Abstract
The objective of this investigation was to determine the role of Pyk2, an intracellular nonreceptor protein tyrosine kinase for postadhesive inflammatory cell migration, on airway inflammation and hyperresponsiveness in immune-sensitized mice. Blockade of Pyk2 was effected by intraperitoneal administration of dominant-negative C-terminal Pyk2 fused to a TAT protein transduction domain (TAT-Pyk2-CT). Ovalbumin challenge elicited infiltration of both eosinophils and lymphocytes into airways, increased mucus-containing epithelial cells, and caused increased airway hyperresponsiveness to methacholine in immune-sensitized mice. Pretreatment with 10 mg/kg TAT-Pyk2-CT intraperitoneally blocked all of these effects and further decreased secretion of Th2 cytokine IL-4, IL-5, and IL-13 into the bronchoalveolar lavage fluid. Intranasal administration of IL-5 caused eosinophil migration into the airway lumen, which was attenuated by systemic pretreatment with TAT-Pyk2-CT. In each paradigm, treatment with control protein TAT-GFP had no blocking effect. We conclude that Pyk2, which is essential for inflammatory cell migration in vitro, regulates airway inflammation, Th2 cytokine secretion, and airway hyperresponsiveness in the ovalbumin-sensitized mice during antigen challenge in vivo.
Keywords: eosinophils, Pyk2, inflammation, lung
CLINICAL RELEVANCE.
This research focuses on the mechanisms by which the protein tyrosine kinase, proline-rich tyrosine kinase 2, regulates leukocyte infiltration in mouse airways after antigen challenge. Data derived from this study should suggest novel approaches for anti-inflammatory therapies in asthma and allergic diseases.
Allergic asthma is characterized by the infiltration of lung tissues by inflammatory cells such as eosinophils, mast cells, and T lymphocytes (1, 2). Several mediators released by these cells cause epithelial damage, leading to airway hyperresponsiveness (AHR) and reversible airway obstruction (3). While the specific role of eosinophils in human asthma remains undefined (4), the ablation of pulmonary eosinophils in ovalbumin (OVA)-sensitized mice (i.e., without concurrent effects on T cell activities) results in a significant decrease in mucus accumulation and abolishes allergen-induced AHR (5). Recently, eosinophils were shown to have an integral role in experimental allergic asthma using two different lines of eosinophil-deficient mice (6, 7). Eosinophils may also have a role airway remodeling (8, 9) and development of AHR (10–12). The adhesion molecules involved in the ligation of eosinophils and endothelial cells (13), chemotactic stimuli for eosinophil recruitment (14, 15), and factors regulating eosinophil survival (16, 17) have been characterized previously. However, the intracellular signal transduction pathways triggered by adhesion receptors and cytokines and chemokines affecting eosinophil migration are less well understood. We have found previously that Pyk2 is not involved in integrin-mediated eosinophil adhesion, the first stage of cell migration. However, β2 integrin adhesion of eosinophils up-regulates Pyk2 activity, and Pyk2 inhibition blocks eosinophil chemotaxis to IL-5 or fMLP in vitro (18). The objective of this investigation was to determine the role of Pyk2 in eosinophil migration in immune-sensitized mice in vivo.
Pyk2 is a member of the focal adhesion kinase (FAK) family that is expressed in the hematopoietic cells (18, 19) that infiltrate the inflamed asthmatic airway. Previous in vivo studies have shown that recruitment of macrophages is reduced in Pyk2-deficient mice after stimulation with chemokine and in response to carageenen (20). Pyk2-deficient mice exhibit a lack of marginal zone B cells in spleen associated with a decreased motility of B lymphocytes in response to a variety of chemokines (21). Prior studies have suggested that inhibition of Pyk2 activation by Src tyrosine kinase inhibitor attenuated IL-8–mediated neutrophil chemotaxis (22). Moreover, overexpression of the kinase-dead mutant of Pyk2 known to prevent Pyk2 enzymatic activity blocked IL-8–induced chemotaxis of HL-60–derived PMN-like cells (22). We have shown that while Pyk2 does not initiate endothelial adhesion of eosinophils, it is essential for eosinophil spreading and migration to various chemoattractants after β2 integrin adhesion in vitro (18). However, the role of Pyk2 in asthma and allergic inflammation in vivo is unknown.
To examine the role of Pyk2 in allergic airway inflammation, we have used a well-characterized OVA mouse model of asthma (23, 24). In these studies, we transduced dominant-negative C-terminal Pyk2 using an HIV-TAT protein transduction domain (TAT-Pyk2-CT) into mice in vivo. Pyk2-CT acted as a dominant negative in that it blocked the tyrosine phosphorylation of endogenous Pyk2 (18, 25, 26). We tested the hypothesis that Pyk2 mediates both antigen-induced airway inflammation and AHR to methacholine. We found that TAT-Pyk2-CT blocked antigen-induced airway inflammation and hyperresponsiveness and reduced Th2 cytokine concentrations in bronchoalveolar lavage (BAL) fluid.
