Abstract
Memory problems are one of the most common symptoms of sport-related mild traumatic brain injury (MTBI), known as concussion. Surprisingly, little research has examined spatial memory in concussed athletes given its importance in athletic environments. Here, we combine functional magnetic resonance imaging (fMRI) with a virtual reality (VR) paradigm designed to investigate the possibility of residual functional deficits in recently concussed but asymptomatic individuals. Specifically, we report performance of spatial memory navigation tasks in a VR environment and fMRI data in 15 athletes suffering from MTBI and 15 neurologically normal, athletically active age matched controls. No differences in performance were observed between these two groups of subjects in terms of success rate (94 and 92%) and time to complete the spatial memory navigation tasks (mean = 19.5 and 19.7 s). Whole brain analysis revealed that similar brain activation patterns were observed during both encoding and retrieval among the groups. However, concussed athletes showed larger cortical networks with additional increases in activity outside of the shared region of interest (ROI) during encoding. Quantitative analysis of blood oxygen level dependent (BOLD) signal revealed that concussed individuals had a significantly larger cluster size during encoding at parietal cortex, right dorsolateral prefrontal cortex, and right hippocampus. In addition, there was a significantly larger BOLD signal percent change at the right hippocampus. Neither cluster size nor BOLD signal percent change at shared ROIs was different between groups during retrieval. These major findings are discussed with respect to current hypotheses regarding the neural mechanism responsible for alteration of brain functions in a clinical setting.
Keywords: Concussion, fMRI, Virtual reality, Spatial memory, Spatial navigation
Introduction
Concussion in sport, otherwise known as mild traumatic brain injury (MTBI) has been referred to as both the most common and the most puzzling type of traumatic brain injury (Shaw 2002; Cantu 2006). It is seen during recreational activities, transportation accidents, and other situations in which the brain accelerates (or decelerates) differentially in relation to the skull. The phenomenon of concussion is puzzling that there exists no consistent set of theoretical studies which clearly articulates its pathophysiology. Further, there is little evidence to suggest that current structural imaging techniques [e.g., magnetic resonance imaging (MRI)] can identify structural changes in the brain following concussion (cf., Schrader et al. 2009). However, a variety of functional deficits observed using brain imaging studies have been noted in concussion (Ptito et al. 2007).
A recent state-of-the-art conference directed at sports concussion in Zurich (2008) emphasized the need to explore residual long-lasting abnormalities and their neural underpinnings in clinically asymptomatic athletes (McCrory et al. 2009, Consensus Statement on Concussion in Sport 3rd International Conference, 2009). One important reason for this need is growing evidence suggesting persistent and diverse individually based symptomatology including deficits of executive functions (Lovell et al. 2003; Reddy and Collins 2009), and/or abnormal behavioral and neural profiles (Guskiewicz 2003; Guskiewicz and Mihalik 2006; Lovell et al. 2007; Slobounov et al. 2007, 2008, 2009) in subjects suffering from even a single episode of MTBI. Research on abnormal neuropsychological profiles in concussed individuals has primarily focused on deficits in working memory, attention, information processing speed, known to be linked to the frontal lobe (McAllister et al. 1999, 2001, 2006; Chen et al. 2004, 2007). Surprisingly, no reports on spatial memory deficits in concussed individuals have been published. This seemed strange to us given the spatial requirements of most sporting events.
As one navigates through space a variety of cortical systems are involved. Both behavioral and neuroimaging studies suggest that distinct brain areas become involved according to the nature of the task (Hartley et al. 2003; Janzen and Weststeijn 2007; Voermans et al. 2004). Utilizing research suggesting two distinct memory systems in navigation, one for route direction and one for relevant object location, Janzen and Weststeijn (2007) suggested that the inferior parietal gyrus, the anterior cingulated gyrus, and the right caudate nucleus are involved in coding of route direction whereas the parahippocampal gyri distinguish between relevant and irrelevant landmarks. A similar distinction based on both human and animal data suggests that the hippocampus is involved in acquiring a cognitive map of the environment whereas the caudate nucleus is involved in learning place appropriate responses leading to habitual behavior (Voermans et al. 2004). Another similar distinction has been made between wayfinding in humans which involves adopting a novel route and route following which involves following a previously used route suggests differential involvement of the hippocampus for the first and the caudate for the second (Hartley et al. 2003).
Brain imaging research with regards to alteration of cognitive functions in concussed individuals, particularly functional magnetic resonance imaging (fMRI), has revealed confusing and contradictory findings. For example, McAllister et al. (2001) have shown enhanced, primarily at prefrontal cortex (PFC), and a spread of blood oxygen level dependent (BOLD) responses in concussed subjects successfully performing cognitive tasks. Similarly, Jantzen et al. (2004) showed increased activation in the parietal and lateral frontal and cerebellar regions in concussed subjects when compared with pre-injury in absence of changes in cognitive performance. In contrast, Chen et al. (2004) reported the opposite findings suggesting a reduction of fMRI BOLD in the mid-dorsolateral PFC in symptomatic concussed subjects along with poorer performance on the working memory tasks. More recently, however, this group (Chen et al. 2007) reported additional activation in concussed subjects in the posterior brain regions including the left temporal lobe that were not present in the normal controls. Whether these contradictory results are related to the performance differences between normal controls and concussed individuals, working memory and baseline task differences and/or different contrasts used in these studies (e.g., subtraction of the experimental and baseline tasks) is still an open question. Further, there is a debate in the literature regarding the values of brain imaging data in clinical assessment of concussion among athletes (Consensus, Zurich, 2008). Therefore, there is a need to document consistent and atypical brain activation patterns in concussed individuals and to consider how these could be used in the clinical assessment of concussion.
In the present study, we aimed to demonstrate that residual functional abnormities may be observed in asymptomatic subjects who have recently suffered from even a single episode of sport-related concussion. Theoretically, this will help to clarify the nature of cortical activation following concussion. Specifically, we designed a fMRI study that incorporates spatial processing tasks using a virtual reality (VR) environment. The VR environment allows one to develop tasks in which the individual can control and manipulate movement along with the sense of self-motion while retaining the body fixation requirements of the fMRI environment (see Burgess et al. 2002). We developed VR-based spatial memory tasks including encoding and retrieval conditions enabling (a) the subjects to experience the sense of presence (Jancke et al. 2009) and (b) to track the changing brain activation patterns in both normal controls and concussed individuals during the exposure to these VR-driven spatial memory task conditions.
Materials and method
Subjects
For this fMRI study, 15 neurologically normal student-athletes with no history of MTBI (mean age 21.3 years) and 15 student-athletes (mean age 20.8 years) recently suffered from sport-related MTBI (collegiate rugby, ice hockey and soccer players) were recruited. The sample was 70% male and 30% female. All injured subjects suffered from grade 1 MTBI (Cantu Data Driven Revised Concussion Grading Guideline, 2006). Grade 1 MTBI was assigned to the cases in which there was no loss of consciousness (LOC), and post-traumatic amnesia was under 30 min. Inclusion criteria for this fMRI study were also the commonly accepted clinical symptoms of MTBI, such as: complaints of loss of concentration, dizziness, fatigue, headache, irritability, visual disturbances, and light sensitivity (Bryant and Harvey 1999). The initial assessment of MTBI was made on the field by Medical Personnel, and then, the detailed brain injury diagnosis was made based on physical, neuropsychological, and neurological examinations performed at the Penn State Center for Sport Medicine, PSU. MTBI subjects were scanned within 30 days post-injury. It should be noted that all MTBI subjects were clinically asymptomatic within 10 days post-injury and cleared for sport participation by the medical practitioners at the Penn State Center for Sport Medicine based upon neurological assessments (Co-operative Ataxia Rating Scale, World Federation of Neurology, Trouillas et al. 1997) as well as clinical symptoms resolution. All subjects were right handed according to Edinburgh Handedness Inventory (Oldfield 1971). The subjects signed an informed consent form and the protocol was approved by the Institutional Review Board of the Pennsylvania State University.
Virtual environment
The experimental paradigm used in this study consisted of a virtual corridor (Fig. 1) in which participants (a) were shown the navigation route to encode (E); (b) navigated randomly (RN); and (c) navigated purposefully with the goal of reaching specific target room location (active navigation, AN) using an MRI-compatible high-resolution joystick (http://www.magconcept.com). The subjects “moved” around using their right thumb to freely navigate in forward, backward, and side-to-side directions. During scanning, the subjects watched the visual scenes in a supine posture within the MRI scanner via a mirror mounted on the head coil. The virtual corridor was generated by a VTC Open GL developing kit (InnovativeVR, Inc, USA), which provided a realistic VR environment and sense of presence to the subjects.
