Abstract
Purpose
Maspin is a tumor suppressor protein that has been reported to stimulate the cell death of cancer and inhibit the metastasis of cancer. The present study aimed to explore the survival pathway by which maspin modulates the resistance of human lung cancer cells to chemotherapeutic drugs, and the consequences of maspin gene therapy in an animal model.
Materials and Methods
NCI-H157 and A549 cells were transfected with either a mock vector (pCMVTaq4C), maspin (pCMV-maspin), siControl or siMaspin. RT-PCR and Western blot analysis were performed to study the expressions of survival proteins in lung cancer. cDNA microarray analysis was carried out to compare the maspin-modulated gene expression between the xenograft tumors derived from the lung cancer cells that were stably transfected with pCMVTaq4C or pCMV-maspin. Maspin gene therapy was performed by intra-tumoral injections of pCMVTaq4C or pCMV-maspin into the pre-established subcutaneous tumors in nude mice.
Results
Maspin significantly decreased the survival to doxorubicin and etoposide, whereas did not affect the survival to cisplatin in the NCI-H157 cells. Interestingly, transfection with a maspin plasmid resulted in a significant reduction of the phosphorylation of Akt in the NCI-H157 cells, whereas knockdown of maspin increased the phosphorylation of Akt in the A549 cells. Microarray analysis of the xenograft tumors revealed a specific gene expression profile, demonstrating that maspin is associated with the differential expressions of PTEN and IGF2R. Direct transfer of pCMV-maspin into the tumor significantly retarded the tumor growth in the animal experiments (p=0.0048).
Conclusion
Lung cancer cells lacking maspin could be resistant to chemotherapeutic drugs such as doxorubicin or etoposide, at least in part by maintaining Akt phosphorylation.
Keywords: SERPIN-B5, Akt, Survival, Microarray analysis
Introduction
Maspin is a 42 kDa protein with high sequence homology to the members of the serpin protease inhibitor family (1-3), yet maspin does not have protease inhibitory activity (4). The expression of maspin is frequently lost in human lung cancer (5), and the genetic polymorphism of Maspin has been recently reported (6). In terms of function, substantial evidence supports a role for maspin in apoptosis (7) and metastasis (8-10), whereas the published data on the role of maspin for the survival of lung cancer cells is relatively limited. Since resistance to chemotherapeutic drugs is one of the major obstacles in the treatment of cancer, we investigated whether a maspin expression influences the cellular responses to chemotherapeutic drugs in lung cancer cells.
Even though apoptosis is the predominant mechanism by which cancer cells die in chemotherapy, the survival pathway may antagonize cancer cell death by such signals as growth factor and Akt (11). In view of the recent findings that a specific pattern of resistance to chemotherapy may occur depending on the genetic or epigenetic abnormalities of cancer cells (12-17), this present work demonstrated a resistance of lung cancer cells to cell death in the context of a maspin expression. cDNA microarray analysis was performed to identify the genes that are differentially expressed according to a maspin expression in vivo. Western blot analysis also showed that the expressions of survival-related genes are modulated by maspin. This is the first report to indicate that the maspin in lung cancer cells may modulate Akt phosphorylation and chemoresistance.
Materials and Methods
1 Materials
The chemicals, protease inhibitors and chemotherapeutic drugs were purchased from Sigma Chemical Co. (St Louis, MO). Fetal bovine serum and the tissue-culture media were purchased from Life Technologies, Inc. (Gaithersburg, MA). The enhanced chemiluminescence detection kit was from Amersham Corp. (Arlington Heights, IL). The reactive proteins were visualized using an ECL kit (Amersham Life Sciences, UK).
Antibodies for maspin, active caspase-3, PARP, Erk, p-Erk, Akt and pAkt were obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
2 The cell-growth and viability assays
The NCI-H157 and A549 human lung carcinoma cell lines were maintained in RPMI 1640 (Life Technologies, Gaithersburg, MD), 1 mM glutamate, 100 U/mL penicillin, 100 ng/mL streptomycin and 10% FCS. The cells were cultured at 37℃ in a humidified atmosphere of 5% CO2. Cell viability was evaluated by trypan blue exclusion assay.
