Summary
Focal adhesion kinase (FAK) is a cytoplasmic tyrosine kinase identified as a key mediator of intracellular signaling by integrins, a major family of cell surface receptors for extracellular matrix, in the regulation of different cellular functions in a variety of cells. Upon activation by integrins through disruption of an auto-inhibitory mechanism, FAK undergoes autophosphorylation and forms a complex with Src and other cellular proteins to trigger downstream signaling through its kinase activity or scaffolding function. A number of integrins are identified as surface markers for mammary stem cells (MaSCs) and both integrins and FAK are found to play crucial roles in the maintenance of MaSCs in studies using mouse models, suggesting that integrin signaling through FAK may serve as a functional marker for MaSCs. Consistent with previous studies linking increased expression and activation of FAK to human breast cancer, these findings suggest a novel cellular mechanism of FAK promotion of mammary tumorigenesis by maintaining the pools of MaSCs as targets of oncogenic transformation. Furthermore, FAK inactivation in mouse models of breast cancer also reduced the pool of mammary cancer stem cells (MaCSCs), decreased their self-renewal in vitro, and compromised their tumorigenicity and maintenance in vivo, suggesting a potential role of integrin signaling through FAK in breast cancer growth and progression through its functions in MaCSCs. This review discusses these recent advances and future studies into the mechanism of integrin signaling through FAK in breast cancer through regulation of MaCSCs that may lead to development of novel therapies for this deadly disease.
Keywords: FAK, mammary stem cells, signaling, mouse models
Introduction
Breast cancer is the most common malignancy among women in the United States and around the world. Both inherited and environmental factors contribute to the high frequency of breast cancer. Research in the last several decades have illuminated the roles of multiple oncogenes, tumor suppressor genes and their associated signaling pathways in the development and progression of breast cancer and other malignancies (1, 2). Early detection and novel therapies based on these mechanistic understanding have significantly improved the diagnosis and treatment of breast cancer in recent years. However, breast cancer remains as a major health threat given its high incidence as well as being the second leading cause of cancer-related death in American women (from the National Cancer Institute, at http://www.cancer.gov)(3).
A major conceptual advance in cancer research recently is the proposed role of cancer stem cells (CSCs) in the initiation and progression of breast and other cancers (4–7). Experimental support for the CSC hypothesis was first provided by studies in human leukemia when Dick and colleagues showed that a small population of leukemic stem cells could transfer the disease to the recipient mice in transplantation (8, 9). The CSC model was extended to the solid tumors by identifying a subpopulation of highly tumorigenic cells with stem cell properties from human breast cancers and other tissue malignancies (10–17). According to the CSC model, while the conventional therapies could destroy the bulk of the tumor mass, even a small amount of residual CSCs could lead to recurrence of the cancer due to their stem cell like ability for self-renewal and differentiation (5). Furthermore, there is evidence suggesting that CSCs are more resistant to conventional cancer therapies compared to the bulk of cells in the tumor mass (18–22), which could further decrease the effectiveness of conventional treatment strategies. Thus, the CSC model suggests that at least part of the problems with the current treatments for breast and other cancers is the possibility of not targeting and eradicating the right cells (i.e. CSCs) in the tumor (4–7).
Given the critical role of mammary CSCs (MaCSCs) in breast cancer development and progression, increasing research is directed at the characterization of key signaling molecules and pathways that regulate self-renewal and maintenance of MaCSCs in order to gain insights into the mechanisms of mammary carcinogenesis and to develop novel treatment strategies targeting the MaCSC pool. These studies suggested that a number of developmental signaling pathways such as Hedgehog, Wnt and Notch pathways play important roles in regulation of MaCSCs in addition to their well-characterized functions in a variety of developmental processes including in normal tissue stem cells (e.g. mammary stem cells [MaSCs]) (4–7). The integrin family of cell surface receptors and their major intracellular signaling mediator focal adhesion kinase (FAK) also emerged as key regulators of MaCSCs and MaSCs in breast cancer in recent studies. This review will focus on these recent advance on the role of integrin signaling through FAK in the regulation of MaCSCs, and the readers are referred to a number of other excellent review articles for general discussion on CSCs and the role of other important signaling pathways in the regulation of CSCs (4–7) and for discussion on the role of FAK in cancer development and progression in general (80, 81).
Integrin signaling through FAK
Integrins are a family of cell surface receptors involved in mediating cellular interactions with extracellular matrix (ECM) as well as cell-cell interactions (23, 24). Each integrin is a heterodimeric protein complex consisting of an α and a β subunit, both of which are transmembrane glycoproteins with a single membrane-spanning segment and generally a short cytoplasmic domain. Eighteen α subunits and 8 β subunits are found in the human genome, which is known to assemble into 24 distinct integrins. The extracellular domain of the α and β subunits associate to form the headpiece, which determine the specificity for ECM ligands. The binding of ECM to integrins induces integrin clustering at focal adhesions and formation of multiprotein complexes consisting of cytoskeletal and signaling molecules at the cytoplasmic domain of integrins (24, 25). Hence, integrins provide a physical link between ECM and actin cytoskeleton and intracellular signaling molecules at focal adhesions, which allows the bidirectional transmission of mechanical and biochemical signals across the plasma membrane to regulate a variety of cellular functions, including adhesion, migration, survival, growth and differentiation.
Integrins have been shown to regulate multiple intracellular signaling pathways through their coupling to cytoplasmic kinases, small GTPases, and scaffolding proteins as well as interaction and modulation of other receptors at the cell surface (23, 24). One of the earliest identified and most prominent components of integrin signaling is FAK, which is a nonreceptor tyrosine kinase predominantly localized in focal adhesions of adherent cells (26–30). FAK was identified in the early 1990s as one of the major substrates of viral oncogene v-Src (31, 32) and the first protein whose tyrosine phosphorylation is dependent on integrin-mediated cell adhesion in adherent cells (33–35). These early studies showing stimulation of FAK activation and phosphorylation by integrin-mediated cell adhesion and oncogenic transformation provided a plausible molecular mechanism for anchorage-independent growth of cancer cells, one of their major hallmarks (33). Since these initial findings 18 years ago, numerous studies have linked FAK-mediated signaling pathways to breast and other cancers as well as a variety of different biological and disease processes.
