Abstract
The Hedgehog (Hh) signaling pathway affects fetal testis growth. Recently, we described the dynamic cellular production of Hh signaling pathway components in juvenile and adult rodent testes. The Hh signaling is understood to regulate cord formation in the fetal testis, but minimal knowledge exists regarding how Hh signaling impacts the postnatal testis. To investigate this, we employed hanging drop cultures, which are used routinely in embryoid body formation. This approach has the advantage of using small media volume, and we examined its suitability for short-term culture of both murine embryonic gonads and adult testis tubules. The effects of cyclopamine, a specific Hh signaling inhibitor, were examined following culture of Embryonic Day 11.5 urogenital ridges (as control) and adult seminiferous tubule fragments for 24–48 h using histological, cell proliferation, and gene expression analyses. Cultured embryonic testes displayed generally normal cord structure, anti-Müllerian hormone (Amh) expression, and cell proliferation; known Hh target gene expression (Gli1, osteopontin, official symbol Spp1, and Amh) was altered in response to cyclopamine. Cultured adult tubules exhibited some loss of seminiferous epithelium organization over 48 h. Spermatogonia continued to proliferate, however, and no significant loss of viability was noted overall. Addition of cyclopamine significantly affected levels of Gli1, Igfbp6, Ccnd2 (cyclin D2), Ccnb1 (cyclin B1), Spp1, Kit, and Amh mRNAs; these genes have been shown previously to be expressed in Sertoli and germ cells. These novel results identify Hh target genes in the testis and demonstrate this signaling pathway likely affects cell survival and differentiation in the context of normal adult testis.
Keywords: Gli1, hedgehog, organ culture, spermatogenesis, testis
Potential target genes of hedgehog signaling were identified in the fetal gonad and adult testis tubules.
INTRODUCTION
Testis development is initiated by expression of the male determining factor, Sry, which induces a specific population of somatic gonadal cells to differentiate as Sertoli cells. Testis cords form as the result of a layer of peritubular myoid cells surrounding and enclosing the Sertoli and germ cell lineages. In males, these cords are established during embryonic development and persist throughout life, with spermatogenesis taking place inside these cords.
Hedgehog (Hh) family members are secreted proteins that influence the growth of a myriad of tissues during development. Three Hh homologues have been identified in vertebrates, but only desert hedgehog (Dhh) is synthesized in the testis [1]. Each Hh molecule acts through two transmembrane receptors that regulate a signaling cascade, eventually leading to the nuclear translocation of the Hh-responsive transcription factors, the GLIs [2]. Of particular importance during development of the male embryonic gonad is the activity of the Dhh gene. Expression of the Dhh gene begins in mouse pre-Sertoli cells at Embryonic Day (E) 11.5, being among the earliest genes to be expressed after Sry [3], and this expression persists through adulthood.
Male mice genetically null for the Dhh gene are infertile, characterized by a lack of mature sperm and the presence of obvious testicular defects in utero, whereas females show no apparent phenotype [4]. Dhh-null males display peritubular cell abnormalities and severely impaired spermatogenesis. Close examination of the Dhh-null fetal testis revealed that these defects are evident as early as E13.5, when the production and full organization of the basal lamina that defines the embryonic seminiferous cords first fails to occur [5]. Mutations in the DHH gene have been identified in three cases of human 46,XY complete pure gonadal dysgenesis [6], a primary defect in gonadal formation. These individuals develop bilateral streak gonads, have normal Müllerian ducts, and contain female external genitalia, further implicating DHH in the testis-determining pathway.
Consistent with a role in seminiferous cord formation, cyclopamine treatment of murine E11.5 gonads in culture caused gonads to display scarce and disorganized laminin deposition compared to controls [7]. Cyclopamine, an antagonist of one of the Hh transmembrane receptors, also caused defects in fetal Leydig cell differentiation, as shown by reduced expression of side-chain cleavage enzyme; therefore, this treatment has been successfully used to manipulate gonadal development in culture. However, because the constitutive absence of Dhh in the fetal and postnatal testis results in a failure of proper testicular development, a different approach is required to gain insight regarding the role of Dhh in the adult testis.
Our understanding of testis development has been facilitated in part by in vitro culture investigations and much of the published in vitro data generated through standard culture techniques or agar mold-based systems [8, 9], which are excellent for the preservation of embryonic gonad morphology and testis cord development. These methods, however, require a minimum of 200 μl of media and thus facilitate diffusion of locally secreted factors away from their site of production and likely action. The cost of supplying exogenous agents in these systems also can be problematic, given the requirement for relatively large volumes of factor.
