30.7, 27.2, 26.7, 21.6, 9.7. HRMS (EI) m/e calcd for C24H33N2O [M+H]+ 365.2593, obsd 365.2580.
In Vitro Bioassays and Receptor Binding Assays
The GPI19 and MVD20 bioassays were carried out as reported in detail elsewhere.21,22 A dose-response curve was determined with [Leu5]enkephalin as standard for each ileum and vas preparation, and IC50 values of the compounds being tested were normalized according to a published procedure.23 Ke-values for naloxone as antagonist were determined from the ratio of IC50 values obtained with an agonist in the presence and absence of a fixed naloxone concentration (20 nM).24 Opioid receptor binding studies were performed as described in detail elsewhere.21 Binding affinities for μ and δ opioid receptors were determined by displacing, respectively, [3H]DAMGO (Multiple Peptide Systems, San Diego, CA) and [3H]DSLET (Multiple Peptide Systems) from rat brain membrane binding sites, and κ opioid receptor affinities were measured by displacement of [3H]U69,593 (Amersham) from guinea pig brain membrane binding sites. Incubations were performed for 2h at 0°C with [3H]DAMGO, [3H]DSLET, and [3H]U69,593 at respective concentrations of 0.72, 0.78, and 0.80 nM. IC50 values were determined from log-dose displacement curves, and Ki values were calculated from the IC50 values by means of the equation of Cheng and Prusoff,25 using values of 1.3, 2.6 and 2.9 nM for the dissociation constants of [3H]DAMGO, [3H]DSLET, and [3H]U69,593, respectively. The free energy change, ΔG, associated with ligand-receptor interactions was calculated using the equation ΔG = − 2.303 RT log Ki, which under physiological conditions (T = 310° K) is approximated (in kcal/mol) by ΔG = −1.4 log Ki.
Theoretical Conformational Analyses and Receptor Docking Studies
All calculations were performed using the SYBYL software version 7.0 (Tripos Associates, St. Louis, MO). The Tripos force field was used for energy calculations. A dielectric constant of 78 was used for the conformational analysis of the isolated ligands in vacuo. A stepwise approach was used to determine the low-energy conformations of the fentanyl analogues.26 First, the cyclohexane ring or piperidine ring was retrieved from the fragment library. The piperidine ring was in the charged (protonated) form. Functional groups were attached to the six-membered ring in each of four different arrangements: axial-axial, axial-equatorial, equatorial-axial and equatorial-equatorial, resulting in four different starting structures for each compound studied. For each structure, a systematic conformational grid search was performed to identify low-energy structures. Each exocyclic rotatable bond was rotated in 30° increments over all space. Energies were calculated and the obtained conformers were grouped into low-energy families. The lowest-energy member of each family was minimized and the resulting conformations were ranked according to energy. The lowest-energy conformations of all compounds have the cyclohexane or piperidine ring in the chair conformation. In the lowest-energy conformation of the cis isomer of the ‘carba’-analogue (8a), the larger substituent on the cyclohexane ring is equatorial and the smaller substituent is axial. The lowest-energy conformation of the trans isomer of the ‘carba’-analogue (8b) has both substituents on the cyclohexane ring in the equatorial position. In the lowest-energy conformations of compounds 1 and 10 both substituents on the piperidine ring are equatorial.
The model of the μ opioid receptor in the activated state, constructed by Mosberg et al. by homology modeling based on the crystal structure of rhodopsin10 (http://mosberglab.phar.umich.edu/resources/), was used in the docking studies. A dielectric constant of 1 was used for the receptor docking studies as well as in all subsequent calculations involving receptor-ligand complexes. Flexible docking of the lowest-energy conformation of each compound was performed using the software program GLIDE (Schrödinger LLC). Each of the resulting ligand-receptor complexes was minimized using the conjugate gradient approach.27 Molecular dynamics simulations of 100 ps at 300 K were performed in order to assess the stability of each complex. In each case no significant change in the complex structure was observed during the simulation.
Supplementary Material
Acknowledgments
This work was financially supported by the U.S. National Institutes of Health (Grant DA-004443) and the Canadian Institutes of Health Research (Grant MOP-89716).
Footnotes
Dedicated to the memory of Ralph F. Hirschmann
Abbreviations: DAMGO, H-Tyr-D-Ala-Gly-NαMePhe-Gly-ol; Dhp, 3-(2,6-dimethyl-4- hydroxyphenyl)propanoic acid; DMAP, 4-(dimethylamino)-pyridine; Dmt, 2′,6′-dimethyltyrosine; DSLET, H-Tyr-D-Ser-Gly-Phe-Leu-Thr-OH; GPI, guinea pig ileum; LENK, [Leu5]enkephalin; (2S)-Mdp, (2S)-2-methyl-3-(2,6-dimethyl-4- hydroxyphenyl)propanoic acid; (3S)-Mdp, (3S)-3-methyl-3-(2,6-dimethyl-4- hydroxyphenyl)propanoic acid; MVD, mouse vas deferens; 2-Nal, 3-(2-naphthyl)-Lalanine; TEA, triethylamine; TFA, trifluoroacetic acid; THF, tetrahydrofuran; TMH, transmembrane helix; U69,593, (5α,7α,8β-(—)-N-methyl-N-[7-(1-pyrrolidinyl)-1- oxaspiro[4.5]dec-8-yl]benzeneacetamide.