FAK and its related kinase Pyk2 constitute a subfamily of cytoplasmic tyrosine, which is structurally distinct from other nonreceptor tyrosine kinases in its lack of Src homology 2 (SH2) and SH3 domains. While FAK is widely expressed in many tissues and cell types, Pyk2 has a more restricted expression mainly in nervous and blood systems (26–30). FAK is highly conserved with greater than 95% amino acid identity across different mammalian species and chicken (36). It is composed of a central kinase domain flanked by an N-terminal FERM (protein 4.1, ezrin, radixin and moesin homology) domain and a C-terminal domain containing the focal adhesion targeting (FAT) sequence responsible for FAK’s localization to focal adhesions. In the inactive state (e.g. in suspended cells), the amino-terminal FERM domain contacts the central kinase domain directly through an intra-molecular interaction, which blocks access to FAK catalytic cleft and sequesters its activation loop as well as the key autophosphorylation site Y397 (37–40). During activation, FERM domain is displaced by an activating protein (e.g. integrin β cytoplasmic domain which can interact with FERM domain (41) or other activators), which is associated with a conformational change of FAK (42) allowing rapid autophosphorylation of Y397 and its exposure for binding other proteins including Src family kinases.
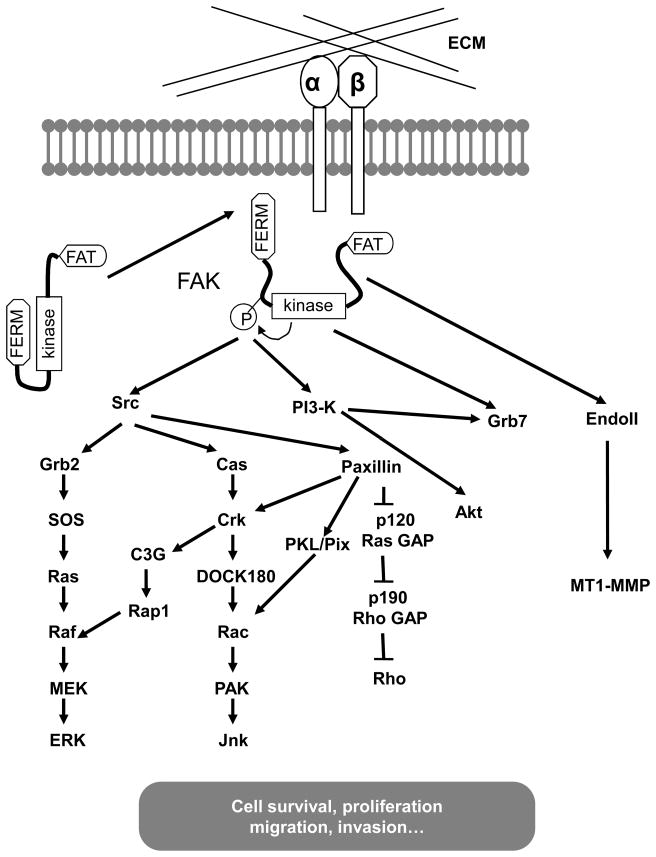
Upon its activation by integrin-mediated cell adhesion or other stimuli, FAK becomes associated with several SH2 domain containing molecules including Src (43, 44) and p85 subunit of PI3K (45, 46) through its autophosporylated Y397 residue. FAK binding to the SH2 domain of Src displaces Src Y527 binding to it, relieving the auto-inhibitory interaction and leading to activation of Src. Conversely, activated Src phosphorylates additional sites on FAK, including residues Y576 and Y577 in FAK’s kinase activation loop, leading to further increased activity of FAK, and Y925 to promote binding of adaptor molecule Grb2 to mediate activation of Ras-MAPK signaling (47). FAK association and activation of PI3K through autophosphorylated Y397 leads to increased production of 3′-phosphorylated phospholipid (48), which can activate Akt kinase to inhibit apoptosis by regulating various cell death machinery proteins (49, 50). In addition to its function as a tyrosine kinase, FAK also serves as a scaffolding protein to allow efficient Src phosphorylation of several other molecules bound to FAK. The C-terminal region of FAK contains a number of protein-protein interacting sites, including two proline-rich regions, which serve as binding sites for a variety of SH3 domain-containing proteins including p130Cas (51) and endophilin A2 (52). FAK interaction with p130Cas has been demonstrated to play a crucial role in the regulation of cell migration and breast cancer progression (51, 53–56). FAK interaction with endophilin A2 and its phosphorylation by FAK/Src complex reduces its interaction with dynamin and decreases endocytosis of MT1-MMP, leading to increased accumulation of MT1-MMP on tumor cell surface and enhanced invasiveness of cancer cells (52). The major FAK-mediated integrin signaling pathways are summarized in Fig. 1, many of which have been shown to regulate breast cancer development and progression based on previous research (26–30) and some of them may do so through their regulation of MaCSCs and MaSCs as suggested by recent studies (57, 58).
Fig. 1. FAK mediated integrin signaling pathways.
Integrin-mediated cell adhesion to ECM activates FAK by disruption of an auto-inhibitory interaction of the kinase and amino terminal FERM domain. The activated FAK undergoes autophosphorylation and binds to Src and other intracellular signaling molecules to trigger multiple downstream pathways to regulate different cellular functions such as survival, proliferation, migration and invasion.
Role of integrin signaling through FAK in MaSCs
The mammary epithelium undergoes dynamic changes in morphology and function during puberty, pregnancy, lactation, and involution. Based on studies in past decades, compelling evidence indicates the existence of MaSCs capable of self-renewal and differentiation into the various cell lineages comprising functional mammary glands (59–62). A single retrovirally-tagged MaSC was shown to give rise to a complete mammary gland upon serial transplantation (59). The β1 and β4 integrins are expressed in mammary epithelium with preferentially higher levels in the basal layer than the luminal epithelial cells (63). While ablation of β4 integrin did not affect the normal development of mammary epithelium, the overexpression of a dominant negative mutant of β1 integrin (64), or the conditional knockout (KO) of β1 integrin in either luminal epithelial cells (65, 66) or basal cells (57) significantly perturbed ductal outgrowth and alveologenesis. Interestingly, populations enriched in MaSCs have been isolated from mice using cell surface markers CD24 and β1 (CD29) or α6 (CD49f) integrins in recent studies (67, 68). Further analysis of these populations revealed that they are basal epithelial cells and are negative for steroid hormone receptor ERα (69). These studies suggest that MaSCs reside in the basal compartment of the mammary epithelium, and that integrin-ECM interactions may play essential roles in MaSCs. In agreement with the idea that integrins serve as “functional” markers for MaSCs (i.e. have a function in the regulation of MaSCs rather than simply as a surface marker), a recent study has shown that deletion of β1 integrin in basal epithelial cells significantly impaired the regeneration potential of MaSCs (57).
