Abstract
Cellulase production was investigated in pH-controlled cultures of Acremonium cellulolyticus. The response to culture pH was investigated for three cellulolytic enzymes, carbomethyl cellulase (CMCase), avicelase, and β-glucosidase. Avicelase and β-glucosidase showed similar profiles, with maximum activity in cultures at pH 5.5–6. The CMCase activity was highest in a pH 4 culture. At an acidic pH, the ratios of CMCase and avicelase activity to cellulase activity defined by filter paper unit were high, but at a neutral pH, the β-glucosidase ratio was high. The pH 6.0 culture showed the highest cellulase activity within the range of pH 3.5–6.5 cultures. The saccharification activity from A. cellulolyticus was compared to those of the cellulolytic enzymes from other species. The A. cellulolyticus culture broth had a saccharification yield comparable to those of the Trichoderma enzymes GC220 and Cellulosin T2, under conditions with the same cellulase activity. The saccharification yields from Solka floc, Avicel, and waste paper, measured as the percent of released reducing sugar to dried substrate, were greater than 80% after 96 h of reaction. The yields were 16% from carboxymethylcellulose and 26% from wood chip refiner. Thus, the A. cellulolyticus enzymes were suitable for converting cellulolytic biomass to reducing sugars for biomass ethanol production. This study is a step toward the establishment of an efficient system to reutilize cellulolytic biomass.
Keywords: Cellulase, Acremonium cellulolyticus, Biomass, Saccharification, Lignocellulose
Introduction
Biomass, which is a lignocellulosic material from sources such as agricultural waste and woody materials, represents an abundant renewable energy source. Many researchers have focused on producing value-added materials from biomass, and new technologies have been developed [1–3]. However, before biomass can be used, pretreatment is necessary to break the lignocellulose down into its three major polymeric constituents: cellulose, hemicelluloses, and lignin. Different pretreatment methods such as steam explosion [4, 5], wet oxidation [6, 7], and microwaves [8] have been proposed for lignocellulose fractionation, in which most of the hemicellulose and lignin degradation products are dissolved, and the cellulose is recovered in the solid fraction. The cellulose is either used for paper production or is further hydrolyzed by cellulolytic enzymes or acids to readily usable carbon and energy in the fermentation process. Although cost-efficient acids can be used as hydrolysis agents, they are not environmentally friendly because they require high temperatures and generate acidic waste. Producing cost-efficient cellulolytic enzymes from microorganisms is thus an important focus of research. However, the current technologies have not been adopted yet because of the high cost of commercial enzymes.
Many well-studied microorganisms produce cellulolytic enzymes; for example, the genus Trichoderma produces cellulolytic enzymes with relatively high enzymatic activity. However, the Trichoderma enzymes alone cannot effectively hydrolyze cellulose biomass, because of their enzymatic composition. The saccharification activity of enzymes is particularly important for producing reducing sugars, especially glucose, from cellulolytic biomass. This ability depends on the composition of the enzymes, particularly the crystalline cellulose-hydrolyzing enzyme and β-glucosidase.
Acremonium cellulolyticus, which was isolated in 1987 and subsequently improved to increase performance, produces both enzymes, in addition to CMCase and small amounts of xylanase, β-1,3-glucanase, and amylase [9]. Twelve distinct endo-cellulases and four β-glucosidases have been purified from A. cellulolyticus. One of the endo-cellulases possesses a potent ability to produce glucose from cellobiose [10]. Thus, the A. cellulolyticus enzymatic activity effectively hydrolyzes cellulolytic biomass. Indeed, the commercial enzymes produced from A. cellulolyticus have high hydrolytic activity against cellulolytic wastes, as compared to the Trichoderma enzymes [11]. In spite of this performance, few studies on the use of A. cellulolyticus for cellulase production have been reported [9–14], although the results have demonstrated its unique enzyme composition and relatively high cellulase activity [9]. Nonetheless, the activity obtained in the reported studies was insufficient for the hydrolysis of industrial amounts of cellulolytic biomass.
