Abstract
Candida albicans is an increasingly important pulmonary fungal pathogen. Resident alveolar macrophages are important in host defense against opportunistic fungal infections. Activation of Group IVA cytosolic phospholipase A2α (cPLA2α) in macrophages initiates arachidonic acid (AA) release for production of eicosanoids, which regulate inflammation and immune responses. We investigated the ability of C. albicans to activate cPLA2α in unprimed alveolar macrophages and after priming with granulocyte macrophage colony-stimulating factor (GM-CSF), which regulates alveolar macrophage maturation. AA was released within minutes by GM-CSF–primed but not unprimed alveolar macrophages in response to C. albicans, and was blocked by soluble glucan phosphate (S-GP). The expression of the β-glucan receptor dectin-1 was increased in GM-CSF–primed macrophages, and AA release from GM-CSF–primed dectin-1−/− alveolar macrophages was reduced to basal levels. The enhanced activation of extracellular signal–regulated kinases and phosphorylation of cPLA2α on Ser-505 that occurred in GM-CSF–primed macrophages were reduced by MEK1 and Syk inhibitors, which also suppressed AA release. At later times after C. albicans infection (6 h), unprimed and GM-CSF–primed macrophages released similar levels of AA. The expression of cyclooxygenase 2 and prostanoid production at 6 hours was higher in GM-CSF–primed macrophages, but the responses were not dependent on dectin-1. However, dectin-1 contributed to the C. albicans–stimulated increase in TNF-α production that occurred in GM-CSF–primed macrophages. The results demonstrate that dectin-1 mediates the acute activation of cPLA2α in GM-CSF–primed alveolar macrophages, but not in the more delayed phase of AA release and GM-CSF–dependent prostanoid production.
Keywords: cytosolic phospholipase A2, dectin-1, alveolar macrophages, granulocyte macrophage colony-stimulating factor, arachidonic acid
CLINICAL RELEVANCE.
Our findings provide insight into the regulation of alveolar macrophage responses to the opportunistic fungal pathogen Candida albicans. The importance of granulocyte macrophage colony-stimulating factor priming for lipid mediator production by alveolar macrophages is shown, and the role of dectin-1 is investigated.
Candida albicans is an opportunistic fungal pathogen of humans, with the capacity to cause both disseminated and mucosal infection. These infections are an increasing problem in immunocompromised patients and are associated with high incidence of mortality and morbidity (1). A variety of fungi including C. albicans cause respiratory disease that occurs during immunosuppressive therapy, bone marrow and organ transplants, and HIV infection (2). In the immunocompetent host C. albicans is cleared from the lung by alveolar macrophages (3). However, during experimental disseminated Candidiasis, alveolar macrophages may contribute to acute lung injury (4). Elucidating how C. albicans interacts with and activates macrophages is important for understanding the regulation of host responses to fungal infection.
Microorganisms bind to pattern recognition receptors (PRR) such as Toll-like receptors (TLR) and C-type lectin receptors on macrophages, triggering the production of cytokines, reactive oxygen species, and lipid mediators (5, 6). There has been considerable interest in how specific PRRs promote cytokine production, but much less is known about the regulation of lipid mediator production by microorganisms. Eicosanoid production is initiated by the release of AA from membrane phospholipids by Group IVA cytosolic phospholipase A2 (cPLA2α) (7, 8). cPLA2α is regulated post-translationally by increases in intracellular calcium levels and phosphorylation on Ser-505 by mitogen-activated protein kinases (7). AA is metabolized by 5-lipoxygenase to leukotrienes and by constitutive cyclooxygenase (COX)1 or inducible COX2 for prostanoid production (9). Eicosanoids are secreted and act locally through cell surface G protein–coupled receptors. They can have diverse roles in regulating innate immunity by promoting acute inflammatory responses such as increased vascular permeability, by recruiting leukocytes to the site of infection, by regulating phagocytosis and cytokine production, and by affecting lymphocyte function (9–12).
Macrophage responses to C. albicans are initiated by engagement of TLRs and C-type lectin receptors by the fungal cell wall carbohydrates glucans and mannans (13–15). The C-type lectin-like receptor dectin-1 is the major β-glucan receptor on macrophages (14, 16). It is a type II transmembrane receptor containing a single extracellular C-type lectin-like domain and an immunoreceptor tyrosine-based activation motif in its cytoplasmic tail (17). The objective of this study was to determine the role of dectin-1 in mediating AA release and inflammatory mediator production by alveolar macrophages in response to C. albicans.
MATERIALS AND METHODS
Materials
Balb/c and ICR mice were obtained from Harlan Sprague-Dawley. Dectin-1−/− knockout mice (Clec7a−/−) were generated as previously described and backcrossed onto the 129 sv/ev strain (14). cPLA2α−/− mice were generated as described previously (18) and backcrossed onto a Balb/c background for 10 generations. All mice were used between 8 and 12 weeks of age for macrophage isolation. Animal studies were approved by the Institutional Animal Care and Use Committee at National Jewish Health. Recombinant mouse granulocyte macrophage colony-stimulating factor (GM-CSF) was from R&D Systems (Minneapolis, MN). Zymosan was procured from Sigma (St. Louis, MO) and boiled in endotoxin-free phosphate-buffered saline (PBS) three times before use. Fluorescein isothiocyanate (FITC)-labeled zymosan particles and Alexa Fluor 488 carboxylic acid, succinimidyl ester were obtained from Invitrogen. Particulate β-glucan was purified from Saccharomyces cerevisiae and structurally characterized by NMR (19). Endotoxin-free water-soluble glucan phosphate (S-GP) was prepared from particulate β-glucan as previously described (20). [5,6,8,9,11,12,14,15-3H]AA (specific activity 100 Ci/mmol) was from PerkinElmer Life Sciences (Waltham, MA). Eicosanoid standards used in mass spectrometry were purchased form Cayman Chemical Co. (Ann Arbor, MI). Hanks' balanced salts solution was purchased from Invitrogen (Carlsbad, CA). Human serum albumin (endotoxin-free) was obtained from Intergen (Burlington, MA). Polyclonal antibody to cPLA2α was raised as described (21). Antibodies to phosphorylated extracellular signal–regulated kinases (ERKs), p38, cPLA2α (Ser-505) and total ERKs were obtained from Cell Signaling Technology, Inc. (Danvers, MA). Polyclonal antibodies to murine cyclooxygenase (COX) 1, COX2 (Cayman Chemical Co) and β-tubulin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) were used. U0126, SB202190, Syk inhibitor (Cat. # 574,711) and piceatannol were procured from Calbiochem (Gibbstown, NJ). Mouse TNF-α Cytoset ELISA kit was purchased from BioSource (Camarillo, CA).
Isolation and Culture of Mouse Alveolar Macrophages
Murine alveolar macrophages were isolated by bronchoalveolar lavage as described (22) with some modifications. Briefly, the mice were killed by CO2 asphyxiation. The trachea of each mouse was cannulated following a midline neck incision, and the lungs were lavaged 10 times with 1.0 ml of warm (37°C) Ca+2/Mg+2-free Hanks' balanced salts solution (pH 7.4) containing 2.5 mM Hepes, 0.5 mM EGTA, and 4.4 mM NaHCO3. Alveolar macrophages were centrifuged at 600 × g for 5 minutes and resuspended in serum-free Ham's F12K containing 0.1% human serum albumin, 100 mg/ml streptomycin sulfate, 100 units/ml penicillin G, and 0.29 mg/ml glutamine (complete media). The cells were plated at a density of 0.5–1.0 × 105/cm2/250 μl. After incubation for 2 hours at 37°C in 5% CO2, macrophages were washed three times with complete media to remove unattached cells.