In the present study, we tested a method employing a significantly smaller volume of culture than has been used previously in studies of embryonic development to determine its potential for the culture of murine embryonic gonads and adult testis tubule fragments. Hanging drop cultures are used routinely to enable aggregation of embryonic stem cells to form embryoid bodies. To our knowledge, however, the application of this system for studies of testis development and function has not been reported. We hypothesized that this approach would make it is possible to study the influence of growth factors and other molecules on testicular cells in a small volume of media while maintaining the concentration of other locally produced factors and, presumably, cellular functions within this complex tissue.
Culture of intact murine embryonic gonads and of adult testis tubule fragments for up to 48 h was used to test the impact of reduced Hh signaling on cell proliferation, viability, and gene expression. Gonadal cells were observed to develop in a manner similar to that observed in vivo, indicating that the hanging drop organ-culture system is useful for the study of regulatory mechanisms relating to gonad and germ cell development. Genes previously identified as downstream targets of Hh signaling, including the benchmark gene, Gli1, were surveyed to validate this approach and to provide the first identification of Hh downstream targets in the fetal and adult mouse testis.
MATERIALS AND METHODS
Animals
For data relating to the adult testis, BALB/c male mice were analyzed. Swiss mice were used to generate data for embryonic analyses. Time-mated Swiss female mice and BALB/c adult male mice were obtained from Monash University Central Animal Services. The animals were killed by decapitation (fetuses) or by cervical dissociation (adult), and tissues were removed. Fetal testes and ovarian tissues were collected from Swiss mouse embryos staged by fore- and hindlimb morphology. All investigations conformed to the NHMRC/CSIRO/AAC Code of Practice for the Care and Use of Animals for Experimental Purposes and were approved by the Monash University Standing Committee on Ethics in Animal Experimentation.
Embryonic Gonad Culture
Urogenital ridges were dissected from embryos isolated from time-mated adult Swiss female mice. The E11.5 embryos were sexed by PCR genotyping for Sry and UbeXl [10]. The urogenital ridges were cultured in 30-μl hanging drops in Dulbecco minimal Eagle medium (DMEM; Gibco) and 10% fetal calf serum at 37°C for 48 h with 5% CO2/95% air. Hanging drops were prepared by pipetting 30 μl of media onto the inner surface of the lid of a 90- × 14-mm Petri dish. To inhibit Hh signaling, cyclopamine (final concentration, 25 μM; Calbiochem) was added to the culture medium at a concentration chosen based on previous mouse embryonic gonad cultures [7, 11]. An equivalent volume of ethanol (solvent for cyclopamine) was added for control drops. Dissected urogenital ridges were carefully transferred to hanging drops by collecting each ridge in 2 μl of media using a pipette and then placing each into a drop. Of the two urogenital ridges per animal, one was treated with cyclopamine, and the other was used as the untreated control. Each dish lid contained approximately 20 drops. Once all gonads were placed in drops, the lid was carefully inverted. The bottom of the Petri dish was filled with approximately 50 ml of dH20 to prevent drop evaporation. After 48 h in culture, tissue samples for RNA preparation were snap-frozen immediately after collection and stored at −80°C until use. Tissues for histological analysis were fixed in Bouin solution for 1 h immediately after collection, dehydrated through a graded ethanol series, embedded in paraffin wax, and sectioned (thickness, 4 μm). All experiments were performed a minimum of three times. Urogenital ridges also were cultured in agar blocks to allow morphological comparison between hanging drop- and agar block-cultured gonads. Urogenital ridges were dissected and sexed as described above and were cultured at the air-surface interface on 1.5% agar/DMEM blocks over 48 h at 37°C in 5% CO2/95% air [8]. The blocks were rested in 200 μl of DMEM plus 10% fetal calf serum in organ culture dishes, with the media replaced after 24 h.
Adult Mouse Seminiferous Tubule Culture
Dissected testes were placed in media and decapsulated using 12-cm spring scissors with 12-mm extrafine blades (World Precision Instruments). Tubules were dissociated by gently pulling them apart using fine forceps and then cut into 5-mm fragments using an Ophthalmic scalpel (Micro Feather). Tubule fragments were cultured in 30-μl hanging drops in DMEM and 0.1% BSA at 32°C for up to 48 h with 5% CO2/95% air. Hanging drops were prepared as described above. Up to five tubule fragments were cultured in each hanging drop. Cyclopamine (final concentration, 100 μM) was added to the culture medium to inhibit Hh signaling. A higher concentration of cyclopamine was used in the adult tubule cultures, because preliminary experiments indicated that it resulted in significant Gli1 downregulation without an observable adverse affect on tissue morphology or viability. An equivalent volume of ethanol was added to control samples. Tubule fragments were transferred to drops by hooking tubules over the tip of the forceps, taking care to avoid squeezing the tubules with the forceps. Cultures were performed as described above, as were tissue sample collection and processing.