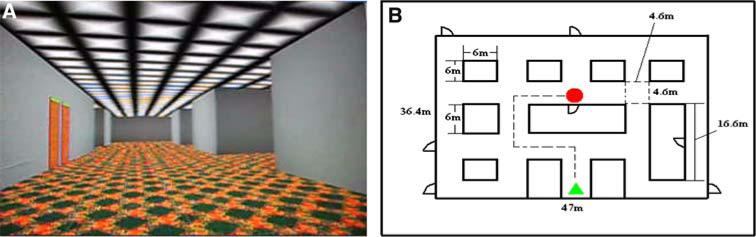
Fig. 1.
a View of the virtual corridor used for navigation tasks under study; and b floor plan, and a sample of the route for one of the runs. The subjects were instructed to reproduce (e.g., retrieval) the previously shown routes (e.g., encoding) via navigating through virtual corridor by MRI-compatible joystick and to find the target location. It should be noted that starting position was the same for all runs
Procedure
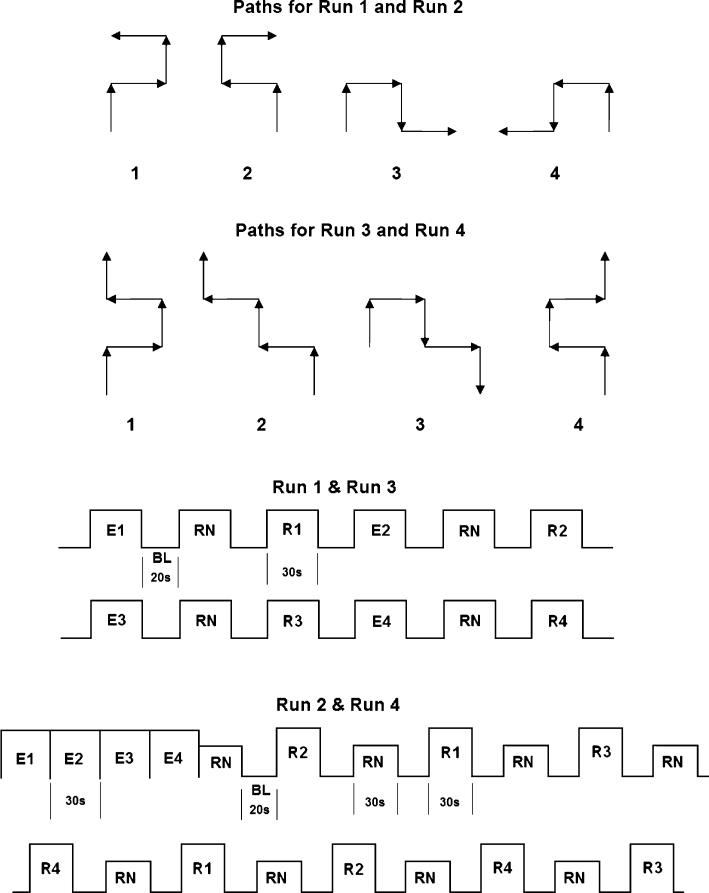
After being positioned in the scanner, the subjects practiced freely navigating (RN) in the virtual corridor. This scene was different than that used during actual experiment and was designed to familiarize them with joystick operations. There were four different runs of graduated memory load during the actual scanning with all of them consisting of (a) encoding (E), in which the subject was shown the navigation route to the target room; (b) retrieval (R), in which the subject was requested to navigate via VR corridor and reproduce the exact route to the target room; (c) free/random navigation (RN); and (d) baseline (BL), in which the subject was requested to track a white cross on black background via a joystick operated by the right thumb similar to previous conditions.
Schematics of four runs under study and required routes are shown in Fig. 3. In run 1, there were four different routes consisting of three turns presented to the subject to encode (E, 30 s) and retrieve (R, 30 s), combined with random navigation (RN, 30 s) and baseline (BL, 20 s) in block designs. The duration of run 1 was 10 min.
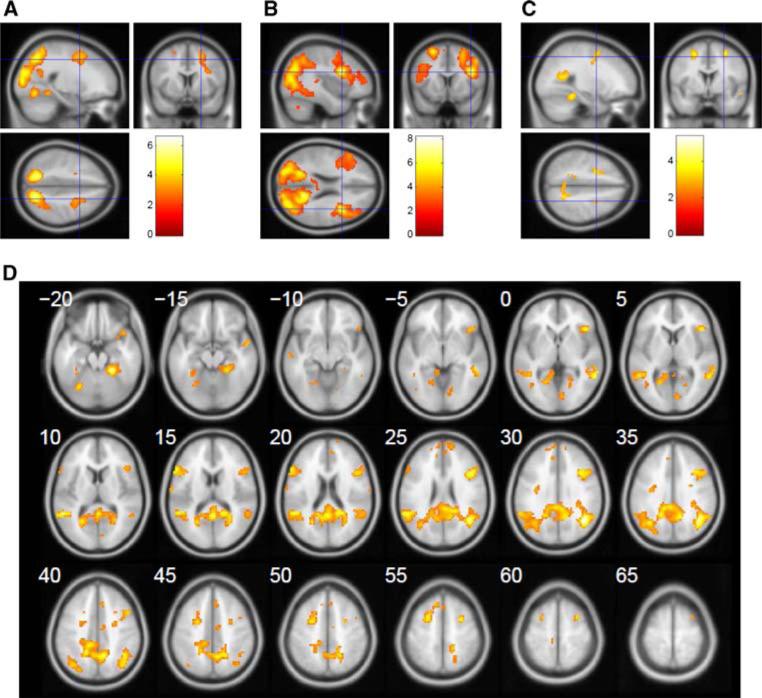
Fig. 3.
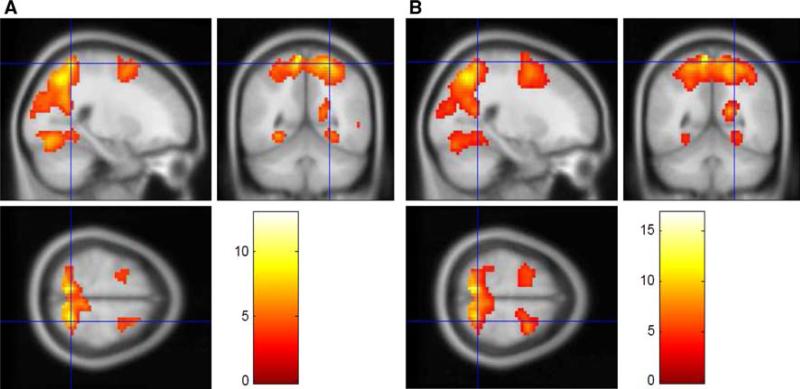
Sagittal, coronal and axial sections showing activation patterns during a encoding (E–BL) versus retrieval (R–RN) contrast, normal controls; b encoding (E–BL) versus retrieval (R–RN), contrast, MTBI subjects; c encoding MTBI–normal controls contrast. d Transversal images series of the contrast in (c). Note, significantly larger activation at several regions including the lateral extrastriate visual cortex V3 (33, –76, 19), extending to V2 (–21, –85, 22); premotor cortex bilaterally (–24, 5, 58 and 30, 2, 55); and right DL-PFC (45, 38, 22) during encoding in both groups. Also note additional activation during encoding in cerebellum (33, –64, –29) and left DL-PFC (–51, 23, 22) in MTBI subjects (c). These identified peaks were not present in normal control group. The regions shown are threshold at p < 0.001, uncorrected to show subthreshold extent of the activated regions. The color bar indicates the t statistic associated with each voxel and z score equivalent
In run 2, there were the same four routes, consisting of three turns, used in run 1 that were presented to the subject in one single block before other conditions (see Fig. 2 for details). This run 2 lasted for 16 min 12 s.
Fig. 2.
The routes (paths) for navigation tasks and sequences of stimuli presentation in block design used in four runs under study. E encoding, RN random navigation; R retrieval; BL baseline. Time of encoding and retrieval (30 s) and well as for baseline (20 s) was constant for all four runs
Runs 3 and 4 were identical in structures with runs 1 and 2. The only difference is that there were four turns in each route. The total time of scanning including four runs was 58.4 min. The order of runs was randomized between subjects.
