Abstract
Mechanosensory hair cells in the chick inner ear synapse onto afferent neurons of the statoacoustic ganglion (SAG). During development these neurons extend a central process to the brain and a peripheral process into one of eight sensory organs. A combination of cues, including chemoattractants and chemorepellents, direct otic axons to their peripheral targets. As a first step in evaluating the role of known axon guidance molecules, Slits and Robos, we examined expression of their transcripts in the chick inner ear from embryonic day 2–11 (Hamburger and Hamilton stages 14–37). Robo2 and slit2 are in migrating neuroblasts and the SAG, while both slits and robos are in the otic epithelium. We speculate that this family of signaling molecules may be involved in repulsion, first of otic neuroblasts and then of otic axons. Later our expression data revealed a potentially novel role for these molecules in maintaining sensory/nonsensory boundaries.
Keywords: auditory, axon guidance, chicken embryo, inner ear, robo, slit, vestibular
INTRODUCTION
In vertebrates the otic placode, which is a region of thickened epithelial cells adjacent to the hindbrain, gives rise to the inner ear. During development the otic placode invaginates to form the otic cup, which then closes to form the otocyst (Hilfer et al., 1989). Cells from the ventral wall of the otocyst migrate into the underlying mesenchyme (Carney and Silver, 1983), eventually forming the neurons of the eighth cranial ganglion that innervate the sensory hair cells of the inner ear. Thus the otic placode gives rise to the cells that make up the sensory and non-sensory tissues of the inner ear and also the associated afferent neurons.
The avian inner ear houses eight sensory organs, including seven vestibular organs and one auditory organ (Knowlton, 1967). The vestibular organs include three cristae, which detect angular acceleration, and four maculae, which detect linear acceleration. The cristae are located in the ampullae of the semicircular canals. The one auditory organ, the basilar papilla, shares the cochlear duct with the lagenar macula. The other 3 maculae are located in the utricular and saccular recesses.
The mechanosensory hair cells within each sensory organ contact the peripheral processes of bipolar afferent neurons whose cell bodies reside in cranial ganglia. The central process of each afferent extends to the brainstem nuclei. The auditory basilar papilla of the chicken connects to afferent neurons whose cell bodies reside in the auditory ganglion. All remaining sensory organs, with the exception of the lagenar macula, connect to vestibular afferent neurons whose cell bodies reside in the vestibular ganglion. Neurons dispersed along the medial edge of the auditory ganglion project to the distal tip of the cochlear duct to contact the lagenar macula (Fischer et al., 1994). In addition to their afferents, hair cells are innervated by efferent neurons whose cell bodies are located in the hindbrain pontine reticular formation and around the superior olive (Strutz and Schmidt, 1982; Code and Carr, 1994).
Relatively little is known about the decision points and cues that regulate the establishment of the peripheral projections of auditory and vestibular ganglion neurons. Current thought is that a combination of global and local cues guide vestibular and auditory axons to their targets (Rubel and Fritzsch, 2002). Several highly conserved families of axon guidance molecules, including Ephs/ephrins (Siddiqui and Cramer, 2005), semaphorins (Chilton and Guthrie, 2003), and Slits (Holmes and Niswander, 2001), have been detected in the chick inner ear during development. In EphB2 mutant mice, otic efferents are temporarily delayed and disoriented as they cross the midline enroute to the inner ear (Cowan et al., 2000). The semaphorin receptor, neuropilin1, plays an important role mediating a dorsal stop signal for vestibular afferents (Gu et al., 2003). Furthermore, neurotrophins have been shown to be involved in inner ear axon guidance and survival (Tessarollo et al., 2004). Erb2 (neuregulin receptor) and a member of the forkhead family of transcription factors (foxg1) also appear to be involved either directly or indirectly in guidance of otic axons (Morris et al., 2006; Pauley et al., 2006). Recently, several morphogens, best known for their roles in tissue patterning during early development, have been added to the growing list of molecules involved in axon guidance in other systems (Bovolenta, 2005; Charron and Tessier-Lavigne, 2005). Examples include fibroblast growth factors (FGF), sonic hedgehog (SHH), bone morphogenetic protein (BMP), and members of the Wnt family, which are all present in or near the otic tissue during peripheral axon outgrowth. Together these studies suggest that many different types of guidance cues and mechanisms could be working together to direct axons to their targets in the inner ear (for recent reviews see Pauley et al., 2005; Webber and Raz, 2006; Fekete and Campero, 2007).
Members of the Slit/Robo signaling family are known for their classical roles in axon guidance, yet these molecules are also involved in axon branching, axon elongation, axon fasciculation, and cell migration in a variety of vertebrate and invertebrate systems (reviewed by Brose and Tessier-Lavigne, 2000; Wong et al., 2002). Roundabout (Robo) proteins, conserved members of the Ig superfamily, were first characterized in Drosophila for their role in axon guidance at the central nervous system midline (Seeger et al., 1993; Kidd et al., 1998). Slit proteins, which are secreted by midline glia (Rothberg et al., 1990), were identified as midline repellents for Robo receptors (Kidd et al., 1999; Li et al., 1999). An excellent review of the role of Slit/Robo in commissural axon pathfinding can be found in Dickson and Gilestro (2006).
The Slit protein is proteolytically cleaved, resulting in several isoforms that have different effects on neurons and elicit specific responses depending on the axonal population examined (Wang et al., 1999; Nguyen Ba-Charvet et al., 2001; reviewed by Wong et al., 2002). In vitro there is promiscuous binding of Slits to different Robos in mammals and Drosophila (Brose et al., 1999; Yuan et al., 1999; Rajagopalan et al., 2000; Marillat et al., 2002). Robo availability in Drosophila is controlled by the protein commissureless (Comm), which is expressed on the surface of midline cells and serves to downregulate Robo by targeting it to the lysosome (Kidd et al., 1998; Huber et al., 2003). A vertebrate homologue of Comm has not been identified. However, recent data suggests that a third member of the Robo family, named Robo3/Rig1, may function to regulate Robo responsiveness to repulsion at the CNS midline (Sabatier et al., 2004). In humans, Robo1 mutations are linked to dyslexia (Hannula-Jouppi et al., 2005) and Robo3 mutations lead to horizontal gaze with progressive scoliosis (Jen et al., 2004).
In the chicken, two Robo homologues and three Slits have been identified (Bashaw and Goodman, 1999; Li et al., 1999; Vargesson et al., 2001). A previous study showed the presence of slit2 and slit3 in the developing chick otocyst by whole mount in situ hybridization on embryonic days (E) 3.5–4 (Hamburger and Hamilton stages 17–24) (Holmes and Niswander, 2001), but the detailed expression pattern in the ear was incomplete.
