Abstract
Objective
To test the hypothesis that similar differences in psychopathology are present across cultures among men and women with schizophrenia (SZ).
Methods
Sex based differences were tested systematically in two independent samples from the Northeastern USA and North India using the same procedures. The clinical variables were obtained from five interview instruments.
Results
Among the US participants, the number of significant differences exceeded chance predictions (15/240 variables significant at p<0.02, 6.25%; expected number of significant differences: 5). Similarly, a greater than expected number of variables differed significantly between men and women among the Indian subjects (13/230 differences at p<0.02, 5.65%; expected: 5). One of these variables significantly differed in both samples (lifetime abuse of cannabis). When multivariate analyses were conducted in the combined US and Indian samples sex based differences remained for only four variables: course of the illness, history of inappropriate emotions, marital status and number of children.
Conclusion
Sex based differences in SZ/schizoaffective disorder are present in the USA and India at greater than chance probabilities. The majority of the variables differ across the samples. The biological underpinnings of these variables need further investigation.
Keywords: Schizophrenia, Gender, Sex, Psychopathology, Cross-cultural
Introduction
There has been extensive interest in sex differences in schizophrenia (SZ). The research runs the gamut from large scale epidemiological studies to smaller studies describing diagnostic and clinical differences. Some studies, including meta-analyses, suggest a modest increase in the incidence and prevalence of SZ among men.1-3 More substantive differences in clinical features, particularly severity have been documented repeatedly. Women with SZ report affective symptoms, auditory hallucinations and persecutory delusions more frequently, with lower prevalence of smoking and substance abuse.4 These authors also reported that male patients had more negative symptoms and cognitive deficits. Though a prospective, double blind placebo controlled trial comparing 24 male and 20 female patients did not find significant sex-based differences,5 an extensive review suggested that optimal antipsychotic maintenance regimens differ between women and men.6 A review of 76 studies on tardive dyskinesia (TD), published through 1989 suggested a significantly higher prevalence in women (26.6%) than in men (21.6%), with a tendency for more severe TD among women.7
There is substantial evidence that men with SZ suffer a more severe form of the illness and a more malignant course than women.8 Women also have significantly better social function.4,9-11 Men are more likely to have an earlier age at onset, a variable correlated with more severe illness.12-14 Sex significantly influenced global measures of function in a two year follow-up of 200 patients.15 A systematic meta-analysis of twenty three studies detected a highly significant association between male sex and deficit SZ [pooled odds ratio (OR)=1.75].16 Women with SZ exhibited a less deteriorated course of illness.17 Not all studies have consistently reported a more severe form of illness among men.11 Ten year follow up of 141 first admitted patients and patients with schizophreniform disorder reported no significant difference between men and women in clinical outcome.18 Other conflicting results have also been reported.19,20 These differences likely reflect the heterogeneity of presentation of SZ, the likely impact of sample size variation and even subtle diagnostic differences.21
Under the assumption that the sex differences reflect the impact of factors that are inherent to the disorder, many investigators have explored biological factors. Some studies have revealed differences in imaging variables22,23 as well as chromosome related differences.24 The most extensive focus has been on hormonal differences, possibly related to dopamine dysfunction.14,25-28
It has been suggested that the later onset among women may be related to a protective effect of estrogens.28 Convincing biological explanations for sex related clinical differences have not been obtained, possibly because such explanations do not take into account diverse influences, including social variables.4,29-31
Almost all these data originate from Caucasian samples, but there is some evidence for ethnic variation with regard to these differences. For example, the well established sex difference in age at onset has not been detected in four studies from the Indian sub-continent.32-35 Another group found that Asian patients had lower prevalence of TD than North American, European, and African patients.7 Thus, cross-ethnic studies may provide additional insights into the sex differences in SZ.
We have analyzed differences among men and women in relation to clinical variation in two large independent samples, one recruited from north eastern USA and another from northern India. Both samples were recruited and evaluated using the same designs, enabling meaningful comparisons.
Methods
Study design
We aimed to identify variables with sex based differences that were consistent across the Indian and US samples. To reduce the likelihood of false positive results, analyses were conducted in two stages. In view of likely cross-national differences, we initially analyzed the samples separately using univariate analyses (Stage I). Only variables that remained significant following conservative Bonferoni corrections were retained for further analysis. These variables were used as dependent variables in multivariate analyses that included site as well as key demographic and clinical variables as covariates (Stage II).