MRI data acquisition
Functional and anatomical images were acquired on a 3.0 Tesla Siemens Trio whole-body scanner (Siemens, Erlangen, Germany) using a 12 channel head coil. The anatomical images of fMRI, T1, and T2 were acquired in the axial plane parallel with the anterior and posterior commissure axes covering the entire brain. Three-dimensional isotropic T1-weighted magnetization prepared rapid gradient echo (MP-RAGE 0.9 mm × 0.9 mm × 0.9 mm resolution, TE = 3.46 ms, TR = 2,300 ms, TI = 900 ms, flip angle = 9°, 160 slices, iPAT = none, NSA = 1, and FOV = 220 mm). Two-dimensional spoiled gradient recalled echo (SPGR 0.8 mm × 0.8 mm × 5 mm resolution, TE = 4 ms, TR = 328 ms, TI = 900 ms, flip angle = 15°, 30 slices, iPAT = none, NSA = 4, and FOV = 200 mm). Two-dimensional T2-weighted fast spin echo (0.7 mm × 0.7 mm × 5 mm resolution, TE = 90 ms, TR = 6,000 ms, flip angles = 90° and 120°, ETL = 18 and 30 slices, iPAT = GRAPPA, acceleration factor = 2, NSA = 1, and FOV = 220 mm). Identical two-dimensional BOLD echo planar fMRI images were obtained for each experimental task (3.1 mm × 3.1 mm × 5 mm resolution, TE = 25 ms, TR = 2,000 ms, EPI factor = 64, flip angle = 79°, 30 slices, iPAT = none, NSA = 1, and FOV = 220 mm). The functional images were matched to the structural SPGR images.
Assessment of “sense of presence” and subjective task complexity
All subjects were requested to report the illusion of forward self-motion and the sense of presence during VR navigation in the MR scanner following each task (Jancke et al. 2009). It should be noted that sense of presence is understood to refer the subjective felling of being in a virtual environment while being unaware of one's real location and surroundings as well as the technology that deliver the stream of virtual input to the senses (Wirth et al. 2007). Subjects were instructed to rate the strength of presence on a scale ranged from 0 (no presence) to 10 (very strong presence), similar to Witmer and Singer (1998). Paired t tests were used for the statistical analysis of differences in ratings that were given to two groups (MTBI and normal controls). In addition, all subjects were requested to report their subjective rating of runs complexity in the order from 1 to 4. All t tests were performed including Bonferroni correction with statistical set at p < 0.05.
Analysis of MRI data
Image processing and statistical analysis were conducted with Statistical Parameter Mapping (Friston et al. 1995) version 8 (2008, Wellcome Department of Cognitive Neurology, London UK; http://www.fil.ion.ucl.ac.uk/spm). All volumes obtained were used for data analysis. Pre-processing with SPM8 included realignment, co-registration, and spatial normalization (template of Montreal Neurological Institute, MNI). Then, a Gaussian filter of 8 mm full width at half maximum was applied to smooth the data spatially. Cerebral activation was rendered either onto T1 brain slices or on the surface of a standard MNI brain.
For the statistical analysis of regional differences in cerebral activation, all conditions including encoding (E), successful and unsuccessful retrievals (RS, RF), random navigation (RN), and baseline (BL) were input into the categorical general linear model (GLM) design at the subject level (Friston et al. 1995). Various contrasts between different tasks were computed for each subject. The probability threshold was set at p < 0.001, uncorrected. The minimum cluster extent (K) was set at 50 contiguous voxels. Those contrast images were then used in the second-level within-group analysis to create contrasts between normal controls and MTBI groups. One-sample t test was used to obtain activation pattern for each group. Then, we analyzed the activation differences between normal controls and MTBI subjects by a full factorial analysis. Contrasts: [(E > BL)TBI > (E > BL)NC], [(RS > BL)TBI > (RS > BL)NC], [(RS > RN)TBI > (RS > RN)NC], [(E > RS)TBI > (E > RS)NC], and vice versa were calculated in the second-level analysis. Xjview (http://www.alivelearn.net-/xjview/), a SPM viewer toolbox, was used to view and generate the sectional images.
Region of interest (ROI) analysis was derived using the SPM 2 toolbox: PickAtlas (Maldjian et al. 2003) and Mars-BaR (Brett et al. 2002). First, we generated ROI masks of occipital cortex, parietal cortex, premotor cortex, dorsolateral PFC (DL-PFC), and hippocampus with PickAtlas. Second, those masks were used to find the activated voxel clusters of various conditions or contrasts. For conditions E and R, we collected the cluster sizes and percent BOLD signal changes of the ROIs for each subject using the Mars-BaR. Within-group statistical analysis was based on one-sample t tests to find the significant differences between normal controls and MTBI subjects. The probability threshold was set at p < 0.001, uncorrected.
Results
Behavioral data
No differences were observed in terms of the accuracy of task performance as well as the time to complete the spatial memory navigation task between groups. Specifically, the success rate of task performance (i.e., accurate navigation to target location for all four runs under study) was 94.2 and 92.9% for concussed subjects and normal controls, respectively. Two-way ANOVA revealed that the main effect of group was not significant [F(1,88) = 1.62, p = 0.93]. Similarly, the main effect of run was also not significant, F(3,88) = 1.17, p = 0.2, indicating that success rate was not affected by differential memory load. Similarly, averaged time to successfully reach the target location was not significantly different between groups (i.e., 19.51 and 19.76 s, respectively). Two-way ANOVA revealed that the main effect of group was not significant (p > 0.05). However, the main effect of run was significant, F(3,88) = 21.41, p < 0.05, which is not surprising considering the different number of turns to reach the target in runs 1 and 2 versus runs 3 and 4.
Sense of presence and subjective rate of runs complexity
Both concussed and normal control subject reports indicated that navigation via immersive VR environment induced a strong sense of presence. The scores for sense of presence were 6.8 (±2.4) for normal controls and 7.4 (±2.7) for concussed subjects. Rating for the sense of presence was not significantly different between groups (p > 0.05). Ninety-five percent of normal controls and 92% of concussed subjects graded the degree of task complexity in the order of run 1, run 3, run 2, and run 4. That said, all of the subjects under study reported that amount of mental effort was not significantly different during these four runs.
fMRI data (group analysis)
Whole brain analysis comparing cerebral activation between the two major conditions (i.e., encoding and retrieval) with respect to baseline/random navigation revealed significant increase during encoding in several brain regions, including the lateral extrastriate visual cortex (with putative area V3) extending into the visual area V2, bilateral parietal cortex, precuneus bilaterally, and right DL-PFC. The anatomical location of the activation peaks was identified by superposing activation maps onto high resolution anatomical MRI. Table 1 summarizes the location of the activation peaks expressed in standardized stereotaxic coordinates of Talairach and Tournoux, their t values, as well as their anatomical loci for both groups of subjects. Clearly, overall increased activation in several brain regions during encoding versus retrieval was similar for both normal controls and concussed individuals.
Table 1.