As a first step in evaluating the role of Slit/Robo interactions during otic axon outgrowth, we studied their spatial and temporal expression in the embryonic chick inner ear. We have used in situ hybridization of serial cryosections to detect slit1, slit2, slit3, robo1 and robo2 transcripts from E2-11. These expression patterns were compared to Bmp4, which was used as a marker for prosensory patches in the inner ear (Wu and Oh, 1996). Axonal trajectories were labeled with a neurofilament antibody.
METHODS
Embryos
White Leghorn chicken embryos were immersion fixed in 4% paraformaldehyde in phosphate buffered saline (PBS) at 4°C overnight, washed in PBS, dehydrated in 10% followed by 15% sucrose in PBS, embedded in Tissue Freezing Media (Triangle Biomedical Sciences) and frozen. Transverse or horizontal sections of 10–15µm thickness were collected onto Superfrost Plus slides (Fisher Scientific) and stored at −80°C until processed.
Staging
Embryos were staged according to Hamburger and Hamilton (1951). For the purposes of discussing probe expression in the ear we have divided the stages into the following groups: stages 14–15 (E2); stages 16–19 (E2.5–3); stages 20–21 (E3–3.5); stages 22–25 (E3.5–4.5); stages 27–30 (E5–6.5); stages 31–37 (E7–11). Slit and robo analysis was based on the following number of tests (individual in situ run of a particular probe on a given embryo): stages 14–15, 22 tests; stages 16–19, 34 tests; stages 20–21, 20 tests; stages 22–25, 30 tests; stages 27–30, 50 tests; stages 31–37, 36 tests.
RNA Probes
Chicken cDNA partial sequences for robo1, robo2, slit1, slit2, slit3 (Vargesson et al., 2001) and full length Bmp4 (Roberts et al., 1995) were used to generate digoxigenin-labeled sense and antisense riboprobes. All sense probes were clean with the exception of slit1-sense, which gave non-specific background staining in 3 out of 6 embryos tested.
In-Situ Hybridization
In situ hybridizations were performed on frozen sections as previously described (Sanchez-Calderon et al., 2004). Briefly, sections were post-fixed in 4 % paraformaldehyde in PBS, treated with Proteinase K (1µg/ml) for 10 minutes, and incubated overnight in probe at 72°C. Probes were detected using anti-digoxigenin-alkaline phosphatase (1:3500; Roche) and a color reaction was performed with BM Purple alkaline phosphatase substrate (Roche).
Dual-label Immunohistochemistry
Following in situ hybridization, sections were post-fixed in 4% paraformaldehyde in PBS, washed in PBS, and blocked in 10% calf serum/ 0.05% TritonX 100/ 0.05% sodium azide in PBS. Specimens were labeled with anti-neurofilament antibody (mouse monoclonal 3A10, 1:20 of culture supernatant, Developmental Studies Hybridoma Bank (DSHB), University of Iowa) in blocking solution at 4°C overnight. In some cases, adjacent sections were labeled with anti-GATA-3 (mouse monoclonal HG3-31, 1:100, Santa Cruz), anti-human HuC/D (mouse moloclonal 16A11, 1:50, Invitrogen) or anti-Islet1 (mouse monoclonal 40.2D6, 1:100 of culture supernatant, DSHB). Anti-neurofilament and anti-GATA-3 were detected with a biotinylated anti-mouse IgG secondary antibody (1:250; Vector Laboratories) followed by standard ABC kit (Vector Laboratories) and diaminobenzidine histochemistry. HuC/D and Islet1 antibodies were detected with AlexaFluor anti-mouse 586 IgG2b and AlexaFluor anti-mouse 488 IgG1 (Invitrogen) respectively. A SPOT Flex camera mounted on a Nikon E800 photomicroscope was used to capture images of sections.
RESULTS
Robo probes were reliable, with strong signals visible in parts of the brain that were used as positive controls. In comparing the gene expression patterns of slits1, -2, and -3, we find the slit2 probe to be most reliable in showing a high signal-to-noise. Slit1 has the highest background and slit3 often has the lowest signal. We can be confident that the slit probes are not cross-reacting because each has a distinctly different expression pattern in the brain. In most samples, probes expressed in the inner ear appear strongest in the apical portions of the epithelium. We begin by describing expression in the otic ganglia at all stages. This is followed by inner ear expression in relation to sensory domains, with results divided into five stages. The last section is focused on the endolymphatic duct.
Statoacoustic Ganglion
Otic neuroblasts delaminate from the antero-ventral otocyst and migrate anteriorly and medially to form the SAG (Hemond and Morest, 1991). During these stages (14–21), when neuroblasts are migrating and gangliogenesis is occurring, robo2 and slit2 show the most distinct expression patterns in the neural lineage. As the neurons differentiate, robo2 expression predominates and is therefore the best candidate for receiving environmental Slit signals as guidance cues. Also evident during the stages of axon outgrowth is a curious subset of cells in the ganglion expressing slit2, followed by a later expression of slit1. We begin by describing expression of the transcripts for the receptors, followed by the ligands.