C. albicans Culture
C. albicans (ATCC 10261) was streaked on fresh Sabouraud dextrose agar plates and grown overnight at 37°C. Cells were scraped from the plate, washed twice, and suspended in endotoxin-free PBS before counting with a hemocytometer.
AA Release Assays and Eicosanoid Measurements
Alveolar macrophages, cultured as described above, were incubated overnight in complete medium containing 0.2 μCi [3H]AA/well/250 μl with or without 20 ng/ml recombinant mouse GM-CSF. The cells were then washed three times, incubated in complete medium, and stimulated with agonists for the times indicated. The culture medium was removed and centrifuged at 15,000 rpm for 15 minutes. Cells were solubilized with 0.1% Triton X-100 and the amount of radioactivity in the culture media and cells determined by scintillation counting. The amount of radioactivity released is expressed as a percentage of the total radioactivity (culture medium plus cell-associated).
In some experiments the levels of AA and its oxygenated metabolites were determined by mass spectrometry. The culture medium was stored at −80°C, thawed, and mixed with an equal volume of cold methanol. Just before analysis, the samples were diluted in water to a final methanol concentration of less than 15% and then extracted using a solid phase extraction cartridge (Strata Polymeric Reversed Phase 60 mg/ml; Phenomenex, Torrance, CA). The eluate (1 ml of methanol) was dried and reconstituted in 75 μl of high-performance liquid chromatography (HPLC) solvent A (8.3 mM acetic acid and buffered to pH 5.7 with NH4OH) and 25 μl of solvent B (acetonitrile/methanol, 65/35, vol/vol). An aliquot of each sample (50 μl) was injected into an HPLC and metabolites separated on a C18 column (Ascentis 15 cm × 2.1 mm, 5 μm; Supelco, Bellefonte, PA) eluted at a flow rate of 200 μl/minute with a linear gradient from 25% to 75% solvent B in 13 minutes then increased to 98% in 2 minutes and held for 11 minutes. The HPLC system was directly interfaced into the electrospray ionization source of a triple quadrapole mass spectrometer (Sciex API 3000; PE-Sciex, Thornhill, ON, Canada) where mass spectrometric analyses were performed in the negative ion mode using multiple reaction monitoring (MRM) of the specific transitions: d4 prostaglandin E2 (PGE2) m/z 355→ 275, d4 thromboxane B2 (TXB2) m/z 373→ 173, d8AA m/z 311→213, PGE2 m/z 351 →271, TXB2 m/z 369→169, AA m/z 303→205. Quantitation was performed using a standard isotope dilution curve as described (23).
Zymosan-Binding and C. albicans–Recognition Assays
In vitro zymosan-binding assays were performed as previously described (16). In brief, overnight cultures of alveolar macrophages were cooled to 4°C and washed three times with pre-chilled complete medium. FITC-Zymosan was added to the macrophages at a ratio of 25 particles/cell for 1 hour on ice. To test the effect of blocking the β-glucan receptor, S-GP (0.1 mg/ml) was added to the chilled cells 30 minutes before the addition of zymosan. After 1 hour of incubation, cells were washed three times to remove unbound FITC-zymosan and then lysed with 3% Triton X-100. FITC-zymosan in lysates was quantified at wavelengths of 495 nm (excitation) and 520 nm (emission) using an LS55 spectrofluorometer (PerkinElmer Life Sciences).
The binding and uptake of C. albicans by alveolar macrophages (recognition assay) was measured as previously described (14). Alveolar macrophages were plated at 2 × 105/cm2 (48-well plate) in complete medium with or without GM-CSF overnight. The cells were washed three times with complete medium and then incubated for 30 minutes at 37°C with Alexa Fluor 488–labeled live C. albicans (multiplicity of infection [moi] 20:1, yeast:macrophage), prepared as described previously (24). The macrophages were washed three times to remove unbound C. albicans and then cultured in complete medium for 1 hour at 37°C. Macrophages were lysed with 3% Triton X-100 and fluorescence was measured as described above.
RNA Isolation and Semiquantitative RT-PCR
Total RNA was isolated from unprimed and GM-CSF–primed cells using the Qiagen (Valencia, CA) RNeasy kit. cDNA was synthesized using Bio-Rad's (Hercules, CA) iScript cDNA synthesis kit using forward 5′-CTCTGCCTACCTAGGGCCCTGTGAAGC-3′ and reverse 3′-CACACCATCTTTATATTCTCACATAC-5′ primers for mouse dectin-1. PCR was performed using 12.5 ng of cDNA for each 20-μl reaction. The number of cycles for amplification of dectin-1 in the linear range was determined. The relative expression of the long (dectin-1A) and the short (dectin-1B) splice variants was determined by densitometric analysis relative to 18 s rRNA.
Western Blotting
Macrophages were washed with PBS and then scraped into ice-cold lysis buffer: 50 mM Hepes, pH 7.4, 150 mM sodium chloride, 1.5 mM magnesium chloride, 10% glycerol, 1% Triton X-100, 1 mM EGTA, 200 μM sodium vanadate, 10 mM tetrasodium pyrophosphate, 100 mM sodium fluoride, 300 nM p-nitrophenyl phosphate, 1 mM PMSF, 10 μg/ml leupeptin, and 10 μg/ml aprotinin. After incubation on ice for 30 minutes, lysates were centrifuged at 15,000 rpm for 15 minutes and protein concentration in the supernatant determined by the bicinchoninic acid method. Lysates were boiled for 5 minutes after addition of Laemmli electrophoresis sample buffer (5×), and then proteins (15–25 μg total protein) were separated on 10% sodium dodecyl sulfate (SDS)-polyacrylamide gels. After electrophoresis, samples were transferred to nitrocellulose membrane, blocked for 1 hour, and then incubated overnight at 4°C with primary antibodies in 20 mM Tris-HCl, pH 7.6, 137 mM NaCl, and 0.05% Tween containing 5% nonfat milk. The membranes were then incubated with horseradish peroxidase–conjugated secondary antibodies for 30 minutes at room temperature. The immunoreactive proteins were detected using the Amersham ECL system (Piscataway, NJ).
TNF-α Quantification
Secretion of TNF-α into the culture medium was measured with Mouse TNFα Cytoset ELISA kit according to the manufacturer's instructions (BioSource, Camarillo, CA).
Statistical Analysis
Data were analyzed in GraphPad using unpaired t test to obtain two-tailed P values. Comparisons were considered significantly different when P < 0.05.