Significant results of fMRI analysis for encoding (E) versus baseline (BL) contrasts
| Anatomical area | BA | Cluster |
Peak |
Coordinates | ||||
|---|---|---|---|---|---|---|---|---|
| p (cor) | K | p (unc) | p (cor) | t score | p (unc) | [x, y, z] (mm) | ||
| Normal control groupa | ||||||||
| Visual cortex V3 | 19 | 0.0000 | 754 | 0.0000 | 0.0028 | 8.40 | 0.0000 | [33; –76; 19] |
| Visual cortex V2 | 18 | 0.0118 | 7.44 | 0.0000 | [–21; –85; 22] | |||
| Visual cortex V1 | 17 | 0.0194 | 7.10 | 0.0000 | [0; –82; –5] | |||
| Premotor cortex (R) | 6 | 0.0000 | 125 | 0.0000 | 0.0107 | 7.51 | 0.0000 | [30; 2; 55] |
| DL-PFC (R) | 46 | 0.0425 | 6.58 | 0.0000 | [45; 38; 22] | |||
| DL-PFC (R) | 9 | 0.2282 | 5.45 | 0.0000 | [36; 5; 43] | |||
| Premotor cortex (L) | 6 | 0.0399 | 92 | 0.0088 | 0.0132 | 7.37 | 0.0000 | [–24; 5; 58] |
| Parietal lobe (R) | 7 | 0.0000 | 325 | 0.0000 | 0.051 | 6.46 | 0.0000 | [18; –55; 64] |
| Precuneus (R) | 7 | 0.054 | 6.42 | 0.0000 | [18; –73; 49] | |||
| Parietal lobe (L) | N/A | 0.0000 | 70 | 0.0010 | 0.085 | 6.12 | 0.0000 | [–15; –61; 46] |
| Lateral ventricle | N/A | 0.7701 | 11 | 0.3169 | 0.897 | 4.08 | 0.0004 | [–21; –46; –11] |
| MTBI groupb | ||||||||
| Middle frontal gyrus (R) | N/A | 0.0000 | 1207 | 0.0000 | 0.0000 | 13.80 | 0.0000 | [30; 2; 55] |
| DL-PFC (R) | 46 | 0.0001 | 10.90 | 0.0000 | [36; 17; 31] | |||
| Premotor cortex (R) | 6 | 0.0001 | 8.91 | 0.0000 | [36; 9; 55] | |||
| Premotor cortex (L) | 6 | 0.0000 | 435 | 0.0000 | 0.0000 | 13.20 | 0.0000 | [–24; 5; 58] |
| Visual cortex V1 | 17 | 0.0000 | 5515 | 0.0000 | 0.0002 | 10.01 | 0.0000 | [0; –82; –5] |
| Visual cortex V1 | 17 | 0.0012 | 8.89 | 0.0000 | [6; –85; 7] | |||
| Visual cortex V2 | 18 | 0.0014 | 8.82 | 0.0000 | [–6; –88; 7] | |||
| Precuneus (R) | 7 | 0.0000 | 283 | 0.0000 | 0.0002 | 9.91 | 0.0000 | [21; –55; 46] |
| Calcarine (L) | N/A | 0.0000 | 23 | 0.0002 | 0.0010 | 9.01 | 0.0000 | [–21; –61; 7] |
| Sub-gyral | N/A | 0.0035 | 8.27 | 0.0000 | [–24; –61; 19] | |||
| DL-PFC (L) | 45 | 0.0170 | 33 | 0.0180 | 0.0000 | 5.54 | 0.0000 | [–51; 23; 22] |
| Precuneus (L) | 7 | 0.0000 | 28 | 0.0001 | 0.0033 | 8.31 | 0.0000 | [–18; –76; 37] |
| Mid. occipital Lobe (L) | N/A | 0.0049 | 8.05 | 0.0000 | [–30; –73; 25] | |||
| Cerebellum | N/A | 0.0036 | 11 | 0.3565 | 0.0062 | 7.89 | 0.0000 | [33; –64; –29] |
Coordinates refer to the Montreal Neurological Institute MRI template. Results threshold is set at p < 0.0001, uncorrected
BA, Brodmann areas; K, cluster sizes in number of voxels; p (cor), FWE-corrected p value; p (unc), uncorrected p value
Significant clusters and peaks from the normal control group analysis
Significant clusters and peaks from the MTBI group
There were shared areas of activation during encoding versus retrieval in both normal controls and concussed subjects (see also Fig. 3a, b). However, direct comparison between groups revealed that the concussed subjects show significantly increased activation of right DL-PFC (42, 14, 36), and left parietal cortex (–36, –64, 37) during encoding. Moreover, there was additional activation of the left DL-PFC (–42, 14, 36) and cerebellum (19, –38, –19) during encoding that was not observed in normal controls (see also Fig. 3c). Detailed coordinates of these additional clusters in concussed subjects are specified in Table 1b.
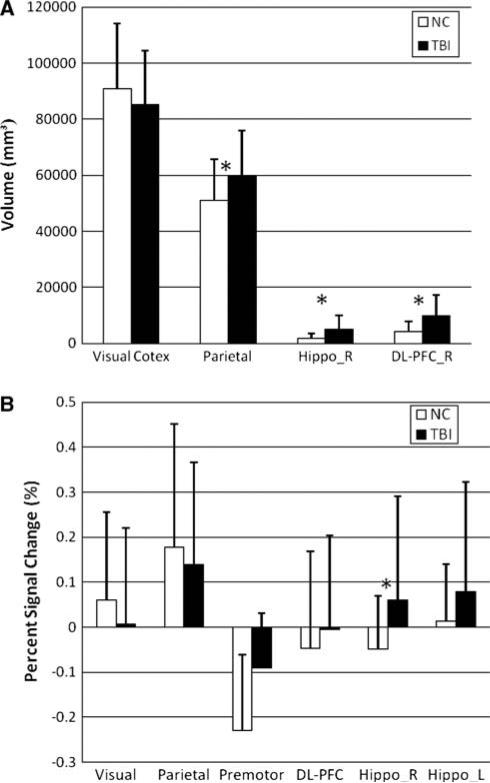
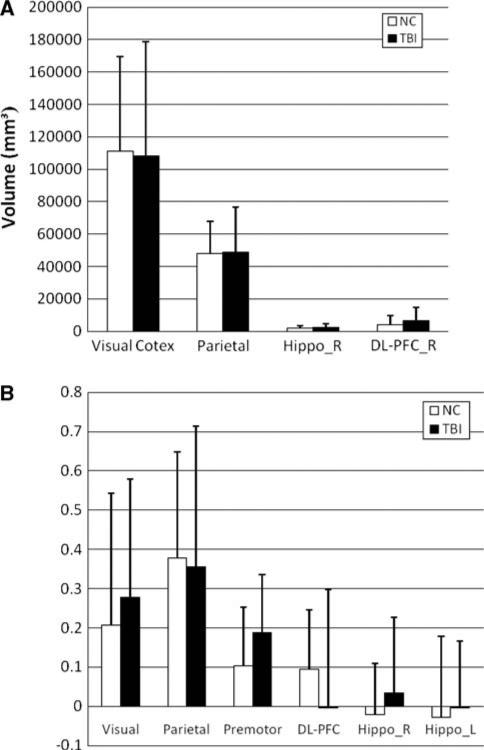
Despite the overall similarity in the topographical activation patterns of these two groups, visual and statistical inspection of peaks suggested that the concussed athletes showed larger encoding related brain activation networks with respect to baseline. Statistically, there were significantly larger cluster sizes around the shared regions of activation during encoding in MTBI subjects (e.g., parietal cortex, right DL-PFC, p < 0.001, see Fig. 4a). The cluster size around another common region of activation (e.g., visual cortex) was not significantly different during encoding in MTBI group (p > 0.05). BOLD signal percent change from baseline for share regions of activation during encoding is shown in Fig. 4b. Statistically, there were no significant differences observed between groups at most of the shared areas of activation (p > 0.05), except that of the hippocampus (p < 0.001).
Fig. 4.
a Cluster size changes during encoding at certain ROI for normal controls and MTBI groups; Note a significantly larger cluster size asterisk at parietal cortex, right hippocampus and right DL-PFC in MTBI subjects; b BOLD signal % change during encoding at shared areas of activation for normal control and MTBI subjects. Note a significant increase of BOLD signal in MTBI subjects at hippocampus
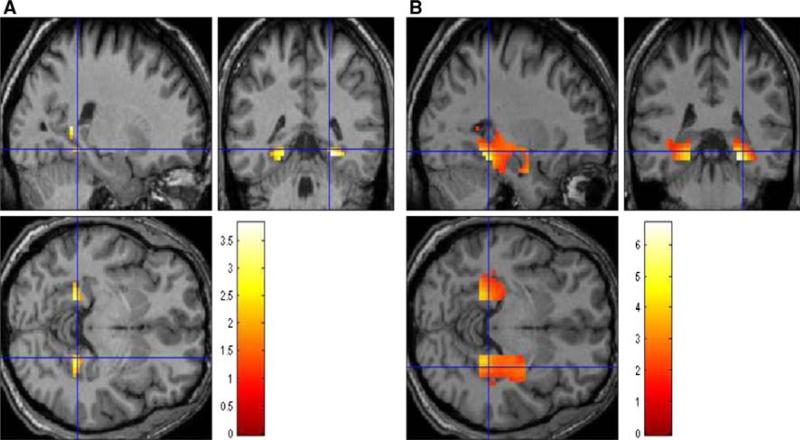
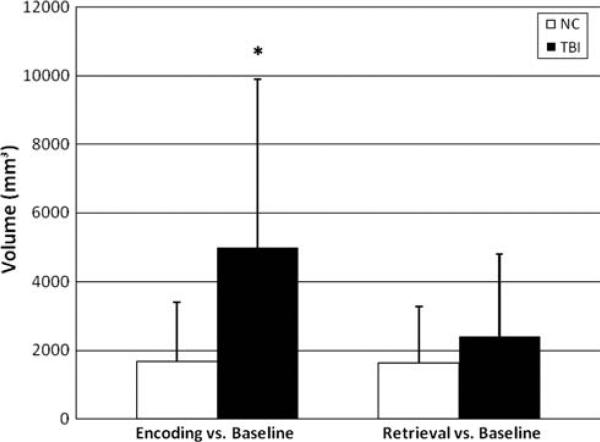
Results of complementary group analysis on activation of hippocampus ROI is shown in Fig. 5. Visually, there was bilateral hippocampal activation during encoding in both groups. Despite this similarity, statistically, there was significantly larger BOLD signal percent change in right hippocampus in concussed subjects (p < 0.001). Moreover, the cluster size around the hippocampal ROI was significantly larger in concussed subjects (p < 0.001, see also Fig. 6). Neither cluster sizes nor BOLD signal percent change were different between groups during retrieval (p > 0.05).