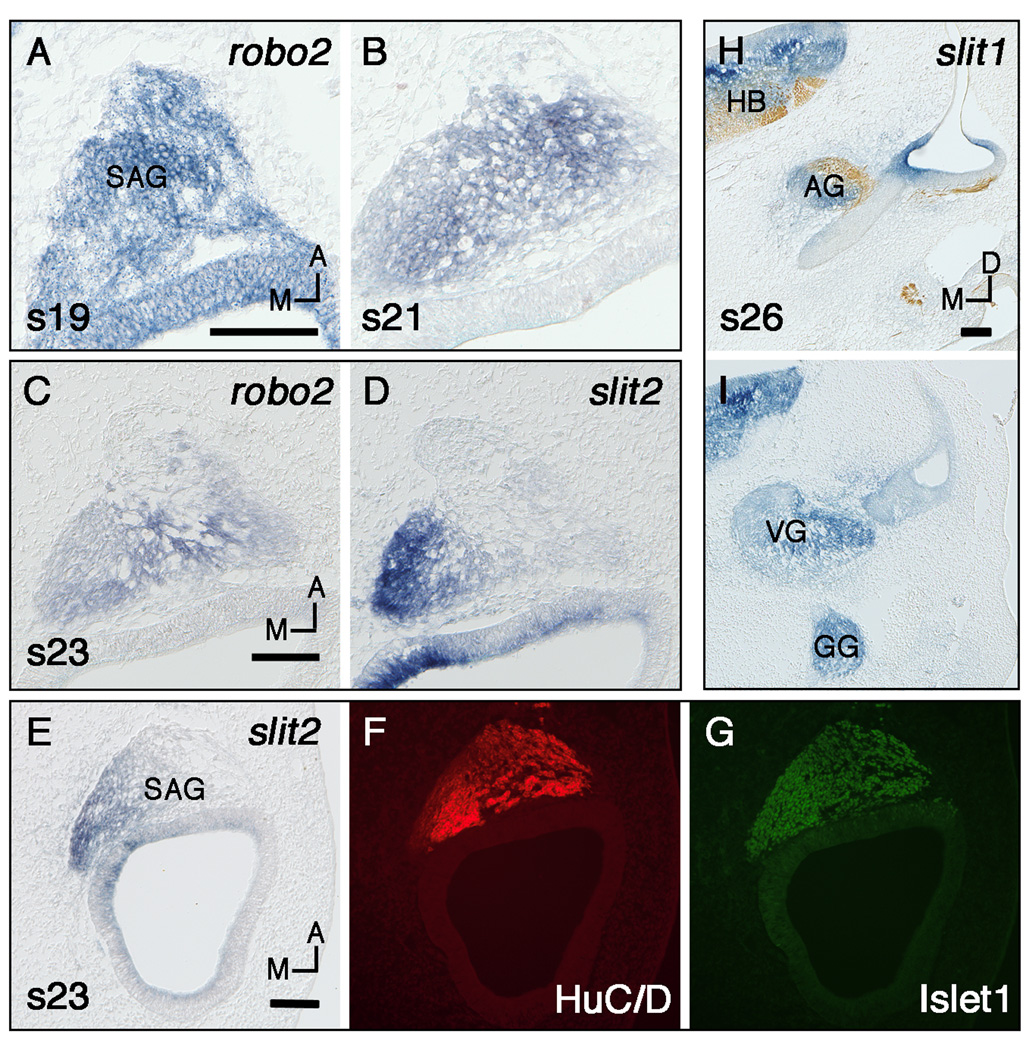
At stage 15, robo2 is expressed in the migrating neuroblasts and SAG; this expression is more distinct at stages 16–19 (Fig. 1A, shown at stage 19). Expression continues in the young SAG at stages 21–23 (Fig. 1B, 1C). Both auditory and vestibular ganglia continue to show robust expression of robo2 for a prolonged period (for an example of late expression, see the auditory ganglion in Fig. 2R). Ganglion expression of robo2 is not downregulated until stage 30.
Fig. 1.
Expression of slits and robos in the statoacoustic ganglion (SAG) determined by in situ hybridization on frozen sections in either the horizontal (A–G) or transverse plane (H–I). Section H is double labeled with a neurofilament antibody. A,B: Robo2 expression in the SAG at stages 19 (A) and 21 (B). C,D: Stage 23 serial sections labeled with robo2 (C) and slit2 (D). E–G: Stage 23 serial sections labeled with slit2 (E) or double labeled with HuC/D and Islet1 antibodies (F,G respectively). H,I: slit1 expression in the auditory (H), vestibular (I), and geniculate ganglia (I) at stage 26. Scale Bars, 100µm. Orientation: A, anterior; D, dorsal; M, medial. Abbreviations: AG, auditory ganglion; GG, geniculate ganglion; HB, hindbrain; VG, vestibular ganglion.
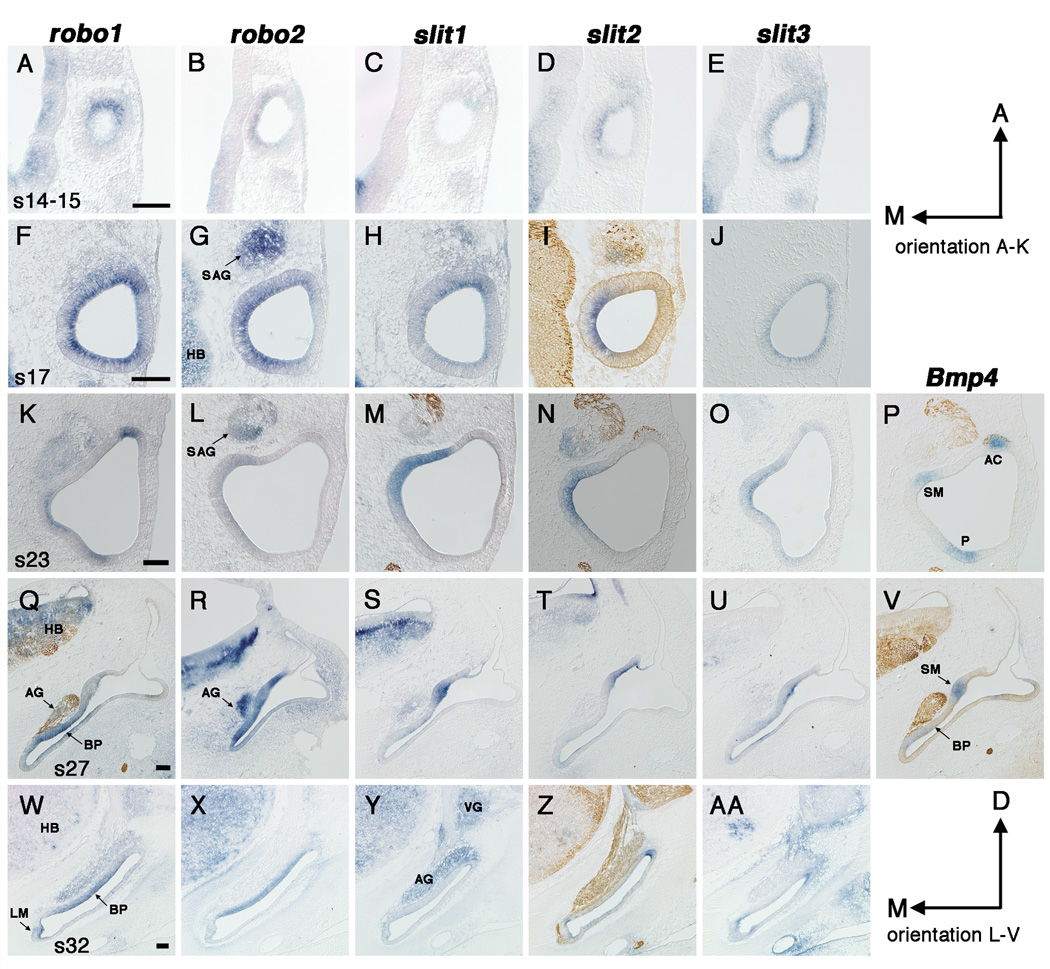
Fig. 2.