Consistent with it being a key intracellular mediator of signal transduction by integrins, several lines of evidence suggest that FAK may also play an important role in the regulation of MaSCs. It was shown recently that human MaSCs and progenitor cells can form mammospheres in suspension culture and propagated in vitro (70). Previous studies showed that most of primary MaECs undergoes apoptosis upon detachment (a process termed anoikis), the ability of MaSCs to propagate in suspension culture suggests that they can survive and proliferate in an anchorage-independent manner. Interestingly, MDCK cells become resistance to anoikis after expression of the constitutively active FAK by gene transfer (71). As resistance to anoikis is a prerequisite for mammosphere formation, these results together suggest that selective activation of FAK in MaSCs may be important for their self-renewal and maintenance in vitro and possibly in vivo. Consistent with such a possibility, we have shown previously that deletion of FAK in MaECs caused a severe lobulo-alveolar hypoplasia and lactational deficiency due to significantly decreased proliferation and differentiation of MaECs (72), implicating a role for FAK in MaSCs as the rapid expansion of the mammary gland in pregnancy and lactation requires a functional pool of MaSCs. Direct analysis of these mice using the newly identified markers showed that ablation of FAK significantly reduced the content of MaSCs in vivo. Furthermore, FAK-null MaSCs exhibited decreased self-renewal as determined by mammosphere assays in vitro as well as limiting dilution transplantation assays in vivo, suggesting that inactivation of FAK severely impairs the self-renewal of MaSCs responsible for the their decreased content in FAK conditional KO mice (Luo and Guan, unpublished results). These recent studies provided a more direct evidence for a role of FAK in MaSCs.
Role of FAK regulation of MaSCs in mammary tumorigenesis
The potential link of FAK to breast cancer was first established by the findings that FAK expression at both mRNA and protein levels were significantly elevated in invasive and metastatic breast tumor specimens in comparison to paired normal tissues, suggesting a role of FAK in promoting breast cancer invasion and metastasis (73). Subsequent studies showed that FAK expression was minimal in benign breast epithelium but was strongly positive in ductal carcinoma in situ (DCIS), suggesting that FAK overexpression is not restricted to the invasive phenotype, but rather appears to be an early event in breast tumorigenesis (74, 75). In a large population-based study of breast tumor samples, high FAK expression was shown to be associated with an aggressive phenotype exemplified by high mitotic index, estrogen and progesterone receptor negativity, and HER-2/neu overexpression (76). FAK expression is required for the early phase of lung metastasis of mammary adenocarcinoma in a rat syngeneic xenograft model (77). Furthermore, intrinsic FAK activity controls orthotopic breast carcinoma metastasis through the regulation of urokinase plasminogen activator expression (78), and promotes a MAPK-associated angiogenic switch during breast tumor progression (79). Therefore, these studies using clinical samples of breast cancer as well as experimental models strongly implicate a important role of FAK in the development and progression of breast cancer (80, 81).
One important prediction of the CSC hypothesis is that reduced pools of stem/progenitor cells in the normal tissue should substantially decrease the probability of cancer formation in the corresponding tissue (4, 5). As inactivation of FAK as well as β1 integrin significantly compromised self-renewal of MaSCs leading to their reduced pool (57)(also Luo and Guan, unpublished results), raising the interesting possibility that integrin signaling through FAK may promote mammary tumorigenesis through regulation of MaSCs. Indeed, very recent studies by several groups including us showed that ablation of FAK suppressed mammary tumorigenesis and progression in mouse models of breast cancer (56, 58, 82, 83). Furthermore, our studies demonstrated directly that deletion of FAK reduced the pool of MaCSCs in primary tumors developed in FAK conditional KO mice (58). These studies suggest a causal role of FAK in promoting breast cancer in vivo and also lend further support for the CSC hypothesis.
In addition to breast cancer, McLean et al have shown recently that inactivation of FAK in the epidermis significantly suppressed both tumor formation and malignant progression in the skin (84). It would be interesting to determine whether deletion of FAK in the epidermis also reduces the pool of epidermal stem cells as a mechanism of suppression of tumor formation and progression. While this possibility has not been directly tested, it is worthwhile to note that inactivation of FAK in keratinocytes did not affect their survival and proliferation in vitro (84), this is in contrast to the findings from us and others that FAK deletion in MaECs significantly decreased proliferation of MaECs and mammary tumor cells both in vitro and in vivo (56, 58, 72, 82, 83). Thus, it remains possible that integrin signaling through FAK may play a preferential role in MaSCs in breast cancer development while affecting the formation and/or progression of cancer through other mechanisms in the skin and other tissues.
FAK promotion of breast cancer progression through regulation of MaCSCs
Accumulating evidence from both clinical and experimental studies strongly support a role of FAK in the progression and metastasis of breast and other cancers (80, 81). The role of integrin signaling through FAK in promoting cell survival and proliferation contributes to tumor growth and metastasis by enabling tumor cells to survive in different environments and to colonize in distal organs. Several FAK signaling pathways have also been well characterized to promote migration and invasion of different cells, thus facilitating tumor angiogenesis and metastasis (see Fig. 1). One pathway involves FAK complex formation with Src and subsequent phosphorylation of the adaptor molecule Cas by the FAK/Src complex (53, 55, 85–87) to promote cell migration via a downstream signaling route including Crk, Dock180, and Rac (55, 88). A second mechanism of FAK promotion of cell migration involves its interactions with PI3K and an adaptor molecule Grb7 (89, 90). FAK has been shown to directly phosphorylate Grb7 in a manner dependent on the production of 3′-phosphorylated phosphoinositides by PI3K to promote cell migration (89–91). In addition, FAK has also been shown to promote cell migration through direct modulation of key proteins involving in the remodeling of the actin cytoskeleton, including the Rho subfamily of small GTPases (92–94), N-WASP (95), and the Arp2/3 complex (96).