3 Transfection of the lung cancer cells
The cells were transfected with siRNA or plasmids using the Effectene (Qiagen, Valencia, CA) or Amaxa electroporation system (Amaxa, Gaithersburg, MD), according to the supplier's protocol. For pCMV-maspin, full-length maspin cDNA was cloned into the pCMVTaq4C cells (Invitrogen, Carlsbad, CA). For obtaining stable maspin expressing cells, the cells were incubated for 48 h after transfection, then the medium was changed and the transfected cells were selected for 2 weeks in the presence of geneticin. The SMART pool small interfering RNAs (siRNA) to maspin and the negative control siRNA were from Dharmacon (Lafayette, CO).
4 Western blot analysis
After the experimental treatments, the cells were harvested and the cell pellets were washed with ice-cold phosphate-buffered saline and then lysed in lysis buffer containing 10 mM Tris (pH 7.5), 150 mM NaCl, 10 mM ethylenediaminetetraacetic acid (EDTA), 1% sodium dodecyl sulfate (SDS), 1 mM sodium orthovanadate and a mixture of protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 1 µg/mL pepstatin A, 2 µg/mL aprotinin). The lysates were sonicated for 10 s, centrifuged for 20 min at 20,000×g and then stored at -70℃. Equal amounts (25 µg) of the cell lysates were resolved by 12% SDS-PAGE and next subjected to Western blot analysis using an enhanced chemiluminescence system (Amersham Corp., Arlington Heights, IL).
5 Animals and the tumor model
Four-week-old, 19 to 22 g female BALB/c nude mice (Nihon Clea, Osaka, Japan) were used and these were housed at the Institute of Laboratory Animals. NCI-H157 cells (5×106) were inoculated subcutaneously into the nude mice. After the tumor size reached between 60 to 70 mm3 in volume, the animals were divided into two groups with nine mice per group, and these animals received intra-tumoral injections of control vector or pCMV-maspin (50 µg in 50 µL PBS). The tumor volume was measured during the administration period for 23 days. The tumor sizes in two dimensions were measured with calipers and the volumes were calculated with the formula (L×W2)×0.5, where L is length and W is width. T-tests were used to compare the difference of tumor size on day 23 between the tumors with and without maspin. The mice were maintained and sacrificed according to institutional guidelines, and the study procedures were approved by the Institutional Committee on the Use and Care of Animals and Recombinant DNA Research.
6 cDNA microarray analysis
A cDNA microarray containing 17,448 sequence-verified human cDNA clones was purchased from GenomicTree Inc. (Seoul, Korea). The target cDNA probes were synthesized and hybridizations were performed as previously described (18). After hybridization, the microarrays were washed and then immediately dried in a microarray centrifuge (GenomicTree Inc.). All the microarray hybridizations were performed in duplicate, and the collected data was averaged.