Expression of slits and robos in the developing chick inner ear from stage 14 (E2) to stage 32 (E7.5). In situ hybridizations on frozen sections in the horizontal (A–P) or transverse plane (Q–AA). Some sections (I,M,N,P,Q,V,Z) are double labeled with a neurofilament antibody. Prosensory regions are identified by Bmp4 expression (P,V). A–E: Stage 14 (A–C) or stage 15 (D,E), A,C and D,E are serial sections. F–J: Stage 17, F–H are serial sections. K–P: Stage 23, L,M and N,P are serial sections. Q–V: Stage 27, S–V are serial sections. W–AA: Stage 32, W,X,Z and Y,AA are serial sections. Scale Bars, 100µm. Orientation: A, anterior; D, dorsal; M, medial. Abbreviations: AC, anterior crista; AG, auditory ganglion; BP, basilar papilla; E, embryonic day; LM, lagenar macula; HB, hindbrain; P, posterior Bmp4-positive focus; SAG, statoacoustic ganglion; SM, saccular macula; VG, vestibular ganglion.
Slit2 expression appears in a subset of cells along the posteromedial edge of the SAG at stage 15; expression strengthens and persists in subsequent stages (Fig. 1D, shown at stage 23). At stages 27–34 this subset of slit2-expressing cells persists near the posteromedial edge of the vestibular ganglion. This location resembles that occupied by the neural crest-derived neurons of the proximal or root ganglion of cranial nerve VII (D'Amico-Martel and Noden, 1983). During stages 35–37 the robust slit2 expression at the edge of the vestibular ganglion disappears.
To ascertain whether the slit2-expressing cells of the SAG are developing neurons, sections were double-labeled with HuC/D and Islet1 antibodies and compared to adjacent sections labeled with slit2 probe at stage 23. Results show that the Islet1 domain is larger but completely overlaps with the slit2 domain (compare Fig. 1E to 1G) while the HuC/D domain does not always totally overlap with the slit2 domain (compare Fig. 1E to 1F).
Slit1 expression becomes obvious in the SAG at stage 25–26 (Fig. 1H,I); this increase in expression level is seen after the SAG has segregated into its two components, the vestibular ganglion and cochleolagenar (here called auditory) ganglion (D'Amico-Martel and Noden, 1983). Expression of slit1 in both the auditory and vestibular ganglia is present until at least stage 32 (Fig. 2Y).
Otic cup stages 14–15
During stages 14–15 the otic cup is deepening and beginning to close (Brigande et al., 2000), while at the same time neuroblasts are starting to delaminate from the ventral region of the otic epithelium. The most distinct expression of all 5 probes is that of robo1. It appears as a small focus in the anteroventral otic cup at stage 14 (Fig. 2A); this corresponds to the approximate position of neuroblast delamination. By comparison, robo2 is not as restricted as robo1 at stage 14; instead robo2 is expressed along the entire medial wall (Fig. 2B). Slit1 is confined to a small antero-ventro-lateral domain at stages 14 (Fig. 2C), however this domain is extremely weak compared to slit1 expression in the brain. Slit2 expression is first detected in the ear at stage 15. It is restricted to the medial otic cup as soon as it appears (Fig. 2D). Distinct slit3 expression also appears in the ear at stage 15. Slit3 is expressed in the entire otic epithelium and is strongest in the ventral ear (Fig. 2E).
Otocyst stages 16–19
At stage 16 (51–56 hours), the otic cup is almost completely closed and by stage 17 otic cup closure leads to the formation of the otocyst (Brigande et al., 2000). During this time neuroblast migration from the ventral otocyst is at a peak (Hemond and Morest, 1991). At stage 16, robo1 appears to be upregulated and is now expressed throughout the entire otocyst. Yet, as early as stage 17 expression begins to weaken in the lateral wall of the otocyst (Fig. 2F). Robo2 appears to upregulate at stages 16–19. It continues to show asymmetry in expression, being weaker in the lateral wall (Fig. 2G).
At stages 16–19, slit1 expression has broadened and it now appears to be expressed in the entire otocyst, sometimes showing stronger expression anteriorly; this expression is still weak compared to that in the brain. Slit2 has the most restricted domain in comparison to either the robos or the other slits, and continues to be confined to the medial quadrant from stages 16–19 (Fig. 2I). Slit3 remains expressed in the entire otocyst at these stages (Fig. 2J).
Prosensory formation stages 20–21
We use a Bmp4 probe to map the incipient sensory organs prior to their overt differentiation. At stage 19 Bmp4 expression identifies two prosensory foci; one anterior and one posterior (Wu and Oh, 1996). The anterior domain will give rise to the anterior cristae while the posterior domain will give rise to the posterior cristae, basilar papilla, lagena macula and macula neglecta. By stage 20, Bmp4 expression marks a third focus in the medial otocyst that will become the saccular macula.
Expression patterns for stages 20–21 (data not shown) are very similar to those of stages 22–23 described below and shown in Fig. 2K–P. At stages 20–21, robo1 expression is now associated with the Bmp4-positive prosensory regions. It becomes restricted to the anterior Bmp4-positive focus, while it encompasses the posterior Bmp4-positive focus, but is larger than it. Robos are expressed in the ventral otocyst in what may become the basilar papilla/lagena macula.
Beginning at stage 20, all three slit probes show similar expression domains except where noted. Slits are restricted to the medial otocyst where they encompass at least part of the future saccular macula. This expression pattern appears strongest at the base of the endolymphatic duct, a location that is presumed to be nonsensory and that also expresses robos. Slits are expressed in the ventral-most otocyst along with robos at these stages.
Stages 22–25
The lateral crista and utricular macula are identifiable with Bmp4 by stage 24 (Wu and Oh, 1996). From stages 22–25, robo1 continues to overlap with all Bmp4 expression domains, yet in the medial ear its expression is not as focused as Bmp4 (compare Fig. 2K with 2P). In contrast, robo2 expression is strongest in the medial ear (Fig. 2L) and begins to upregulate in the cristae and basilar papilla around stage 25.
Slit1 and slit2 continue their robust expression in and above the saccular macula in the medial ear (Fig. 2M,N). By stage 25, weaker expression of both slits extends anteriorly to encompass the newly-formed utricular macula primordium (data not shown). At stage 23, slit3 expression is broad ventrally where it encompasses the entire cochlear duct (data not shown). In the central ear this slit3 domain extends dorsally along the medial wall (Fig. 2O), where it overlaps with slit1 and slit2. In toto, the slit3 domain may encompass a number of prosensory patches but is not restricted to them.
Stages 27–30
At stage 27, robo1 is most strongly expressed in all of the Bmp4-positive sensory foci (shown in the basilar papilla, Fig. 2Q). In the stage 29 cochlear duct, we can now detect a separation of the two sensory organs, the lagenar macula and the basilar papilla, based on their expression of robo1. When developed for a longer time, robo1 is also present in the lateral wall of the cochlear duct, with a sharp border where the cochlea meets the saccular chamber. Robo2 is primarily expressed along the medial wall of the cochlear duct, including the regions that will give rise to the basilar papilla and saccular macula (Fig. 2R).