Fig. 5.
ROI hippocampus showing the sagittal, coronal and axial sections during encoding (E–BL); a normal controls; and b MTBI subjects. Bilateral activation of hippocampus is present in both groups. Note an increased hippocampal region of activation in MTBI subjects
Fig. 6.
Focusing on hippocampal area, cluster sizes differences during encoding (E–BL) and retrieval (R–BL) between groups. Note a significantly larger cluster size in MTBI subjects only during encoding
BOLD analysis during retrieval condition in both normal controls and MTBI group revealed an increased activation in several shared regions including occipital area (V1, V2 and V3), precuneus bilaterally, parital cortex bilateral, and premotor cortex bilaterally (see also Fig. 7; details are presented in Table 2). Percent BOLD signal change during retrieval versus baseline was not significant between groups at the shared regions of activation (e.g., visual cortex, parietal cortex, premotor cortex; p > 0.05). Similarly, the clusters size differences around these shared ROI were not significant among groups (p > 0.05; see also Fig. 8).
Fig. 7.
Sagittal, coronal and axial sections showing activation patterns during a retrieval (R–RN) contrast, normal controls; b retrieval (R–RN), contrast, MTBI subjects. Significant change in activation regions including the lateral extrastriate visual cortex V3 (–30, 85, 16), extending to V2 (–15, 91, 16); premotor cortex bilaterally (–27, –7, 55 and 21, 5, 58); during retrieval in both groups. The regions shown are threshold at p < 0.001, uncorrected to show subthreshold extent of the activated regions. The color bar indicates the t statistic associated with each voxel and z score equivalent
Table 2.
Significant results of fMRI analysis for retrieval (R) versus baseline (BL)
| Anatomical area | BA | Cluster |
Peak |
Coordinates | ||||
|---|---|---|---|---|---|---|---|---|
| p (cor) | K | p (unc) | p (cor) | t score | p (unc) | [x, y, z] (mm) | ||
| Normal control groupa | ||||||||
| Parietal lobe (L) | N/A | 0.0000 | 321 | 0.0000 | 0.0000 | 13.0699 | 0.0000 | [–18; –67; 55] |
| Precuneus (L) | N/A | 0.0002 | 10.0057 | 0.0000 | [–9; –55; 64] | |||
| Visual cortex V3 | 19 | 0.012 | 7.26 | 0.0000 | [–30; –85; 16] | |||
| Visual cortex V2 | 18 | 0.024 | 6.79 | 0.0000 | [–15; –91; 16] | |||
| Parietal lobe (R) | N/A | 0.0000 | 389 | 0.0000 | 0.0001 | 10.2524 | 0.0000 | [18; –70; 49] |
| Somatosensory area (R) | 7 | 0.0004 | 9.5417 | 0.0000 | [18; –55; 64] | |||
| Precuneus (R) | N/A | 0.0013 | 8.8183 | 0.0000 | [12; –64; 58] | |||
| Visual cortex V2 | 18 | 0.0000 | 167 | 0.0000 | 0.0005 | 9.4005 | 0.0000 | [3; –94; 7] |
| Visual cortex V2 | 18 | 0.0033 | 8.1573 | 0.0000 | [15; –94; 13] | |||
| Angular gyrus | 39 | 0.0000 | 117 | 0.0000 | 0.0007 | 9.2601 | 0.0000 | [36; –76; 28] |
| Fusiform gyrus | 19 | 0.0018 | 8.5804 | 0.0000 | [–21; –64; –11] | |||
| Cerebellum | N/A | 0.0136 | 7.1734 | 0.0000 | [–30; –64; –23] | |||
| Visual cortex V2 | 18 | 0.0000 | 41 | 0.0008 | 0.0106 | 7.342 | 0.0000 | [24; –76; –14] |
| Premotor cortex(R) | 6 | 0.0070 | 53 | 0.0100 | 0.065 | 6.13 | 0.0000 | [21; 5; 58] |
| Premotor cortex (L) | 6 | 0.1360 | 11 | 0.2020 | 0.326 | 5.03 | 0.0000 | [–27; –7; 55] |
| MTBI groupb | ||||||||
| Parietal lobe (L) | N/A | 0.0000 | 945 | 0.0000 | 0.0000 | 16.8451 | 0.0000 | [–18; –67; 55] |
| Somatosensory area (R) | 7 | 0.0000 | 14.3684 | 0.0000 | [21; –64; 49] | |||
| Precuneus (L) | N/A | 0.0000 | 13.3314 | 0.0000 | [–9; –58; 64] | |||
| Precuneus (R) | N/A | 0.0000 | 13.63 | 0.0000 | [18; –70; 52] | |||
| Cuneus | N/A | 0.0000 | 166 | 0.0000 | 0.0000 | 12.2792 | 0.0000 | [6; –79; 1] |
| Visual cortex V2 | 18 | 0.0001 | 10.4125 | 0.0000 | [3; –91; 4] | |||
| Angular gyrus | 39 | 0.0000 | 71 | 0.0000 | 0.0003 | 9.8377 | 0.0000 | [36; –73; 31] |
| Visual cortex V3 | 19 | 0.0015 | 8.7286 | 0.0000 | [42; –79; 16] | |||
| Visual cortex V3 | 19 | 0.0000 | 31 | 0.0003 | 0.0003 | 9.7178 | 0.0000 | [–21; –64; –14] |
| Cerebellum | N/A | 0.0011 | 8.94 | 0.0000 | [–30; –67; –23] | |||
| Premotor cortex (R) | 6 | 0.0000 | 136 | 0.0007 | 0.0006 | 9.3099 | 0.0000 | [30; –1; 58] |
| Calcarine (R) | N/A | 0.0002 | 11 | 0.0172 | 0.0006 | 9.3064 | 0.0000 | [18; –55; 16] |
| Premotor cortex (L) | 6 | 0.0000 | 89 | 0.0044 | 0.0019 | 8.553 | 0.0000 | [–27; –4; 55] |
| Visual cortex V3 | 19 | 0.0001 | 15 | 0.0068 | 0.0024 | 8.3746 | 0.0000 | [–27; –88; 13] |
Coordinates refer to the Montreal Neurological Institute MRI template. Results threshold is set at p < 0.0001, uncorrected
BA, Brodmann areas; K, cluster sizes in number of voxels; p (cor), FWE-corrected p value; p (unc), uncorrected p value
Significant clusters and peaks from the normal control group analysis
Significant clusters and peaks from the MTBI group
Fig. 8.
a Cluster size changes during retrieval at certain ROI for normal controls and MTBI groups; b BOLD signal % change during retrieval at shared areas of activation for normal control and MTBI subjects. Note no significant differences between groups observed for both cluster size and BOLD signal percent change (p > 0.05)
Discussion
It is important to better articulate the functional alterations of spatial memory processes in MTBI since concussions are often found in sporting events which require a high degree of spatial processing (i.e., rugby, ice hockey, soccer, etc.). Accordingly, in the present study, we utilized a VR environment requiring navigation to the destination location in individuals with and without concussions in order to study the neural underpinning of encoding and retrieval of spatial information. We were particularly interested in differential involvement of the brain regions and alteration of cortical networks in concussed individuals as reflected in fMRI data. The first goal we explored was to articulate the cortical areas involved in the encoding of spatial information. To answer this question, we compared the “active encoding” task requiring the subjects to memorize various spatial routes with a passive one (baseline task) or just navigate randomly within virtual environment by moving the joystick without a goal. The second goal we explored was to articulate the cortical areas involved in the retrieval of spatial information via examining the brain activation patterns during navigation via VR environment. The third goal was to articulate the manner in which individuals with and without concussion performed the spatial navigation tasks.