Thymidine Assay
To assess the effect of Hh signaling inhibition on cell proliferation, the incorporation of [3H]thymidine in adult seminiferous tubules, in the presence and absence of cyclopamine, was measured. Adult seminiferous tubule cultures were set up as described above. The [3H]thymidine (0.5 μCi/hanging drop) was added for the final 20 h of culture. Extraction of DNA from the tubules was performed using the DNeasy Tissue kit (Qiagen), and DNA was quantitated by spectrophotometry. Radioactivity was measured using a 1900TR Liquid Scintillation Analyzer (Packard).
Cell Viability Assay
To assess viability of cells in culture, the CellTiter 96 AQueous Non-Radioactive Cell Proliferation Assay (Promega) was used. This assay is based on the conversion of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) into formazan by dehydrogenase enzymes found in metabolically active cells. The amount of formazan produced is directly proportional to the number of living cells in culture and is measured by the amount of absorbance at 490 nm. Briefly, MTS/PMS (phenazine methosulfate) solution was added to each hanging drop (1 μl of MTS/PMS solution per 5 μl of media) and incubated for 1.5 h, after which the absorbance was read on a Labsystems Mulitscan RC plate reader.
Immunohistochemistry
Immunohistochemistry was performed as described previously [12], beginning with antigen retrieval in 50 mM glycine (pH 3.5; >90°C maintained for 8 min). The primary antibody, mouse anti-PCNA (M0879; DAKO) or anti-AMH (sc-6886; Santa Cruz Biotechnology, Inc.), was applied by overnight incubation in 0.1% BSA/PBS. Subsequent steps were performed at room temperature, with PBS washes between incubations. Primary antibody binding was detected using a biotinylated rabbit anti-mouse (E0354; DAKO) or rabbit anti-goat (E0466; DAKO) (1:500 dilution, 1 h) and then the Vectastain Elite ABC kit (Vector Laboratories) according to the manufacturer's instructions. Antibody binding was detected as a brown precipitate following development with 3,3′-diaminobenzidine tetrahydrochloride (Sigma), and Harris Hematoxylin was used as counterstain. The sections were mounted under glass coverslips in Depex (BDH Laboratories).
TUNEL Analysis
Apoptotic cells were detected by TUNEL assay using the ApopTag Peroxidase In Situ Apoptosis Detection Kit (Chemicon). The procedure was carried out according to the manufacturer's instructions on sections. Briefly, sections were incubated in equilibration buffer for 15 min, followed by terminal deoxynucleotidyl transferase (supplied with kit) enzyme under plastic coverslips for 1 h at 37°C. After removal of coverslips, the slides were washed in preheated Stop wash solution for 30 min at 37°C. Sections were blocked using CAS Block (Zymed) for 30 min, followed by a 30-min incubation with the antidigoxigenin antibody (supplied with kit). Sections were developed and processed as described for immunohistochemistry.
Real-Time PCR
Isolation of RNA from cultured E11.5 gonads and adult testis tubule segments was performed using the RNeasy RNA extraction kit (Qiagen), and contaminating DNA was removed using the DNA-free kit (Ambion). The RNA was quantified using the Quant-iT RiboGreen RNA Assay kit (Molecular Probes, Invitrogen). Complementary DNA was synthesized using Superscript III Reverse Transcriptase (Life Technologies) with random hexamer primers according to the manufacturer's instructions.
Each 500-ng sample of RNA was used in each 20-μl RT reaction. The PCR samples were prepared in a final volume of 10 μl using SYBR-Green PCR master mix (Roche Diagnostics). The PCR, which was performed in the LightCycler 2.0 Instrument (Roche Molecular Biochemicals), used the following primers: Amh: forward (F), 5′-gcagttgctagtcctacatc-3′; reverse (R), 5′-tcatccgcgtgaaacagcg-3′; Kit: F, 5′-tcatcgagtgtgatgggaaa-3′; R, 5′-ggtgacttgtttcaggcaca; Ccnb1: F, 5′-agatggagatgaagattctcagagttct-3′; R, 5′-gacgtcaacctctccgacttta-3′; Ccnd2: F, 5′-cctcacgacttcattgagca-3′; R, 5′-atgctgctcttgacggaact-3′; Gli1: F, 5′-ggaagtcctattcacgccttga-3′; R, 5′-caaccttcttcttgctcacacatgtaag-3′; Igfbp6: F, 5′-cagagaccggcagaagaatc-3′; R, 5′-gcttccttgaccatctggag-3′; Spp1: F, 5′-actttcactccaatcgtcccta-3′; R, 5′-tgtggcatcaggatactgttca-3′; Kitl: F, 5′-tggatgacctcgtgttatgc-3′; R, 5′-tcagatgccaccataaagtcc-3′; Ddx4: F: 5′-ggtccaaaagtgacatatataccc-3′; R, 5′-ttggttgatcagttctcgagt-3′; 18s: F, 5′-gtaacccgttgaaccccatt-3′; R, 5′-ccatccaatcggtagtagcg-3′. Each PCR reaction was performed in triplicate, with negative controls, in which water replaced the reverse-transcribed template (included for each primer pair to ascertain whether PCR amplification of contaminating DNA had occurred). Candidate gene mRNA levels were normalized to that of 18s RNA. Statistical analysis was performed using the Student unpaired t-test. The PCR products were verified by size fractionation on 1% agarose gels and visualized under ultraviolet light.