The medial (saccule) expression domain of slits-1, -2 and –3 is still extremely pronounced at stage 27 (compare Fig. 2S–U to Fig. 2V). For the first time, an upregulation of all three slits in the flanking nonsensory regions of the other sensory organs becomes apparent. Over the next few stages, this pattern becomes increasingly obvious with the strongest probe, slit2. Its prosensory expression diminishes (with the exception of the saccule) and expression flanking auditory and vestibular sensory patches strengthens. In the cochlear duct, slit expression is present in the basilar papilla, but appears much stronger in the flanking nonsensory territories along both neural and abneural edges (Fig. 3C).
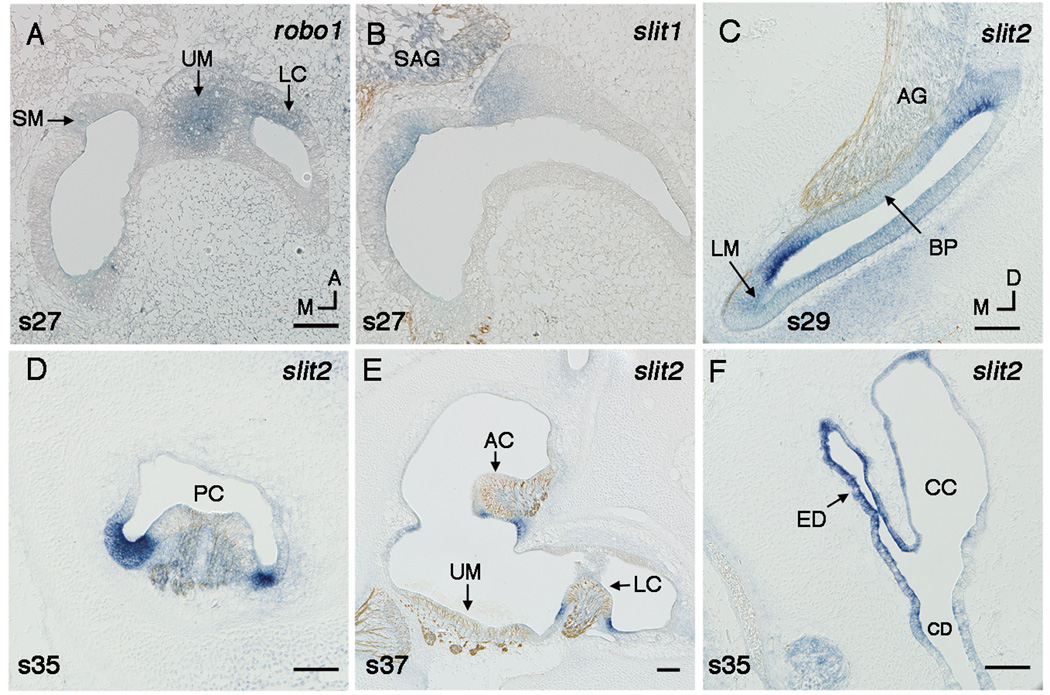
Fig. 3.
A,B: Robo1 (A) and slit1 (B) in the utricular macula at stage 27 shown by in situ hybridzation on horizonal sections. C–E: Slit2 flanks sensory territories shown in transverse sections. Sections (B,C,E) are double labeled with a neurofilament antibody. Slit2 flanks the auditory basilar papilla (BP) at stage 29 (C). Slit2 flanks the vestibular sensory epithelia, including the posterior crista (PC) at stage 35 (D), and the anterior crista (AC), and the lateral crista (LC) at stage 37 (E). Slit2 is expressed in the endolymphatic duct (ED) at stage 35 (F). Scale Bars, 100µm. Orientation: A, anterior; D, dorsal; M, medial. Abbreviations: CC, common crus; CD, cochlear duct; SM, saccular macula; SAG, statoacoustic ganglion; UM, utricular macula.
There is one additional intriguing expression domain for slit1 in the utricular macula. At stage 27, slit1 appears to be restricted to the medial half of the utricular macula. Identifying the edges of the utricular macula at this stage was not reliable using Bmp4 expression or neurofilament labeling. We therefore compared slit1 (Fig. 3B) expression to robo1 (Fig. 3A), which is expressed in all of the sensory organs at this stage. Slit1 overlaps with the medial domain of robo1 in the utricular macula, suggesting it is not expressed in the entire sensory organ. The abrupt lateral edge of the domain could reflect a boundary between neurogenic (medial) and non-neurogenic (lateral) utricular domains described in this approximate position in the mouse (Raft et al., 2007). Alternatively, it could be marking the location of the future striola, thus placing Slit1 in a position to differentially regulate axonal innervation on each side of the striola. We were unable to identify the exact location of the striola with an anti-Gata3 antibody, as the striola is not immunopositive until stage 38 (Lillevali et al., 2007).
Stages 31–37
During these stages, robo1 continues to be expressed in sensory regions, showing the most robust expression in the basilar papilla (Fig. 2W). Robo2 remains expressed in the basilar papilla (Fig. 2X), while weaker expression is seen in the maculae. Weak slit1 expression can sometimes be seen in the saccule (data not shown), but overall expression in the otic epithelium during these stages is difficult to distinguish from background. Slit2 expression flanking auditory and vestibular sensory regions strengthens at these stages and continues through stage 37, the latest stage examined (Fig. 2Z and Fig. 3C–E). Slit2 also remains detectable in the saccular macula, but becomes weaker by stage 37 (data not shown). Slit3 expression is weak at these stages, but appears to mimic slit2 in flanking the sensory territories; this pattern is most obvious in the cochlear duct (Fig. 2AA). After stage 30, slit3 expression is not seen in the saccular macula.
Endolymphatic Duct
Slits are obviously expressed in the endolymphatic duct and sac during middle and late stages. Slit expression first appears in the endolymphatic duct at stage 27. Slit2 expression (Fig. 3F) is stronger than slit1 and slit3 and persists through stage 37. Robos are also expressed in the endolymphatic duct and sac from stage 27 onwards (data not shown).
DISCUSSION
Using in situ hybridization, we have described the expression of slits and robos in the chick inner ear from stages 14–37 (E2-11). Probe specificity has been confirmed by comparing brainstem and sense probe expression. Our data, which show expression of slits and robos at all stages examined, lead us to speculate that these molecules may have multiple functions during inner ear development.
We first consider whether Slits/Robos are involved in neuronal migration during ear development. In mammals, Slits are known to play a role in the migration of neurons, including neuronal progenitors from the anterior subventricular zone and GABAergic interneurons from the lateral ganglionic eminence (reviewed by Wong et al., 2002). In the chick, neuroblast migration from the ventral otocyst begins at stage 14 (Hemond and Morest, 1991). At this time transcripts for both ligands and receptors are present in the otic epithelium, albeit weakly. This observation leads us to speculate that Slit/Robo signaling may be involved in pushing neuroblasts out of the otocyst, although the data are far from compelling. Once the neuroblasts have left the epithelium, their upregulation of robo2 at stages 15–16 could prime them to use Slit signaling for more classical axon guidance, as discussed next.