Variables used for analysis
Patients with SZ or schizoaffective disorder were recruited from treatment facilities in US and India as described.36,37 The primary recruitment sites were Dr. Ram Manohar Lohia Hospital (RML), a publicly funded tertiary care center providing inpatient and outpatient care New Delhi, India and University of Pittsburgh, USA. Participants were sought among inpatients and outpatients at publicly funded hospitals and private clinics in both cities, so as to sample from the spectrum of care available at each site. Recruiters approached various psychiatrists in different private and public hospitals and requested the psychiatrists to refer willing persons with SZ or schizoaffective patients for the participation. The consented individuals were referred to us and after documenting informed consent, they were enrolled in to the study.
Briefly, potentially eligible patients diagnosed clinically with SZ or psychoses were interviewed using the English or the Hindi versions of the Diagnostic Interview for Genetic Studies (DIGS), a semi-structured interview schedule.38,39 The reliability of the English and the Hindi versions of the DIGS have been investigated.38,39 The DIGS includes sections covering different domains of psychopathology. Each section typically begins with screening questions that enable participants to 'skip out' if initial responses are negative. The following sections of the DIGS were completed routinely: Demographic details, Medical history, Overview of psychiatric illness, Major Depression, Mania/Hypomania, Dysthymia, Alcohol abuse and dependence, Drug abuse and dependence, Psychosis, Comorbidity assessment, Suicidal Behaviour. Onset age of psychosis was assessed in a standardized manner as described in question 64 of psychosis section of the DIGS, in India as well as in US. Additional scales that form part of the DIGS were also administered, namely the Global Assessment Scale,40 the Scale for Assessment of Negative symptoms,41 the Scale for Assessment of Positive Symptoms,42 Schizotypy Interview Schedule (SIS)43 and the OPCRIT.44 Information was also obtained from medical records, as well as from relatives and relevant clinicians. The information was synthesized and presented to board certified psychiatrists for consensus diagnosis. Inter-rater and inter-site diagnostic reliability was monitored throughout the study. All participants provided written informed consent, as required by the University of Pittsburgh Institutional Review Board and the Ethics Committee at Dr Ram Manohar Lohia Hospital, New Delhi.
Statistical analysis
Variables were compared using the chi-square test or the Student's t-test as appropriate. The Bonferroni correction was used to account for multiple comparisons. We further analysed such statistically significant variables, using logistic regression and multinomial regression. The Statistical Package for the Social Sciences versions 14 and 16 (SPSS 14, 16) were used for all analyses.
Results
Stage I: Univariate analyses
In view of 'skip outs' in different parts of the DIGS, the number of participants who responded to individual questions varied. A total of 635 records each with 240 variables from USA and 891 records with 230 variables from India were included for analysis.
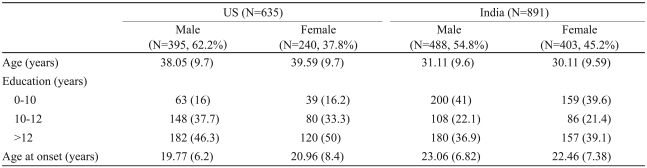
The demographic variables and DSM-IV diagnoses for the participants are provided in Table 1. There were 395 men in the US sample (62.2%) and 488 men in the Indian sample (54.8%). The mean age of the US participants was 38.6 years [standard deviation (SD)=9.7] and that of Indian participants was 30.7 (SD=9.6). The mean age at onset for the US participants was 20.2 years (SD=7.1) and that of Indian participants was 22.8 (SD=7.1). In the US sample, 46.3% of the men and 50% of the women had more than 12 years of education. In the Indian sample, 36.9% of the men and 39.1% of the women had more than 12 years of education.
Table 1.
Demographic and clinical characteristics of the samples
Numbers shown as means. The numbers in brackets indicate percentages for discontinuous variables or standard deviations for continuous variables
Sex based analyses
Age, onset age and education were not significantly different by sex in the US or the Indian sample. Additional comparisons by sex were then conducted.