MATERIALS AND METHODS
Generation of TAT-Pyk2-CT and TAT-GFP Fusion Protein
Plasmid encoding TAT-Pyk2 C terminus (pTAT-Pyk2-CT) was provided by Dr. C. Nathan (Cornell University, New York, NY). pTAT-GFP plasmid was provided by Dr. S. Dowdy (UCSD, San Diego, CA). TAT-Pyk2-CT is a fusion protein in which TAT peptide is fused to the N terminus of the proline-rich C-terminal domain of Pyk2 (amino acid residues 680–1009) (Figure 1A). BL21(DE3)pLysS-competent Escherichia coli was transformed with either pTAT-Pyk2-CT or pTAT-GFP construct, and fusion proteins were produced using a native isolation method (18, 27). Briefly, E. coli cells were sonicated in Tris-buffered saline plus protease inhibitor cocktail (Roche, Indianapolis, IN) and centrifuged, and the resulting supernatant was loaded onto a Ni-ProBond resin column (Invitrogen, Carlsbad, CA). Pure TAT fusion proteins with N-terminal hexa-His tags were eluted with an imidazole gradient, washed, and transferred into 20 mM HEPES (pH 8.0) buffer for storage and use.
Figure 1.
(A) Purified TAT fusion proteins used in this study. Upper panel: Structure of TAT-Pyk2-CT fusion protein. Six His residues and the 11–amino acid TAT peptide precede the C-terminal of the Pyk2 protein. The 11 amino acids of TAT are the protein transduction domain (PTD). Lower panel: TAT-GFP, a TAT control protein. (B) Ovalbumin (OVA)-induced murine model of asthma. Mice were sensitized at Days 0, 7, and 14 and challenged with OVA for 3 consecutive days (Days 21 to 23). TAT-Pyk2-CT or TAT-GFP was intraperitoneally administered 1 hour before each OVA challenge. Bronchoalveolar lavage (BAL) and airway hyperresponsiveness (AHR) were measured 24 hours after last challenge.
Mice
Female C57BL/6 mice, 6 to 8 weeks old, were purchased from the Jackson Laboratory (Bar Harbor, ME) and housed in a pathogen-free biohazard level 2 facility maintained by The University of Chicago Animal Resources Center (Chicago, IL). The studies reported here conform to the principles outlined by the Animal Welfare and the NIH guidelines for the care and use of animals in biomedical research.
Immunization and Airway Challenge with OVA
Mice were sensitized and challenged as described previously (23). Briefly, mice were immunized with 10 μg of OVA and 1.125 mg of aluminum hydroxide (Imgect Alum; Pierce, Rockford, IL) in 0.2 ml of sterile saline intraperitoneally on Days 0, 7, and 14. On Days 21–23, mice were exposed to aerosolized OVA (1%) or saline for 40 minutes (Figure 1B). To examine the effect of TAT-Pyk2-CT on antigen-induced airway reactions, the animals received intraperitoneal injection of TAT-Pyk2-CT or TAT-GFP 1 hour before each OVA challenge. The in vivo distribution of the TAT fusion protein and tissue kinetics in mice have been well studied previously (28, 29). TAT fusion protein is distributed into all tissues in mice, and peak time is approximately 2 hours in blood leukocytes after intraperitoneal injection (23, 30). The animals receiving injection of TAT-Pyk2-CT or TAT-GFP were assigned randomly to the experimental groups consisting of six mice each.
Measurement of Airway Responsiveness to Methacholine
Methacholine challenge was performed 24 hours after the last OVA challenge. Respiratory system resistance (Rrs) was measured through a computer-controlled small-animal ventilator (SAV) (Flexivent; SCIREQ, Montreal, PQ, Canada) as previously described (30). Briefly, mice were anesthetized with 30 mg/kg xylazine and 80 mg/kg ketamine intraperitoneally, and the trachea was cannulated with an 18-gauge metal needle connected to the SAV. Mechanical ventilation was applied, and animals were ventilated quasisinusoidally at a frequency of 120 breaths/minute at a tidal volume of 6 ml/kg. The expiratory valve of the SAV allowed the animal to empty passively through a water trap adjusted to maintain a positive end-expiratory pressure (PEEP) of 2.0 cm H2O. In preliminary experiments, this PEEP was shown to be optimal for the determination of methacholine-induced effects on Rrs (30). Increasing doses of methacholine were infused through a jugular venous catheter at 5-minute intervals.
Collection and Analysis of Bronchoalveolar Fluid Cells
Airway inflammation was assessed 24 hours after the final antigen challenge with OVA. BAL was performed by delivering 0.8 ml cold PBS into the airway through a trachea cannula and gently aspirating the fluid. The lavage was repeated three times to recover a total volume of 2 to 3 ml. The cells were stained with Trypan blue to determine viability and with Turk solution to obtain total nucleated cell counts using a hemocytometer. Cytospin (Cytospin 2; Shandon, Pittsburgh, PA) slides were prepared from the BAL and were then fixed and stained using Diff-Quick (Dade Diagnostics, Aguada, PR). Differential cell counts were determined by counting a minimum of 300 cells/slide using standard morphologic criteria in a single-blind method.
Measurement of Cytokine Concentration in BAL
The concentrations of IL-4, IL-5, IL-13, and IFN-γ in BAL fluid were measured using a Mouse Cytokine Multiplex Panel according to the manufacturer's protocol (Millipore, Billerica, MA). The detection limits were 0.5 pg/ml for IL-4, 1.1 pg/ml for IL-5, 9.8 pg/ml for IL-13, and 1.5 pg/ml for IFN-γ.