RESULTS
GM-CSF Priming of Alveolar Macrophages Promotes cPLA2α-Mediated AA Release in Response to Short-Term C. albicans Infection
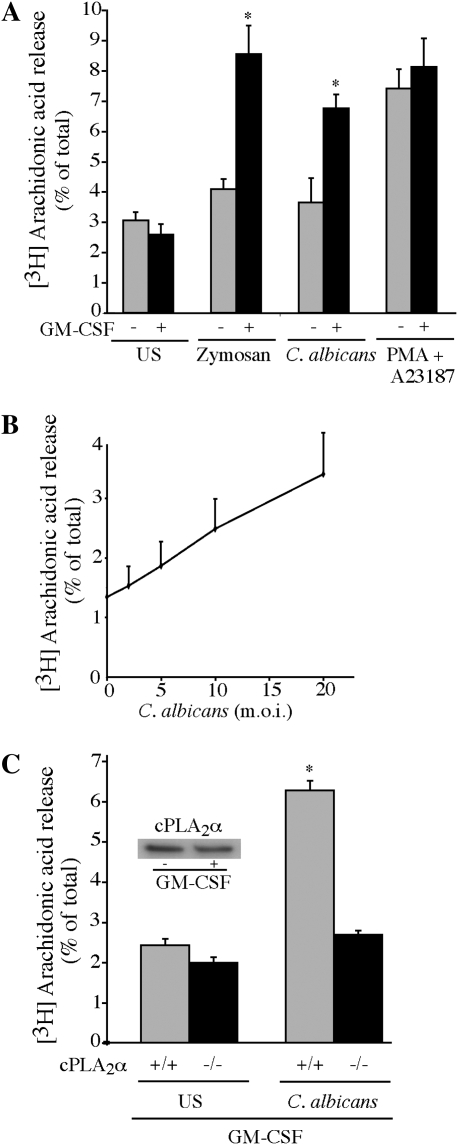
We first investigated the ability of nonopsonized C. albicans to stimulate AA release from unprimed and GM-CSF–primed resident alveolar macrophages. Macrophages from ICR mice were initially used to compare with our previous work studying AA release from resident peritoneal macrophages (8, 25–27). In contrast to our findings with peritoneal macrophages, AA release from unprimed alveolar macrophages was not significantly increased above control values measured 60 minutes after C. albicans infection (Figure 1A). However, there was a significant increase in AA release from GM-CSF–primed macrophages in response to C. albicans and zymosan. In contrast, unprimed and GM-CSF–primed macrophages released similar amounts of AA in response to the combination of A23187 and PMA, which activate cPLA2α in a receptor-independent manner by mobilizing calcium and activation of ERKs, respectively (8). AA release from GM-CSF–primed macrophages increased as the amount of C. albicans was raised from moi 2 to 20 (Figure 1B). GM-CSF–primed alveolar macrophages isolated from cPLA2α−/− mice (Balb/c) failed to release AA in response to C. albicans, confirming an essential role for cPLA2α (Figure 1C). As observed with alveolar macrophages from ICR mice, GM-CSF priming was necessary for C. albicans to stimulate AA release from cPLA2α+/+ alveolar macrophages from Balb/c mice (data not shown). GM-CSF did not act by increasing expression of cPLA2α, since Western blots showed similar levels of cPLA2α protein in unprimed and GM-CSF–primed macrophages (Figure 1C, inset).
Figure 1.
Granulocyte macrophage colony-stimulating factor (GM-CSF) priming augments cPLA2α-mediated arachidonic acid (AA) release from alveolar macrophages treated with Candida albicans. (A) Alveolar macrophages (ICR mice) were incubated overnight in serum-free medium containing [3H]AA either with or without 20 ng/ml GM-CSF, and then stimulated for 60 minutes with zymosan (20 particles/cell), C. albicans (multiplicity of infection [moi] 20), or A23187 (0.5 mg/ml) together with PMA (20 ng/ml). GM-CSF–primed macrophages released significantly (*P < 0.05) more [3H]AA than did unprimed cells. (B) [3H]AA-labeled alveolar macrophages (ICR mice) primed with GM-CSF were stimulated for 60 minutes with C. albicans at an moi of 2 to 20. (C) Alveolar macrophages from cPLA2α+/+ and cPLA2α−/− mice (Balb/c) were labeled with [3H]AA and primed with GM-CSF, followed by 60 minutes of infection with C. albicans (moi, 20). The inset shows a Western blot of cPLA2α in cell lysates of unprimed and GM-CSF–primed cPLA2α+/+ macrophages (Balb/c). GM-CSF–primed cPLA2α+/+ macrophages release significantly (*P < 0.05) more [3H]AA in response to C. albicans than cPLA2α−/− cells. [3H]AA released into the medium from stimulated and unstimulated (US) cells is expressed as a percentage of the total incorporated radioactivity. Data shown are the average ± SEM of three independent experiments.
C. albicans–Stimulated AA Release from GM-CSF–Primed Alveolar Macrophages Is Mediated by the β-Glucan Receptor Dectin-1
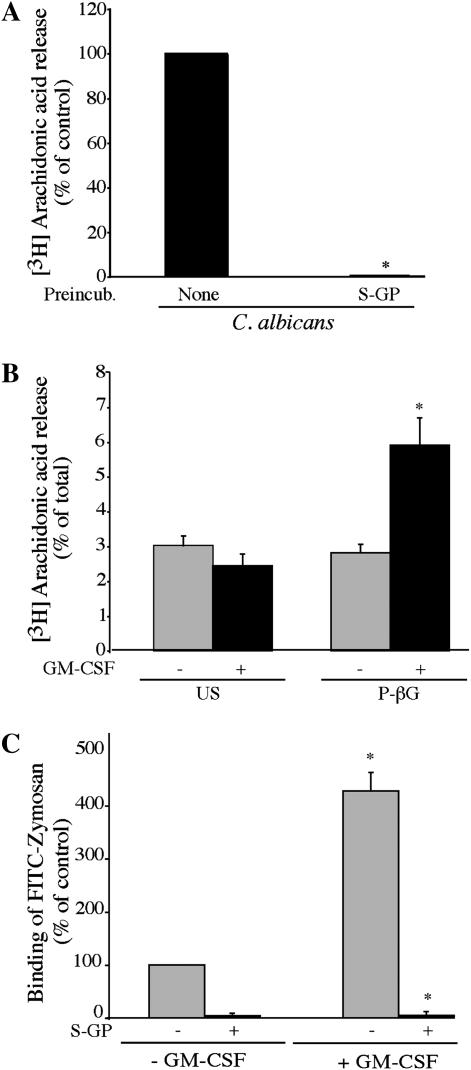
We previously reported that a β-glucan receptor mediates C. albicans–stimulated AA release from resident peritoneal macrophages (27). In addition, priming of macrophages with GM-CSF has been shown to increase expression of the β-glucan receptor dectin-1, and to enhance cytokine production in response to β-glucan particles (28–30). We found that S-GP blocked C. albicans–stimulated AA release from GM-CSF–primed alveolar macrophages (Figure 2A). In addition, particulate-β-glucan (P-βG) stimulated AA release from GM-CSF–primed macrophages but not from unprimed macrophages (Figure 2B). GM-CSF priming enhanced binding of FITC-zymosan to alveolar macrophages, and the increased binding was blocked by S-GP (Figure 2C). Collectively the results suggest that GM-CSF priming up-regulates the expression of a β-glucan receptor on alveolar macrophages that mediates C. albicans–stimulated AA release.
Figure 2.
A β-glucan receptor is involved in regulating AA release from alveolar macrophges. (A) [3H]AA-labeled alveolar macrophages (ICR mice) primed with GM-CSF were incubated for 30 minutes with 100 μg/ml soluble glucan-phosphate (S-GP) before infection for 60 minutes with C. albicans (moi, 20). The amount of [3H]AA released is expressed as a percentage of release from C. albicans–infected macrophages not treated with S-GP (100%). Results are the average of three experiments ± SEM. S-GP significantly (*P < 0.05) blocked [3H]AA release. (B) Unprimed or GM-CSF–primed [3H]AA-labeled alveolar macrophages were left unstimulated (US) or stimulated for 60 minutes with 100 μg/ml particulate β-glucan (P-βG). [3H]AA release from unstimulated (US) and P-βG–stimulated cells is calculated as a percentage of the total incorporated radioactivity. Data are the average ± SEM of three independent experiments. P-βG significantly (*P < 0.05) increases [3H]AA release from GM-CSF–primed cells compared with unprimed cells. (C) Unprimed or GM-CSF–primed alveolar macrophages were pre-incubated for 30 minutes with 100 μg/ml S-GP and then incubated with 25 particles/cell of FITC-zymosan at 4°C. The amount of FITC-zymosan binding is expressed as a percentage of binding to unprimed macrophages not treated with S-GP (100%). Results are the average ± SEM of three experiments. FITC-zymosan binding is significantly (*P < 0.05) higher in GM-CSF–primed macrophages relative to unprimed cells, and is significantly (*P < 0.05) blocked by S-GP.