US sample
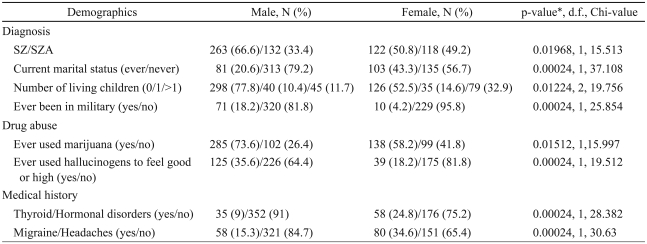
Among the US patients, significant male-female differences exceeded chance predictions (15/240 variables at p=0.02 or better, 6.25%; detailed list in Supplementary Table 1). Following Bonferroni corrections for multiple comparisons, eight variables were significantly different (Table 2, Supplementary Table 1). SZ was the more frequent diagnosis among men (66.6%). Among women, SZ and schizoaffective disorder were diagnosed more evenly (50.8% vs. 49.2%). The majority of male patients did not have children (77.8%), while proportionately more women (47.5%) had one or more children. A higher proportion of men (18.2%) had joined the military (71 participants). Cannabis abuse was more common among men (73.6%) than women (58.2%). Men were more likely to be unmarried (79.2% vs. 56.7%). Women were more likely to report the presence of thyroid or other hormonal disorders (male : female; 9% : 24.8%) or migraine headaches (male : female; 15.3% : 34.6%).
Table 2.
US sample: variables with significant gender differences following corrections for multiple comparisons
*p-values corrected for multiple comparisons. SZ: schizophrenia, SZA: schizoaffective disorder
Indian sample
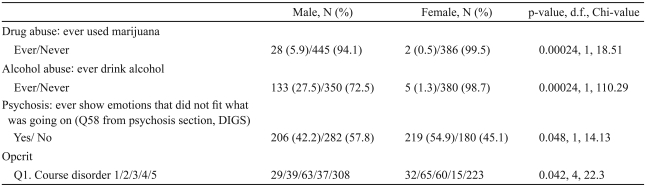
Four variables were significantly different following Bonferroni corrections (Table 3, Supplementary Table 2). Drug or alcohol consumption was more common among men than women. Women were more frequent in the category of 'showing emotions that did not fit for what was going on' (men : women; 42.2% : 54.9%). Compared to women, more men were categorized as having continuous chronic illness with deterioration (Opcrit rating scale, Q1).
Table 3.
Indian sample: variables with significant gender differences following corrections for multiple comparisons
Only variables that would remain significant following Bonferroni corrections are listed. *p-values corrected for multiple comparisons. 1: single episode with good recovery, 2: multiple episodes with good recovery between episodes, 3: multiple episodes with partial recovery between episodes, 4: continuous chronic illness without/deterioration, 5: continuous chronic illness with deterioration. DIGS: Diagnostic Interview for Genetic Studies
Stage II: Post-hoc multivariate analyses
After completing the univariate analyses, we conducted posthoc multivariate analyses to further explore the observed differences, in the presence of significant covariates. Only Bonferoni corrected significant variables from the Indian or the US samples were included as outcome variables (Table 1-3). In the combined US and Indian samples, age, age at onset and education were included as covariates, with sex and nationality as the independent variables. We included age at onset as an index of illness severity. The following variables were not included for post-hoc analyses due to lack of sufficient sample cell sizes in one or both samples: thyroid diseases, 'ever been in military', 'ever used marijuana', 'ever used hallucinogen to feel good or high', 'ever consumed alcohol regularly'.
Sex continued to be a significant predictor for four variables: course of the disorder [p=0.00023; OR 0.66, 95% confidence interval (CI) 1.808, 1.984]; life time history of 'showing emotions that did not fit for what was going on' (p=0.00047; OR 0.638, 95% CI 0.496, 0.821), marital status (p=0.0011; OR 0.662, 95% CI 1.225, 2.255), number of living children (p=0.000001; OR 0.472, 95% CI 0.368, 0.606).
Discussion
Several variables with sex related differences were noted in the US and the Indian samples, but four were noted across both samples through multivariate analyses. They include the course of the illness, history of inappropriate emotions, marital status and number of children. The consistency across very diverse settings noted for these variables likely underscores their importance in the presentation of SZ. Others have reported cross-national or ethnic variation in the course of the disorder,8,17 but the differences with regard to inappropriate emotions are novel, to our knowledge. We have previously reported on sex based differences in marital status and number of children in the USA, as well as India.32 The samples analyzed in the present study overlap with the published data, so the present results should not be considered to be replications of our earlier report. Nevertheless, the consistency in sex based procreational indices reflects an important aspect of the disorder. The lack of overlap between the US and Indian samples for several other variables requires further investigation. Multivariate analyses revealed sex by nationality interactions for several of these variables (data not shown). It is possible that they are impacted by cultural, environmental or genetic differences across the sites.