Lung Histology
Lungs removed from the chest cavity were fixed by injection of 4% buffered paraformaldehyde into the tracheal cannula at a pressure of 20 cm H2O and immersed in paraformaldehyde for 24 hours. Lobes were sectioned sagittally, embedded in paraffin, cut into 5-μm sections, and stained with hematoxylin and eosin (H&E) for histologic analysis. Additional sections were stained with Alcian blue/PAS to identify mucus-containing cells. The severity of peribronchial inflammation was graded semiquantitatively for the following features: 0, normal; 1, few cells; 2, a ring of inflammatory cells 1 cell layer deep; 3, a ring of inflammatory cells 2 to 4 cells deep; 4, a ring of inflammatory cells of more than 4 cells deep. The numerical scores for the abundance of PAS-positive mucus-containing cell in each airway were determined as follows: 0, less than 5% PAS-positive cells; 1, 5 to 25%; 2, 25 to 50%; 3, 50 to 75%; 4, more than 75% (31).
Determination of IL-5 Production by OVA-Restimulated Cells of the Lung and Thoracic Lymph Node In Vitro
Cytokine production by OVA-restimulated T cells in lung draining lymph nodes was determined as described (32, 33). Mice were sensitized and challenged with OVA as above and single-cell suspensions of thoracic lymph nodes of each mouse were prepared 24 hours after the final aerosol challenge. Thoracic lymph nodes were removed from the paratracheal and parabronchial region, transferred to cold PBS, and gently homogenized on a 70-μm cell strainer to obtain a single-cell suspension. The cells were washed and resuspended in culture medium (RPMI 1640 containing 10% heat-inactivated fetal calf serum, 1% glutamax I, 50 μg/ml gentamycin, and 50 μM β-mercaptoethanol). Cells were cultured with TAT-Pyk2-CT, TAT-GFP, and 100 μg/ml OVA at 4 × 105 cells/well for 48 hours, and supernatants were harvested and stored at −20°C until cytokine levels were determined by sandwich ELISA as described (32). The detection limit for IL-5 is 20 pg/ml.
IL-5–Induced Airway Accumulation of Eosinophils
TAT-Pyk2-CT (1 or 10 mg/kg) or TAT-GFP was administered intraperitoneally 1 hour before challenge with IL-5. Animals were anesthetized with 4.4 mg/kg xylazine and 65 mg/kg intraperitoneal ketamine and then challenged intranasally with 100 μl of 100 ng/ml mouse IL-5 dissolved in PBS through a 24-g catheter. BAL was performed 12 hours after IL-5 instillation as described above.
Statistical Analysis
Variation between three or more groups was analyzed using Kruskal-Wallis ANOVA followed by Mann-Whitney U-test. Statistical significance was claimed whenever P < 0.05.
RESULTS
Effect of TAT-Pyk2-CT on Antigen-Induced Airway Inflammation
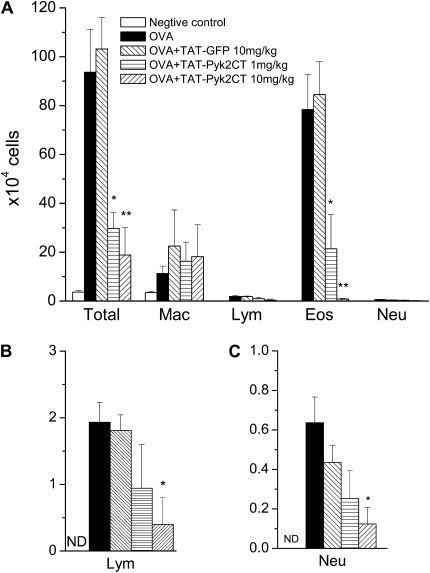
We have previously shown in vitro that TAT-Pyk2-CT blocked endogenous Pyk2 activation in eosinophils caused by integrin adhesion and also blocked subsequent spreading and migration of eosinophils caused by IL-5, PAF, or fMLP (18). To determine the effect of TAT-Pyk2-CT on airway inflammation in vivo, mice were sensitized and challenged by OVA, and BAL fluid was collected 24 hours after the last OVA aerosol challenge; total and differential cell counts then were performed. OVA inhalation significantly increased eosinophil (P < 0.01) and lymphocyte (P < 0.01) numbers in BAL fluid (Figure 2). Eosinophils were absent in BAL fluid collected from the saline-challenged group. After OVA challenge, the number of eosinophils in the BAL fluid increased to 78.4 ± 14.3 × 104 cells (P < 0.01). Treatment of mice with 1 to 10 mg/kg TAT-Pyk2-CT blocked the infiltration of eosinophils 24 hours after OVA challenge in a concentration-dependent manner. Eosinophil number decreased to 21.4 ± 13.9 × 104 in OVA-challenged mice pretreated with 1 mg/kg TAT-Pyk2-CT (P < 0.05), and further decreased to 0.8 ± 0.3 × 104 in mice pretreated with 10 mg/kg TAT-Pyk2-CT (P < 0.01). By contrast, TAT-GFP had no inhibitory effect on OVA-induced eosinophil count in BAL fluid. In further control experiments, mice were pretreated with 10 mg/kg TAT-Pyk2-CT, which was heat-inactivated at 56°C for 1 hour (34, 35). Heat-inactivated TAT-Pyk2-CT had no inhibitory effect on OVA-induced eosinophil count in BAL fluid either (data not shown).
Figure 2.