Recent studies using mouse models of breast cancer provided direct in vivo evidence for the role of FAK in promoting breast cancer progression (56, 72, 82, 83). In one report, Lahlou et al showed that conditional KO of FAK in MaECs blocked mammary tumor progression in a model where the efficiency of Cre-mediated FAK deletion in MaECs was estimated at 64.3% (82). Under this relatively low excision efficiency, mammary carcinomas developed in the FAK conditional KO mice all express FAK, while FAK-null MaECs, although present in premalignant mammary hyperplasia, failed to progress to advanced carcinomas and subsequent metastases, suggesting a critical role of FAK in promoting mammary tumor progression. Using a different MMTV-Cre transgenic mouse strain with a higher deletion efficiency of 96.4%, Pylayeva et al reported that deletion of FAK in MaECs significantly suppressed both mammary tumorigenesis and progression (56). Interestingly, this study also indicated that virtually all the primary and lung metastatic tumor lesions found in the FAK conditional KO mice expressed FAK, suggesting that they had originated from the minority of MaECs that had not undergone Cre-mediated deletion of FAK. They also demonstrated a critical role of FAK signaling pathway through Cas in the regulation of mammary tumor invasion in vitro as well as tumorigenicity in vivo.
Although the above study indicated an important role of FAK signaling in mammary tumorigenesis and progression, the fact that FAK is expressed in all malignant primary tumors and metastatic nodules derived in the FAK conditional KO mice prevented analysis of a potential role of FAK in promotion of breast cancer progression through regulation of MaCSCs in vivo. Our studies used a third MMTV-Cre transgenic mouse line that confers Cre-mediated recombination at early embryonic stage to obtain 100% of FAK deletion in the MaECs (72). In this model, we found that deletion of FAK in MaECs significantly suppressed mammary tumor formation, growth and metastasis (58). Mammary tumors were eventually developed in FAK conditional KO mice, but with decreased multiplicity and growth retardation, and they did not express FAK. Similar results and the absence of FAK in PyMT-induced mammary tumors of FAK conditional KO mice was also reported by another group (83). Using our mouse model that completely ablates FAK expression in mammary tumor cells, we showed that inactivation of FAK reduced the pool of MaCSCs in primary tumors developed in FAK conditional KO mice, decreased their self-renewal in vitro, and compromised their tumorigenicity and maintenance in vivo (58).
In MMTV-PyMT tumor model, MaCSCs isolated based on markers of CD24, CD29 and CD61 have been shown to have higher migratory activity compared to corresponding non-stem-like cells (97). By using ALDH activity as a marker for MaCSCs, we also showed a significantly higher migration for ALDH+ cells compared to unsorted and ALDH− cells. Moreover, we found that the migration of FAK-null ALDH+ cells is decreased by about 70% relative to ALDH+ cells from control mice, suggesting an important role of FAK in the regulation of migration of MaCSCs (58). These observations of the reduced migration of FAK-null MaCSCs is very interesting as this may suggest a more direct role of FAK in metastasis through its regulation of MaCSCs migration besides influencing the survival and expansion of metastasized MaCSCs in new location through controlling their self-renewal.
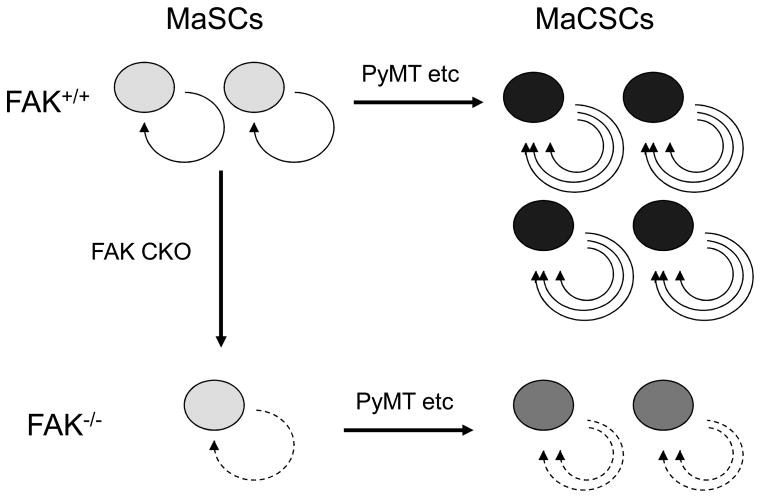
Given the widely recognized role of mammary and other CSCs in cancer initiation and progression (4–7), these studies using mouse models provide a novel cellular mechanism of integrin signaling through FAK in promoting breast cancer. Inactivation of FAK may inhibit mammary tumorigenesis by reducing the self-renewal and available pool of MaSCs and block the growth and progression of breast cancer by impairing self-renewal, migration and tumorigenicity of MaCSCs. A working model for the potential regulation of breast cancer by integrin signaling through FAK is summarized in Fig. 2.
Fig. 2. Inactivation of FAK suppresses breast cancer development and progression caused by deficient self-renewal and decreased pool of MaSCs and MaCSCs in mouse models.
In the normal mammary glands, integrin signaling through FAK contributes to the self-renewal of MaSC (light grey), which can be transformed by oncogenes such as PyMT to form MaCSCs (black) with significantly increased self-renewal and tumorigenecity (more circular lines). MaEC-specific deletion of FAK (FAK CKO) results in deficient self-renewal (broken circular lines) and reduced pool of MaSCs. The reduced pool of MaSCs may contribute to the decreased mammary tumorigenesis (i.e. reduced frequency of MaCSCs formation). The FAK-null MaCSCs (dark grey) also exhibit deficient self-renewal and tumorigenecity (broken circular lines). The deficient self-renewal and the reduced pool of MaCSCs could account for the suppressed growth and progression of mammary tumors developed in these mice.
Concluding remarks and perspectives
Since its initial identification as a key mediator of integrin signaling (32–35, 98), a large body of studies in the last 18 years have clearly established an important role for FAK in breast cancer development and progression (26–30, 80, 81). Moreover, these studies also illustrated multiple signaling pathways mediated by FAK through its interactions and phosphorylation of other intracellular molecules in the regulation of various cellular functions (26–30). Emerging evidence suggests that integrin signaling through FAK may promote breast cancer through the regulation of MaCSCs and MaSCs. It will be interesting to determine which of the FAK signaling pathways play important roles in the regulation of self-renewal and other activities of MaCSCs, and whether any of these pathways play differential functions in the regulation of MaSCs. The potential differences in the regulation of MaCSCs and MaSCs by specific FAK signaling pathways may be exploited to develop treatments to eliminate MaCSCs but not harming MaSCs for effective new therapies of breast cancer. In addition, it would be interesting to determine the potential cross-talks between integrin-FAK signaling and other signaling pathways involved in the regulation of MaCSCs and MaSCs. These include a number of well-characterized developmental signaling pathways including Notch, Wnt, and hedgehog pathways (4–7). These studies may suggest that the use of a combination of inhibitors for multiple signaling pathways might be more effective than blockade of a single pathway to eradicate MaCSCs.