Supporting Information Available: NMR data for stereochemical assignment. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Rees DC, Hunter JC. Opioid Receptors. In: Emmet JC, editor. Comprehensive Medicinal Chemistry. Pergamon Press; New York: 1990. pp. 805–846. [Google Scholar]
- 2.Schiller PW, Berezowska I, Nguyen TMD, Schmidt R, Lemieux C, Chung NN, Falcone-Hindley ML, Yao W, Liu J, Iwama S, Smith AB, III, Hirschmann RF. Novel Ligands Lacking a Positive Charge for the δ- and μ-Opioid Receptors. J Med Chem. 2000;43:551–559. doi: 10.1021/jm990461z. [DOI] [PubMed] [Google Scholar]
- 3.Lu Y, Nguyen TMD, Weltrowska G, Berezowska I, Lemieux C, Chung NN, Schiller PW. [2′,6′-Dimethyltyrosine1]Dynorphin A(1–11)-NH2 Analogues Lacking an N-Terminal Amino Group: Potent and Selective κ Opioid Antagonists. J Med Chem. 2001;44:3048–3053. doi: 10.1021/jm0101186. [DOI] [PubMed] [Google Scholar]
- 4.Schiller PW, Weltrowska G, Nguyen TMD, Lemieux C, Chung NN, Lu Y. Conversion of δ-, κ- and μ-Receptor Selective Opioid Peptide Agonists into δ-, κ- and μ-Selective Antagonists. Life Sci. 2003;73:691–698. doi: 10.1016/s0024-3205(03)00389-8. [DOI] [PubMed] [Google Scholar]
- 5.Weltrowska G, Lu Y, Lemieux C, Chung NN, Schiller PW. A Novel Cyclic Enkephalin Analogue with Potent Opioid Antagonist Activity. Bioorg Med Chem Lett. 2004;14:4731–4733. doi: 10.1016/j.bmcl.2004.06.077. [DOI] [PubMed] [Google Scholar]
- 6.Berezowska I, Lemieux C, Chung NN, Wilkes BC, Schiller PW. Cyclic Opioid Peptide Agonists and Antagonists Obtained Via Ring-Closing Metathesis. Chem Biol Drug Des. 2009;74:329–334. doi: 10.1111/j.1747-0285.2009.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li JG, Chen C, Yin J, Rice K, Zhang Y, Matecka D, de Riel JK, DesJarlais RL, Liu-Chen LY. Asp147 in the Third Transmembrane Helix of the Rat μ Opioid Receptor Forms Ion-Pairing With Morphine and Naltrexone. Life Sci. 1999;65:175–185. doi: 10.1016/s0024-3205(99)00234-9. [DOI] [PubMed] [Google Scholar]
- 8.Dardonville C, Fernandez-Fernandez C, Gibbons SL, Ryan GJ, Jagerovic N, Gabilondo AM, Meana JJ, Callado LF. Synthesis and Pharmacological Studies of New Hybrid Derivatives of Fentanyl Active at the μ-Opioid Receptor and I2-Imidazoline Binding Sites. Bioorg Med Chem. 2006;14:6570–6580. doi: 10.1016/j.bmc.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Rosowsky A, Papoulis AT, Forsch RA, Queener SF. Synthesis and Antiparasitic and Antitumor Activity of 2,4-Diamino-6-(arylmethyl)-5,6,7,8- tetrahydroquinazoline Analogues of Piritrexim. J Med Chem. 1999;42:1007–1017. doi: 10.1021/jm980572i. [DOI] [PubMed] [Google Scholar]
- 10.Fowler CB, Pogozheva JD, Lomize AL, Le Vine H, III, Mosberg HI. Complex of an Active μ-Opioid Receptor with a Cyclic Peptide Agonist Modeled from Experimental Constraints. Biochemistry. 2004;43:15796–15810. doi: 10.1021/bi048413q. [DOI] [PubMed] [Google Scholar]
- 11.Kane BE, Svensson B, Ferguson DM. Molecular Recognition of Opioid Receptor Ligands. AAPS J. 2006;8:E126–E137. doi: 10.1208/aapsj080115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pogozheva ID, Lomize AL, Mosberg HI. Opioid Receptor Three-Dimensional Structures from Distance Geometry Calculations With Hydrogen Bonding Constraints. Biophys J. 1998;75:612–634. doi: 10.1016/S0006-3495(98)77552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subramanian G, Paterlini MG, Portoghese PS, Ferguson DM. Molecular Docking Reveals a Novel Binding Site Model for Fentanyl at the μ-Opioid Receptor. J Med Chem. 2000;43:381–391. doi: 10.1021/jm9903702. [DOI] [PubMed] [Google Scholar]