The aim of this research was to achieve the efficient, industrial-scale production of cellulolytic enzymes using the filamentous fungus Ferm P-18508 A. cellulolyticus C-1, which is a hyperproducing mutant of the original strain, A. cellulolyticus Y-94. This paper focuses on the response to the culture pH of the CMCase, β-glucosidase, and avicelase enzymes of A. cellulolyticus. The culture broths were subjected to a saccharification test to assess the enzymatic performance.
Materials and Methods
Strains and Culture Media
Ferm P-18508 A. cellulolyticus C-1 was seeded on potato dextrose agar slants, incubated at 30 °C for 3–5 days until colonies grew, and then stored at 4 °C. The colonies were colored brown to reddish-brown and had short white hyphae and no spores.
The preculture medium contained 40 g/l cellulose powder, 24 g/l KH2PO4, 10 g/l corn steep liquor, 1 g/l Tween 80, 5 g/l (NH4)2SO4, 4.7 g/l potassium tartrate, 1.2 g/l MgSO4·7H2O, 0.01 g/l ZnSO4·7H2O, 0.01 g/l MnSO4·6H2O, 0.01 g/l CuSO4·7H2O, and 2 g/l urea, pH 4.0 [15]. Solka floc (CAS no. 9004-34-6, Fibers Sales & Development Co. Urbana, OH, USA) was used as the cellulose powder. The cellulase production medium contained 50 g/l cellulose powder, 1 g/l KH2PO4, 1 g/l Tween 80, 5 g/l (NH4)2SO4, 4.7 g/l potassium tartrate, 1.2 g/l MgSO4·7H2O, 0.01 g/l ZnSO4·7H2O, 0.01 g/l MnSO4·6H2O, 0.01 g/l CuSO4·7H2O, and 4 g/l urea.
For flask cultures, one colony was inoculated into an L-shaped test tube containing 5 ml of preculture medium. The preculture was incubated for 65 h at 30 °C at 90 shakes per minute (spm) in a reciprocal shaker (SJ-10R, TAITEC Co., Ltd., Japan). For cellulase production in flasks, a 2.5 ml portion of the preculture was inoculated into a 500-ml Erlenmeyer flask containing 50 ml of production medium. The cultures were incubated for 5–8 days at 30 °C at 220 rpm in a rotary shaker (Takasaki Sci. Instru. Co., Japan).
pH-Controlled Cellulase Production
A 3-l bioreactor (MD-400, Marubishi, Co., Ltd., Japan) with a 1.5 l working volume was used for pH-controlled cellulase production. Distilled water was used for all components of the production medium, and the system was sterilized at 121 °C for 20 min. Solka floc and Tween 80 were mixed in 500 ml of distilled water and sterilized separately to prevent blockage of the bioreactor air nozzles and to avoid excess bubble formation in the medium. The appropriate amount of urea, in 50 ml of distilled water, was filtered with a 0.45 µm filter. The sterilized Solka floc, Tween 80, and urea were added to the bioreactor before inoculation.
Two colonies were inoculated into 500-ml Erlenmeyer flasks containing 50 ml of preculture medium and were cultured at 30 °C, 220 rpm for 96 h. An inoculum of 150 ml was added to the bioreactor and was cultured at 30 °C. The pH was controlled at 3.5, 4.0, 4.5, 5.0, 5.5, 6.0, or 6.5 with 5% NH4OH and 2 N H2SO4. Air was supplied at 1 volume of air per volume of medium per minute, with an agitation rate of 600 rpm. An anti-foam agent (Silicone 72, KM-70, Shin-Etsu Chemical Co. Ltd. Japan) was added in small amounts as needed. Each experiment was carried out in triplicate; data are presented as averages.