In complementary to further analysis of intracellular pathways, it would also be interesting to explore the role of integrin signaling through FAK in the regulation of MaCSCs in the context of influence of tumor microenvironments on these cells. Although it is well known that niches play crucial roles in many tissue stem cells as well as CSCs (99–101), very little is known about how extrinsic factors (i.e. niche) control maintenance and self-renewal of MaCSCs. Given their likely roles as “functional” markers, integrins and their signaling through FAK (i.e. activation of FAK) may play an essential function in mediating regulation of MaSCs and MaCSCs by the mammary stroma and the tumor microenvironments, respectively, which may provide the niches crucial for the self renewal of the stem cells. In a recent report, interestingly, formation of fibronectin-rich patches (pre-Metastasis niche) initiated by the VEGFR1+ bone marrow-derived hematopoietic progenitor cells were observed in the target organs of cancer metastasis (102), suggesting the possibility that integrin signaling through FAK in MaCSCs upon adhesion to fibronectin patch may facilitate the survival and self-renewal of metastasized MaCSCs in the target organs.
Given the highly conserved sequence and functions of FAK and its signaling pathways between mouse and human, it is very likely that FAK signaling pathways involved in the regulation of MaCSCs in mouse models also play crucial roles in human MaCSCs. Nevertheless, it would be important to confirm that FAK signaling plays a role in human MaCSCs, which may account for the observations of an correlation between FAK activation and malignant progression of human breast cancer in previous studies (80, 81), and to elucidate the molecular mechanisms and downstream effectors of FAK signaling in human MaCSCs. Conversely, although MaCSCs were first described in human breast tumors (10), the origins of these highly tumorigenic cells (e.g. whether derived from normal MaSCs) remain obscure and are difficult to determine in human tumors. Mouse models of breast cancer provide an excellent system to address the cellular origins of MaCSCs, one of the important issues in the CSC model with great implications in breast cancer treatments. The major advantages of using mouse models include the relative ease of genetic manipulation (KO and knockin approaches), well established methods to isolate primary MaECs and tumor cells followed by transplantation into syngeneic recipient mice after manipulation in vitro (e.g. gene transduction by recombinant lentiviruses), and the well-characterized specific cell surface markers for distinct subpopulations of the mammary epithelial hierarchy including MaSCs and progenitor cells (67, 68, 103). The use of syngeneic mouse models allows one to study mammary tumor development and progression in animals with intact immune system, which contains both suppressive and promoting activities for breast cancer (104–106), as well as the right microenvironments which could also influence mammary tumor cells in both positive and negative manners (107, 108). In short, future studies using a combination of approaches including mouse models as well as human breast cancer samples will generate significant insights into the mechanisms by which key signaling proteins and pathways regulate self-renewal and maintenance of MaCSCs, and will significantly advance our understanding of the molecular and cellular mechanisms of breast cancer. These studies will also contribute to the development of novel therapies targeting the MaCSC pool to eradicate this deadly disease.
Acknowledgments
Work in author’s laboratory is supported by NIH grants.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell. 2009;136:823–837. doi: 10.1016/j.cell.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Althuis MD, Dozier JM, Anderson WF, Devesa SS, Brinton LA. Global trends in breast cancer incidence and mortality 1973–1997. International journal of epidemiology. 2005;34:405–412. doi: 10.1093/ije/dyh414. [DOI] [PubMed] [Google Scholar]
- 4.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annual review of medicine. 2007;58:267–284. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 5.Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea--a paradigm shift. Cancer Res. 2006;66:1883–1890. doi: 10.1158/0008-5472.CAN-05-3153. discussion 1895–1886. [DOI] [PubMed] [Google Scholar]
- 6.Rosen JM, Jordan CT. The increasing complexity of the cancer stem cell paradigm. Science. 2009;324:1670–1673. doi: 10.1126/science.1171837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stingl J, Caldas C. Molecular heterogeneity of breast carcinomas and the cancer stem cell hypothesis. Nat Rev Cancer. 2007;7:791–799. doi: 10.1038/nrc2212. [DOI] [PubMed] [Google Scholar]
- 8.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 9.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 10.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 13.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 14.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 15.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 16.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 17.Malanchi I, Peinado H, Kassen D, Hussenet T, Metzger D, Chambon P, Huber M, Hohl D, Cano A, Birchmeier W, Huelsken J. Cutaneous cancer stem cell maintenance is dependent on beta-catenin signalling. Nature. 2008;452:650–653. doi: 10.1038/nature06835. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC, Wong H, Rosen J, Chang JC. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. Journal of the National Cancer Institute. 2008;100:672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 19.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 20.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 21.Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, Joshua B, Kaplan MJ, Wapnir I, Dirbas FM, Somlo G, Garberoglio C, Paz B, Shen J, Lau SK, Quake SR, Brown JM, Weissman IL, Clarke MF. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woodward WA, Chen MS, Behbod F, Alfaro MP, Buchholz TA, Rosen JM. WNT/beta-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc Natl Acad Sci U S A. 2007;104:618–623. doi: 10.1073/pnas.0606599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 24.Hynes R. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 25.Miranti CK, Brugge JS. Sensing the environment: a historical perspective on integrin signal transduction. Nat Cell Biol. 2002;4:E83–90. doi: 10.1038/ncb0402-e83. [DOI] [PubMed] [Google Scholar]