Results
1 The maspin expression modulates chemoresistance in lung cancer cells
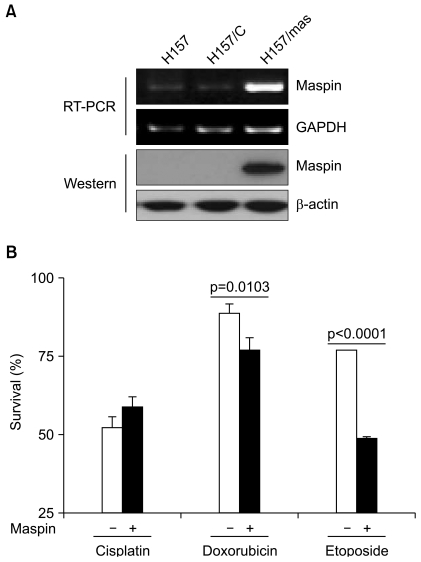
To study the chemoresistance-associated role of maspin, we generated a stable maspin expressing system in NCI-H157 human lung cancer cells. The ectopic expression of transfected maspin was confirmed by RT-PCR and Western blot analysis (Fig. 1A). We compared the response of the maspin-transfected NCI-H157 (H157/mas) cells to the chemotherapy drugs with that of the empty vector-transfected control (H157/C) cells. The maspin-expressing cells displayed a significant decrease in survival to doxorubicin, with the survival decreasing from 89.1±2.7% in the control H157/C cells compared with 77.2±3.7% in the maspin-expressing H157/mas cells (p=0.0103, Fig. 1B). Similar to that observed for doxorubicin, the maspin-expressing cells displayed a significant decrease in survival to etoposide compared with that of the control cells. Specifically, the control cells displayed a survival of 77.1±0.4% compared with a survival of 48.7±1.6% for the maspin-expressing cells (p<0.0001), suggesting that maspin induced chemosensitivity to etoposide in these cells (Fig. 1B). However, treatment with cisplatin resulted a survival rate of 51.8±3.3% in the control cells, whereas the survival rate was 58.9±3.5% in the maspin-expressing NCI-H157 cells (p>0.05).
Fig. 1.
The ectopic expression of maspin decreases the rate of survival to drug-induced cell death. (A) RT-PCR and Western blot analysis demonstrating the ectopic expression of recombinant maspin in the NCI-H157 cells. (B) The effect of a stable maspin expression on the cytotoxicity of cisplain (50 µM), doxorubicin (5 µM) and etoposide (10 µM) in the NCI-H157 cells. Cells (1×105/well) were plated in 6-well plates and they were treated with drugs for 24 h on the following day. Trypan blue staining was performed to detect the live cells and cell survival is expressed as the ratio of the number of viable cells following a given treatment to the number of untreated cells×100 (%). Bars, SD.
2 PARP cleavage and caspase-3 activation are suppressed by knockdown of the maspin expression
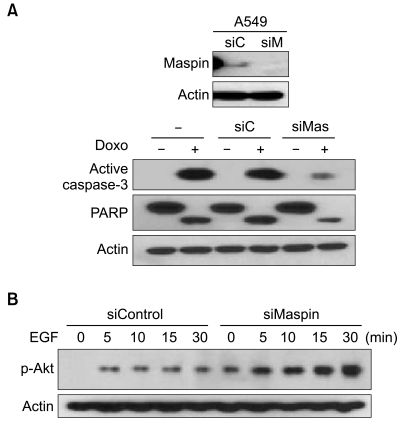
To examine whether the observed survival difference induced by maspin was due to modulation of a component in the apoptosis pathway, we analyzed caspase-3 activation and PARP cleavage according to the knockdown of the maspin expression after the treatment with drugs. The PARP assays demonstrated cleavage of the PARP protein when the A549 cells were treated with doxorubicin (Fig. 2A). However, in agreement with the cell survival data, the maspin-knockdown cells were relatively resistant to PARP cleavage and caspase-3 activation as compared with that of the parental or mock cells.
Fig. 2.
Maspin knockdown modulates the phosphorylation of Akt and drug-induced apoptosis. (A) Active caspase-3 and PARP cleavage demonstrates reduction of active caspase-3 and the cleaved PARP fragment in the siMaspin-transfected (siMas, 50 nM) A549 cells, as compared with the parental (-), and siControl-transfected cells (siC, 50 µM), after the treatment with 5 µM doxorubicin. The identical blot was reprobed with β-actin as a control for loading. (B) Western blot analysis demonstrating the induction of phosphorylated Akt in the siMaspin (50 nM) - transfected A549 cells after EGF (10 nM) treatment.
3 Akt phosphorylation is induced by knockdown of the maspin expression
To examine whether the observed survival difference induced by maspin was due to modulation of a component in the survival pathway, we analyzed the phosphorylated Akt levels according to knockdown of the maspin expression after exposure to EGF. Western blot analysis demonstrated an increase of the phosphory-lated Akt in the siMaspin-transfected cells (Fig. 2B). Notice that even without the addition of EGF, a phosphorylated Akt level was already induced in the maspin-knockdown cells.