(A) Effect of intraperitoneal administration of TAT-Pyk2-CT on airway inflammation after OVA sensitization and challenge. Mice were injected with TAT-Pyk2-CT or TAT-GFP 1 hour before each OVA challenge, and inflammatory cells in BAL fluid were collected 24 hours after last OVA challenge. Saline-challenged animals serve as negative controls. OVA-challenged mice serve as positive controls. (B) Lymphocytes and (C) neutrophils in BAL also were recorded on a different scale. Each bar represents the mean ± SEM of four to six mice. *P < 0.05 and **P < 0.01 compared with OVA+ buffer group. N.D.: not detectable.
Lymphocytes were absent in BAL fluid from the saline-challenged control. After OVA challenge, the number of lymphocytes in the BAL fluid increased to 1.9 ± 0.3 × 104 (P < 0.01, Figure 2B). TAT-Pyk2-CT blocked the increase in lymphocytes in the airway lumen after OVA challenge. The number of lymphocytes decreased to 0.9 ± 0.6 × 104 at 1 mg/kg and 0.4 ± 0.4 × 104 at 10 mg/kg TAT-Pyk2-CT (P < 0.05 versus OVA controls). TAT-GFP had no inhibitory effect on OVA-induced lymphocyte count in BAL fluid.
Neutrophils were absent in BAL fluid from the saline-challenged control. After OVA challenge, the number of neutrophils in the BAL fluid modestly increased to 0.6 ± 0.1 × 104 (P < 0.01, Figure 2C). TAT-Pyk2-CT blocked the increase in neutrophils in the airway lumen after OVA challenge. The number of neutrophils decreased to 0.3 ± 0.1 × 104 at 1 mg/kg and 0.1 ± 0.1 × 104 at 10 mg/kg TAT-Pyk2-CT (P < 0.05 versus OVA controls). TAT-GFP had no inhibitory effect on OVA-induced lymphocyte count in BAL fluid.
Macrophages were the dominant cells in BAL from saline-challenged mice. OVA challenge also increased the number of macrophages in BAL. However, neither pretreatment with TAT-Pyk2-CT nor TAT-GFP reduced macrophage count in BAL.
Antigen-Induced Th2 Cytokine Secretion in BAL Fluid
To evaluate the role of Th2 cytokines in OVA-induced airway responses, we measured the concentrations of IL-5, IL-13, and IL-4 in BAL fluid. OVA inhalation caused a significant increase in the concentration of IL-5, IL-13, and IL-4, which was concentration-dependently inhibited by TAT-Pyk2-CT. There was no significant reduction of these Th2 cell cytokines in control mice receiving TAT-GFP (Figure 3). There was no detectable concentration of IFN-γ in the BAL after either saline or OVA challenge or pretreatment with TAT fusion proteins.
Figure 3.
Effect of TAT-Pyk2-CT on Th2 cytokine secretion after OVA challenge. BAL fluids were collected 24 hours after the last OVA challenge. Negative controls are saline-challenged animals. Mice were injected with buffer, TAT-Pyk2-CT, or TAT-GFP 1 hour before each OVA challenge, and BAL fluid was collected 24 hours after last OVA challenge. Each bar represents the mean ± SEM of four to six mice. *P < 0.05 and **P < 0.01 compared with OVA-alone group. Con: negative controls; N.D.: not detectable.
Effect of TAT-Pyk2-CT on Airway Histology
In comparison to saline-challenged negative control mice (Figure 4A), OVA challenge induced inflammatory cell infiltration (largely eosinophils and mononuclear cells) in the lung interstitium around the airways and pulmonary blood vessels (Figure 4B, positive control group). Pretreatment 1 hour before OVA challenge with 10 mg/kg TAT-GFP did not block inflammatory cell infiltration in lung tissues after OVA challenge (Figure 4C). However, pretreatment with 10 mg/kg TAT-Pyk2-CT blocked cell infiltration at 24 hours after last challenge (Figure 4D). Figure 4I is the semiquantitative peribronchial inflammatory scores summarized from these H&E-stained slides.
Figure 4.
Histologic examination of lung tissues. The lung sections were obtained from (A, E) saline- or (B, F) OVA-challenged mice, or OVA-challenged mice pretreated with (C, G) 10 mg/kg TAT-GFP or (D, H) TAT-Pyk2-CT. Sections were stained with (A–D) hematoxylin and eosin for morphologic analysis of inflammation or (E–H) Alcian blue/PAS for analysis of mucin-containing cells. Semiquantitative analysis of (I) the severity of peribronchial inflammation and (J) the abundance of PAS-positive mucus-containing cells is described in Materials and Methods. Tissue was examined by light microscopy (original magnification: ×400). **P < 0.01 compared with the OVA-alone group.
There are no mucus-containing epithelial cells in the airways of saline-challenged negative control mice (Figure 4E). OVA challenge induced the expression of mucus-containing epithelial cells in the airways (Figure 4F, positive control). Pretreated with 10 mg/kg TAT-Pyk2-CT (Figure 4H), but not TAT-GFP (Figure 4G), reduced the number of mucus-containing epithelial cells in the airways of OVA-challenged mice. Figure 4J is the semiquantitative analysis of the abundance of PAS-containing cells summarized from these PAS-stained slides. Heat-inactivated TAT-Pyk2-CT at 10 mg/kg had no inhibitory effect on either OVA-induced lung inflammation or mucus cell hyperplasia (data not shown).