Outgrowth of otic axons from the SAG is underway on E2.5 (Kuratani et al., 1988). At stage 19 (68–72 hours), fibers first reach the anterior crista (Hemond and Morest, 1991). Vestibular afferents invade the otic sensory epithelium on E4 (Von Bartheld et al., 1991), while the majority of auditory afferents invade on E5–7 (Whitehead and Morest, 1985). Approximately two days later, on E6 (vestibular) and E8–9 (auditory), otic afferents synapse on hair cells (Ginzberg and Gilula, 1980; Whitehead and Morest, 1985) .
Based on our expression data, we speculate that Slits/Robos may be guiding different modalities during specific stages of inner ear development. Presumably, the first axons to emerge from the ganglion at stage 17 will become the vestibular cristae afferents. At this time, nearby slit-expressing cells in the anterior and medial otic epithelium are in a position to repel the pioneer robo-expressing axons, assuming the transcripts reflect protein expression. This repulsion could divert the axons laterally and posteriorly to innervate the more distant sensory organs that are specified first, particularly the cristae. Later emerging vestibular afferents would then innervate the more proximal organs that are specified shortly thereafter, such as the maculae. Yet, since slit expression in the medial otocyst continues well after the peripheral processes of otic axons are finished pathfinding, we must consider how the slit-expressing saccular macula ever becomes innervated in this scenario. One possibility is that axons innervating the saccular macula are unresponsive to Slit because they either fail to express, or downregulate Robo proteins.
We consider a second role for Slit/Robo signaling during otic axon guidance a little later in development (E5–7), when auditory (Whitehead and Morest, 1985) and (presumably) lagenar afferents are pathfinding in the periphery. Now, slit expression in the medial otocyst may act to keep robo-expressing cochlear axons from projecting into innervated vestibular sensory organs, forcing them ventrally towards the cochlear duct.
Yet a third role for Slit/Robo signaling from E5 onwards is suggested by our data showing slit2 flanking the auditory and vestibular sensory organs. At these stages, we speculate that Slit2 could channel auditory or late-arriving vestibular axons into the sensory epithelium. The approximate 2-day waiting period between the time afferents arrive at their peripheral targets, and when they synapse on hair cells (Ginzberg and Gilula, 1980; Whitehead and Morest, 1985), leads us to also consider that Slit2 flanking the sensory epithelium keeps otic axons out of the non-sensory territories as they wait to synapse with hair cells.
Staying with the same general hypothesis, that Slits in the otocyst repel Robo2-expressing otic axons, yet another possibility is that Slits repel the central processes of SAG neurons, forcing them away from the periphery and towards the hindbrain. A recent study shows that Slit/Robo signaling has dual functions in sensory neurons; one stimulates branching in their peripheral processes and another induces a repellent response in their central processes (Ma and Tessier-Lavigne, 2007). The versatility of Slit/Robo within one sensory system strengthens the idea that this signaling family could play multiple roles in the inner ear during development. However, it seems unlikely that Slits and Robos are involved in peripheral axon branching in the inner ear since slits are not robustly expressed in the sensory epithelium (except for the saccular macula) at the correct times.
Additional intriguing expression patterns, such as slit2 along the edge of the SAG and robos in the otic sensory epithelium, lead to even further questions about the roles of Slits/Robos during inner ear development. One plausible function for slit2 in the vicinity of the SAG may be to segregate different populations of neurons (for example, SAG vs. facial or auditory vs. vestibular). A related idea is that Slits in the otocyst repel non-otic axons that are navigating in the vicinity of the otocyst. A role for Robos in the sensory epithelium is harder to reconcile with the known functions of Slits/Robos in other systems. However, the apposition of Slits and Robos leads us to hypothesize a novel role for these molecules in the maintenance of sensory/non-sensory boundaries. Specifically, this arrangement could limit or prevent cell mixing as the ear continues its substantial growth during the second half of embryogenesis (Bissonnette and Fekete, 1996). This would be similar to the dual roles of Eph-ephrin signaling in both axon guidance and hindbrain boundary formation (reviewed by Wilkinson, 2001). These hypotheses, along with the others stated above, await experimental investigation.
In summary, the Slit/Robo signaling family is known to have multiple functions during vertebrate and invertebrate development (Brose and Tessier-Lavigne, 2000; Wong et al., 2002). As a first step in evaluating the role of Slits and Robos during chicken ear development we examined their transcripts from E2-11. Our results suggest that slits and robos are expressed at the correct time and location to potentially play multiple roles during inner ear development. We speculate that Slit and Robo interactions could be involved in neuroblast migration from the otocyst, axon repulsion of otic or non-otic neurons, and/or the maintenance of sensory-nonsensory boundaries. Yet regardless of their purpose, the prolonged and very specific expression of slit and robo transcripts in the ear strongly suggests the involvement of the proteins in ear development. Studies are now needed to determine their functional role in the inner ear.
ACKNOWLEDGEMENTS
The authors would like to thank Ed Laufer for the slit and robo plasmids, Doris Wu for the Bmp4 RNA probe, Ulrike Sienknecht for technical advice and Pei Xin Lim and Deborah Biesemeier for technical assistance.