The behavioral data were used as a manipulation check to ensure that the fMRI differences observed in our study were reflective of the manner in which the normal controls and the individuals with concussion “cortically” performed the tasks and not the results of performance alone. First, both groups reflected a similar sense of presence in the VR environment which suggests that they were able to actively be a part of the VR situation and not be distracted by external mechanical characteristics of the scanner environment such as noise or a personal sense of discomfort. Second, both groups showed similar performance abilities in terms of accuracy and timing to reach the target location. These behavioral findings helped us to rule out alternative explanations and to view the fMRI data as reflective of manner in which the normal control and concussion groups cognitively processed the tasks.
There are several findings of interest from this study. First, the whole brain analysis comparing cerebral activation between the two major conditions (i.e., encoding and retrieval) with respect to baseline/random navigation revealed significant increases during encoding in the several brain regions, including the lateral extrastriate visual cortex (with putative area V3) extending into the visual area V2, bilateral parietal cortex, precuneus bilaterally, and right DL-PFC. This was true for both normal controls and concussed individuals. This observation is consistent with the visual requirements of the spatial navigation task as well as the previously reported evidence that shows involvement of the frontal areas in all types of spatial memory (Kessels et al. 2000a, b). An increased activation during encoding versus retrieval may reflect the enhanced “cerebral challenge” during encoding to meet the task demands (Hillary et al. 2006). Additionally, percent BOLD signal change during retrieval versus baseline was not significant for both groups at the shared regions of activation. Similarly, the cluster size differences around these common ROI during retrieval were not significant among groups. In fact, the brain-injured patients with more pronounced encoding deficits had poorer recovery rate than those with retrieval deficits. Future research may expand these findings since successful retrieval is a component of the default mode of the brain, but successful encoding is not (Daselaar et al. 2009).
Second, despite the common areas of activation during encoding in both normal controls and concussed subjects, the concussed subjects have significantly larger activation at right DL-PFC and left parietal cortex. Moreover, we observed an additional activation of cerebellum and left DL-PFC during encoding in MTBI subjects that was not observed in normal controls. This is consistent with a variety of studies showing enhanced PFC involvement in working memory with clinical populations (see Hillary 2008 for a theoretical overview). In fact, in healthy individuals, activity in the PFC is reduced as performance improves and the task becomes routine. In our study, despite the overall similarity in the topographical activation patterns, its visual inspection suggested that the concussed athletes showed larger encoding related cortical networks. Statistically, there was significantly larger cluster size around the share regions of activation during encoding in MTBI subjects (e.g., parietal cortex, right DL-PFC) but not the visual cortex. This is particularly intriguing in that performance and timing was similar for both groups. Whether the concussed group recruited additional cerebral resources to perform at the same level of competency as the control group remains an open question. For example, the PFC has been shown to be involved in working memory related to a top-down control of attention in terms of internal representations of sensory stimuli and motor plans that are stored in more posterior regions (Curtis and D'Esposito 2003). Thus, future research can better articulate which aspect of cognitive processing is compromised during concussion. It is also intriguing to note that larger cortical networks also emerge during memory tasks in older adults (see Dennis and Cabeza 2007 for an overview).
Third, in terms of percent BOLD signal change from baseline during encoding, there were no significant differences observed between the groups at most of the shared areas of activation except for the hippocampus. Visually, there was bilateral hippocampal activation during encoding in both groups. Further, despite this similar pattern of activation, there was statically significantly larger BOLD signal percent change in right hippocampus in concussed subjects. Moreover, the cluster size around the hippocampal ROI was significantly larger in concussed subjects. The overall hippocampal activity is consistent with results from a variety of studies showing hippocampus activity during the processing of spatial information (Hayes et al. 2004; Iaria et al. 2007; Parslow et al. 2004).
An increased right hippocampal activity in concussed individual during encoding is intriguing considering the number of previous studies suggesting that successful wayfinding individuals would preferentially engage the right posterior hippocampus during navigation task performance (McNamara and Shelton 2003). Also, hippocampal activation was suggested as a reflection of the formation of mental representation of the environment (i.e., cognitive map) that plays a critical role during human navigation (Iaria et al. 2007). That said, it should be noted that characterizing the functions of the hippocampus with respect to human topological orientation is still a matter of controversy (see Burgess et al. 2002 for review), which is beyond the scope of this report.
Overall, our major findings are consistent with majority of previous reports indicating increased neural activity and/ or more elaborate neural network (i.e., dispersion) with additional neural recruitment of DL-PFC in clinical sample associated with performance of cognitive tasks. It should be noted, however, that a previous report indicated a reduction of working memory-related BOLD signal at the right DLPFC in symptomatic concussed subjects with persistent post-concussive symptoms (Chen et al. 2004). There are several possible candidates to explain the enlargement of cerebral activation in absence of encoding/retrieval deficits in asymptomatic concussed individuals, including brain reorganization and/or compensation (Audoin et al. 2003 McAllister et al. 2001; Pantano et al. 2006), generalized cortical disinhibition (Chiaravalloti et al. 2005; Hillary et al. 2003), task-related navigation network (Burgess et al. 2002), and more lately cognitive control accommodating neural dysfunction (Hillary 2008) hypotheses.
Specifically, one possibility is that the larger cluster sizes found in individuals with concussion represent initial attempts by the brain to imitate a process of cortical reorganization. Within the scope of this hypothesis, additional areas of recruitment reflect presumably permanent changes in the brain functional network enabling the individual to perform the task at the same high level of competency. For example, in longitudinal fMRI studies of stroke patients, an initial hyperactivation is observed which becomes more focal after recovery (see Ward 2005 for an overview). Likewise, Sanchez-Carrion et al. (2008) studied the patients after severe diffuse traumatic injury and normal controls over a 6 month interval. Similar to our study, they found no MRI evidence of focal lesions. However, significant changes in brain activation over time were observed in their patients, but not in controls. Difference between TBI and controls had decreased significantly after 6 months. It is premature for us to fully accept the brain reorganization hypothesis to explain our major findings since neither a longitudinal study design was implemented nor do we have data available to determine whether the changes we observed in concussed individuals are permanent or transitory. Indeed, future studies need to employ longitudinal designs to test the brain reorganization hypothesis, in general, and to address important questions regarding the time course, magnitude, and nature of the brain dysfunctions in clinical subjects’ population, in specific (Hillary 2008).
The compensation hypothesis also assumes the enlarged recruitment of neural resources to facilitate the task performance. However, these alterations are seen as transitory and should be observed during the period of cerebral challenge, as opposed to permanent injury-specific changes in the functional network. Moreover, in order to test this hypothesis, various degrees of cerebral challenge cognitive tasks should be implemented in a manner that increased activations in clinical subjects and normal controls are tied to parametric increases in the task load (see Perlstein et al. 2004 for details). In our study, although differential spatial memory load during encoding was implemented, we observed neither facilitated task performance nor differential patterns of brain activation as a function of memory load in both groups of subjects. Therefore, our findings do not explicitly support the compensation hypothesis.
In conclusion, it is feasible to suggest that our major findings favor a task-related navigation network hypothesis. The common regions of activation during encoding and retrieval of spatial information may indicate a consistent brain network, including visual cortex, parietal cortex, DLPFC, and hippocampal cortices, which is in charge of competency of spatial navigation tasks. Either structural/functional disruption of connectivity or deficits within some focal clusters in this network may be caused by a concussive blow. In fact, departure from small-world network, as evidenced by reduced clustering coeYcient (Cp) as an index of local structure and increased path length (Lp) as an index of graph integration, has been recently documented in a number of clinical studies (Zhou et al. 2008; Rosenbaum et al. 2008) including concussion (Cao and Slobounov 2009). Moreover, there is direct evidence suggesting that track-specific variation in microstructural white matter integrity among subjects suffering from concussion (DTI study) can account for the variation in performance of cognitive tasks (Niogi et al. 2008). Also, there is evidence of focal neuronal loss and altered cellular metabolism (magnetic resonance spectroscopy studies) within 30 days post-injury in normally appearing, based on conventional MRI, concussed individuals (Garnett et al. 2000, 2001; Bluml and Brooks 2006; Kirov et al. 2007; Tollard et al. 2009). The need for eYcient performance of navigation task during this cerebral challenge may give rise to the hyperactivation of focal clusters and to recruitment of additional cerebral resources. In fact, both cluster size and BOLD signal percent increase at common ROI during encoding were documented in our study. Whether the observed alteration of task-related navigation network is relatively transient in acute state of brain injury or long-term persistent functional abnormality is yet to be determined.