Saccharification Yields from A. cellulolyticus Culture Broth
To investigate the enzymatic performance in A. cellulolyticus culture broth, saccharification experiments were conducted on culture supernatants. Two commercial enzymes were used for comparison: GC 220 from Trichoderma reesei (Genencor International Inc., CA, USA) and Cellulosin T2 from Trichoderma viride (HBI Enzymes Inc., Osaka, Japan). The filter paper unit (FPU) activities of GC 220 and Cellulosin T2 were 443.3 and 0.9 FPU/mg of enzyme, respectively, and were adjusted to 15 FPU/g-dry biomass before use. Solka floc, Avicel, carboxymethylcellulose (CMC), waste office paper, and waste wood chip refiner were used to evaluate the saccharification efficiency. Waste paper was cut with a standard office shredder into 2 mm × 1.5 cm rectangles and was used for enzymatic hydrolysis without further pretreatment [16]. Waste wood chips were broken down to 12-mm mesh particles and were pre-treated with 2% sulfuric acid at 170 °C for 10 min. Chips were ground into a powder using a Recycle Refiner (Aikawa Iron Works Co., Shizuoka, Japan).
Sterilized flasks containing 50 ml of 0.1 M acetate buffer (pH 4.5) and 1.5 g dried substrate were incubated at 45 °C for 10 min. Hydrolysis of the substrate was initiated by the addition of commercial cellulase powders or A. cellulolyticus culture broth. Reactions were conducted in 500-ml Erlenmeyer flasks using a reciprocal shaker at 110 spm for 96 h at 45 °C. Samples were boiled to terminate the reactions, and the reaction mixtures were centrifuged at 10,000 rpm for 5 min. Reducing sugars and glucose concentrations were measured in the supernatants. The saccharification yield (%) of substrates to reducing sugar (x) was calculated as:
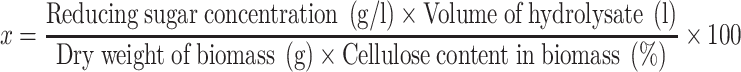 |
where the cellulose contents in office paper and wood chips were 80.3% and 49.9%, respectively [17, 18].
Analytical Methods
Total cellulase activity was determined using the IUPAC standard procedure with Whatman no. 1 filter paper [19, 20] and is expressed in FPU. Cellulase components were analyzed as follows. Avicel (1% w/v, Sigma-Aldrich Chem. GmbH) in acetate buffer (50 mM, pH 5.5) and the diluted enzyme solution were mixed in equal volumes and were reacted at 30 °C for 120 min with shaking (160 spm), with sampling every 15 min. A 180 µl aliquot of CMS (1% w/v, Sigma-Aldrich Chem.) was mixed with 20 μl of diluted enzyme solution in acetate buffer (50 mM, pH 5.5) and was reacted at 40 °C for 15 min with sampling every 3 min. The released reduced sugars were analyzed using the Somogyi-Nelson method [21]. One unit of enzyme activity was defined as the amount required to produce 1 μmol of reducing sugars in 1 min. The β-glucosidase activity was measured using glucose β-para-nitrophenyl (Glcβ-pNP) as substrate. Aliquots (25 µl) of Glcβ-pNP (10 mM) and the diluted enzyme solution were mixed in acetate buffer (50 mM, pH 5.5) and then reacted at 40 °C for 10 min. Samples were obtained every 2 min. The reaction was stopped with 190 μl of Na2CO3 (1 M), and the absorbance at 405 nm was measured. One unit of enzyme activity was defined as 1 μmol of pNP released per 1 min.
Due to the difficulty of separating the mycelium from the medium, the cell weight was measured using the inner nucleic acid (INA) method, and the dry cell weight (DCW) was calculated using the equation, 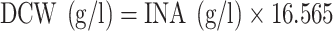 .
.
Supernatant protein concentrations were determined with a Bio-Rad Protein Assay kit (Bio-Rad Lab., USA). The concentration of reducing sugars in the medium was analyzed using the dinitrosalicylic acid method [22] and HPLC (PU-980, JASCO, Co. Ltd., Japan) with Shim-pack CLC-NH2 columns (Shimadzu Co., Kyoto, Japan) and a mobile phase of a 75% acetonitrile solution at a flow rate of 2 ml/min. Detection was accomplished with a refractive index detector (RI-930, JASCO, Co. Ltd., Tokyo, Japan). The glucose concentration was measured using a glucose kit (Wako Co. Ltd., Japan).