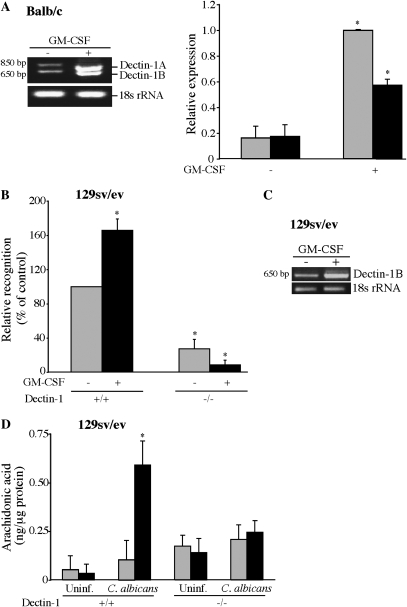
GM-CSF priming increased the expression of dectin-1A and the shorter splice variant dectin-1B as determined by semiquantitative RT-PCR of RNA isolated from alveolar macrophages from Balb/c mice (Figure 3A). GM-CSF also increased dectin-1 expression in alveolar macrophages from ICR mice, which also express both dectin-1 variants (data not shown). A comparison of alveolar macrophages isolated from wild-type and dectin-1−/− mice (129 sv/ev) demonstrated that GM-CSF priming significantly enhanced the recognition of C. albicans by dectin-1+/+ alveolar macrophages, and recognition is significantly lower in dectin-1−/− macrophages (Figure 3B). As previously reported, the 129 sv/ev mouse strain primarily expresses the smaller dectin-1 splice variant (dectin-1B) (31). This splice variant was increased in dectin-1+/+ alveolar macrophages (129 sv/ev) by GM-CSF priming (Figure 3C). C. albicans did not stimulate AA release (quantified by mass spectrometry) from unprimed dectin-1+/+ macrophages, but GM-CSF priming significantly enhanced AA release from dectin-1+/+ but not from dectin-1−/− alveolar macrophages (Figure 3D). The results imply a role for dectin-1 in mediating cPLA2α activation in GM-CSF–primed alveolar macrophages.
Figure 3.
Dectin-1 up-regulation by GM-CSF contributes to C. albicans recognition and AA release. (A) RNA was isolated from unprimed and GM-CSF–primed alveolar macrophages (Balb/c) and levels of dectin-1 expression determined by semiquantitative RT-PCR relative to 18 s rRNA. Densitometric analysis of dectin-1 mRNA relative to 18 s rRNA of three experiments ± SEM is shown in the histogram on the right. GM-CSF priming significantly (*P < 0.05) increased expression of both dectin-1 isoforms. Shaded bars, dectin-1A; solid bars, dectin-1B. (B) Dectin-1+/+ and dectin-1−/− alveolar macrophages (129 sv/ev) were incubated with Alexa-fluor–labeled C. albicans (moi, 20) to determine the effect of GM-CSF priming on recognition of C. albicans. Recognition (measured as relative fluorescence units) is expressed as the percentage of unprimed dectin-1+/+ control macrophages (100%). Results are the average of three experiments ± SEM. GM-CSF–primed dectin-1+/+ macrophages show significantly (*P < 0.05) more C. albicans recognition than unprimed dectin-1+/+ macrophages. Recognition by dectin-1−/− unprimed or GM-CSF–primed macrophages is significantly lower (*P < 0.05) than the corresponding dectin-1+/+ macrophages. (C) Levels of dectin-1 expression were determined by semiquantitative RT-PCR relative to 18 s rRNA in unprimed and GM-CSF–primed dectin-1+/+ alveolar macrophages (129 sv/ev). (D) AA released by unprimed (shaded bars) and GM-CSF–primed (solid bars) dectin-1+/+ and dectin-1−/− alveolar macrophages after stimulation with C. albicans for 60 minutes was quantified by mass spectrometry. Results are the average of three experiments ± SEM. GM-CSF priming significantly (*P < 0.05) increases AA release from C. albicans–infected dectin-1+/+ macrophages but not dectin-1−/− macrophages.
Dectin-1 Contributes to ERK Activation in GM-CSF–Primed Alveolar Macrophages
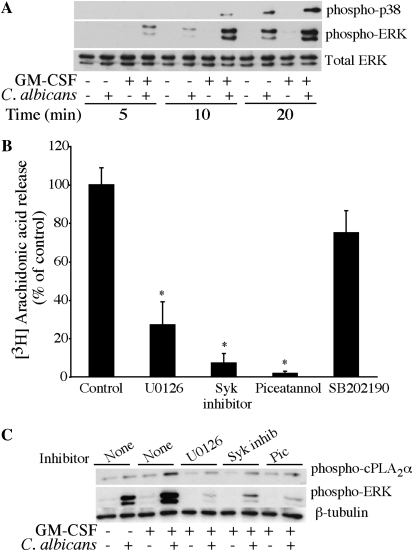
Mitogen-activated protein kinases p38 and p42/p44 ERKs regulate cPLA2α activation by phosphorylation of Ser-505 (32, 33). Activation of p38 and ERKs occurred to a greater extent in GM-CSF–primed macrophages than in unprimed cells, and was evident by 10 minutes for p38 and by 5 minutes for ERKs after C. albicans addition (Figure 4A). By 20 minutes after C. albicans addition, there was significant activation of MAPKs in unprimed macrophages, although it remained lower than in GM-CSF–primed macrophages. The dectin-1 ITAM-like motif mediates signaling through activation of spleen tyrosine kinase (Syk) (34–36). We investigated whether activation of MAPKs and Syk were necessary for cPLA2α activation and AA release in response to C. albicans by treating cells with inhibitors of these pathways. Incubation of GM-CSF–primed alveolar macrophages with the MEK1 inhibitor U0126, but not the p38 inhibitor SB202190, significantly blocked AA release in response to C. albicans (Figure 4B). Two structurally different Syk inhibitors (Syk inhibitor and piceatannol) also inhibited C. albicans–stimulated AA release from GM-CSF–primed alveolar macrophages. The effect of the MEK1 and Syk inhibitors on ERK activation and phosphorylation of cPLA2α on Ser-505 was investigated by Western blotting using phosphospecific antibodies (Figure 4C). The enhanced ERK activation that occurs in GM-CSF–primed macrophages stimulated with C. albicans, was completely inhibited by U0126 as expected and also by the two Syk inhibitors, suggesting that Syk is upstream of ERKs. Phosphorylation of cPLA2α on Ser-505 occurred to the greatest extent in GM-CSF–primed macrophages stimulated with C. albicans and was blocked by U0126 and the Syk inhibitors to levels observed in unprimed macrophages (Figure 4C).
Figure 4.