Grant sponsor: NIH DC002756
REFERENCES
- Bashaw GJ, Goodman CS. Chimeric axon guidance receptors: the cytoplasmic domains of slit and netrin receptors specify attraction versus repulsion. Cell. 1999;97:917–926. doi: 10.1016/s0092-8674(00)80803-x. [DOI] [PubMed] [Google Scholar]
- Bissonnette JP, Fekete DM. Standard atlas of the gross anatomy of the developing inner ear of the chicken. J Comp Neurol. 1996;368:620–630. doi: 10.1002/(SICI)1096-9861(19960513)368:4<620::AID-CNE12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Bovolenta P. Morphogen signaling at the vertebrate growth cone: a few cases or a general strategy? J Neurobiol. 2005;64:405–416. doi: 10.1002/neu.20161. [DOI] [PubMed] [Google Scholar]
- Brigande JV, Iten LE, Fekete DM. A fate map of chick otic cup closure reveals lineage boundaries in the dorsal otocyst. Dev Biol. 2000;227:256–270. doi: 10.1006/dbio.2000.9914. [DOI] [PubMed] [Google Scholar]
- Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell. 1999;96:795–806. doi: 10.1016/s0092-8674(00)80590-5. [DOI] [PubMed] [Google Scholar]
- Brose K, Tessier-Lavigne M. Slit proteins: key regulators of axon guidance, axonal branching, and cell migration. Curr Opin Neurobiol. 2000;10:95–102. doi: 10.1016/s0959-4388(99)00066-5. [DOI] [PubMed] [Google Scholar]
- Carney PR, Silver J. Studies on cell migration and axon guidance in the developing distal auditory system of the mouse. J Comp Neurol. 1983;215:359–369. doi: 10.1002/cne.902150402. [DOI] [PubMed] [Google Scholar]
- Charron F, Tessier-Lavigne M. Novel brain wiring functions for classical morphogens: a role as graded positional cues in axon guidance. Development. 2005;132:2251–2262. doi: 10.1242/dev.01830. [DOI] [PubMed] [Google Scholar]
- Chilton JK, Guthrie S. Cranial expression of class 3 secreted semaphorins and their neuropilin receptors. Dev Dyn. 2003;228:726–733. doi: 10.1002/dvdy.10396. [DOI] [PubMed] [Google Scholar]
- Code RA, Carr CE. Choline acetyltransferase-immunoreactive cochlear efferent neurons in the chick auditory brainstem. J Comp Neurol. 1994;340:161–173. doi: 10.1002/cne.903400203. [DOI] [PubMed] [Google Scholar]
- Cowan CA, Yokoyama N, Bianchi LM, Henkemeyer M, Fritzsch B. EphB2 guides axons at the midline and is necessary for normal vestibular function. Neuron. 2000;26:417–430. doi: 10.1016/s0896-6273(00)81174-5. [DOI] [PubMed] [Google Scholar]
- D'Amico-Martel A, Noden DM. Contributions of placodal and neural crest cells to avian cranial peripheral ganglia. Am J Anat. 1983;166:445–468. doi: 10.1002/aja.1001660406. [DOI] [PubMed] [Google Scholar]
- Dickson BJ, Gilestro GF. Regulation of commissural axon pathfinding by slit and its Robo receptors. Annu Rev Cell Dev Biol. 2006;22:651–675. doi: 10.1146/annurev.cellbio.21.090704.151234. [DOI] [PubMed] [Google Scholar]
- Fekete DM, Campero AM. Axon guidance in the inner ear. Int J Dev Biol. 2007;51:549–556. doi: 10.1387/ijdb.072341df. [DOI] [PubMed] [Google Scholar]
- Fischer FP, Eisensamer B, Manley GA. Cochlear and lagenar ganglia of the chicken. J Morphol. 1994;220:71–83. doi: 10.1002/jmor.1052200107. [DOI] [PubMed] [Google Scholar]
- Ginzberg RD, Gilula NB. Synaptogenesis in the vestibular sensory epithelium of the chick embryo. J Neurocytol. 1980;9:405–424. doi: 10.1007/BF01181545. [DOI] [PubMed] [Google Scholar]
- Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, Kolodkin AL, Ginty DD. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell. 2003;5:45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphology. 1951;88:49–91. [PubMed] [Google Scholar]
- Hannula-Jouppi K, Kaminen-Ahola N, Taipale M, Eklund R, Nopola-Hemmi J, Kaariainen H, Kere J. The axon guidance receptor gene ROBO1 is a candidate gene for developmental dyslexia. PLoS Genet. 2005;1:e50. doi: 10.1371/journal.pgen.0010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemond SG, Morest DK. Ganglion formation from the otic placode and the otic crest in the chick embryo: mitosis, migration, and the basal lamina. Anat Embryol (Berl) 1991;184:1–13. doi: 10.1007/BF01744256. [DOI] [PubMed] [Google Scholar]
- Hilfer SR, Esteves RA, Sanzo JF. Invagination of the otic placode: normal development and experimental manipulation. J Exp Zool. 1989;251:253–264. doi: 10.1002/jez.1402510213. [DOI] [PubMed] [Google Scholar]
- Holmes G, Niswander L. Expression of slit-2 and slit-3 during chick development. Dev Dyn. 2001;222:301–307. doi: 10.1002/dvdy.1182. [DOI] [PubMed] [Google Scholar]
- Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu Rev Neurosci. 2003;26:509–563. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- Jen JC, Chan WM, Bosley TM, Wan J, Carr JR, Rub U, Shattuck D, Salamon G, Kudo LC, Ou J, Lin DD, Salih MA, Kansu T, Al Dhalaan H, Al Zayed Z, MacDonald DB, Stigsby B, Plaitakis A, Dretakis EK, Gottlob I, Pieh C, Traboulsi EI, Wang Q, Wang L, Andrews C, Yamada K, Demer JL, Karim S, Alger JR, Geschwind DH, Deller T, Sicotte NL, Nelson SF, Baloh RW, Engle EC. Mutations in a human ROBO gene disrupt hindbrain axon pathway crossing and morphogenesis. Science. 2004;304:1509–1513. doi: 10.1126/science.1096437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the robo receptor in Drosophila. Cell. 1999;96:785–794. doi: 10.1016/s0092-8674(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Kidd T, Brose K, Mitchell KJ, Fetter RD, Tessier-Lavigne M, Goodman CS, Tear G. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell. 1998;92:205–215. doi: 10.1016/s0092-8674(00)80915-0. [DOI] [PubMed] [Google Scholar]
- Knowlton VY. Correlation of the development of membranous and bony labyrinths, acoustic ganglia, nerves, and brain centers in the chick embryo. J Morphol. 1967;121:179–208. [Google Scholar]
- Kuratani S, Tanaka S, Ishikawa Y, Zukeran C. Early development of the facial nerve in the chick embryo with special reference to the development of the chorda tympani. Am J Anat. 1988;182:169–182. doi: 10.1002/aja.1001820207. [DOI] [PubMed] [Google Scholar]
- Li HS, Chen JH, Wu W, Fagaly T, Zhou L, Yuan W, Dupuis S, Jiang ZH, Nash W, Gick C, Ornitz DM, Wu JY, Rao Y. Vertebrate slit, a secreted ligand for the transmembrane protein roundabout, is a repellent for olfactory bulb axons. Cell. 1999;96:807–818. doi: 10.1016/s0092-8674(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Lillevali K, Haugas M, Pituello F, Salminen M. Comparative analysis of Gata3 and Gata2 expression during chicken inner ear development. Dev Dyn. 2007;236:306–313. doi: 10.1002/dvdy.21011. [DOI] [PubMed] [Google Scholar]