Results
Effect of Phosphate on Cellulase Production from A. cellulolyticus C1
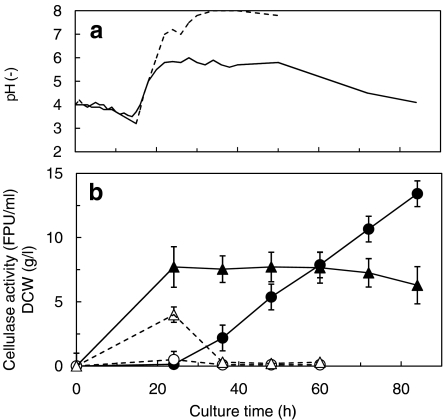
When A. cellulolyticus was cultured in medium with an initial monophotassium phosphate concentration of 24 g/l, only 0.8 g/l phosphate was consumed (data not shown). This indicated that approximately 1 g/l phosphate is sufficient for an A. cellulolyticus culture. This was confirmed with cultures containing initial phosphate concentrations of 24 and 1 g/l (Fig. 1). When the initial phosphate concentration was 1 g/l, the pH increased to 8 after 36 h of culture, and the cellulase activity was below 0.5 FPU/ml. However, at 24 g/l initial phosphate, the pH was maintained at 5.5–6.0 during cellulase production, and the cellulase activity increased with the culture time, reaching 13.2 FPU/ml at 84 h. The dry cell weight was 2.5 times higher than that obtained with 1 g/l initial phosphate. This indicates that the phosphate in the medium played an important role as a buffer in culture pH maintenance, suggesting that pH control is required for cellulase production in medium with weak buffering capacity.
Fig. 1.
pH profiles (a) and cellulase activity and dry cell weight (b) in batch cultures of A. cellulolyticus C1 in a 3-l bioreactor. Dotted and solid lines in a denote the pH values of cultures with initial monopotassium phosphate concentrations of 1 and 24 g/l, respectively. Closed circles and closed triangles in b indicate cellulase activity and dry cell weight, respectively, when the initial monopotassium phosphate concentration was 24 g/l; open circles and open triangles in b indicate cellulase activity and dry cell weight, respectively, when the initial monopotassium phosphate concentration was 1 g/l
Cellulase Production in pH-Controlled Cultures
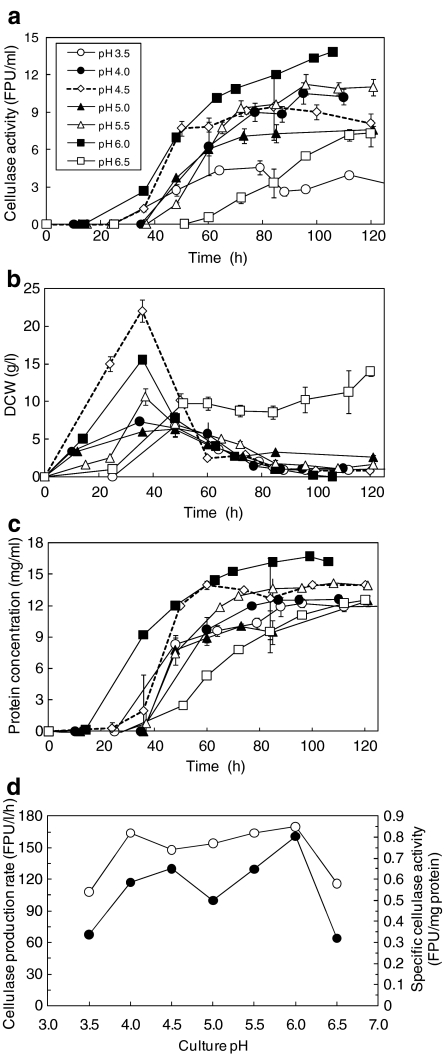
The culture pH was controlled from 3.5 to 6.5, using sulfuric acid and ammonium water. The cellulase activity was highest in the pH 6.0 culture (13.8 FPU/ml; Fig. 2a). At pH values of 4.0 and 5.5, the cellulase activity was 10.5 FPU/ml. In the pH 4.5-controlled culture, the cellulase activity was initially similar to those of the pH 4.0- and 5.5-controlled cultures, but decreased and ended at 7 FPU/ml. In the pH 6.5- and 3.5-controlled cultures, the cellulase production was significantly lower than those in other cultures. The dry cell weight increased until 36 h, but then decreased in all except the pH 6.5-controlled culture (Fig. 2b). At an acidic pH (pH 4.5 only), the dry cell weight reached 22.5 g/l, before decreasing sharply.