Extracellular signal–regulated kinases (ERKs) and Syk regulate C. albicans–stimulated AA release from GM-CSF–primed alveolar macrophages. (A) Cell lysates of unprimed and GM-CSF–primed alveolar macrophages were prepared at the indicated times after infection with C. albicans (moi, 20). Activation of ERKs or p38 was determined by Western blotting using phosphospecific antibodies. Sample loading was determined using total ERK antibodies. (B) [3H]AA-labeled alveolar macrophages (Balb/c) primed with GM-CSF were preincubated with vehicle (DMSO), 10 μM U0126, 1μM Syk inhibitor, 25 μM piceatannol, or 10 μM SB202190 for 30 minutes followed by infection (moi, 20) with C. albicans for 60 minutes. The amount of [3H]AA released into the media is expressed as a percentage of release from control macrophages not treated with inhibitors set at 100% after subtracting AA release from unstimulated macrophages. Results are the average of three experiments ± SEM. [3H]AA release is significantly lower (*P < 0.05) from macrophages treated with U0126, Syk inhibitor, or piceatannol compared with untreated control cells. (C) Unprimed and GM-CSF–primed alveolar macrophages were pretreated with or without kinase inhibitors (as described in B above) and then stimulated for 10 minutes with C. albicans (moi, 20). Activation of ERKs and phosphorylation of cPLA2α on Ser-505 was determined by Western blotting using phosphospecific antibodies. Sample loading was determined using antibodies to β-tubulin. Results are representative of three independent experiments.
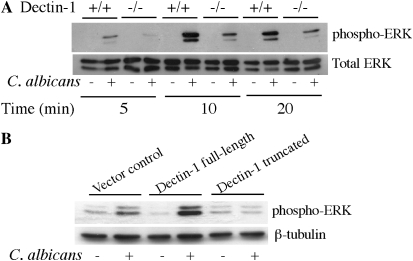
The results suggest that up-regulation of dectin-1 by GM-CSF leads to greater activation of ERKs, which contribute to cPLA2α activation and AA release by phosphorylating Ser-505. The role of dectin-1 in mediating ERK activation was investigated further by comparing responses in dectin-1+/+ and dectin-1−/− alveolar macrophages. There was less C. albicans–stimulated ERK activation in GM-CSF–primed alveolar macrophage from dectin-1−/− mice than from wild-type mice, suggesting that dectin-1 contributes to ERK activation (Figure 5A). However, the results are not conclusive, since there is less recognition of C. albicans by dectin-1−/− alveolar macrophages. Another approach was to compare ERK activation in RAW264.7 macrophages overexpressing either full-length dectin-1 or truncated dectin-1 that lacks the cytoplasmic tail containing the ITAM-like motif but contains the β-glucan–binding domain expressed on the cell surface (37). We have previously reported that RAW264.7 cells overexpressing full-length dectin-1, but not truncated dectin-1, release significantly greater amounts of AA release in response to C. albicans compared with vector control cells (27). Activation of ERKs in response to C. albicans was enhanced in RAW264.7 cells overexpressing full-length dectin-1 compared with vector control cells (Figure 5B). However, ERKs were not activated by C. albicans in macrophages overexpressing truncated dectin-1, suggesting that the ITAM motif mediates ERK activation.
Figure 5.
Dectin-1 regulates ERK activation. (A) GM-CSF–primed dectin-1+/+ and dectin-1−/− alveolar macrophages were infected with C. albicans (moi, 20) for 10 minutes. Activation of ERKs was determined by Western blotting using phosphospecific antibodies. Sample loading was determined using antibodies to total ERKs. Results are representative of three independent experiments. (B) RAW264.7 cells overexpressing full-length dectin-1, truncated dectin-1, and vector controls were infected with C. albicans (moi, 20) for 10 minutes. Activation of ERKs was determined by Western blotting using phosphospecific antibodies. Sample loading was determined using antibodies to β-tubulin. Results are representative of three independent experiments.
COX2 Expression and Prostanoid Production in GM-CSF–Primed Macrophages Is Dectin-1 Independent
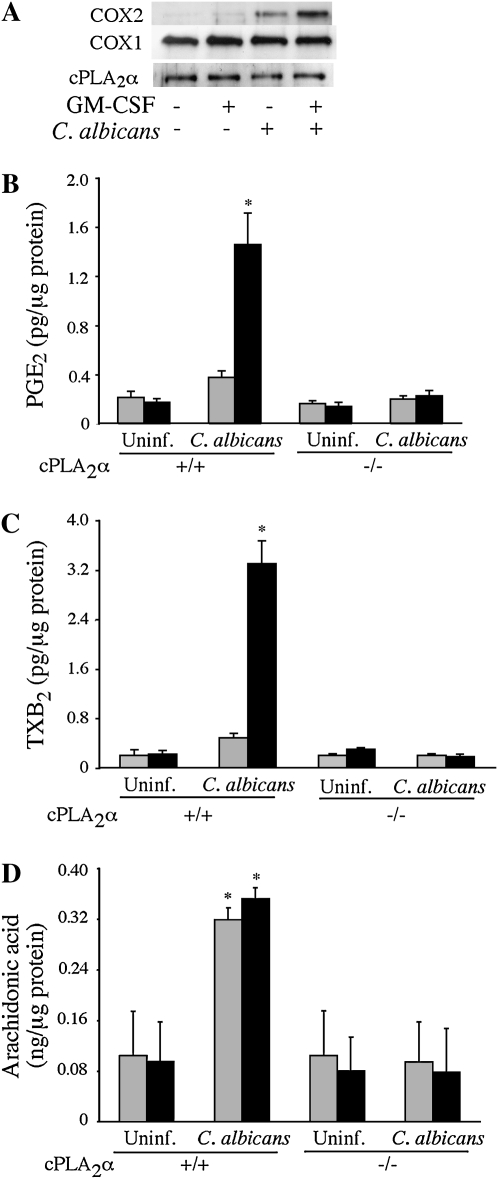
To determine the effect of GM-CSF priming on the ability of C. albicans to induce COX2 expression and eicosanoid production, C. albicans was used at a lower moi of 2 for a 6-hour incubation. A preliminary experiment showed that COX2 was weakly expressed at 3 hours and increased by 6 hours after C. albicans infection (data not shown). As shown in Figure 6A, GM-CSF–primed macrophages expressed higher levels of COX2 than unprimed cells in response to C. albicans, but the levels of COX1 and cPLA2α were unaffected. The increased COX2 expression correlated with significantly more production of PGE2 and TXB2 by GM-CSF–primed macrophages in response to C. albicans (Figures 6B and 6C). In contrast to our findings after a 1-hour infection with C. albicans showing that GM-CSF priming was required for AA release, by 6 hours similar levels of AA were found in the culture medium of unprimed and GM-CSF–primed macrophages (Figure 6D). C. albicans did not stimulate release of AA and production of eicosanoids from unprimed or GM-CSF–primed cPLA2α−/− macrophages, confirming an essential role for cPLA2α (Figures 6B–6D).
Figure 6.
GM-CSF priming enhances COX2 expression and prostanoid production in response to C. albicans. (A) Levels of COX2, COX1, and cPLA2α were determined by Western blot analysis of cell lysates prepared from unprimed (shaded bars) and GM-CSF–primed (solid bars) cPLA2α+/+ alveolar macrophages stimulated with or without C. albicans (moi, 2) for 6 hours. The Western blot results are representative of three independent experiments. (B–D) Unprimed and GM-CSF–primed cPLA2α+/+ and cPLA2α−/− alveolar macrophages (Balb/c) were infected with C. albicans (moi, 2) for 6 hours. Culture medium was collected and analyzed for levels of (B) PGE2, (C) TXB2, and (D) AA by mass spectrometry. Data are the average ± SEM of three experiments. GM-CSF–primed cPLA2α+/+ macrophages release significantly more (*P < 0.05) PGE2 (B) and TXB2 (C) than unprimed cPLA2α+/+ macrophages, and significantly more than cPLA2α−/− macrophages in response to C. albicans infection. C. albicans–infected cPLA2α+/+ macrophages (primed and unprimed) release significantly more (*P < 0.05) AA than cPLA2α−/− cells.