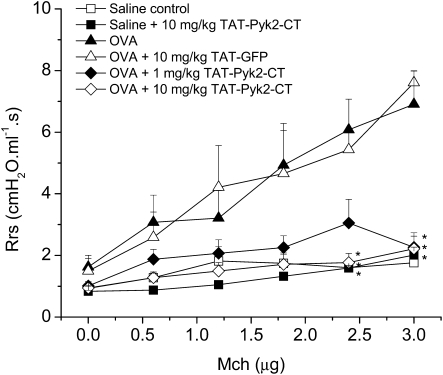
Antigen-Induced AHR
Antigen-induced AHR to methacholine was attenuated by 1 and 10 mg/kg TAT-Pyk2-CT (Figure 5). Rrs was comparable 24 hours after the last OVA challenge in each group before methacholine challenge (dose zero). Inhalation with OVA caused substantial increase in methacholine responsiveness compared with mice receiving saline inhalation (negative control mice). TAT-Pyk2-CT—but not TAT-GFP—inhibited the AHR to methacholine in a concentration-dependent manner in OVA-sensitized and -challenged mice. TAT-Pyk2-CT at 10 mg/kg had no inhibitory effect on baseline Rrs to methacholine in saline-sensitized and saline-challenged mice.
Figure 5.
Effect of TAT-Pyk2-CT on antigen-induced AHR to methacholine in immunized mice after three OVA challenges on Days 21, 22, and 23. Mice were injected with TAT-Pyk2-CT or TAT-GFP 1 hour before each OVA challenge, and airway responsiveness to increasing doses of methacholine was measured 24 hours after the last OVA challenge. Negative controls are saline-challenged animals and positive controls are OVA-challenged animals. Results are expressed as the mean ± SEM of four to six mice. *P < 0.05 compared with OVA-challenged positive control group.
Cytokine Production by OVA-Restimulated Cells of Thoracic Lymph Nodes In Vitro
To examine T cell responses on antigen-specific restimulation, single-cell suspensions of thoracic lymph nodes of each mouse were prepared 24 hours after the final OVA aerosol challenge. IL-5 was undetectable in the medium-alone group. Upon restimulation with OVA, the amount of IL-5 in cell cultures was increased to 619.3 ± 79.4 pg/ml (Figure 6). Cells cultured with 2 μM TAT-Pyk2-CT or 2 μM TAT-GFP all contained high concentrations of IL-5. However, IL-4 was undetectable in these cell cultures. Thus, TAT-Pyk2-CT had no effect on IL-5 production from the lung draining lymph node cells upon in vitro restimulation.
Figure 6.
IL-5 production by OVA restimulated bronchial lymph node cells after treatment with TAT-Pyk2-CT or TAT-GFP. Bronchial lymph node cells were harvested 24 hours after final OVA aerosol challenge and restimulated in vitro with 100 μg/ml OVA together with 2 μM TAT-Pyk2-CT or TAT-GFP. IL-5 concentration was measured by sandwich ELISA assay. Results are expressed as the mean ± SEM of three mice.
Effect of TAT-Pyk2-CT on IL-5–Induced Lung Inflammation
Further studies were performed to determine the role of Pyk2 in IL-5–induced eosinophil migration into airways. Mice were pretreated with 1 and 10 mg/kg TAT-Pyk2-CT or 10 mg/kg TAT-GFP 1 hour before intranasal challenge with murine recombinant IL-5, and BAL was collected 12 hours after IL-5 challenge (Figure 7A). Eosinophils were not detected in BAL fluid in PBS-challenged control animals. The eosinophil count in BAL fluid was 276.0 ± 18.8 × 103 at 12 hours after intranasal challenge with murine IL-5 (P < 0.01 versus PBS controls; Figure 7B). Pretreatment with TAT-Pyk2-CT caused concentration-dependent blockade of BAL eosinophilia to 132.8 ± 17.9 × 103 in mice pretreated with 1 mg/kg TAT-Pyk2-CT (P < 0.01 versus IL-5 alone) and 4.3 ± 1.5 × 103 in mice pretreated with 10 mg/kg TAT-Pyk2-CT (P < 0.01 versus IL-5 alone). TAT-GFP had no effect on eosinophil infiltration into BAL caused by IL-5 challenge (P = NS versus IL-5 alone).
Figure 7.
(A) Model of IL-5–induced eosinophil infiltration in BAL. Mice were pretreated intraperitoneally 1 hour before intranasal challenge with mouse recombinant IL-5. BAL was performed 12 hours after IL-5 challenge. (B) Effects of TAT-Pyk2-CT on eosinophil accumulation in BAL fluid 12 hours after IL-5 challenge. Cell counts were obtained from animals receiving IL-5 + either TAT-GFP control or TAT-Pyk2-CT. Each bar represents the mean ± SEM of three mice. **P < 0.01 compared with IL-5 alone group. N.D.: not detectable.