RESULTS
Analysis of E11.5 Gonads after Culture in Hanging Drops
The architecture of the E11.5 gonads cultured in hanging drops for up to 48 h was well-preserved in 7 of 10 cultured urogenital ridges, and testicular cells appeared to develop at a rate similar to that observed in vivo (Fig. 1, a–c and g–i). The E11.5 gonads cultured for 24 and 48 h were similar to the E12.5 and E13.5 fetal gonads, respectively, on inspection of histological specimens. Proliferation and apoptosis were not assessed in cyclopamine-treated fetal gonads, because it has been shown previously that cyclopamine treatment does not affect proliferation or apoptosis at this stage of gonadogenesis [11].
FIG. 1.
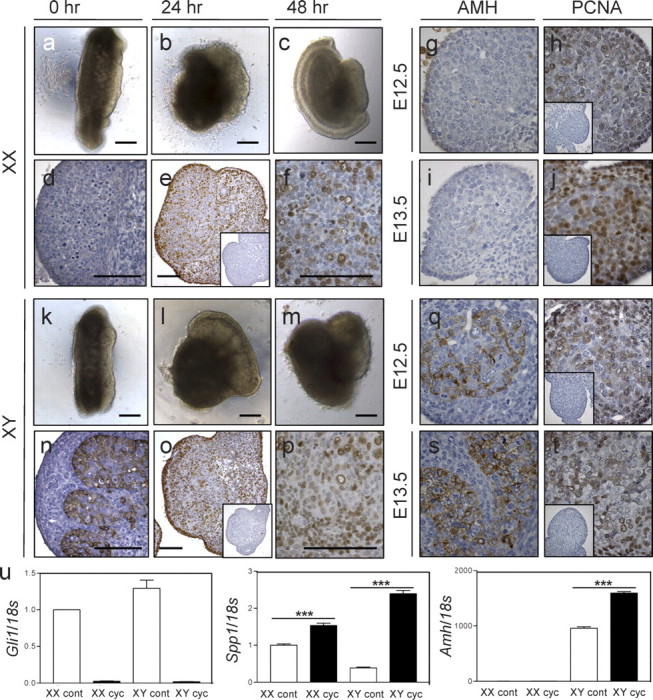
Embryonic Day (E) 11.5 gonads before and after culture in hanging drops for up to 48 h. Shown are E11.5 XX (a) and XY (k) gonads before culture, after 24 h of culture (b, l), and after 48 h of culture (c–f, m–p). AMH (d, n) and PCNA (e, f, o, p) immunostaining of E11.5 gonads cultured for 48 h is shown as well. Insets show control sections using no primary antibody. AMH and PCNA staining in normal gonads is shown in the panels on the right as a comparison. AMH staining is shown in normal XX E12.5 (g) and E13.5 (i) gonads and XY E12.5 (q) and E13.5 (s) gonads, whereas PCNA staining is shown in normal XX E12.5 (h) and E13.5 (j) gonads and XY E12.5 (r) and E13.5 (t). Real-time PCR was used to measure mRNA levels (u) of Gli1 (n = 4 shown) as well as Spp1 and Amh (n = 1 shown here) in control (cont) and cyclopamine-treated (cyc) gonads (k). Real-time data were normalized to 18s levels. XX control values were normalized to one. Error bars represent the SEM. Asterisks indicate statistical significance (***P < 0.001). Bars = 100 μm, all panels and insets; scale bars in panels d and n apply to g–j and q–t, respectively.
In cultured male urogenital ridges, seminiferous cords are clearly visible after 48 h in culture, and Sertoli cells express markers such as AMH (Fig. 1n), as seen at E12.5 and E13.5 (Fig. 1, q and s), suggesting that development within drops mirrors in vivo differentiation. As expected, AMH protein is not detected in female gonads after culture (Fig. 1d). The PCNA immunostaining revealed that cell proliferation occurs throughout the urogenital ridge after 48 h of culture (Fig. 1, e, f, o, and p), and staining appears to be similar to that observed in E12.5 (Fig. 1, h and r) and E13.5 (Fig. 1, j and t) gonads.