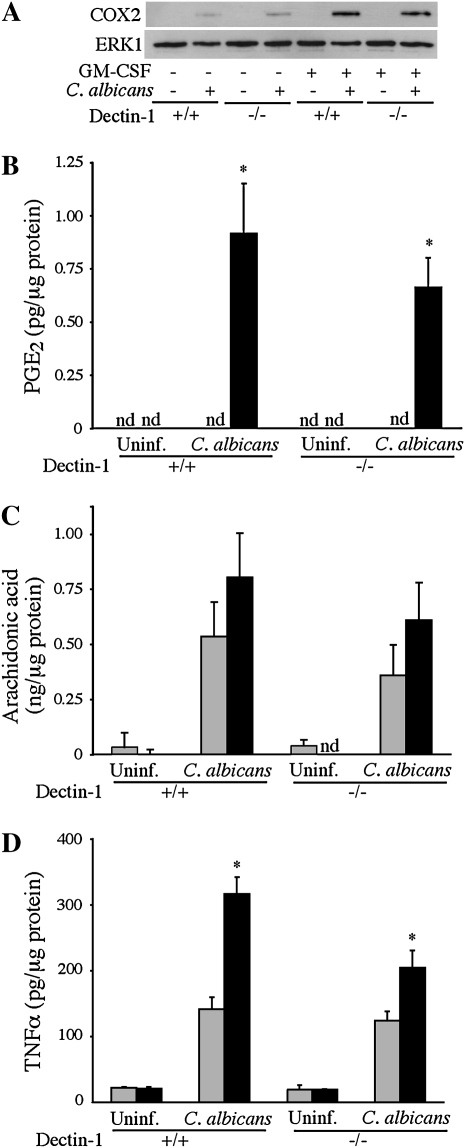
A comparison of dectin-1+/+ and dectin-1−/− alveolar macrophages showed that the increased expression of COX2 and PGE2 production in GM-CSF–primed macrophages treated with C. albicans for 6 hours was dectin-1 independent (Figures 7A and 7B). As observed in alveolar macrophages from Balb/c mice, similar levels of AA were found in the culture medium of unprimed and GM-CSF–primed dectin-1+/+ macrophages treated with C. albicans for 6 hours, and were not significantly different in dectin-1−/− macrophages (Figure 7C). However, the increased TNF-α production that occurred in GM-CSF–primed alveolar macrophages 6 hours after C. albicans addition was in part dependent on dectin-1 (Figure 7D).
Figure 7.
Dectin-1 does not regulate COX2 expression or prostanoid production in GM-CSF–primed alveolar macrophages stimulated with C. albicans, but contributes to TNF-α production. Unprimed (shaded bars) and GM-CSF–primed (solid bars) dectin-1+/+ and dectin-1−/− macrophages were incubated with C. albicans (moi, 2) for 6 hours or left uninfected (Uninf.). (A) Levels of COX2 in cell lysates were determined by Western blot analysis and ERK1 probed as a loading control. The Western blot results are representative of three independent experiments. Levels of (B) PGE2 and (C) AA in the culture media were quantified by mass spectrometry, and amounts of TNF-α (D) determined by ELISA. Data are the average ± SEM of three experiments. GM-CSF–primed dectin-1+/+ and dectin-1−/− macrophages produced significantly more (*P < 0.05) PGE2 (B) than unprimed cells in response to C. albicans. Significantly more (*P < 0.05) TNF-α (D) was produced by GM-CSF–primed dectin-1+/+ macrophages than unprimed cells. TNF-α levels from GM-CSF–primed dectin-1−/− cells were significantly less (*P < 0.05) than those from GM-CSF–primed dectin-1+/+ cells.
DISCUSSION
Alveolar macrophages participate in the initial clearance of C. albicans from lungs of mice and in preventing the dissemination of C. albicans from lungs of immunocompromised mice (3, 38). It is important to understand how microbial pathogens affect the function of alveolar macrophages, since they are sentinel cells of the airway for initiating immune responses to infection. Little is known about the mechanisms involved in activation of cPLA2α for lipid mediator production in response to specific pathogens. Eicosanoids are a diverse family of lipid mediators that act locally through G protein–coupled receptors to exert biological responses. The leukotrienes induce proinflammatory responses such as recruitment of inflammatory cells, increases in vascular permeability, and bronchoconstriction (9). The cyclooxygenase metabolites prostaglandins and thromboxane have proinflammatory and immunosuppressive effects (9, 39). We have previously reported that live, nonopsonized C. albicans potently stimulates cPLA2α-mediated release of AA from unprimed resident peritoneal macrophages through a β-glucan receptor (27). Although resident mouse alveolar macrophages have been reported to express relatively high levels of the β-glucan receptor dectin-1 (40), C. albicans did not stimulate significant amounts of AA from unprimed alveolar macrophages. GM-CSF priming enhanced AA release that correlated with increased expression of dectin-1. However, despite increased expression of dectin-1 in GM-CSF–primed alveolar macrophages, the amount of AA release from alveolar macrophages is considerably lower than from resident peritoneal macrophages and requires higher amounts of C. albicans (27). It has recently been reported that dectin-1 signaling is highly variable in different macrophages populations that is not necessarily related to the levels of dectin-1 expression (30). The results implicate other cell type–specific regulatory pathways that either suppress cPLA2α in alveolar macrophages or enhance its activation in peritoneal macrophages in response to C. albicans. The lung is exposed to environmental insults including microorganisms, and alveolar macrophages are more refractory to microbial stimulation to prevent excess inflammation in the lung, although they can be activated to become effector cells (41–43).
GM-CSF plays an important role in lung homeostasis and maintaining macrophage differentiation (44). GM-CSF−/− mice specifically develop a lung abnormality, alveolar proteinosis, due to defective clearance of pulmonary surfactant (45, 46). GM-CSF also functions in regulating host defense in the lung since GM-CSF−/− mice are more susceptible to microbial infections (44). Alveolar macrophages from GM-CSF−/− mice have lower expression of pattern recognition receptors, reduced phagocytic capacity and microbial killing (44). However, GM-CSF expression is increased at sites of inflammation and may contribute to lung inflammation (47). We found that the levels of dectin-1 expression were higher in alveolar macrophages incubated overnight with GM-CSF compared with unprimed cells. This was not due to GM-CSF maintaining dectin-1 expression levels, but rather increasing dectin-1 expression compared with levels found in freshly isolated alveolar macrophages (data not shown). The level of dectin-1 mRNA expression was similar in freshly isolated alveolar macrophages and unprimed macrophages incubated overnight (data not shown). This suggests that dectin-1 expression may increase in the lung during inflammation due to increased production of GM-CSF.
Our results demonstrate that the increase in dectin-1 expression in GM-CSF–primed macrophages is essential for the early activation of cPLA2α and AA release that occurs within 1 hour of C. albicans stimulation. Dectin-1 promotes both the recognition of C. albicans and contributes to generating the signals for activation of cPLA2α. GM-CSF priming enhances activation of p38 and ERKs by C. albicans in alveolar macrophages. Although both of these MAPKs can contribute to cPLA2α activation by phosphorylating Ser-505, our results demonstrate that activation of ERKs, but not p38, is required for C. albicans–stimulated AA release. Syk inhibitors blocked ERK activation, cPLA2α phosphorylation, and C. albicans–stimulated AA release from GM-CSF–primed alveolar macrophages, consistent with a role for dectin-1. The cytoplasmic tail of dectin-1 contains an ITAM-like motif that is required for signaling through Syk (34–36). We found that RAW264.7 cells expressing truncated dectin-1 lacking the cytoplasmic tail fail to activate ERKs in response to C. albicans, in contrast to RAW264.7 cells expressing wild-type dectin-1. The lack of ERK activation could account for the inability of C. albicans to stimulate AA release from RAW264.7 cells expressing the truncated form of dectin-1 (27).