- 26.Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 27.Schlaepfer DD, Mitra SK. Multiple connections link FAK to cell motility and invasion. Curr Opin Genet Dev. 2004;14:92–101. doi: 10.1016/j.gde.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Siesser PM, Hanks SK. The signaling and biological implications of FAK overexpression in cancer. Clin Cancer Res. 2006;12:3233–3237. doi: 10.1158/1078-0432.CCR-06-0456. [DOI] [PubMed] [Google Scholar]
- 29.Schaller MD. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim Biophys Acta. 2001;1540:1–21. doi: 10.1016/s0167-4889(01)00123-9. [DOI] [PubMed] [Google Scholar]
- 30.Cohen LA, Guan JL. Mechanisms of focal adhesion kinase regulation. Curr Cancer Drug Targets. 2005;5:629–643. doi: 10.2174/156800905774932798. [DOI] [PubMed] [Google Scholar]
- 31.Kanner SB, Reynolds AB, Vines RR, Parsons JT. Monoclonal antibodies to individual tyrosine-phosphorylated protein substrates of oncogene-encoded tyrosine kinases. Proc Natl Acad Sci U S A. 1990;87:3328–3332. doi: 10.1073/pnas.87.9.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaller MD, Borgman CA, Cobb BS, Vines RR, Reynolds AB, Parsons JT. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci U S A. 1992;89:5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guan JL, Shalloway D. Regulation of focal adhesion-associated protein tyrosine kinase by both cellular adhesion and oncogenic transformation. Nature. 1992;358:690–692. doi: 10.1038/358690a0. [DOI] [PubMed] [Google Scholar]
- 34.Guan JL, Trevithick JE, Hynes RO. Fibronectin/integrin interaction induces tyrosine phosphorylation of a 120-kDa protein. Cell Regul. 1991;2:951–964. doi: 10.1091/mbc.2.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kornberg LJ, Earp HS, Turner CE, Prockop C, Juliano RL. Signal transduction by integrins: increased protein tyrosine phosphorylation caused by clustering of beta 1 integrins. Proc Natl Acad Sci U S A. 1991;88:8392–8396. doi: 10.1073/pnas.88.19.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitney GS, Chan PY, Blake J, Cosand WL, Neubauer MG, Aruffo A, Kanner SB. Human T and B lymphocytes express a structurally conserved focal adhesion kinase, pp125FAK. DNA Cell Biol. 1993;12:823–830. doi: 10.1089/dna.1993.12.823. [DOI] [PubMed] [Google Scholar]
- 37.Lietha D, Cai X, Ceccarelli DF, Li Y, Schaller MD, Eck MJ. Structural basis for the autoinhibition of focal adhesion kinase. Cell. 2007;129:1177–1187. doi: 10.1016/j.cell.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dunty JM, Gabarra-Niecko V, King ML, Ceccarelli DFJ, Eck MJ, Schaller MD. FERM Domain Interaction Promotes FAK Signaling. Mol Cell Biol. 2004;24:5353–5368. doi: 10.1128/MCB.24.12.5353-5368.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cooper LA, Shen TL, Guan JL. Regulation of focal adhesion kinase by its amino-terminal domain through an autoinhibitory interaction. Mol Cell Biol. 2003;23:8030–8041. doi: 10.1128/MCB.23.22.8030-8041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen LA, Guan JL. Residues within the first subdomain of the FERM-like domain in focal adhesion kinase are important in its regulation. J Biol Chem. 2005;280:8197–8207. doi: 10.1074/jbc.M412021200. [DOI] [PubMed] [Google Scholar]
- 41.Schaller MD, Otey CA, Hildebrand JD, Parsons JT. Focal adhesion kinase and paxillin bind to peptides mimicking beta integrin cytoplasmic domains. J Cell Biol. 1995;130:1181–1187. doi: 10.1083/jcb.130.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai X, Lietha D, Ceccarelli DF, Karginov AV, Rajfur Z, Jacobson K, Hahn KM, Eck MJ, Schaller MD. Spatial and temporal regulation of focal adhesion kinase activity in living cells. Mol Cell Biol. 2008;28:201–214. doi: 10.1128/MCB.01324-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2- dependent binding of pp60src. Mol Cell Biol. 1994;14:1680–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xing Z, Chen HC, Nowlen JK, Taylor SJ, Shalloway D, Guan JL. Direct interaction of v-Src with the focal adhesion kinase mediated by the Src SH2 domain. Mol Biol Cell. 1994;5:413–421. doi: 10.1091/mbc.5.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen HC, Guan JL. Association of focal adhesion kinase with its potential substrate phosphatidylinositol 3-kinase. Proc Natl Acad Sci U S A. 1994;91:10148–10152. doi: 10.1073/pnas.91.21.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen HC, Appeddu PA, Isoda H, Guan JL. Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3-kinase. J Biol Chem. 1996;271:26329–26334. doi: 10.1074/jbc.271.42.26329. [DOI] [PubMed] [Google Scholar]
- 47.Schlaepfer DD, Hanks SK, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- 48.Reiske HR, Kao SC, Cary LA, Guan JL, Lai JF, Chen HC. Requirement of phosphatidylinositol 3-kinase in focal adhesion kinase- promoted cell migration. J Biol Chem. 1999;274:12361–12366. doi: 10.1074/jbc.274.18.12361. [DOI] [PubMed] [Google Scholar]
- 49.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 50.Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer cell. 2003;4:257–262. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 51.Polte TR, Hanks SK. Interaction between focal adhesion kinase and Crk-associated tyrosine kinase substrate p130Cas. Proc Natl Acad Sci U S A. 1995;92:10678–10682. doi: 10.1073/pnas.92.23.10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu X, Gan B, Yoo Y, Guan JL. FAK-mediated src phosphorylation of endophilin A2 inhibits endocytosis of MT1-MMP and promotes ECM degradation. Dev Cell. 2005;9:185–196. doi: 10.1016/j.devcel.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 53.Cary LA, Han DC, Polte TR, Hanks SK, Guan JL. Identification of p130Cas as a mediator of focal adhesion kinase- promoted cell migration. J Cell Biol. 1998;140:211–221. doi: 10.1083/jcb.140.