In cultures where testicular architecture was perturbed, substantial increases in mesonephros size were observed, whereas growth of the gonad was visibly restricted (data not shown). These samples were excluded from further analysis.
We also compared the morphology of gonads cultured in hanging drops with those cultured in agar blocks, a standard in vitro technique for embryonic gonad culture (Supplemental Fig. 1, available online at www.biolreprod.org). In general, the agar block-cultured male and female gonads remain straighter (Supplemental Fig. 1, a, c, e, and g) after 24 and 48 h compared with those cultured in hanging drops (Fig. 1, b, d, f, and h). The hanging drop gonads became more rounded over the culture period. Both techniques, however, allow gonad growth, and the gonad and mesonephros are easily distinguishable at each time point.
To rule out depletion of media in hanging drops, we repeatedly tested the volume of hanging drops before and after 24 and 48 h of culture at 37°C. No changes in volume were observed over this period of time (data not shown).
Identification of Hh Signaling Target Genes in the Embryonic Gonad
The suitability of hanging drop cultures for studying the effect of exogenous molecules on gonadal development was assessed by adding a selective inhibitor of the Hh signaling pathway to the culture system, and quantitative real-time PCR was used to determine whether downstream Hh target gene expression was altered in treated embryonic gonads. Cyclopamine selectively inhibits Hh signaling and has been used to alter gonadal development in culture [7, 11]; hence, it was an excellent molecule to use for testing the viability of the hanging drop system.
A significantly lower level of Gli1 mRNA was measured in XX and XY embryonic gonads treated with cyclopamine compared to control samples (Fig. 1u). Spp1 (osteopontin) and Amh also were affected by reduced Hh signaling. In three independent experiments, the measured level of Amh was always significantly higher in XY gonads after cyclopamine treatment when compared to untreated controls (Fig. 1u); the change varied between a 1.2- and 2-fold increase. Similarly, osteopontin was significantly increased in cyclopamine-treated XX gonads (between 1.5- and 2.8-fold measured increase) and XY gonads (between 1.3- and 6-fold increase) in each of three experiments. Because of the variability of the difference between experiments, a representative graph from one culture is shown in Figure 1.
Analysis of Adult Seminiferous Tubules Cultured in Hanging Drops
Adult mouse tubules cultured for up to 48 h displayed overall maintenance of seminiferous epithelial organization. The PCNA immunostaining revealed cell proliferation in the tubules after 48 h of culture; however, a quantitative reduction in cell proliferation was observed after 24 h of culture (Fig. 2e). Beyond 48 h of culture, seminiferous tubules appeared more disorganized, and an increase in germ cell loss was noted (data not shown). Tubule viability after 48 h probably is limited by the absence of signals from the interstitial compartment, which is removed when tubules are separated.
FIG. 2.
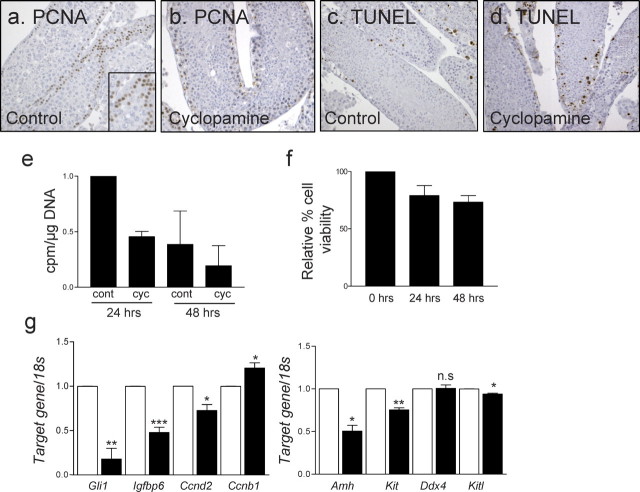
Effect of cyclopamine on cultured adult testis tubules. Shown are PCNA immunostaining of control (a) and cyclopamine-treated adult (b) testis tubules cultured for 24 h as well as TUNEL staining of control (c) and cyclopamine-treated (d) adult testis tubules cultured for 24 h. Thymidine assay using 24- and 48-h cultures, comparing control and cyclopamine-treated tubules, also is shown (e). Counts are normalized to amount of DNA to account for differences in cell number. Value of the 24-h control was set to one. A graph representing one experiment is shown here. Measurement of cell viability of adult tubule fragments over time in culture is depicted (f), with the value of freshly collected tubules (0 h) set at 100%. Real-time PCR was used to measure candidate gene mRNAs in control and cyclopamine-treated tubules cultured for 48 h (g). Error bars represent the SEM of three separate experiments. Asterisks indicate statistical significance (*P < 0.05, **P < 0.01, ***P < 0.001). n.s., not significant. Original magnification ×40 (a–d) and ×63 (inset in a).