DISCUSSION
We have reported previously that Pyk2, which has no role in initiating β2-integrin adhesion of eosinophils to endothelial ligands, subsequently regulates eosinophil spreading and chemotaxis in Transwell plates after adhesion in vitro (18). Pyk2 regulates the formation of lamellapodia and filapodia as well as chemotaxis to IL-5, PAF, and fMLP (18). The objective of this investigation was to determine if these prior preliminary observations would translate to reduction in AHR in an immune-sensitized mouse in vivo. Accordingly, specific isolation of each affected site is not possible in vivo. Thus, we did not establish which cells were predominantly targeted by TAT-Pyk2-CT. In this study, the biological role of Pyk2 in asthmatic response in vivo was explored by using TAT-mediated protein transduction of dominant-negative Pyk2 into antigen-sensitized and -challenged mice. Our results showed that Pyk2 inhibition by TAT-Pyk2-CT concentration-dependently attenuated antigen-induced airway infiltration of eosinophils, lymphocytes, and neutrophils, reduced the number of mucus-containing cells in the airway lumen, reduced Th2 cytokine concentrations in BAL, and blocked AHR to methacholine. These findings suggest that Pyk2 signaling likely has a regulatory role in antigen-induced airway inflammation and hyperresponsiveness in mice.
Our data demonstrate that TAT-Pyk2-CT blocks eosinophil infiltration into the airways in antigen-sensitized and -challenged mice, as shown by a significant reduction in total cell counts and eosinophil counts in BAL fluid (Figure 2). Similarly, tissue eosinophilia was also inhibited, as revealed by a significant reduction of inflammatory cell infiltration by histologic examination (Figure 4). Eosinophil transmigration into the airways is a multi-phasic process that is regulated by Th2 cytokines and chemokines in combination with adhesion molecules expressed on both eosinophils and endothelial cells (36, 37). Eosinophil migration into the airways caused by IL-5 challenge was also blocked by Pyk2 inhibition (Figure 7). Together with our in vitro findings (18), these results suggest that Pyk2 regulates the intrinsic mobility of eosinophils.
Our results also show that IL-5 concentrations in BAL fluid from TAT-Pyk2-CT–treated mice were substantially reduced versus TAT-GFP controls (Figure 3). This finding is associated with decreased airway infiltration with eosinophils after OVA challenge (Figure 4). The contribution of Pyk2 expressed in vascular endothelial cells to the migration of eosinophils is currently unknown. Recent studies have found that inhibition of endothelial Pyk2 results in decreased transmigration of human neutrophils (38) and that Pyk2 regulates the phosphorylation of adherens junction proteins and disassembly of endothelial junctions, which allow the passage of leukocytes. Hence, the observed reduction in airway eosinophils by TAT-Pyk2-CT may be a result of composite effects of reduction in Th2 cytokine production and reduction of eosinophil motility via Pyk2 pathway inhibition.
Th2 cytokines are produced by bronchial epithelial cells, tissue mast cells, and alveolar macrophages, as well as infiltrated inflammatory cells such as lymphocytes and eosinophils. Our data show that TAT-Pyk2-CT significantly reduced the concentration of IL-4, IL-5, and IL-13 in BAL fluids 24 hours after OVA challenge in immune-sensitized and -challenged mice (Figure 3). The reduced Th2 cytokine concentration in BAL in TAT-Pyk2-CT–treated mice is not due to inhibition of Th2 differentiation, as TAT-Pyk2-CT had no effect on Th2 cell in vitro differentiation as measured by IL-5 production after OVA restimulation (Figure 6). The specific cells targeted by TAT-Pyk2-CT in vivo currently are unknown. Our data also show that TAT-Pyk2-CT inhibits OVA-induced goblet cell hyperplasia (Figure 4) and AHR to intravenous methacholine (Figure 5). The observed reduction of mucus-producing cells and AHR corresponds to the blockade of secretion of Th2 cytokines (IL-4, IL-5, and IL-13; Figure 3) and tissue eosinophilia (Figure 4) caused by TAT-Pyk2-CT.
It is important to recognize specific limitations of our findings. Our data were obtained in mice with experimentally induced antigen-mediated AHR. Although the histologic changes in these airways resemble those of human asthma, these data cannot be extrapolated directly to the human state. Nonetheless, the regulatory role of Pyk2 in the chemotaxis of human eosinophils has been validated by our in vitro studies (18). The functional interference of TAT-Pyk2-CT on Pyk2 activity in mouse eosinophils ex vivo cannot be confirmed in the current study because sufficient eosinophils could not be obtained from bone marrow or blood of individual mice. However, the functional interference of TAT-Pyk2-CT on endogenous Pyk2 activity in both human blood neutrophils and eosinophils has been determined previously (18, 25). We also found that Pyk2 inhibition attenuated three Th2 cytokines commonly associated with human AHR in vivo; however, the precise mechanisms by which Pyk2 regulates Th2 cytokine secretion in vivo are not defined in the current studies, and further investigation is warranted. Finally, Pyk2 inhibition blocked BAL eosinophilia, mucous gland hyperplasia, and AHR—conditions that are characteristic of the asthmatic state in human as well.
We conclude that blockade of Pyk2 in immune-sensitized mice (1) attenuates Th2 cytokine concentration in the BAL; (2) blocks migration of eosinophils, lymphocytes, and neutrophils into airways; and (3) blocks consequent AHR to methacholine. To our knowledge, this is the first report demonstrating that Pyk2 may have a potential role in the immune regulation of AHR in the antigen-sensitized state in mice.
Acknowledgments
The authors thank Dr. Carl Nathan (Cornell University) for providing pTAT-Pyk2-CT plasmid and Dr. Steven Dowdy (University of California San Diego, La Jolla, CA) for providing pTAT-GFP expression plasmid.