Many of the sex based differences noted in the US sample are consistent with earlier reports. Two thirds of the US male participants were diagnosed with SZ, the remaining men being diagnosed with schizoaffective disorder. Men were more likely to be unmarried, to have fewer children than women and to have served in the military. They had more hospitalizations than women. In developed countries, sex has previously been reported to be significantly associated with diagnosis2,21; marital status2,11,13,18,19,32 and number of living children.32 Men are reported to have more hospitalizations and longer hospital stays than women.10,11,45 Premenopausal women appear to experience an overall better course of the illness compared to men.26 The sex based differences noted in our sample are thus consistent with several prior reports.3,4,11,26,31,45-48 These differences are postulated to be due to biological differences, including hormonal changes.4,28,31 Others have suggested proteins encoded by sex chromosomes [Monoamine oxidase (MAO) or candidates gene variants24 as causal].
Our Indian sample was ascertained and evaluated in the same manner as our US sample. It was substantially larger than the US sample. However, fewer variables differed significantly in the Indian sample following correction for multiple comparisons. Some variables showed sex related differences in both samples, though they did not remain significant following Bonferoni corrections (Table 2, 3; supplementary Table 1, 2). For example, migraine, as well as thyroid disorders was reported more often by women in the US sample. Thyroid disorders were also more commonly reported by Indian women, but migraine was not associated with gender in India. Thyroid disorders and migraine are more frequent among women in population based surveys,49-51 so the sex differences observed here may not be related to SZ per se. Grandiose delusions were more common among men in both samples. Chu and colleagues52 reported that men with SZ were slower, hypoactive, and grandiose, while Galdos53 did not report such associations in a UK sample.
More men than women were likely to have used alcohol at least once in the Indian sample, consistent with Indian cultural traditions. A similar difference in lifetime alcohol use was not observed in the US sample, though US male patients were more likely to have used alcohol for at least 6 months on a regular basis. In both the samples, women were more likely to rate positive for the variable, 'returned to normal self at least two months' (Q66, Psychosis section of DIGS). This is consistent with some earlier reports-Salem and Kring31 documented that men had poorer course and medication response, poorer premorbid social and intellectual functioning.
Some limitations of the present study need to be considered. Though we aimed for representative samples, neither the US nor the Indian samples were ascertained randomly. Thus, neither sample can be considered representative of patients in the US or India. As there were no exclusions based on gender, however, the gender related differences noted here are unlikely to reflect selection bias. Medications very likely impact on many of the variables listed here, and men are known to receive higher doses of medications than women.31 As accurate medication data were not available, we included age at onset in our multivariate analyses. As this variable is correlated with illness severity, we reasoned that it could function as a crude index of illness severity. In addition, we analyzed psychopathology in relation to lifetime occurrence.
Conclusion
Four variables appear to be consistently observed across both samples, after accounting for key clinical and demographic variables. Different patterns of sex related differences were observed among US and Indian patients with regard to other variables, suggesting an impact of environmental/cultural factors.