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vuori K, Hirai H, Aizawa S, Ruoslahti E. Introduction of p130cas signaling complex formation upon integrin- mediated cell adhesion: a role for Src family kinases. Mol Cell Biol. 1996;16:2606–2613. doi: 10.1128/mcb.16.6.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klemke RL, Leng J, Molander R, Brooks PC, Vuori K, Cheresh DA. CAS/Crk coupling serves as a “molecular switch” for induction of cell migration. J Cell Biol. 1998;140:961–972. doi: 10.1083/jcb.140.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pylayeva Y, Gillen KM, Gerald W, Beggs HE, Reichardt LF, Giancotti FG. Ras- and PI3K-dependent breast tumorigenesis in mice and humans requires focal adhesion kinase signaling. J Clin Invest. 2009;119:252–266. doi: 10.1172/JCI37160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taddei I, Deugnier MA, Faraldo MM, Petit V, Bouvard D, Medina D, Fassler R, Thiery JP, Glukhova MA. beta1 Integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nat Cell Biol. 2008 doi: 10.1038/ncb1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luo M, Fan H, Nagy T, Wei H, Wang C, Liu S, Wicha MS, Guan JL. Mammary epithelial-specific ablation of the focal adhesion kinase suppresses mammary tumorigenesis by affecting mammary cancer stem/progenitor cells. Cancer Res. 2009;69:466–474. doi: 10.1158/0008-5472.CAN-08-3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kordon EC, Smith GH. An entire functional mammary gland may comprise the progeny from a single cell. 1998:1921–1930. doi: 10.1242/dev.125.10.1921. [DOI] [PubMed] [Google Scholar]
- 60.Smith GH, Medina D. A morphologically distinct candidate for an epithelial stem cell in mouse mammary gland. J Cell Sci. 1988;90(Pt 1):173–183. doi: 10.1242/jcs.90.1.173. [DOI] [PubMed] [Google Scholar]
- 61.Stingl J, Raouf A, Emerman JT, Eaves CJ. Epithelial progenitors in the normal human mammary gland. Journal of mammary gland biology and neoplasia. 2005;10:49–59. doi: 10.1007/s10911-005-2540-7. [DOI] [PubMed] [Google Scholar]
- 62.Visvader JE, Lindeman GJ. Mammary Stem Cells and Mammopoiesis. 2006:9798–9801. doi: 10.1158/0008-5472.CAN-06-2254. [DOI] [PubMed] [Google Scholar]
- 63.Taddei I, Faraldo MM, Teuliere J, Deugnier MA, Thiery JP, Glukhova MA. Integrins in mammary gland development and differentiation of mammary epithelium. Journal of mammary gland biology and neoplasia. 2003;8:383–394. doi: 10.1023/B:JOMG.0000017426.74915.b9. [DOI] [PubMed] [Google Scholar]
- 64.Faraldo MM, Deugnier MA, Lukashev M, Thiery JP, Glukhova MA. Perturbation of beta1-integrin function alters the development of murine mammary gland. Embo J. 1998;17:2139–2147. doi: 10.1093/emboj/17.8.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li N, Zhang Y, Naylor MJ, Schatzmann F, Maurer F, Wintermantel T, Schuetz G, Mueller U, Streuli CH, Hynes NE. {beta}1 integrins regulate mammary gland proliferation and maintain the integrity of mammary alveoli. EMBO J. 2005;24:1942–1953. doi: 10.1038/sj.emboj.7600674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Naylor MJ, Li N, Cheung J, Lowe ET, Lambert E, Marlow R, Wang P, Schatzmann F, Wintermantel T, Schuetz G, Clarke AR, Mueller U, Hynes NE, Streuli CH. Ablation of {beta}1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J Cell Biol. 2005;171:717–728. doi: 10.1083/jcb.200503144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shackleton M, Vaillant Fo, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat M-L, Wu L, Lindeman GJ, Visvader JE. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 68.Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 69.Asselin-Labat ML, Shackleton M, Stingl J, Vaillant F, Forrest NC, Eaves CJ, Visvader JE, Lindeman GJ. Steroid hormone receptor status of mouse mammary stem cells. Journal of the National Cancer Institute. 2006;98:1011–1014. doi: 10.1093/jnci/djj267. [DOI] [PubMed] [Google Scholar]
- 70.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frisch SM, Vuori K, Ruoslahti E, Chan-Hui PY. Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol. 1996;134:793–799. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nagy T, Wei H, Shen TL, Peng X, Liang CC, Gan B, Guan JL. Mammary Epithelial-specific Deletion of the Focal Adhesion Kinase Gene Leads to Severe Lobulo-Alveolar Hypoplasia and Secretory Immaturity of the Murine Mammary Gland. J Biol Chem. 2007;282:31766–31776. doi: 10.1074/jbc.M705403200. [DOI] [PubMed] [Google Scholar]
- 73.Owens LV, Xu L, Craven RJ, Dent GA, Weiner TM, Kornberg L, Liu ET, Cance WG. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res. 1995;55:2752–2755. [PubMed] [Google Scholar]
- 74.Oktay MH, Oktay K, Hamele-Bena D, Buyuk A, Koss LG. Focal adhesion kinase as a marker of malignant phenotype in breast and cervical carcinomas. Hum Pathol. 2003;34:240–245. doi: 10.1053/hupa.2003.40. [DOI] [PubMed] [Google Scholar]
- 75.Lightfoot HM, Jr, Lark A, Livasy CA, Moore DT, Cowan D, Dressler L, Craven RJ, Cance WG. Upregulation of focal adhesion kinase (FAK) expression in ductal carcinoma in situ (DCIS) is an early event in breast tumorigenesis. Breast Cancer Res Treat. 2004;88:109–116. doi: 10.1007/s10549-004-1022-8. [DOI] [PubMed] [Google Scholar]
- 76.Lark AL, Livasy CA, Dressler L, Moore DT, Millikan RC, Geradts J, Iacocca M, Cowan D, Little D, Craven RJ, Cance W. High focal adhesion kinase expression in invasive breast carcinomas is associated with an aggressive phenotype. Mod Pathol. 2005;18:1289–1294. doi: 10.1038/modpathol.3800424. [DOI] [PubMed] [Google Scholar]
- 77.van Nimwegen MJ, Verkoeijen S, van Buren L, Burg D, van de Water B. Requirement for Focal Adhesion Kinase in the Early Phase of Mammary Adenocarcinoma Lung Metastasis Formation. 2005:4698–4706. doi: 10.1158/0008-5472.CAN-04-4126. [DOI] [PubMed] [Google Scholar]
- 78.Mitra SK, Lim ST, Chi A, Schlaepfer DD. Intrinsic focal adhesion kinase activity controls orthotopic breast carcinoma metastasis via the regulation of urokinase plasminogen activator expression in a syngeneic tumor model. Oncogene. 2006;25:4429–4440. doi: 10.1038/sj.onc.1209482. [DOI] [PubMed] [Google Scholar]
- 79.Mitra SK, Mikolon D, Molina JE, Hsia DA, Hanson DA, Chi A, Lim ST, Bernard-Trifilo JA, Ilic D, Stupack DG, Cheresh DA, Schlaepfer DD. Intrinsic FAK activity and Y925 phosphorylation facilitate an angiogenic switch in tumors. Oncogene. 2006;25:5969–5984. doi: 10.1038/sj.onc.1209588. [DOI] [PubMed] [Google Scholar]