The viability of tubules also was assessed using a cell viability kit, which is based on the conversion of MTS to formazan by dehydrogenase enzymes in metabolically active cells. Although a minor decrease in viability was observed from time zero, the change was not statistically significant at 24 and 48 h in culture (Fig. 2f).
Adult Seminiferous Tubules Exhibit Cyclopamine-Responsiveness
Adult seminiferous tubule fragments cultured in hanging drops for up to 48 h were used to test the impact of reduced Hh signaling on adult mouse seminiferous tubules. Cyclopamine was administered to inhibit Hh signaling. Assessment of cell proliferation by both PCNA immunohistochemistry and [3H]thymidine incorporation revealed reduced cell proliferation in tubules cultured with cyclopamine relative to vehicle controls (Fig. 2, a, b, and e). The TUNEL analysis was used to detect apoptotic cells, and no difference in the frequency of cells undergoing apoptosis between control and cyclopamine-treated seminiferous tubules was apparent (Fig. 2, c and d).
To identify downstream Hh target genes relevant to the adult testis, real-time PCR measurements were used to compare target gene mRNA levels between control and cyclopamine-treated tubules (Fig. 2g). Ddx4 (also known as Mvh) and Kitl are markers for germ cells and Sertoli cells, respectively. The Ddx4 mRNA levels were measured to test for any changes in germ cell number. No significant difference in Ddx4 mRNA levels was found between control and cyclopamine-treated samples, suggesting that cyclopamine treatment did not affect germ cell number. The established Hh target gene, Gli1, is significantly downregulated in cyclopamine-treated tubules, confirming inhibition of the Hh pathway in this system, as reported previously for cyclopamine treatment in other systems [13–15]. We have identified genes such as Igfbp6, Ccnd2, Amh, and Kit that showed significant downregulation, suggesting Dhh signaling normally induces their expression. Ccnb1 showed a significant increase in expression in cyclopamine-treated tubules compared to controls, indicating that it normally is inhibited by Dhh.
DISCUSSION
In the present study, we have developed a novel approach for embryonic and adult mouse testis organ culture and have validated its application for short-term studies of factor affects on gene expression. Using this hanging drop approach, adapted from work with embryonic stem cell-derived embryoid bodies [16], it will be possible to survey the influence of growth factors and other molecules on testicular cells in a small volume using limited amounts of tissue. We propose that the drops serve to maintain a high local concentration of endogenous factors and, thus, to sustain tissue function. In addition, the high ratio of surface area to volume facilitates gas exchange.
Testicular cells in the E11.5 gonad appear to follow their normal differentiation pathways in these cultures over 48 h relative to what is observed in vivo. The PCNA staining of cultured fetal gonads demonstrated that both germ and somatic cells continued to proliferate, supporting histological observations that the tissue is healthy and that the cells are, at least on a gross level, following their normal developmental program. Seven out of 10 embryonic testes appeared to be normal after 48 h in culture when histological specimens were examined; hence, the system does support the growth of healthy gonads in the majority of tissue placed in culture. We observed that urogenital ridges placed in the drop with gonad and mesonephros laying side-by-side rather than one on top of the other tended to retain their morphology. This could be because the side-by-side orientation more closely mimics the physical relationship between the two tissues in vivo [17].
This side-by-side orientation is mimicked by gonads cultured in agar blocks, one of the standard embryonic urogenital ridge culture techniques, as seen in Supplemental Figure 1. Only slight differences, however, were found in the morphology of gonads cultured in agar blocks when compared to those cultured in hanging drops. The block methodology allowed the gonad to grow in an elongated fashion rather than with the slight rounding that was seen in the hanging drop gonads. This rounding most likely resulted from the gonad sitting in the bottom of the drop; however, this did not appear to hamper gonad growth. The main advantage of using hanging drops over agar blocks is the ability to maintain treatment or local factor concentrations. In cultures with agar mold blocks, locally produced factors can easily diffuse away from the gonad, and almost 7-fold more media is required in comparison to that with hanging drops. Clearly, both methodologies are viable options for in vitro culture, and with the adaptation of this technique, researchers now have more choice in how experiments are performed.