Acknowledgments
This study was supported by NIH Grant RO1 NS056227-01A2 “Identification of Athletes at Risk for Traumatic Brain Injury” awarded to Dr. Slobounov, PI. We would like to thank Elena Slobounov for VR programming and Dr. Susan Lemieux for fMRI design development. All human studies have been approved by the The Pennsylvania State University IRB and all subjects gave their informed consent prior to their inclusion in the study.
Contributor Information
Semyon M. Slobounov, Department of Kinesiology, The Pennsylvania State University, 19 Recreation Building, University Park, PA 16802, USA Center for Sport Medicine and Hershey Medical School, The Pennsylvania State University, University Park, USA.
K. Zhang, Department of Kinesiology, The Pennsylvania State University, 19 Recreation Building, University Park, PA 16802, USA
D. Pennell, Chandlee Laboratory, Social, Life and Engineering Sciences Imaging Center, The Pennsylvania State University, University Park, PA 16802, USA
W. Ray, Department of Psychology, The Pennsylvania State University, 612 Moore Building, University Park, PA 16802, USA
B. Johnson, Department of Kinesiology, The Pennsylvania State University, 19 Recreation Building, University Park, PA 16802, USA
W. Sebastianelli, Center for Sport Medicine and Hershey Medical School, The Pennsylvania State University, University Park, USA
References
- Audoin B, Ibarrola D, Ranjeva JP, Confort-Gouny S, Malikova I, Ali-Cherif A, Pelletier J, Cozzone P. Compensatory cortical activation observed by fMRI during a cognitive task at the earliest stage of MS. Hum Brain Mapp. 2003;20:51–58. doi: 10.1002/hbm.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluml S, Brooks W. Magnetic resonance spectroscopy of traumatic brain injury and concussion. In: Slobounov S, Sebastianelli W, editors. Foundations of sport-related brain injuries. Spinger; NY: 2006. pp. 197–220. [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox. Presented at the 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. 2–6 June; 2002. [abstract] Available on CD-ROM in Neuroimage. [Google Scholar]
- Bryant R, Harvey A. Postconcussive symptoms and posttraumatic stress disorder after mind traumatic brain injury. J Nerv Ment Dis. 1999;187:302–305. doi: 10.1097/00005053-199905000-00006. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire A, O'Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Cantu R. Concussion classification: ongoing controversy. In: Slobounov S, Sebastianelli W, editors. Foundations of sport-related brain injuries. Springer; NY: 2006. pp. 87–111. [Google Scholar]
- Cao C, Slobounov S. Alteration of cortical functional connectivity as a result of traumatic brain injury revealed by graph theory, ICA and sLORETA analyses of EEG signals. IEEE. 2009 doi: 10.1109/TNSRE.2009.2027704. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JK, Johnston KM, Frey S, Petrides M, Worsley K, Ptito A. Functional abnormalities in symptomatic concussed athletes: an fMRI study. Neuroimage. 2004;22:68–82. doi: 10.1016/j.neuroimage.2003.12.032. [DOI] [PubMed] [Google Scholar]
- Chen J-K, Johnston K, Collie A, McCrory P, Ptito A. A validation of the post concussion symptom scale in the assessment of complex concussion using cognitive testing and functional MRI. J Neurosurg Psychiatry. 2007;78:1231–1238. doi: 10.1136/jnnp.2006.110395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaravalloti N, Hillary F, Ricker J, Christodoulou C, Kalnin A, Liu WC, et al. Cerebral activation patterns during working memory performance in multiple sclerosis using fMRI. J Clin Exp Neuropsychol. 2005;27(1):33–54. doi: 10.1080/138033990513609. [DOI] [PubMed] [Google Scholar]
- Curtis C, D'Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci. 2003;7:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- Daselaar S, Prince S, Dennis N, Hayes S, Kim H, Cabeza R. Posterior midline and ventral parietal activity is associated with retrieval success and encoding failure. Front Hum Neurosci. 2009;3:1–10. doi: 10.3389/neuro.09.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis N, Cabeza R. Neuroimaging of healthy cognitive aging. In: Craik F, Salthouse T, editors. The handbook of aging and cognition. 3rd edn. Psychology Press; New York: 2007. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, POline J-P, Frithh CD, Frackowiak RSJ. Statistical parametric maps in functional neuroimaging. A general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Garnett MR, Blamire AM, Corkill RG, et al. Early proton magnetic resonance spectroscopy in normal-appearing brain correlates with outcome in patients following traumatic brain injury. Brain. 2000;123:2040–2054. doi: 10.1093/brain/123.10.2046. [DOI] [PubMed] [Google Scholar]
- Garnett MR, Corkill RG, Blamire AM, et al. Altered cellular metabolism following traumatic brain injury: a magnetic resonance spectroscopy study. J Neurotrauma. 2001;18:231–240. doi: 10.1089/08977150151070838. [DOI] [PubMed] [Google Scholar]
- Guskiewicz K. Assessment of postural stability following sport-related concussion. Curr Sport Med Rep. 2003;2(1):24–30. doi: 10.1249/00149619-200302000-00006. [DOI] [PubMed] [Google Scholar]
- Guskiewicz K, Mihalik J. The biomechanics and pathomechanics of sport-related concussion: looking at history to build the future. In: Slobounov S, Sebastianelli W, editors. Foundations of sport-related brain injuries. Springer; NY: 2006. pp. 65–84. [Google Scholar]
- Hartley T, Maguire E, Spiers H, Burgess N. The well-worn route and the path less traveled: distinct neural bases of route following and wayfinding in humans. Neuron. 2003;37:877–888. doi: 10.1016/s0896-6273(03)00095-3. [DOI] [PubMed] [Google Scholar]
- Hayes S, Ryan L, Schnyer D, Nadel L. An fMRI study of episodic memory: retrieval of object, spatial and temporal information. Behav Neurosci. 2004;118:885–896. doi: 10.1037/0735-7044.118.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillary FG. Neuroimaging of working memory dysfunction and the dilemma with brain reorganization hypotheses. J Int Neuropsychol Soc. 2008;14(4):526–534. doi: 10.1017/S1355617708080788. [DOI] [PubMed] [Google Scholar]
- Hillary FG, Chiaravalloti ND, Ricker JH, Steffener J, Bly BM, Lange G, et al. An investigation of working memory rehearsal in multiple sclerosis using fMRI. J Clin Exp Neuropsychol. 2003;25(7):965–978. doi: 10.1076/jcen.25.7.965.16490. [DOI] [PubMed] [Google Scholar]
- Hillary FG, Genova HM, Chiaravalloti ND, DeLuca J. Prefrontal modulation of working memory performance in brain injury and disease. Hum Brain Mapp. 2006;27:837–847. doi: 10.1002/hbm.20226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaria G, Chen J, Guariglia C, Ptito A, Petrides M. Retrosplenial and hippocampal brain regions in human navigation: complementary functional contributions to the formation and use of cognitive maps. Eur J Neurosci. 2007;25:890–899. doi: 10.1111/j.1460-9568.2007.05371.x. [DOI] [PubMed] [Google Scholar]
- Jancke L, Cheetham M, Baumgartner T. Virtual reality on the role or prefrontal cortex in adults and children. Front Neurosci. 2009;3(1):52–59. doi: 10.3389/neuro.01.006.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen KL, Anderson B, Steinberg FL, et al. A prospective functional MR imaging study of mild traumatic brain injury in collegiate football players. Am J Neuroradiol. 2004;25:738–745. [PMC free article] [PubMed] [Google Scholar]
- Janzen G, Weststeijn C. Neural representation of object location and route direction: an evernt-related fMRI study. Brain Res. 2007;1165:116–125. doi: 10.1016/j.brainres.2007.05.074. [DOI] [PubMed] [Google Scholar]