Fig. 2.
Time courses of cellulase activity (a), dry cell weight (b), and protein concentration (c) in cultures with pH-controlled at 3.5 (open circles), 4.0 (closed circles), 4.5 (open rhombuses), 5.0 (closed triangles), 5.5 (open triangles), 6.0 (closed squares), and 6.5 (open squares). d pH response of the cellulase production rate (closed circles) and the specific cellulase activity (open circles). The cellulase production rate and the specific cellulase activity were calculated from the data shown in a and c
The protein concentration profiles (Fig. 2c) were similar to those of the cellulase activity. The maximum specific cellulase activities based on the extracellular protein concentration were in the range of 0.75–0.85 FPU/mg protein for all except the pH 3.5- and 6.5-controlled cultures (Fig. 2d). The highest production rate, 160.9 FPU/l/h, was achieved in the pH 6.0-controlled culture (Fig. 2d). The values of the pH 4.0- and 4.5-controlled cultures were 117 and 130 FPU/l/h, respectively, which were higher than those of the other cultures. Interestingly, the production rate of the pH 5.0-controlled culture fell to 100 FPU/l/h. When the culture pH was controlled between 3.5 and 6.5, the cellulase production rate showed two peaks, at pH 4.0–4.5 and 6.0 (Fig. 2d). The traces of the pH responses of the specific cellulase activity and the cellulase production rate were found to be M-shaped, which is the first such finding for cellulase production using A. cellulolyticus.
Cellulase Activity in pH-Controlled Cultures
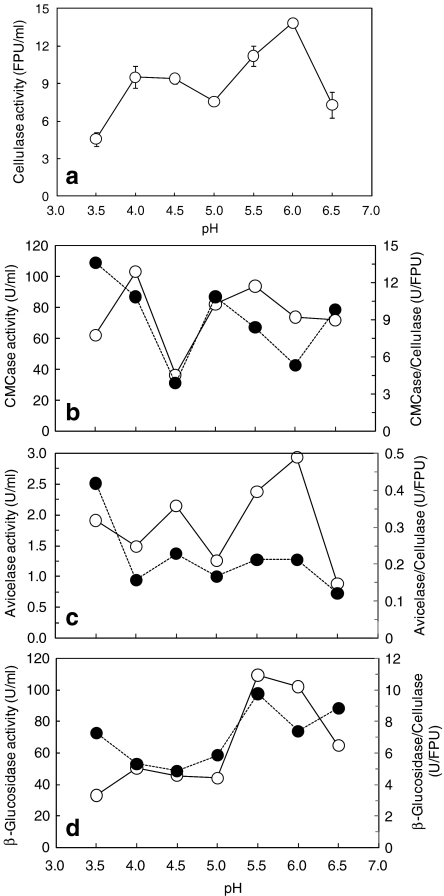
The cellulase activity also showed two peaks, at pH 4.0–4.5 and 6.0 (Fig. 3a). To investigate the cellulase response to culture pH, three enzyme activities were analyzed. The CMCase activity was maximal in the pH 4.0- and 5.5-controlled cultures, but lowest in the pH 4.5 culture (Fig. 3b). The avicelase activity showed two peaks in the pH 4.5- and 6.0-controlled cultures (Fig. 3c). The β-glucosidase activity was maximal in the pH-5.5 and 6.0-controlled cultures and was low at the acidic pH and at pH 6.5 (Fig. 3d). The first peak shown in the pH 4.0- and 4.5-controlled cultures (Fig. 3a) was due to the high CMCase activity; the second peak (pH 6.0-controlled culture) was caused by the high activities of avicelase and β-glucosidase. In the pH 4.5- and 5.0-controlled cultures, either the CMCase or avicelase activity was low, along with the β-glucosidase. These results reflect the fact that the cellulase activity represents a combination of three enzymatic activities that are sensitive to the pH of the culture.