Over 48 h in culture, the normally highly ordered arrangement of adult testis tubules became disrupted. Spermatogonia, however, continued to stain positive for PCNA, as expected for this mitotic cell type, and overall cell viability did not decrease significantly. Thus, despite the loss of architectural integrity and maintenance of normal interstitial and vascular cell types that occurs in tubule cultures such as these, this approach proved to be useful for short-term interrogation of the processes and target genes affected by Hh pathway activity in the adult mouse testis that were not previously known. The results of cyclopamine inhibition indicate that Hh signaling positively influences cell proliferation in the adult mouse testis. Recent reports from other systems implicate Hh signaling in modulation of selected cell-cycle regulators, antiapoptotic factors, and cell signaling proteins. Gli1 is a well-known and robust Hh target gene; it was significantly downregulated in both ages of cyclopamine-treated tissues examined, confirming that inhibition of Hh pathway activity had been achieved. The other candidate genes used for the present study were selected based on prior evidence of their regulation by Hh. The Sonic hedgehog-mediated regulation of Igfbp6, Ccnd2, and Spp1 has been demonstrated previously in the fetal prostate [18], whereas regulation of Amh was measured in C3H/10T1/2 cells [19]. In addition, Gli1 consensus DNA-binding sequences have been identified in the 5′ regions of Ccnd2, Igfbp6, and Spp1 [20].
Some of these genes were differently regulated by Hh inhibition at the two stages of development tested, with Amh being upregulated in fetal gonads and downregulated in adult testis tubules. It is not clear why this is the case, but because the three mammalian GLI transcription factors display target gene specificity [21], their relative abundance may be expected to vary and, thereby, dictate the complement of genes expressed within each cell type, resulting in differences such as these.
The present results have identified clear candidates for DHH target genes in the fetal and adult mouse testis in vivo, and the data also support the conclusion that hanging drops can be used to study the short-term effect of exogenous molecules on testis development. These results implicate Hh signaling in the regulation of molecules that mediate important cellular processes in the testis, thus highlighting the potential consequences of dysregulated Hh signaling. Cyclin D2 is required for spermatogonial differentiation in the adult mouse testis [22]. Testes of Ccnd2−/− mice exhibit hypoplasia and, consequently, a 2.0- to 2.9-fold reduced sperm count [23]. The KIT receptor function is critical for normal spermatogenesis and is required for spermatogonial differentiation [24]. Of the genes identified in the present study, Igfbp6 [25], Ccnd2 [25], and Kit [25, 26] are commonly overexpressed in testicular germ cell tumors, indicating a potential link between altered Hh activity and tumor development in the testis. In addition, the finding that target genes analyzed in the present study are expressed in both Sertoli and germ cells suggests that both lineages are targets of Hh activity in the fetal and adult testis. Further analyses will identify how perturbation of Hh signaling, either directly or indirectly through factors such as these, contributes to defects in spermatogenesis and reduced fertility observed in the absence of Hh signaling.
Supplementary Material
Footnotes
1Supported by the Australian Research Council (#348239) and by a fellowship (#384108) to K.L.L. from the National Health and Medical Research Council of Australia. A.S. is supported by a Monash University Graduate Scholarship and an Australian Research Council Centre of Excellence Scholarship.
REFERENCES
- Szczepny A, Hime GR, Loveland KL. Expression of hedgehog signaling components in adult mouse testis. Dev Dyn 2006; 235: 3063 3070 [DOI] [PubMed] [Google Scholar]
- Hooper JE, Scott MP. Communicating with hedgehogs. Nat Rev Mol Cell Biol 2005; 6: 306 317 [DOI] [PubMed] [Google Scholar]
- Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol 1995; 172: 126 138 [DOI] [PubMed] [Google Scholar]
- Bitgood MJ, Shen L, McMahon AP. Sertoli cell signaling by desert hedgehog regulates the male germline. Curr Biol 1996; 6: 298 304 [DOI] [PubMed] [Google Scholar]
- Pierucci-Alves F, Clark AM, Russell LD. A developmental study of the desert hedgehog-null mouse testis. Biol Reprod 2001; 65: 1392 1402 [DOI] [PubMed] [Google Scholar]