4 Microarray analysis reveals maspin targets that may account for the differential survival
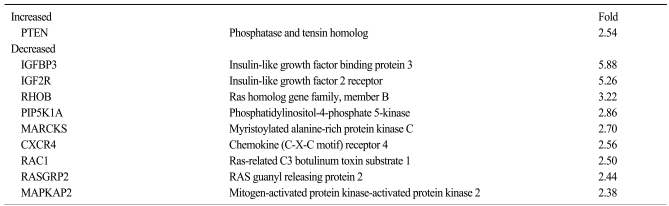
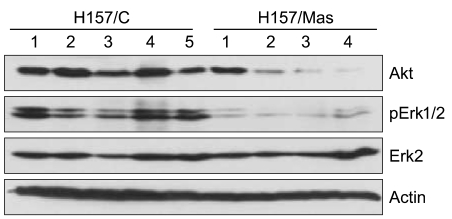
We examined the target genes by which the ability of maspin to modulate the survival of lung cancer cells might be mediated. To identify potential target genes, a microarray analysis was carried out and the differential gene expressions were compared between the tumors derived from the H157/C and H157/mas cells. Dozens of survival-associated genes were identified with at least a 2-fold difference in expression in the H157/mas tumors. Among these, IGF2R, which is involved in the growth factor signaling pathways, was down-regulated in the H157/mas tumors (Table 1). Interestingly, PTEN, an inhibitory protein of Akt, was induced by maspin, implicating that the reduced survival of H157/mas tumor cells could be caused by modulation of these proteins. To further confirm the microarray data, we performed Western blot analysis and examined whether or not a maspin expression might affect the survival pathway in vivo. As shown in Fig. 3, the expressions of Akt and phosphorylated Erk were significantly downregulated in the tumors generated by the H157/mas cells, indicating that maspin modulates these proteins, which may subsequently affect cell survival.
Table 1.
List of the genes that were up- or down-regulated by maspin
The fold indicates the level of induction or reduction over the maspin-expression in the xenograft tumors derived from NCI-H157 cells. Note: The hybridization signals for genes that showed a significant detection in two samples were considered in the comparison analysis.
Fig. 3.
Western blot analysis of the survival-related proteins in the total cell lysates from the tumors derived from the mock-transfected (H157/C, 5 samples) and maspin over-expressing (H157/mas, 4 samples) cells. The identical blot was reprobed with β-actin as a control for loading.
5 The effect of maspin gene therapy on the growth of established tumor
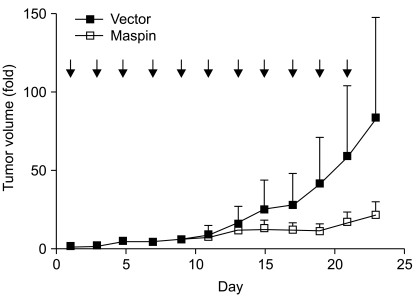
Finally, we investigated whether maspin gene transfer could affect tumor growth in vivo. First, the subcutaneous tumors were established after injection of NCI-H157 cells into the athymic nude mice. When the mice had an average tumor volume in the range of 60 to 70 mm3, the plasmid DNAs were injected every other day. During the administration period, the tumors in the control group continued to grow. The control group formed tumors with an average increase of 83-fold over 23 days. In contrast, the maspin plasmid-injected group developed tumors that averaged a 20-fold increase during the same period (p=0.0048, Fig. 4), demonstrating that maspin could indeed affect in vivo tumor progression in lung cancer.
Fig. 4.
The effect of maspin DNA transfer on the established tumor. A pCMV-maspin or control plasmid was given every other day (arrow) into the subcutaneous tumor that had been established after the injection of NCI-H157 cells (5×106 cells). The plasmid was administered as a mixture of 50 µg DNA in 50 µL PBS per injection. Nine mice were used for each group. p=0.0048, t-test comparing the control plasmid injected xenographs to the pCMV-maspin injected xenografts on day 23. Bars, SEM.