The release of AA early after infection (1 h) with C. albicans is higher in GM-CSF–primed alveolar macrophages and is dectin-1 dependent. However, by 6 hours of infection the level of AA in the culture medium is equivalent in unprimed and GM-CSF–primed macrophages. The release of AA over the longer time frame is mediated by cPLA2α, but our results show that it is dectin-1 independent. Although GM-CSF–primed alveolar macrophages express higher levels of COX2 and produce more prostanoids than unprimed macrophages, this is not due to the up-regulation of dectin-1. Dectin-1 does, however, play a partial role in regulating some responses at the longer time of infection, since TNF-α levels are significantly lower in GM-CSF–primed dectin-1−/− macrophages. It has been reported that dectin-1 induces signals for cytokine production, primarily during the initial interaction with β-glucan at the cell surface, and that internalization of dectin-1 upon ligand binding attenuates signaling (29, 48, 49). Our results suggest that a similar phenomenon occurs for cPLA2α activation in GM-CSF–primed alveolar macrophages, since AA release is only dependent on dectin-1 early after infection with C. albicans.
GM-CSF priming of alveolar macrophages enhances COX2 expression and prostanoid production but not levels of AA at later times of C. albicans infection. These results suggest that distinct receptors mediate cPLA2α activation in unprimed alveolar macrophages, and COX2 expression in GM-CSF–primed alveolar macrophages, at later times after infection. The receptors involved in promoting the later phase of cPLA2α-mediated AA release and eicosanoid production remain to be identified. The interaction of C. albicans with macrophages is a complex process involving a variety of receptors that differentially function in promoting phagocytosis and signaling for specific cellular responses. In addition to dectin-1, it has recently been reported that the scavenger receptors SCARF1 and CD36 on macrophages mediate the β-glucan–dependent uptake of fungi and cytokine production (50). Once internalized, C. albicans can also engage intracellular receptors such as the mannose receptor and possibly TLR9, adding to the complexity of the regulatory mechanisms for macrophage activation by C. albicans (51, 52).
Acknowledgments
The authors thank Charis Uhlson for mass spectrometry analysis and Dr. Chad Steele for providing the dectin-1 knockout breeder mice.
This work was supported by National Institutes of Health Grants HL34303 (C.C.L., R.C.M.), DK54741 (J.V.B.), GM53522 (D.L.W), and a Medical Research Council Senior Fellowship G0601617 (P.R.T.).
Originally Published in Press as DOI: 10.1165/rcmb.2009-0110OC on June 5, 2009
Conflict of Interest Statement: C.C.L. has received consultancy fees from Asubio Pharmaceuticals, Inc. for less than $10,000. She and her spouse have also received grants from the National Institutes of Health (both for >$100,000). None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 2007;20:133–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yao Z, Liao W. Fungal respiratory disease. Curr Opin Pulm Med 2006;12:222–227. [DOI] [PubMed] [Google Scholar]
- 3.Sawyer RT, Harmsen AG. The relative contribution of resident pulmonary alveolar macrophages and inflammatory polymorphonuclear neutrophils in host resistance to pulmonary infection by Candida albicans. Mycopathologia 1989;108:95–105. [DOI] [PubMed] [Google Scholar]
- 4.Kubota Y, Iwasaki Y, Harada H, Yokomura I, Ueda M, Hashimoto S, Nakagawa M. Role of alveolar macrophages in Candida-induced acute lung injury. Clin Diagn Lab Immunol 2001;8:1258–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 2006;124:783–801. [DOI] [PubMed] [Google Scholar]
- 6.Gordon S. Pattern recognition receptors: doubling up for the innate immune response. Cell 2002;111:927–930. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh M, Tucker DE, Burchett SA, Leslie CC. Properties of the group IV phospholipase A2 family. Prog Lipid Res 2006;45:487–510. [DOI] [PubMed] [Google Scholar]
- 8.Gijón MA, Leslie CC. Regulation of arachidonic acid release and cytosolic phospholipase A2 activation. J Leukoc Biol 1999;65:330–336. [DOI] [PubMed] [Google Scholar]
- 9.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 2001;294:1871–1875. [DOI] [PubMed] [Google Scholar]
- 10.Peters-Golden M, Canetti C, Mancuso P, Coffey MJ. Leukotrienes: underappreciated mediators of innate immune responses. J Immunol 2004;173:589–594. [DOI] [PubMed] [Google Scholar]
- 11.Lee SP, Serezanik CH, Medeiros AI, Ballinger MN, Peters-Golden M. Crosstalk between prostaglandin E2 and leukotriene B4 regulates phagocytosis in alveolar macrophages via combinatorial effects on cyclic AMP. J Immunol 2008;182:530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harizi H, Gualde N. The impact of eicosanoids on the crosstalk between innate and adaptive immunity: the key roles of dendritic cells. Tissue Antigens 2005;65:507–514. [DOI] [PubMed] [Google Scholar]
- 13.Netea MG, Brown GD, Kullberg BJ, Gow NAR. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol 2008;6:67–78. [DOI] [PubMed] [Google Scholar]
- 14.Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, et al. Dectin-1 is required for β-glucan recognition and control of fungal infection. Nat Immunol 2007;8:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willment JA, Brown GD. C-type lectin receptors in antifungal immunity. Trends Microbiol 2008;16:27–32. [DOI] [PubMed] [Google Scholar]
- 16.Brown GD, Taylor PR, Reid DM, Willment JA, Williams DL, Martinez-Pomares L, Wong SYC, Gordon S. Dectin-1 is a major β-glucan receptor on macrophages. J Exp Med 2002;196:407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown GD. Dectin-1: A signalling non-TLR pattern-recognition receptor. Nat Rev Immunol 2006;6:33–43. [DOI] [PubMed] [Google Scholar]
- 18.Bonventre JV, Huang Z, Taheri MR, O'Leary E, Li E, Moskowitz MA, Sapirstein A. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature 1997;390:622–625. [DOI] [PubMed] [Google Scholar]
- 19.Ensley H, Tobias B, Pretus HA, McNamee RB, Jones EL, Browder IW, Williams DL. NMR spectral analysis of a water-insoluble (1–3)-β-d-glucan isolated from Saccharomyces cerevisiae. Carbohydr Res 1994;258:307–311. [DOI] [PubMed] [Google Scholar]
- 20.Williams DL, McNamee RB, Jones EL, Pretus HA, Ensley HE, Browder IW, Di Luzio NR. A method for the solubilization of a (1–3)-β-d-glucan isolated from Saccharomyces cerevisiae. Carbohydr Res 1991;219:203–213. [DOI] [PubMed] [Google Scholar]
- 21.de Carvalho MS, McCormack FX, Leslie CC. The 85-kda, arachidonic acid-specific phospholipase A2 is expressed as an activated phosphoprotein in Sf9 cells. Arch Biochem Biophys 1993;306:534–540. [DOI] [PubMed] [Google Scholar]
- 22.Garner RE, Rubanowice K, Sawyer RT, Hudson JA. Secretion of TNF-alpha by alveolar macrophages in response to Candida albicans mannan. J Leukoc Biol 1994;55:161–168. [DOI] [PubMed] [Google Scholar]