- 80.Golubovskaya VM, Cance WG. Focal adhesion kinase and p53 signaling in cancer cells. Int Rev Cytol. 2007;263:103–153. doi: 10.1016/S0074-7696(07)63003-4. [DOI] [PubMed] [Google Scholar]
- 81.McLean GW, Carragher NO, Avizienyte E, Evans J, Brunton VG, Frame MC. The role of focal-adhesion kinase in cancer - a new therapeutic opportunity. Nat Rev Cancer. 2005;5:505–515. doi: 10.1038/nrc1647. [DOI] [PubMed] [Google Scholar]
- 82.Lahlou H, Sanguin-Gendreau V, Zuo D, Cardiff RD, McLean GW, Frame MC, Muller WJ. Mammary epithelial-specific disruption of the focal adhesion kinase blocks mammary tumor progression. Proc Natl Acad Sci U S A. 2007;104:20302–20307. doi: 10.1073/pnas.0710091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Provenzano PP, Inman DR, Eliceiri KW, Beggs HE, Keely PJ. Mammary epithelial-specific disruption of focal adhesion kinase retards tumor formation and metastasis in a transgenic mouse model of human breast cancer. Am J Pathol. 2008;173:1551–1565. doi: 10.2353/ajpath.2008.080308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McLean GW, Komiyama NH, Serrels B, Asano H, Reynolds L, Conti F, Hodivala-Dilke K, Metzger D, Chambon P, Grant SG, Frame MC. Specific deletion of focal adhesion kinase suppresses tumor formation and blocks malignant progression. Genes Dev. 2004;18:2998–3003. doi: 10.1101/gad.316304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cary LA, Chang JF, Guan JL. Stimulation of cell migration by overexpression of focal adhesion kinase and its association with Src and Fyn. J Cell Sci. 1996;109:1787–1794. doi: 10.1242/jcs.109.7.1787. [DOI] [PubMed] [Google Scholar]
- 86.Owen JD, Ruest PJ, Fry DW, Hanks SK. Induced focal adhesion kinase (FAK) expression in FAK-null cells enhances cell spreading and migration requiring both auto- and activation loop phosphorylation sites and inhibits adhesion-dependent tyrosine phosphorylation of Pyk2. Mol Cell Biol. 1999;19:4806–4818. doi: 10.1128/mcb.19.7.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sieg DJ, Hauck CR, Schlaepfer DD. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J Cell Sci. 1999;112:2677–2691. doi: 10.1242/jcs.112.16.2677. [DOI] [PubMed] [Google Scholar]
- 88.Cary LA, Han DC, Polte TR, Hanks SK, Guan JL. Identification of p130Cas as a mediator of focal adhesion kinase-promoted cell migration. J Cell Biol. 1998;140:211–221. doi: 10.1083/jcb.140.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Han DC, Guan JL. Association of focal adhesion kinase with Grb7 and its role in cell migration. J Biol Chem. 1999;274:24425–24430. doi: 10.1074/jbc.274.34.24425. [DOI] [PubMed] [Google Scholar]
- 90.Han DC, Shen TL, Guan JL. Role of Grb7 targeting to focal contacts and its phosphorylation by focal adhesion kinase in regulation of cell migration. J Biol Chem. 2000;275:28911–28917. doi: 10.1074/jbc.M001997200. [DOI] [PubMed] [Google Scholar]
- 91.Chu PY, Huang LY, Hsu CH, Liang CC, Guan JL, Hung TH, Shen TL. Tyrosine phosphorylation of growth factor receptor-bound protein-7 by focal adhesion kinase in the regulation of cell migration, proliferation, and tumorigenesis. J Biol Chem. 2009;284:20215–20226. doi: 10.1074/jbc.M109.018259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen BH, Tzen JT, Bresnick AR, Chen HC. Roles of Rho-associated kinase and myosin light chain kinase in morphological and migratory defects of focal adhesion kinase-null cells. J Biol Chem. 2002;277:33857–33863. doi: 10.1074/jbc.M204429200. [DOI] [PubMed] [Google Scholar]
- 93.Ren XD, Kiosses WB, Sieg DJ, Otey CA, Schlaepfer DD, Schwartz MA. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J Cell Sci. 2000;113(Pt 20):3673–3678. doi: 10.1242/jcs.113.20.3673. [DOI] [PubMed] [Google Scholar]
- 94.Hsia DA, Mitra SK, Hauck CR, Streblow DN, Nelson JA, Ilic D, Huang S, Li E, Nemerow GR, Leng J, Spencer KS, Cheresh DA, Schlaepfer DD. Differential regulation of cell motility and invasion by FAK. J Cell Biol. 2003;160:753–767. doi: 10.1083/jcb.200212114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu X, Suetsugu S, Cooper LA, Takenawa T, Guan JL. Focal adhesion kinase regulation of N-WASP subcellular localization and function. J Biol Chem. 2004;279:9565–9576. doi: 10.1074/jbc.M310739200. [DOI] [PubMed] [Google Scholar]
- 96.Serrels B, Serrels A, Brunton VG, Holt M, McLean GW, Gray CH, Jones GE, Frame MC. Focal adhesion kinase controls actin assembly via a FERM-mediated interaction with the Arp2/3 complex. Nat Cell Biol. 2007;9:1046–1056. doi: 10.1038/ncb1626. [DOI] [PubMed] [Google Scholar]
- 97.Kouros-Mehr H, Bechis SK, Slorach EM, Littlepage LE, Egeblad M, Ewald AJ, Pai SY, Ho IC, Werb Z. GATA-3 links tumor differentiation and dissemination in a luminal breast cancer model. Cancer cell. 2008;13:141–152. doi: 10.1016/j.ccr.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hanks SK, Calalb MB, Harper MC, Patel SK. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc Natl Acad Sci U S A. 1992;89:8487–8491. doi: 10.1073/pnas.89.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res. 2006;66:4553–4557. doi: 10.1158/0008-5472.CAN-05-3986. [DOI] [PubMed] [Google Scholar]
- 100.Perry JM, Li L. Disrupting the stem cell niche: good seeds in bad soil. Cell. 2007;129:1045–1047. doi: 10.1016/j.cell.2007.05.053. [DOI] [PubMed] [Google Scholar]
- 101.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 102.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, Hartley L, Robb L, Grosveld FG, van der Wees J, Lindeman GJ, Visvader JE. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9:201–209. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]
- 104.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 105.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 106.Finn OJ. Cancer immunology. N Engl J Med. 2008;358:2704–2715. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 107.Littlepage LE, Egeblad M, Werb Z. Coevolution of cancer and stromal cellular responses. Cancer cell. 2005;7:499–500. doi: 10.1016/j.ccr.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 108.Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]