- Kessels R, Pastma A, Wijnalda E, Haan E. Frontal-lobe involvement in spatial memory: evidence from PET, fMRI, and lesion studies. Neuropsych Rev. 2000a;10:101–113. doi: 10.1023/a:1009016820717. [DOI] [PubMed] [Google Scholar]
- Kessels RPC, de Haan EHF, Kapelle LJ, Postma A. Varieties of human spatial; memory: a meta-analysis of the effects of hippocampal lesions. Brain Res Rev. 2000b;10:295–303. doi: 10.1016/s0165-0173(01)00058-3. [DOI] [PubMed] [Google Scholar]
- Kirov I, Fleysher L, Babb JS, Silver JM, Grossman RI, Gonen O. Characterizing ‘mild’ in traumatic brain injury with proton MR spectroscopy in the thalamus: initial findings. Brain Inj. 2007;21(11):1147–1154. doi: 10.1080/02699050701630383. [DOI] [PubMed] [Google Scholar]
- Lovell M, Collins M, Iverson G, Field M, Maroon J, Cantu R, Rodell K, Powell J, Fu F. Recovery from concussion in high school athletes. J Neurosurg. 2003;98:296–301. doi: 10.3171/jns.2003.98.2.0296. [DOI] [PubMed] [Google Scholar]
- Lovell MR, Pardini JE, Welling J, Collins MW, Bakal J, Lazar N, Roush R, Eddy WF, Becker JT. Functional brain abnormalities are related to clinical recovery and time to return-to-play in athletes. Neurosurgery. 2007;61(2):359–360. doi: 10.1227/01.NEU.0000279985.94168.7F. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JB, Kraft RA. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McAllister TW, Saykin AJ, Flashman LA, et al. Brain activation during working memory I month after mild traumatic brain injury: a functional MRI study. Neurology. 1999;53:1300–1308. doi: 10.1212/wnl.53.6.1300. [DOI] [PubMed] [Google Scholar]
- McAllister TW, Sparling MB, Flashman LA, Guerin SJ, Mamourian AC, Saykin AJ. Differential working memory load effects after mild traumatic brain injury. Neuroimage. 2001;14(5):1004–1012. doi: 10.1006/nimg.2001.0899. [DOI] [PubMed] [Google Scholar]
- McAllister TW, Flashman LA, McDonald BC, Saykin AJ. Mechanisms of working memory dysfunction after mild and moderate TBI: evidence from functional MRI and neurogenetics. J Neurotrauma. 2006;23:1450–1467. doi: 10.1089/neu.2006.23.1450. [DOI] [PubMed] [Google Scholar]
- McCrory P, Meeuwisse W, Johnston K, Dvorak J, Aubry M, Molloy M, Cantu R. Consensus statement on concussion sport: the 3rd international conference on concussion in sport. Br J Sport Med. 2009;43:176–184. [Google Scholar]
- McNamara T, Shelton A. Cognitive maps and the hippocampus. Trends Cogn Neurosci. 2003;7(8):333–335. doi: 10.1016/s1364-6613(03)00167-0. [DOI] [PubMed] [Google Scholar]
- Niogi SN, Mukherjee P, Ghajar J, Johnson CE, Kolster R, Lee H, Sun M, Zimmerman R, Manley G, McCandliss B. Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain. 2008;131:320–3221. doi: 10.1093/brain/awn247. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pantano P, Mainero C, Caramia F. Functional brain reorganization in multiple sclerosis: evidence from fMRI studies. J Neuroimage. 2006;16:104–114. doi: 10.1111/j.1552-6569.2006.00029.x. [DOI] [PubMed] [Google Scholar]
- Parslow D, Rose D, Brooks B, Fleminger S, et al. Allocentric spatial memory activation of the hippocampal formation measured with fMRI. Neuropsychology. 2004;18:450–461. doi: 10.1037/0894-4105.18.3.450. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Cole MA, Demery JA, Seignourel PJ, Dixit NK, Larson MJ, et al. Parametric manipulation of working memory load in traumatic brain injury: behavioral and neural correlates. J Int Neuropsychol Soc. 2004;10(5):724–741. doi: 10.1017/S1355617704105110. [DOI] [PubMed] [Google Scholar]
- Ptito A, Chen J-K, Johnston K. Contribution of functional magnetic resonance imaging (fMRI) to sport concussion evaluation. Neurorehabilitation. 2007;22:217–227. [PubMed] [Google Scholar]
- Reddy CC, Collins MW. Sports concussion: management and predictors of outcome. Curr Sports Med Rep. 2009;8(1):10–15. doi: 10.1249/JSR.0b013e31819539ca. [DOI] [PubMed] [Google Scholar]
- Rosenbaum R, Furey M, Horwitz B, Grady C. Altered connectivity among emotion-related brain regions during short-term memory in Alzheimer's disease. Neurobiol Aging. 2008;10:724–741. doi: 10.1016/j.neurobiolaging.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Carrion R, Fernandez-Esprjo D, Junque C, Falcon C, Burgallo N, Roig T, Bernabeu M, Torsmos J, Vendrell P. A longitudinal fMRI study of working memory in severe TBI patients with diffuse axonal injury. Neuroimage. 2008;43:421–429. doi: 10.1016/j.neuroimage.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Schrader H, Mickrevičiene D, Gleizniene R, Jakstiene S, Surkiene D, Stovner L, Obelieniene D. Magnetic resonance imaging after most common form of concussion. BMC Med Imag. 2009;9(11) doi: 10.1186/1471-2342-9-11. this article is available from: http://www.biomedcentral.com/1471-2342/9/11. [DOI] [PMC free article] [PubMed]
- Shaw N. The neurophysiology of concussion. Prog Neurobiol. 2002;67:281–344. doi: 10.1016/s0301-0082(02)00018-7. [DOI] [PubMed] [Google Scholar]
- Slobounov S, Sebastianelli W, Cao C, Slobounov E, Newell K. Differential rate of recovery in athletes after first versus and second concussion episodes. Neurosurgery. 2007;61(2):238–244. doi: 10.1227/01.NEU.0000280001.03578.FF. [DOI] [PubMed] [Google Scholar]
- Slobounov S, Cao C, Sebastianelli W, Slobounov E, Newell K. Residual deficits from concussion as revealed by virtual time-to-contact measures of postural stability. Clin Neurophysiol. 2008;119(2):281–289. doi: 10.1016/j.clinph.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Slobounov S, Cao C, Sebastianelli W. Differential effect of single versus recurrent mild traumatic brain injuries on wavelet entropy measures of EEG. Clin Neurophysiol. 2009;120(5):862–867. doi: 10.1016/j.clinph.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollard E, Galanaud D, Perlbarg V, Sanchez-Pena P, Le Fur Y, Abdennour L, Cozzone P, Lehericy S, Chiras J, Puybasset L. Experience of diffusion tensor imaging and 1H spectroscopy for outcome prediction in severe traumatic brain injury: preliminary results. Crit Care Med. 2009;37(4):1448–1455. doi: 10.1097/CCM.0b013e31819cf050. [DOI] [PubMed] [Google Scholar]
- Trouillas P, Tkayanagi T, Hallett M, Currier D, Subramony S, Wessel K, Bryer A, Diener H, Massaquoi S, Gomez C, et al. International cooperative ataxia rating scale for pharmacological assessment of the cerebellar syndrome. J Neurol Sci. 1997;145:205–211. doi: 10.1016/s0022-510x(96)00231-6. [DOI] [PubMed] [Google Scholar]
- Voermans N, Petersson K, Daudey L, Weber B, van Spaendonck K, Kremer H, Fernández Interaction between the human hippocampus and the caudae nucleus during route recognition. Neuron. 2004;43:427–435. doi: 10.1016/j.neuron.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Ward N. Plasticity and the functional reorganization of the human brain. Int J Psychophysiol. 2005;58:158–161. doi: 10.1016/j.ijpsycho.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Wirth W, Hartmann T, Boecking S, Vorderer P, Klimmt C, Schramm H, et al. A process model of the formation of spatial presence experience. Med Psychol. 2007;9:493–525. [Google Scholar]
- Witmer BG, Singer MJ. Measuring presence in virtual environments: a presence questionnaire. Presence Teleop Virtual Environ. 1998;7:225–240. [Google Scholar]
- Zhou Y, Dougherty J, Hubner K, Bai B, Cannon RK, Hutson R. Abnormal connectivity in the posterior cingulate and hippocampus in early Alzheimer's disease and mild cognitive impairment. Alzheimers Dement. 2008;4(4):265–270. doi: 10.1016/j.jalz.2008.04.006. [DOI] [PubMed] [Google Scholar]