- Ma L, Tessier-Lavigne M. Dual branch-promoting and branch-repelling actions of Slit/Robo signaling on peripheral and central branches of developing sensory axons. J Neurosci. 2007;27:6843–6851. doi: 10.1523/JNEUROSCI.1479-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marillat V, Cases O, Nguyen-Ba-Charvet KT, Tessier-Lavigne M, Sotelo C, Chedotal A. Spatiotemporal expression patterns of slit and robo genes in the rat brain. J Comp Neurol. 2002;442:130–155. doi: 10.1002/cne.10068. [DOI] [PubMed] [Google Scholar]
- Morris JK, Maklad A, Hansen LA, Feng F, Sorensen C, Lee KF, Macklin WB, Fritzsch B. A disorganized innervation of the inner ear persists in the absence of ErbB2. Brain Res. 2006;1091:186–199. doi: 10.1016/j.brainres.2006.02.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Ba-Charvet KT, Brose K, Ma L, Wang KH, Marillat V, Sotelo C, Tessier-Lavigne M, Chedotal A. Diversity and specificity of actions of Slit2 proteolytic fragments in axon guidance. J Neurosci. 2001;21:4281–4289. doi: 10.1523/JNEUROSCI.21-12-04281.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauley S, Lai E, Fritzsch B. Foxg1 is required for morphogenesis and histogenesis of the mammalian inner ear. Dev Dyn. 2006 doi: 10.1002/dvdy.20839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauley S, Matei V, Beisel K, Fritzsch B. Wiring the ear to the brain: the molecular basis of neurosensory development, differentiation, and survival. In: Kelley MW, Wu DK, Popper AN, Fay RR, editors. Development of the Inner Ear. New York, NY: Spring; 2005. pp. 85–121. [Google Scholar]
- Raft S, Koundakjian E, Quinones H, Jayasena CS, Goodrich LV, Johnson JE, Segil N, Groves AK. Development In Press. 2007. Cross-regulation of Ngn1 and Math1 coordinates the production of neurons and sensory hair cells during inner ear development. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Vivancos V, Nicolas E, Dickson BJ. Selecting a longitudinal pathway: Robo receptors specify the lateral position of axons in the Drosophila CNS. Cell. 2000;103:1033–1045. doi: 10.1016/s0092-8674(00)00207-5. [DOI] [PubMed] [Google Scholar]
- Roberts DJ, Johnson RL, Burke AC, Nelson CE, Morgan BA, Tabin C. Sonic hedgehog is an endodermal signal inducing Bmp-4 and Hox genes during induction and regionalization of the chick hindgut. Development. 1995;121:3163–3174. doi: 10.1242/dev.121.10.3163. [DOI] [PubMed] [Google Scholar]
- Rothberg JM, Jacobs JR, Goodman CS, Artavanis-Tsakonas S. slit: an extracellular protein necessary for development of midline glia and commissural axon pathways contains both EGF and LRR domains. Genes Dev. 1990;4:2169–2187. doi: 10.1101/gad.4.12a.2169. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Sabatier C, Plump AS, Le M, Brose K, Tamada A, Murakami F, Lee EY, Tessier-Lavigne M. The divergent Robo family protein rig-1/Robo3 is a negative regulator of slit responsiveness required for midline crossing by commissural axons. Cell. 2004;117:157–169. doi: 10.1016/s0092-8674(04)00303-4. [DOI] [PubMed] [Google Scholar]
- Sanchez-Calderon H, Martin-Partido G, Hidalgo-Sanchez M. Otx2, Gbx2, and Fgf8 expression patterns in the chick developing inner ear and their possible roles in otic specification and early innervation. Gene Expr Patterns. 2004;4:659–669. doi: 10.1016/j.modgep.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Seeger M, Tear G, Ferres-Marco D, Goodman CS. Mutations affecting growth cone guidance in Drosophila: genes necessary for guidance toward or away from the midline. Neuron. 1993;10:409–426. doi: 10.1016/0896-6273(93)90330-t. [DOI] [PubMed] [Google Scholar]
- Siddiqui SA, Cramer KS. Differential expression of Eph receptors and ephrins in the cochlear ganglion and eighth cranial nerve of the chick embryo. J Comp Neurol. 2005;482:309–319. doi: 10.1002/cne.20396. [DOI] [PubMed] [Google Scholar]
- Strutz J, Schmidt CL. Acoustic and vestibular efferent neurons in the chicken (Gallus domesticus). A horseradish peroxidase study. Acta Otolaryngol. 1982;94:45–51. doi: 10.3109/00016488209128888. [DOI] [PubMed] [Google Scholar]
- Tessarollo L, Coppola V, Fritzsch B. NT-3 replacement with brain-derived neurotrophic factor redirects vestibular nerve fibers to the cochlea. J Neurosci. 2004;24:2575–2584. doi: 10.1523/JNEUROSCI.5514-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargesson N, Luria V, Messina I, Erskine L, Laufer E. Expression patterns of Slit and Robo family members during vertebrate limb development. Mech Dev. 2001;106:175–180. doi: 10.1016/s0925-4773(01)00430-0. [DOI] [PubMed] [Google Scholar]
- Von Bartheld CS, Patterson SL, Heuer JG, Wheeler EF, Bothwell M, Rubel EW. Expression of nerve growth factor (NGF) receptors in the developing inner ear of chick and rat. Development. 1991;113:455–470. doi: 10.1242/dev.113.2.455. [DOI] [PubMed] [Google Scholar]
- Wang KH, Brose K, Arnott D, Kidd T, Goodman CS, Henzel W, Tessier-Lavigne M. Biochemical purification of a mammalian slit protein as a positive regulator of sensory axon elongation and branching. Cell. 1999;96:771–784. doi: 10.1016/s0092-8674(00)80588-7. [DOI] [PubMed] [Google Scholar]
- Webber A, Raz Y. Axon guidance cues in auditory development. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:390–396. doi: 10.1002/ar.a.20299. [DOI] [PubMed] [Google Scholar]
- Whitehead MC, Morest DK. The development of innervation patterns in the avian cochlea. Neuroscience. 1985;14:255–276. doi: 10.1016/0306-4522(85)90177-0. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG. Multiple roles of EPH receptors and ephrins in neural development. Nat Rev Neurosci. 2001;2:155–164. doi: 10.1038/35058515. [DOI] [PubMed] [Google Scholar]
- Wong K, Park HT, Wu JY, Rao Y. Slit proteins: molecular guidance cues for cells ranging from neurons to leukocytes. Curr Opin Genet Dev. 2002;12:583–591. doi: 10.1016/s0959-437x(02)00343-x. [DOI] [PubMed] [Google Scholar]
- Wu DK, Oh SH. Sensory organ generation in the chick inner ear. J Neurosci. 1996;16:6454–6462. doi: 10.1523/JNEUROSCI.16-20-06454.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W, Zhou L, Chen JH, Wu JY, Rao Y, Ornitz DM. The mouse SLIT family: secreted ligands for ROBO expressed in patterns that suggest a role in morphogenesis and axon guidance. Dev Biol. 1999;212:290–306. doi: 10.1006/dbio.1999.9371. [DOI] [PubMed] [Google Scholar]