This work was supported by National Heart, Lung and Blood Institute Grants HL-92328 (X.Z.), HL-85779 (A.R.L), and by a grant from GlaxoSmithKline Center of Excellence.
Originally Published in Press as DOI: 10.1165/rcmb.2008-0469OC on June 11, 2009
Conflict of Interest Statement: B.S.C. received a grant from the National Institutes of Health (NIH) for $10,001–50,000. X.Z. received grants from GlaxoSmithKline for $50,001–100,000 and from the NIH for more than $100,001. A.R.L. received grants from GlaxoSmithKline for more than $100,001 and from the NIH for more than $100,001. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Shum BO, Rolph MS, Sewell WA. Mechanisms in allergic airway inflammation: lessons from studies in the mouse. Expert Rev Mol Med 2008;10:e15. [DOI] [PubMed] [Google Scholar]
- 2.Lee NA, Gelfand EW, Lee JJ. Pulmonary T cells and eosinophils: coconspirators or independent triggers of allergic respiratory pathology? J Allergy Clin Immunol 2001;107:945–957. [DOI] [PubMed] [Google Scholar]
- 3.Jacobsen EA, Ochkur SI, Lee NA, Lee JJ. Eosinophils and asthma. Curr Allergy Asthma Rep 2007;7:18–26. [DOI] [PubMed] [Google Scholar]
- 4.Leckie MJ, ten Brinke A, Khan J, Diamant Z, O'Connor BJ, Walls CM, Mathur AK, Cowley HC, Chung KF, Djukanovic R, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet 2000;356:2144–2148. [DOI] [PubMed] [Google Scholar]
- 5.Justice JP, Borchers MT, Crosby JR, Hines EM, Shen HH, Ochkur SI, McGarry MP, Lee NA, Lee JJ. Ablation of eosinophils leads to a reduction of allergen-induced pulmonary pathology. Am J Physiol Lung Cell Mol Physiol 2003;284:L169–L178. [DOI] [PubMed] [Google Scholar]
- 6.Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O'Neill KR, Protheroe C, Pero R, Nguyen T, Cormier SA, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science 2004;305:1773–1776. [DOI] [PubMed] [Google Scholar]
- 7.Yu C, Cantor AB, Yang H, Browne C, Wells RA, Fujiwara Y, Orkin SH. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J Exp Med 2002;195:1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH, et al. A critical role for eosinophils in allergic airways remodeling. Science 2004;305:1776–1779. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka H, Komai M, Nagao K, Ishizaki M, Kajiwara D, Takatsu K, Delespesse G, Nagai H. Role of interleukin-5 and eosinophils in allergen-induced airway remodeling in mice. Am J Respir Cell Mol Biol 2004;31:62–68. [DOI] [PubMed] [Google Scholar]
- 10.Humbles AA, Lu B, Friend DS, Okinaga S, Lora J, Al-Garawi A, Martin TR, Gerard NP, Gerard C. The murine CCR3 receptor regulates both the role of eosinophils and mast cells in allergen-induced airway inflammation and hyperresponsiveness. Proc Natl Acad Sci USA 2002;99:1479–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma W, Bryce PJ, Humbles AA, Laouini D, Yalcindag A, Alenius H, Friend DS, Oettgen HC, Gerard C, Geha RS. CCR3 is essential for skin eosinophilia and airway hyperresponsiveness in a murine model of allergic skin inflammation. J Clin Invest 2002;109:621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wegmann M, Goggel R, Sel S, Sel S, Erb KJ, Kalkbrenner F, Renz H, Garn H. Effects of a low-molecular-weight CCR-3 antagonist on chronic experimental asthma. Am J Respir Cell Mol Biol 2007;36:61–67. [DOI] [PubMed] [Google Scholar]
- 13.Barthel SR, Johansson MW, McNamee DM, Mosher DF. Roles of integrin activation in eosinophil function and the eosinophilic inflammation of asthma. J Leukoc Biol 2008;83:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broide D, Sriramarao P. Eosinophil trafficking to sites of allergic inflammation. Immunol Rev 2001;179:163–172. [DOI] [PubMed] [Google Scholar]
- 15.Costa GG, Silva RM, Franco-Penteado CF, Antunes E, Ferreira HH. Interactions between eotaxin and interleukin-5 in the chemotaxis of primed and non-primed human eosinophils. Eur J Pharmacol 2007;566:200–205. [DOI] [PubMed] [Google Scholar]
- 16.Rosas M, Dijkers PF, Lindemans CL, Lammers JW, Koenderman L, Coffer PJ. IL-5-mediated eosinophil survival requires inhibition of GSK-3 and correlates with beta-catenin relocalization. J Leukoc Biol 2006;80:186–195. [DOI] [PubMed] [Google Scholar]
- 17.Simon HU. Molecules involved in the regulation of eosinophil apoptosis. Chem Immunol Allergy 2006;91:49–58. [DOI] [PubMed] [Google Scholar]
- 18.Zhu X, Boetticher E, Wang L, Duan Y, Learoyd J, Leff AR. Proline-rich tyrosine kinase 2 regulates spreading and migration of eosinophils after beta2-integrin adhesion. Am J Respir Cell Mol Biol 2008;39:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avraham S, Avraham H. Characterization of the novel focal adhesion kinase RAFTK in hematopoietic cells. Leuk Lymphoma 1997;27:247–256. [DOI] [PubMed] [Google Scholar]