Discussion
Chemotherapeutic drugs induce apoptosis by affecting the cell-death or survival pathways. Thus, several ongoing clinical trials are currently under investigation to overcome drug resistance by modulation of apoptosis or cell survival (19). In the current study, we have identified maspin as a modulator of doxorubicin and etoposide susceptibility in NCI-H157 lung cancer cells, and we described several possible targets of maspin that might account for the chemosensitivity. Maspin has been implicated in apoptosis (7), as well as in metastasis (8-10). However, to the best of our knowledge, this is the first report indicating that the expression of maspin in lung cancer may play a role in modulating the cell survival pathway. Our data indicates that the decreased expression of maspin in a lung cancer cell line induces resistance to apoptosis. Thus, the loss of a maspin expression may denote a poor prognosis due to the high probability of resistance to therapy.
The exact mechanism of how maspin may modulate cell survival remains unknown. Whatever the mechanisms are, it is intriguing to note that maspin-mediated inhibition of cell death is different depending on the anticancer agents. To identify the putative targets of maspin that may account for the resistance to chemotherapy, a cDNA microarray analysis was performed on the RNA extracted from the tumors derived from the empty vector-transfected and maspin-transfected lung cancer cells. The array identified many transcriptional alterations, and most have no obvious connection to chemoresistance. However, the proteins involved in Akt signaling were recognized. Specifically, the expressions of PTEN and IGF2R were associated with the maspin expression. Considering those studies reporting that the Akt pathway inhibits apoptosis in cancer cells, the up-regulation of PTEN and the down-regulation of IGF2R may alleviate the survival of cancer cells. It has been suggested that Akt may function as an anti-apoptotic survival protein based on the observation that the inactivation of Akt induced cell death in several cancer cells (11). Therefore, the up-regulation of anti-apoptotic proteins through the activation of Akt could be a mechanism for inducing chemoresistance in maspin-lacking cancer cells, although this remains to be demonstrated.
Conclusion
Our study shows that maspin inhibits the survival pathway by inactivating Akt phosphorylation and this influences the response to cell death in lung cancer cells. Therefore, lung cancer cells lacking maspin would be resistant to chemotherapeutic drugs such as doxorubicin or etoposide, implying that treatment strategies based on the level of maspin might improve the efficacy of these chemotherapeutic drugs.
Footnotes
This work was supported by a grant (KRF-2006-312-C00416) from the Korean Research Foundation.
References
- 1.Schneider SS, Schick C, Fish KE, Miller E, Pena JC, Treter SD, et al. A serine proteinase inhibitor locus at 18q21.3 contains a tandem duplication of the human squamous cell carcinoma antigen gene. Proc Natl Acad Sci USA. 1995;92:3147–3151. doi: 10.1073/pnas.92.8.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheng S, Pemberton PA, Sager R. Production, purification, and characterization of recombinant maspin proteins. J Biol Chem. 1994;269:30988–30993. [PubMed] [Google Scholar]
- 3.McGowen R, Biliran H, Jr, Sager R, Sheng S. The surface of prostate carcinoma DU145 cells mediates the inhibition of urokinase-type plasminogen activator by maspin. Cancer Res. 2000;60:4771–4778. [PubMed] [Google Scholar]