Supplementary Material
References
- 1.Aleman A, Kahn RS, Selten JP. Sex differences in the risk of schizophrenia: evidence from meta-analysis. Arch Gen Psychiatry. 2003;60:565–571. doi: 10.1001/archpsyc.60.6.565. [DOI] [PubMed] [Google Scholar]
- 2.Castle DJ, Wessely S, Murray RM. Sex and schizophrenia: effects of diagnostic stringency, and associations with and premorbid variables. Br J Psychiatry. 1993;162:658–664. doi: 10.1192/bjp.162.5.658. [DOI] [PubMed] [Google Scholar]
- 3.Iacono WG, Beiser M. Are males more likely than females to develop schizophrenia? Am J Psychiatry. 1992;149:1070–1074. doi: 10.1176/ajp.149.8.1070. [DOI] [PubMed] [Google Scholar]
- 4.Leung A, Chue P. Sex differences in schizophrenia, a review of the literature. Acta Psychiatr Scand Suppl. 2000;401:3–38. doi: 10.1111/j.0065-1591.2000.0ap25.x. [DOI] [PubMed] [Google Scholar]
- 5.Pinals DA, Malhotra AK, Missar CD, Pickar D, Breier A. Lack of gender differences in neuroleptic response in patients with schizophrenia. Schizophr Res. 1996;22:215–222. doi: 10.1016/s0920-9964(96)00067-9. [DOI] [PubMed] [Google Scholar]
- 6.Seeman MV. Gender differences in the prescribing of antipsychotic drugs. Am J Psychiatry. 2004;161:1324–1333. doi: 10.1176/appi.ajp.161.8.1324. [DOI] [PubMed] [Google Scholar]
- 7.Yassa R, Jeste DV. Gender differences in tardive dyskinesia: a critical review of the literature. Schizophr Bull. 1992;18:701–715. doi: 10.1093/schbul/18.4.701. [DOI] [PubMed] [Google Scholar]
- 8.Lindamer LA, Bailey A, Hawthorne W, Folsom DP, Gilmer TP, Garcia P, et al. Gender differences in characteristics and service use of public mental health patients with schizophrenia. Psychiatr Serv. 2003;54:1407–1409. doi: 10.1176/appi.ps.54.10.1407. [DOI] [PubMed] [Google Scholar]
- 9.Angermeyer MC, Kühn L, Goldstein JM. Gender and the course of schizophrenia: differences in treated outcomes. Schizophr Bull. 1990;16:293–307. doi: 10.1093/schbul/16.2.293. [DOI] [PubMed] [Google Scholar]
- 10.Salokangas RK. Prognostic implications of the sex of schizophrenic patients. Br J Psychiatry. 1983;142:145–151. doi: 10.1192/bjp.142.2.145. [DOI] [PubMed] [Google Scholar]
- 11.Usall J, Araya S, Ochoa S, Busquets E, Gost A, Márquez M. Gender differences in a sample of schizophrenic outpatients. Compr Psychiatry. 2001;42:301–305. doi: 10.1053/comp.2001.24582. [DOI] [PubMed] [Google Scholar]
- 12.Angermeyer MC, Kühn L. Gender differences in age at onset of schizophrenia. An overview. Eur Arch Psychiatry Neurol Sci. 1988;237:351–364. doi: 10.1007/BF00380979. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein JM, Tsuang MT, Faraone SV. Gender and schizophrenia: implications for understanding the heterogeneity of the illness. Psychiatry Res. 1989;28:243–253. doi: 10.1016/0165-1781(89)90205-9. [DOI] [PubMed] [Google Scholar]
- 14.Häfner H, Riecher-Rössler A, An Der Heiden W, Maurer K, Fätkenheuer B, Löffler W. Generating and testing a causal explanation of the gender difference in age at first onset of schizophrenia. Psychol Med. 1993;23:925–940. doi: 10.1017/s0033291700026398. [DOI] [PubMed] [Google Scholar]
- 15.Usall J, Haro JM, Ochoa S, Márquez M, Araya S. Influence of gender on social outcome in schizophrenia. Acta Psychiatr Scand. 2002;106:337–342. doi: 10.1034/j.1600-0447.2002.01351.x. [DOI] [PubMed] [Google Scholar]
- 16.Roy MA, Maziade M, Labbé A, Merette C. Malé gender is associated with deficit schizophrenia: a meta-analysis. Schizophr Res. 2001;47:141–147. doi: 10.1016/s0920-9964(99)00231-5. [DOI] [PubMed] [Google Scholar]
- 17.Bardenstein KK, McGlashan TH. Gender differences in affective, schizoaffective, and schizophrenic disorders. A review. Schizophr Res. 1990;3:159–172. doi: 10.1016/0920-9964(90)90034-5. [DOI] [PubMed] [Google Scholar]
- 18.Opjordsmoen S. Long-term clinical outcome of schizophrenia with special reference to gender differences. Acta Psychiatr Scand. 1991;834:307–313. doi: 10.1111/j.1600-0447.1991.tb05545.x. [DOI] [PubMed] [Google Scholar]