- 14.Essawi MY, Portoghese PS. Synthesis and Evaluation of 1- and 2-Substituted Fentanyl Analogues for Opioid Activity. J Med Chem. 1983;26:348–352. doi: 10.1021/jm00357a007. [DOI] [PubMed] [Google Scholar]
- 15.Jagerovic N, Cano C, Elguero J, Goya P, Callado LF, Meana JJ, Girón R, Abalo R, Ruiz D, Goicoechea C, Martin MA. Long-Acting Fentanyl Analogues: Synthesis and Pharmacology of N-(1-phenylpyrazolyl)-N-(1- phenylalkyl-4-piperidyl)propanamides. Bioorg Med Chem. 2002;10:817–827. doi: 10.1016/s0968-0896(01)00345-5. [DOI] [PubMed] [Google Scholar]
- 16.Burley SK, Petsko GA. Weakly Polar Interactions in Proteins. Adv Protein Chem. 1988;39:125–189. doi: 10.1016/s0065-3233(08)60376-9. [DOI] [PubMed] [Google Scholar]
- 17.Harding WW, Tidgewell K, Byrd N, Cobb H, Dersch CM, Butelman ER, Rothman RB, Prisinzano TE. Neoclerodane Diterpenes As a Novel Scaffold for μ Opioid Receptor Ligands. J Med Chem. 2005;48:4765–4771. doi: 10.1021/jm048963m. [DOI] [PubMed] [Google Scholar]
- 18.Kane BE, McCurdy CR, Ferguson DM. Toward a Structure-Based Model of Salvinorin A Recognition of the κ-Opioid Receptor. J Med Chem. 2008;51:1824–1830. doi: 10.1021/jm701040v. [DOI] [PubMed] [Google Scholar]
- 19.Paton WDM. The Action of Morphine and Related Substances on Contraction and on Acetylcholine Output of Coaxially Stimulated Guinea-Pig Ileum. Br J Pharmacol Chemother. 1957;12:119–127. doi: 10.1111/j.1476-5381.1957.tb01373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henderson G, Hughes J, Kosterlitz HW. A New Example of a Morphine-Sensitive Neuro-Effector Junction: Adrenergic Transmission in the Mouse Vas Deferens. Br J Pharmacol. 1972;46:764–766. doi: 10.1111/j.1476-5381.1972.tb06901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schiller PW, Lipton A, Horrobin DF, Bodanszky M. Unsulfated C-Terminal 7-Peptide of Cholecystokinin: a New Ligand of the Opiate Receptor. Biochem Biophys Res Commun. 1978;85:1332–1338. doi: 10.1016/0006-291x(78)91149-x. [DOI] [PubMed] [Google Scholar]
- 22.DiMaio J, Nguyen TMD, Lemieux C, Schiller PW. Synthesis and Pharmacological Characterization in Vitro of Cyclic Enkephalin Analogues: Effect of Conformational Constraints on Opiate Receptor Selectivity. J Med Chem. 1982;25:1432–1438. doi: 10.1021/jm00354a008. [DOI] [PubMed] [Google Scholar]
- 23.Waterfield AA, Leslie FM, Lord JAH, Ling N, Kosterlitz HW. Opioid Activities of Fragments of β-Endorphin and of Its Leucine65-Analogue. Comparison of the Binding Properties of Methionine- and Leucine-Enkephalin. Eur J Pharmacol. 1979;58:11–18. doi: 10.1016/0014-2999(79)90334-0. [DOI] [PubMed] [Google Scholar]
- 24.Kosterlitz HW, Watt AJ. Kinetic Parameters of Narcotic Agonists and Antagonists with Particular Reference to N-Allylnoroxymorphone (Naloxone) Br J Pharmacol Chemother. 1968;33:266–276. doi: 10.1111/j.1476-5381.1968.tb00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng YC, Prusoff WH. Relationship Between the Inhibition Constant (KI) and the Concentration of Inhibitor which Causes 50 Per Cent Inhibition (I50) of an Enzymatic Reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 26.Wilkes BC, Schiller PW. Conformation-Activity Relationships of Cyclic Dermorphin Analogues. Biopolymers. 1990;29:89–95. doi: 10.1002/bip.360290113. [DOI] [PubMed] [Google Scholar]
- 27.Powell MJD. Restart Procedures for the Conjugate Gradient Method. Mathematical Programming. 1977;12:241–254. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.