Fig. 3.
pH response of cellulase activity (a), CMCase and its ratio to cellulase (b), avicelase and its ratio to cellulase (c), and β-glucosidase and its ratio to cellulase (d). The enzyme activity (straight lines) and the ratio of each enzyme to cellulase (dotted lines) were analyzed at the end of each pH-controlled culture
The effect of the culture pH on the ratios of each enzyme to the cellulase activity is significant (Fig. 3b–d). In the acidic pH-controlled cultures, the ratios of the avicelase and CMCase activities were higher than those of the neutral pH-controlled cultures. On the other hand, the ratio of the β-glucosidase activity was higher at the neutral pH range than those in the acidic pH ranges. This result suggests that the saccharification yield is dependent upon the pH condition of the saccharification reaction.
Saccharification by A. cellulolyticus Culture Supernatants
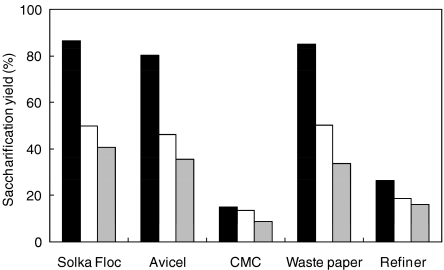
The culture supernatant of A. cellulolyticus and two other commercial enzymes were applied to the hydrolysis of Solka floc, Avicel, CMC, waste paper, and waste wood refiner. The saccharification yields of Solka floc, Avicel, and waste paper were greater than 80% in a 96 h reaction (Fig. 4). The Trichoderma enzymes GC220 and Cellulosin T2 converted 46–50% and 33–40% of the substrate to reducing sugars, respectively. The saccharification yields from CMC and refiner were 8–26%, using the three types of cellulase. The hydrolysis of wood chip refiner was similar among the three enzyme treatments; specifically, it was 26% in the culture supernatant of A. cellulolyticus, 17% in GC220, and 16% in T2 Cellulosin. Since most of the cellulose in wood chips is surrounded by lignin, it is difficult for enzymes to attack, which is one reason why the saccharification yields were lower than those from waste paper.
Fig. 4.
Comparison of the enzymatic performance using Solka floc, Avicel, CMC, waste paper, and waste wood chips refiner. Closed, open, and gray bars denote saccharification yields using culture solutions, GC220, and Cellulosin T2, respectively
The glucose content in the reaction mixture of A. cellulolyticus was 83%. The other 13% of reducing sugars were presumed to be xylose and cellobiose, based on previous research [8, 11]. The culture supernatant of A. cellulolyticus produced large amounts of glucose, while the enzymes from Trichoderma gave a lower glucose ratio. This is consistent with reports from other research groups [13]. Since members of the Trichoderma genus generally produce relatively low levels of β-glucosidase, they do not convert cellulolytic biomass to glucose efficiently. Thus, it is important to have a high glucose concentration in the hydrolysis solution, since it is the only sugar that the microorganisms can easily assimilate. The A. cellulolyticus supernatant had the highest saccharification yield and glucose content of all enzyme mixtures tested. This confirmed that the A. cellulolyticus enzymes have a remarkable ability to hydrolyze cellulolytic biomass.
Discussion
Cellulose-hydrolyzing enzymes, also called cellulolytic enzymes or simply cellulases, can play a crucial role in the environmentally friendly utilization of cellulolytic biomass. Most research on cellulase production has used cellulose powder or cellulolytic biomass as a carbon source, and the cellulase producer is usually Trichoderma sp. [22–25]. Using this organism, corn fiber [24], wheat straw [26], waste newspaper and sorbose, and manure [27–29] have been used for cellulase production. Penicillium brasilianum [30] or Penicillium janthinellum [31] have also been reported as cellulase producers.