- Canto P, Soderlund D, Reyes E, Mendez JP. Mutations in the desert hedgehog (DHH) gene in patients with 46,XY complete pure gonadal dysgenesis. J Clin Endocrinol Metab 2004; 89: 4480 4483 [DOI] [PubMed] [Google Scholar]
- Yao HH, Capel B. Disruption of testis cords by cyclopamine or forskolin reveals independent cellular pathways in testis organogenesis. Dev Biol 2002; 246: 356 365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau J, Nordqvist K, Tilmann C, Lovell-Badge R, Capel B. Male-specific cell migration into the developing gonad. Curr Biol 1997; 7: 958 968 [DOI] [PubMed] [Google Scholar]
- Meehan T, Schlatt S, O'Bryan MK, de Kretser DM, Loveland KL. Regulation of germ cell and Sertoli cell development by activin, follistatin, and FSH. Dev Biol 2000; 220: 225 237 [DOI] [PubMed] [Google Scholar]
- Chuma S, Nakatsuji N. Autonomous transition into meiosis of mouse fetal germ cells in vitro and its inhibition by gp130-mediated signaling. Dev Biol 2001; 229: 468 479 [DOI] [PubMed] [Google Scholar]
- Yao HH, Whoriskey W, Capel B. Desert Hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev 2002; 16: 1433 1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveland KL, Herszfeld D, Chu B, Rames E, Christy E, Briggs LJ, Shakri R, de Kretser DM, Jans DA. Novel low-molecular-weight microtubule-associated protein-2 isoforms contain a functional nuclear localization sequence. J Biol Chem 1999; 274: 19261 19268 [DOI] [PubMed] [Google Scholar]
- Nagase M, Nagase T, Koshima I, Fujita T. Critical time window of hedgehog-dependent angiogenesis in murine yolk sac. Microvasc Res 2006; 71: 85 90 [DOI] [PubMed] [Google Scholar]
- Russell MC, Cowan RG, Harman RM, Walker AL, Quirk SM. The hedgehog signaling pathway in the mouse ovary. Biol Reprod 2007; 77: 226 236 [DOI] [PubMed] [Google Scholar]
- Lamm ML, Catbagan WS, Laciak RJ, Barnett DH, Hebner CM, Gaffield W, Walterhouse D, Iannaccone P, Bushman W. Sonic hedgehog activates mesenchymal Gli1 expression during prostate ductal bud formation. Dev Biol 2002; 249: 349 366 [DOI] [PubMed] [Google Scholar]
- Dang SM, Kyba M, Perlingeiro R, Daley GQ, Zandstra PW. Efficiency of embryoid body formation and hematopoietic development from embryonic stem cells in different culture systems. Biotechnol Bioeng 2002; 78: 442 453 [DOI] [PubMed] [Google Scholar]
- Kaufman MH. The Atlas of Mouse Development. London: Academic Press; 1995. [Google Scholar]
- Lipinski RJ, Cook CH, Barnett DH, Gipp JJ, Peterson RE, Bushman W. Sonic hedgehog signaling regulates the expression of insulin-like growth factor binding protein-6 during fetal prostate development. Dev Dyn 2005; 233: 829 836 [DOI] [PubMed] [Google Scholar]
- Ingram WJ, Wicking CA, Grimmond SM, Forrest AR, Wainwright BJ. Novel genes regulated by sonic hedgehog in pluripotent mesenchymal cells. Oncogene 2002; 21: 8196 8205 [DOI] [PubMed] [Google Scholar]
- Yoon JW, Kita Y, Frank DJ, Majewski RR, Konicek BA, Nobrega MA, Jacob H, Walterhouse D, Iannaccone P. Gene expression profiling leads to identification of GLI1-binding elements in target genes and a role for multiple downstream pathways in GLI1-induced cell transformation. J Biol Chem 2002; 277: 5548 5555 [DOI] [PubMed] [Google Scholar]
- Aza-Blanc P, Lin HY, Ruiz i Altaba A, Kornberg TB. Expression of the vertebrate Gli proteins in Drosophila reveals a distribution of activator and repressor activities. Development 2000; 127: 4293 4301 [DOI] [PubMed] [Google Scholar]
- Beumer TL, Roepers-Gajadien HL, Gademan IS, Kal HB, de Rooij DG. Involvement of the D-type cyclins in germ cell proliferation and differentiation in the mouse. Biol Reprod 2000; 63: 1893 1898 [DOI] [PubMed] [Google Scholar]
- Sicinski P, Donaher JL, Geng Y, Parker SB, Gardner H, Park MY, Robker RL, Richards JS, McGinnis LK, Biggers JD, Eppig JJ, Bronson RT, Elledge SJ, Weinberg RA. Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature 1996; 384: 470 474 [DOI] [PubMed] [Google Scholar]
- Yoshinaga K, Nishikawa S, Ogawa M, Hayashi S, Kunisada T, Fujimoto T, Nishikawa S. Role of c-kit in mouse spermatogenesis: identification of spermatogonia as a specific site of c-kit expression and function. Development 1991; 113: 689 699 [DOI] [PubMed] [Google Scholar]
- Hoei-Hansen CE, Nielsen JE, Almstrup K, Hansen MA, Skakkebaek NE, Rajpert-DeMeyts E, Leffers H. Identification of genes differentially expressed in testes containing carcinoma in situ. Mol Hum Reprod 2004; 10: 423 431 [DOI] [PubMed] [Google Scholar]
- Rajpert-De Meyts E, Skakkebaek NE. Expression of the c-kit protein product in carcinoma-in-situ and invasive testicular germ cell tumors. Int J Androl 1994; 17: 85 92 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.