- 20.Okigaki M, Davis C, Falasca M, Harroch S, Felsenfeld DP, Sheetz MP, Schlessinger J. Pyk2 regulates multiple signaling events crucial for macrophage morphology and migration. Proc Natl Acad Sci USA 2003;100:10740–10745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guinamard R, Okigaki M, Schlessinger J, Ravetch JV. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat Immunol 2000;1:31–36. [DOI] [PubMed] [Google Scholar]
- 22.Di Cioccio V, Strippoli R, Bizzarri C, Troiani G, Cervellera MN, Gloaguen I, Colagrande A, Cattozzo EM, Pagliei S, Santoni A, et al. Key role of proline-rich tyrosine kinase 2 in interleukin-8 (CXCL8/IL-8)-mediated human neutrophil chemotaxis. Immunology 2004;111:407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myou S, Leff AR, Myo S, Boetticher E, Tong J, Meliton AY, Liu J, Munoz NM, Zhu X. Blockade of inflammation and airway hyperresponsiveness in immune-sensitized mice by dominant-negative phosphoinositide 3-kinase-TAT. J Exp Med 2003;198:1573–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duan W, Chan JH, Wong CH, Leung BP, Wong WS. Anti-inflammatory effects of mitogen-activated protein kinase kinase inhibitor U0126 in an asthma mouse model. J Immunol 2004;172:7053–7059. [DOI] [PubMed] [Google Scholar]
- 25.Han H, Fuortes M, Nathan C. Critical role of the carboxyl terminus of proline-rich tyrosine kinase (Pyk2) in the activation of human neutrophils by tumor necrosis factor: separation of signals for the respiratory burst and degranulation. J Exp Med 2003;197:63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dylla SJ, Deyle DR, Theunissen K, Padurean AM, Verfaillie CM. Integrin engagement-induced inhibition of human myelopoiesis is mediated by proline-rich tyrosine kinase 2 gene products. Exp Hematol 2004;32:365–374. [DOI] [PubMed] [Google Scholar]
- 27.Zhu X, Boetticher E, Wang L, Duan Y, Learoyd J, Leff AR. Pyk2 regulates spreading and migration of eosinophils after {beta}2 integrin adhesion. Am J Respir Cell Mol Biol 2008;39:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science 1999;285:1569–1572. [DOI] [PubMed] [Google Scholar]
- 29.Cai SR, Xu G, Becker-Hapak M, Ma M, Dowdy SF, McLeod HL. The kinetics and tissue distribution of protein transduction in mice. Eur J Pharm Sci 2006;27:311–319. [DOI] [PubMed] [Google Scholar]
- 30.Myou S, Zhu X, Myo S, Boetticher E, Meliton AY, Liu J, Munoz NM, Leff AR. Blockade of airway inflammation and hyperresponsiveness by HIV-TAT-dominant negative Ras. J Immunol 2003;171:4379–4384. [DOI] [PubMed] [Google Scholar]
- 31.Ford JG, Rennick D, Donaldson DD, Venkayya R, McArthur C, Hansell E, Kurup VP, Warnock M, Grunig G. Il-13 and IFN-gamma: interactions in lung inflammation. J Immunol 2001;167:1769–1777. [DOI] [PubMed] [Google Scholar]
- 32.Bandukwala HS, Clay BS, Tong J, Mody PD, Cannon JL, Shilling RA, Verbeek JS, Weinstock JV, Solway J, Sperling AI. Signaling through Fc gamma RIII is required for optimal T helper type (Th)2 responses and Th2-mediated airway inflammation. J Exp Med 2007;204:1875–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Oosterhout AJ, Van Esch B, Hofman G, Hofstra CL, Van Ark I, Nijkamp FP, Kapsenberg ML, Savelkoul HF, Weller FR. Allergen immunotherapy inhibits airway eosinophilia and hyperresponsiveness associated with decreased IL-4 production by lymphocytes in a murine model of allergic asthma. Am J Respir Cell Mol Biol 1998;19:622–628. [DOI] [PubMed] [Google Scholar]
- 34.Eum WS, Choung IS, Li MZ, Kang JH, Kim DW, Park J, Kwon HY, Choi SY. HIV-1 Tat-mediated protein transduction of Cu,Zn-superoxide dismutase into pancreatic beta cells in vitro and in vivo. Free Radic Biol Med 2004;37:339–349. [DOI] [PubMed] [Google Scholar]
- 35.Iglesias R, Locovei S, Roque A, Alberto AP, Dahl G, Spray DC, Scemes E. P2X7 receptor-Pannexin1 complex: pharmacology and signaling. Am J Physiol Cell Physiol 2008;295:C752–C760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simson L, Foster PS. Chemokine and cytokine cooperativity: eosinophil migration in the asthmatic response. Immunol Cell Biol 2000;78:415–422. [DOI] [PubMed] [Google Scholar]
- 37.Jia GQ, Gonzalo JA, Hidalgo A, Wagner D, Cybulsky M, Gutierrez-Ramos JC. Selective eosinophil transendothelial migration triggered by eotaxin via modulation of Mac-1/ICAM-1 and VLA-4/VCAM-1 interactions. Int Immunol 1999;11:1–10. [DOI] [PubMed] [Google Scholar]
- 38.Allingham MJ, van Buul JD, Burridge K. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J Immunol 2007;179:4053–4064. [DOI] [PubMed] [Google Scholar]