Yamanobe et al. [9] isolated A. cellulolyticus Y-94 and obtained 5.0 FPU/ml from cellulose powder, which represented remarkably efficient hydrolysis, and a relatively high FPU value. However, these cellulase activities are insufficient for the industrial hydrolysis of cellulolytic biomass. Therefore, to obtain higher cellulase activity, we improved the cell line and optimized the medium for the practical production of cellulase. The optimized medium was used for 50 l scale-up experiments [15], which yielded cultures of Ferm P-18508 A. cellulolyticus C1 that could produce 15.5 FPU/ml of cellulase activity in flask cultures, 17.42 FPU/ml in a 7-l bioreactor, and 13.08 FPU/1ml in a 50-l bioreactor.
A. cellulolyticus produces several cellulases and β-glucosidases [8]. Additionally, one of its endo-cellulase components effectively produces glucose from cellobiose [8]. In this study, CMCase, avicelase, and β-glucosidase were assayed from pH-controlled cultures. Interestingly, avicelase and β-glucosidase showed similar profiles, with maximal activity in the pH 5.5- and 6.0-controlled cultures. The CMCase activity was the highest in the pH 4-controlled culture. The pH 6.0-controlled culture showed the highest cellulase activity among the cultures grown from pH 3.5 to 6.5.
The ratio of CMCase and avicelase to cellulase activity was high in the acidic pH region, but the ratio of β-glucosidase activity was high in the neutral pH region. This indicates that the breakage of the internal bond of cellulose and the cleavage of two to four units from the ends of the exposed chains are effective at an acidic pH. However, the hydrolysis of the cellobiose into individual monosaccharides, such as glucose or other reducing sugars, is more efficient at a neutral pH. The effectiveness of the cellulase performance was determined by the synergistic combination of these three enzymes. Therefore, if the breaking and cleaving reactions of cellulose are performed at an acidic pH, but the hydrolytic reaction to produce the monosaccharide is accomplished at a neutral pH, then the saccharification yield may be improved. These results indicated that these three types of cellulolytic enzymes are responsive to the culture pH. This is the first report describing the pH responses of these three types of enzymes on the cellulase production from A. cellulolyticus.
We plan to convert the reducing sugars obtained from the saccharification of waste biomass into biomass ethanol. Due to environmental concerns, there is demand for alternative resources, and this study is a step toward the establishment of an efficient system for reutilizing cellulolytic biomass.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Ikeda Y, Park E, Okuda N. Bioresource Technology. 2006;97:1030–1035. doi: 10.1016/j.biortech.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 2.Kádár Z, Szengyel Z, Réczey K. Journal of Biological Chemistry. 2004;153:375–380. [Google Scholar]
- 3.Park E, Pham A, Okuda N. Bioresource Technology. 2004;93:77–83. doi: 10.1016/j.biortech.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 4.Montane D, Farriol X, Salvado J, Jollez P, Chornet E. Biomass and Bioenergy. 1998;14:261–276. doi: 10.1016/S0961-9534(97)10045-9. [DOI] [Google Scholar]
- 5.Tengborg C, Galbe M, Zacchi G. Biotechnology Progress. 2001;17:110–117. doi: 10.1021/bp000145+. [DOI] [PubMed] [Google Scholar]