- 23.Hall LM, Murphy RC. Electrospray mass spectrometric analysis of 5-hydroperoxy and 5-hydroxyeicosatetraenoic acids generated by lipid peroxidation of red blood cell ghost phospholipids. J Am Soc Mass Spectrom 1998;9:527–532. [DOI] [PubMed] [Google Scholar]
- 24.de Pedro MA, Young KD, Holtje J-V, Schwarz H. Branching of Escherichia coli cells arises from multiple sites of inert peptidoglycan. J Bacteriol 2003;185:1147–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiu Z-H, Gijón MA, de Carvalho MS, Spencer DM, Leslie CC. The role of calcium and phosphorylation of cytosolic phospholipase A2 in regulating arachidonic acid release in macrophages. J Biol Chem 1998;273:8203–8211. [DOI] [PubMed] [Google Scholar]
- 26.Gijón MA, Spencer DM, Siddiqi AR, Bonventre JV, Leslie CC. Cytosolic phospholipase A2 is required for macrophage arachidonic acid release by agonists that do and do not mobilize calcium: novel role of mitogen-activated protein kinase pathways in cytosolic phospholipase A2 regulation. J Biol Chem 2000;275:20146–20156. [DOI] [PubMed] [Google Scholar]
- 27.Suram S, Brown GD, Ghosh M, Gordon S, Loper R, Taylor PR, Akira S, Uematsu S, Williams DL, Leslie CC. Regulation of cytosolic phospholipase A2 activation and cyclooxygeanse 2 expression in macrophages by the β-glucan receptor. J Biol Chem 2006;9:5506–5514. [DOI] [PubMed] [Google Scholar]
- 28.Willment JA, Lin H-H, Reid DM, Taylor PR, Williams DL, Wong SY, Gordon S, Brown GD. Dectin-1 expression and function are enhanced on alternatively activated and GM-CSF-treated macrophages and are negatively regulated by IL-10, dexamethasone, and lipopolysaccharide. J Immunol 2003;171:4569–4573. [DOI] [PubMed] [Google Scholar]
- 29.Rosas M, Liddiard K, Kimberg M, Faro-Trindade I, McDonald JU, Williams DL, Brown GD, Taylor PR. The induction of inflammation by dectin-1 in vivo is dependent on myeloid cell programming and the progression of phagocytosis. J Immunol 2008;181:3549–3557. [DOI] [PubMed] [Google Scholar]
- 30.Goodridge HS, Shimada T, Wolf AJ, Hsu Y-MS, Becker CA, Lin X, Underhill DM. Differential use of CARD9 by dectin-1 in macrophages and dendritic cells. J Immunol 2009;182:1146–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heinsbroek SEM, Taylor PR, Rosas M, Willment JA, Williams DL, Gordon S, Brown GD. Expression of functionally different dectin-1 isoforms by murine macrophages. J Immunol 2006;176:5513–5518. [DOI] [PubMed] [Google Scholar]
- 32.Kramer RM, Roberts EF, Um SL, Börsch-Haubold AG, Watson SP, Fisher MJ, Jakubowski JA. P38 mitogen-activated protein kinase phosphorylates cytosolic phospholipase A2 (cPLA2) in thrombin-stimulated platelets. J Biol Chem 1996;271:27723–27729. [DOI] [PubMed] [Google Scholar]
- 33.Lin L-L, Wartmann M, Lin AY, Knopf JL, Seth A, Davis RJ. cPLA2 is phosphorylated and activated by MAP kinase. Cell 1993;72:269–278. [DOI] [PubMed] [Google Scholar]
- 34.Rogers NC, Slcak EC, Edwards AD, Nolte MA, Schultz O, Schweighoffer E, Williams DL, Gordon S, Tybulewica VL, Brown GD, et al. Syk-dependent cytokine induction by dectin-1 reveals a novel pattern recognition pathway for C-type lectins. Immunity 2005;22:507–517. [DOI] [PubMed] [Google Scholar]
- 35.Underhill DM, Rossnagle E, Lowell CA, Simmons RM. Dectin-1 activates syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood 2005;106:2543–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dennehy KM, Ferwerda G, Faro-Trindade I, Pyz E, Willment JA, Taylor PR, Kerrigan A, Tsoni SV, Gordon S, Meyer-Wentrup F, et al. Syk kinase is required for collaborative cytokine production induced through dectin-1 and toll-like receptors. Eur J Biochem 2008;38:500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown GD, Herre J, Williams DL Jr, Willment JA, Marshall AS, Gordon S. Dectin-1 mediates the biological effect of β-glucans. J Exp Med 2003;197:1119–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sawyer RT. Experimental pulmonary candidiasis. Mycopathologia 1990;109:99–109. [DOI] [PubMed] [Google Scholar]
- 39.Tilley SL, Coffman TM, Koller BH. Mixed messages: modulation of inflammation and immune responses by prostaglandins and thromboxanes. J Clin Invest 2001;108:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor PR, Brown GD, Reid DM, Willment JA, Martinez-Pomares L, Gordon S, Wong SY. The β-glucan receptor, dectin-1, is predominently expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J Immunol 2002;169:3876–3882. [DOI] [PubMed] [Google Scholar]
- 41.Snelgrove RJ, Goulding J, Didierlaurent AM, Lyonga D, Vekaria S, Edwards L, Gwyer E, Sedgwick JD, Barclay AN, Hussell T. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat Immunol 2008;9:1074–1083. [DOI] [PubMed] [Google Scholar]
- 42.Peters-Golden M. The alveolar macrophage: the forgotten cell in asthma. Am J Respir Cell Mol Biol 2004;31:3–7. [DOI] [PubMed] [Google Scholar]
- 43.Holt PG, Strickland DH, Wikstrom ME, Jahnsen FL. Regulation of immunological homeostasis in the respiratory tract. Nat Immunol 2008;8:142–152. [DOI] [PubMed] [Google Scholar]
- 44.Trapnell BC, Whitsett JA. GM-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage-mediated innate host defense. Annu Rev Physiol 2002;64:775–802. [DOI] [PubMed] [Google Scholar]
- 45.Stanley E, Lieschke GJ, Grail D, Metcalf D, Hodgson G, Gall JA, Maher DW, Cebon J, Sinickas V, Dunn AR. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci USA 1994;91:5592–5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dranoff G, Crawford AD, Sadelain M, Ream B, Rashid A, Bronson RT, Dickersin GR, Bachurski CJ, Mark EL, Whitsett JA, et al. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science 1994;264:713–716. [DOI] [PubMed] [Google Scholar]
- 47.Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol 2008;8:533–544. [DOI] [PubMed] [Google Scholar]
- 48.Hernanz-Falcon P, Joffre O, Williams DL, Reis e Sousa C. Internalization of dectin-1 terminates induction of inflammatory responses. Eur J Immunol 2009;39:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herre J, Marshall AS, Caron E, Edwards AD, Williams DL, Schweighoffer E, Tybulewicz V, Reis e Sousa C, Gordon S, Brown GD. Dectin-1 uses novel mechanisms for yeast phagocytosis in macrophages. Blood 2004;104:4038–4045. [DOI] [PubMed] [Google Scholar]
- 50.Means TK, Mylonakis E, Tampakakis E, Colvin RA, Seung E, Puckett L, Tai MF, Stewart CR, Pukkila-Worley R, Hickman SE, et al. Evolutionarily conserved recognition and innate immunity to fungal pathogens by scavenger receptors SCARF1 and CD36. J Exp Med 2009;206:637–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heinsbroek SEM, Taylor PR, Martinez FO, Martinez-Pomares L, Brown GD, Gordon S. Stage-specific sampling by pattern recognition receptors during Candida albicans phagocytosis. PLoS Pathog 2008;4:e1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van de Veerdonk FL, Netea MG, Jansen TJ, Jacobs L, Verschueren I, Van der Meer JWM, Kullberg BJ. Redundant role of TLR9 for anti-candida host defense. Immunobiology 2008;213:613–620. [DOI] [PubMed] [Google Scholar]