- 4.Pemberton PA, Wong DT, Gibson HL, Kiefer MC, Fitzpatrick PA, Sager R, et al. The tumor suppressor maspin does not undergo the stressed to relaxed transition or inhibit trypsin-like serine proteases. Evidence that maspin is not a protease inhibitory serpin. J Biol Chem. 1995;270:15832–15837. doi: 10.1074/jbc.270.26.15832. [DOI] [PubMed] [Google Scholar]
- 5.Kim S, Han J, Kim J, Park C. Maspin expression is transactivated by p63 and is critical for the modulation of lung cancer progression. Cancer Res. 2004;64:6900–6905. doi: 10.1158/0008-5472.CAN-04-1657. [DOI] [PubMed] [Google Scholar]
- 6.Jang H, Nam ES, Yeom S, Lee KH, Son HJ, Park C. Maspin polymorphism associated with susceptibility to apoptosis and in vivo tumorigenesis. Int J Mol Med. 2008;22:333–338. [PubMed] [Google Scholar]
- 7.Liu J, Yin S, Reddy N, Spencer C, Sheng S. Bax mediates the apoptosis-sensitizing effect of maspin. Cancer Res. 2004;64:1703–1711. doi: 10.1158/0008-5472.can-03-2568. [DOI] [PubMed] [Google Scholar]
- 8.Zou Z, Anisowicz A, Hendrix MJ, Thor A, Neveu M, Sheng S, et al. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994;263:526–529. doi: 10.1126/science.8290962. [DOI] [PubMed] [Google Scholar]
- 9.Hendrix MJ. De-mystifying the mechanism(s) of maspin. Nat Med. 2000;6:374–376. doi: 10.1038/74624. [DOI] [PubMed] [Google Scholar]
- 10.Sheng S, Carey J, Seftor EA, Dias L, Hendrix MJ, Sager R. Maspin acts at the cell membrane to inhibit invasion and motility of mammary and prostatic cancer cells. Proc Natl Acad Sci USA. 1996;93:11669–11674. doi: 10.1073/pnas.93.21.11669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawlor MA, Alessi DR. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J Cell Sci. 2001;114:2903–2910. doi: 10.1242/jcs.114.16.2903. [DOI] [PubMed] [Google Scholar]
- 12.Kang HC, Kim IJ, Park JH, Shin Y, Ku JL, Jung MS, et al. Identification of genes with differential expression in acquired drug-resistant gastric cancer cells using high-density oligonucleotide microarrays. Clin Cancer Res. 2004;10:272–284. doi: 10.1158/1078-0432.ccr-1025-3. [DOI] [PubMed] [Google Scholar]
- 13.Moorehead RA, Singh G. Influence of the proto-oncogene c-fos on cisplatin sensitivity. Biochem Pharmacol. 2000;59:337–345. doi: 10.1016/s0006-2952(99)00333-0. [DOI] [PubMed] [Google Scholar]
- 14.Hettinga JV, Lemstra W, Meijer C, Los G, de Vries EG, Konings AW, et al. Heat-shock protein expression in cisplatin-sensitive and -resistant human tumor cells. Int J Cancer. 1996;67:800–807. doi: 10.1002/(SICI)1097-0215(19960917)67:6<800::AID-IJC8>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 15.Mizutani Y, Fukumoto M, Bonavida B, Yoshida O. Enhancement of sensitivity of urinary bladder tumor cells to cisplatin by c-myc antisense oligonucleotide. Cancer. 1994;74:2546–2554. doi: 10.1002/1097-0142(19941101)74:9<2546::aid-cncr2820740924>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 16.Beale PJ, Rogers P, Boxall F, Sharp SY, Kelland LR. BCL-2 family protein expression and platinum drug resistance in ovarian carcinoma. Br J Cancer. 2000;82:436–440. doi: 10.1054/bjoc.1999.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park C, Lee I, Kang WK. Lovastatin-induced E2F-1 modulation and its effect on gastric cancer cell death. Carcinogenesis. 2001;22:1727–1731. doi: 10.1093/carcin/22.10.1727. [DOI] [PubMed] [Google Scholar]
- 18.Jung HH, Lee JY, Kim JH, Ryu KJ, Kang SA, Park CH, et al. STAT1 and Nmi are downstream targets of Ets-1 transcription factor in MCF-7 human breast cancer cell. FEBS Letters. 2005;579:3941–3946. doi: 10.1016/j.febslet.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Nicholson DW. From bench to clinic with apoptosis-based therapeutic agents. Nature. 2000;407:810–816. doi: 10.1038/35037747. [DOI] [PubMed] [Google Scholar]