- 6.Abbi M, Kuhad RC, Singh A. Process Biochemistry. 1996;31:555–560. doi: 10.1016/S0032-9592(95)00104-2. [DOI] [Google Scholar]
- 7.Vlasenko EY, Ding H, Labavitch JM, Shoemaker SP. Bioresource Technology. 1997;59:109–119. doi: 10.1016/S0960-8524(96)00169-1. [DOI] [Google Scholar]
- 8.Palmarola-Adrados B, Juhasz T, Galbe M, Zacchi G. Biotechnology Progress. 2004;20:474–479. doi: 10.1021/bp034243h. [DOI] [PubMed] [Google Scholar]
- 9.Yamanobe T, Mitsuishi Y, Takasaki Y. Agricultural Biological Chemistry. 1987;51:65–74. [Google Scholar]
- 10.Kansarn S, Matsushita N, Kono T, Okada G. Journal of Applied Glycoscience. 2000;47:177–185. [Google Scholar]
- 11.Park E, Ikeda Y, Okuda N. Biotechnology Bioprocess Engineering. 2002;7:268–274. doi: 10.1007/BF02932835. [DOI] [Google Scholar]
- 12.Yamanobe T, Mitsuishi Y, Yagisawa M. Agricultural Biological Chemistry. 1988;52:2493–2501. [Google Scholar]
- 13.Yamanobe T, Hiraishi J, Kruus I. Agricultural Biological Chemistry. 1990;54:535–536. [Google Scholar]
- 14.Yamanobe T, Mitsuishi Y. Agricultural Biological Chemistry. 1990;54:301–307. [Google Scholar]
- 15.Ikeda Y, Hayashi H, Okuda N, Park EY. Biotechnology Progress. 2007;23:333–338. doi: 10.1021/bp060201s. [DOI] [PubMed] [Google Scholar]
- 16.Park E, Michinaka A, Okuda N. Biotechnology Progress. 2001;17:379–382. doi: 10.1021/bp0100070. [DOI] [PubMed] [Google Scholar]
- 17.Sosulski, K., & Swerhone, B. (1993). US DOE Report NREL-CP-200-5768, 2, 1032–1044.
- 18.Söderström J, Pilcher L, Zacchi G. Biomass and Bioenergy. 2003;24:475–486. doi: 10.1016/S0961-9534(02)00148-4. [DOI] [Google Scholar]
- 19.Ghose TK. Pure & Applied Chemistry. 1987;59:257–268. doi: 10.1351/pac198759020257. [DOI] [Google Scholar]
- 20.Nelson N. Journal of Biological Chemistry. 1944;153:375–380. [Google Scholar]
- 21.Bernfeld P. Methods in Enzymology. 1955;1:149. doi: 10.1016/0076-6879(55)01021-5. [DOI] [Google Scholar]
- 22.Domingues F, Queiroz J, Cabral J, Fonseca L. Enzyme Microbial Technolology. 2000;26:394–401. doi: 10.1016/S0141-0229(99)00166-0. [DOI] [PubMed] [Google Scholar]
- 23.Mountenecourt B, Eveleigh D. Advances in Chemistry Series. 1979;181:289–301. doi: 10.1021/ba-1979-0181.ch014. [DOI] [Google Scholar]
- 24.Gáspár M, Juhász T, Szemgyl Z, Réczey K. Process Biochemistry. 2005;40:1183–1188. doi: 10.1016/j.procbio.2004.04.004. [DOI] [Google Scholar]
- 25.Mandels M, Weber J, Parizek R. Applied Microbiology. 1971;21:152–154. doi: 10.1128/am.21.1.152-154.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chahal P, Chahal D, Lê G. Applied Biochemistry. Biotechnology. 1996;57(58):433–442. [Google Scholar]
- 27.Chen S, Wayman M. Process Biochemistry. 1991;26:93–100. doi: 10.1016/0032-9592(91)80023-I. [DOI] [Google Scholar]
- 28.Wen Z, Liao W, Chen S. Bioresource Technology. 2005;96:491–499. doi: 10.1016/j.biortech.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 29.Wen Z, Liao W, Chen S. Process Biochemistry. 2005;40:3087–3094. doi: 10.1016/j.procbio.2005.03.044. [DOI] [Google Scholar]
- 30.Jørgensen H, Olsson L. Enzyme and Microbial Technology. 2006;38:381–390. doi: 10.1016/j.enzmictec.2005.06.018. [DOI] [Google Scholar]
- 31.Adsul M, Ghule J, Singh R, Shaikh H, Bastawde K, Gokhale D, Varma A. Carbohydrate Polymers. 2004;57:67–72. doi: 10.1016/j.carbpol.2004.04.001. [DOI] [Google Scholar]