- 19.Andia AM, Zisook S, Heaton RK, Hesselink J, Jernigan T, Kuck J, et al. Gender differences in schizophrenia. J Nerv Ment Dis. 1995;183:522–528. doi: 10.1097/00005053-199508000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Shtasel DL, Gur RE, Gallacher F, Heimberg C, Gur RC. Gender differences in the clinical expression of schizophrenia. Schizophr Res. 1992;7:225–231. doi: 10.1016/0920-9964(92)90016-x. [DOI] [PubMed] [Google Scholar]
- 21.Beauchamp G, Gagnon A. Influence of diagnostic classification on gender ratio in schizophrenia - a meta-analysis of youths hospitalized for psychosis. Soc Psychiatry Psychiatr Epidemiol. 2004;39:1017–1022. doi: 10.1007/s00127-004-0844-3. [DOI] [PubMed] [Google Scholar]
- 22.DeLisi LE, Dauphinais ID, Hauser P. Gender differences in the brain: are they relevant to the pathogenesis of schizophrenia? Compr Psychiatry. 1989;30:197–208. doi: 10.1016/0010-440x(89)90038-2. [DOI] [PubMed] [Google Scholar]
- 23.Flor-Henry P. Influence of gender in schizophrenia as related to other psychopathological syndromes. Schizophr Bull. 1990;16:211–227. doi: 10.1093/schbul/16.2.211. [DOI] [PubMed] [Google Scholar]
- 24.DeLisi LE, Crow TJ. Evidence for a sex chromosome locus for schizophrenia. Schizophr Bull. 1989;15:431–440. doi: 10.1093/schbul/15.3.431. [DOI] [PubMed] [Google Scholar]
- 25.Cho JJ, Iannucci FA, Fraile M, Franco J, Alesius TN, Stefano GB. The role of the estrogen in neuroprotection: implications for neurodegenerative diseases. Neuro Endocrinol Lett. 2003;24:141–147. [PubMed] [Google Scholar]
- 26.Halbreich U, Kahn LS. Hormonal aspects of schizophrenias: an overview. Psychoneuroendocrinology. 2003;28(Suppl 2):1–16. doi: 10.1016/s0306-4530(02)00124-5. [DOI] [PubMed] [Google Scholar]
- 27.Seeman MV, Lang M. The role of estrogens in schizophrenia gender differences. Schizophr Bull. 1990;16:185–194. doi: 10.1093/schbul/16.2.185. [DOI] [PubMed] [Google Scholar]
- 28.Hafner H. Prevention and early intervention in schizophrenia: facts and visions. Seishin Shinkeigaku Zasshi. 2002;104:1033–1054. [PubMed] [Google Scholar]
- 29.Nasser EH, Walders N, Jenkins JH. The experience of schizophrenia: what's gender got to do with it? A critical review of the current status of research on schizophrenia. Schizophr Bull. 2002;28:351–362. doi: 10.1093/oxfordjournals.schbul.a006944. [DOI] [PubMed] [Google Scholar]
- 30.Riecher-Rössler A, Häfner H. Gender aspects in schizophrenia: bridging the border between social and biological psychiatry. Acta Psychiatr Scand Suppl. 2000;407:58–62. doi: 10.1034/j.1600-0447.2000.00011.x. [DOI] [PubMed] [Google Scholar]
- 31.Salem JE, Kring AM. The role of gender differences in the reduction of etiologic heterogeneity in schizophrenia. Clin Psychol Rev. 1998;18:795–819. doi: 10.1016/s0272-7358(98)00008-7. [DOI] [PubMed] [Google Scholar]
- 32.Bhatia T, Franzos MA, Wood JA, Nimgaonkar VL, Deshpande SN. Gender and procreation among patients with schizophrenia. Schizophr Res. 2004;68:387–394. doi: 10.1016/j.schres.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Naqvi H, Khan MM, Faizi A. Gender differences in age at onset of schizophrenia. J Coll Physicians Surg Pak. 2005;156:345–348. [PubMed] [Google Scholar]
- 34.Thara R, Rajkumar S. Gender differences in schizophrenia. Results of a follow-up study from India. Schizophr Res. 1992;7:65–70. doi: 10.1016/0920-9964(92)90075-g. [DOI] [PubMed] [Google Scholar]
- 35.Venkatesh BK, Thirthalli J, Naveen MN, Kishorekumar KV, Arunachala U, Venkatasubramanian G, et al. Sex difference in age of onset of schizophrenia: findings from a community-based study in India. World Psychiatry. 2008;7:173–176. doi: 10.1002/j.2051-5545.2008.tb00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deshpande SN, Bhatia T, Wood J, Brar JS, Thelma BK, Ganguli R, et al. Evaluation of familial influences on the course and severity of schizophrenia among US and Indian cases. Soc Psychiatry Psychiatr Epidemiol. 2004;39:369–374. doi: 10.1007/s00127-004-0749-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas P, Mathur P, Gottesman II, Nagpal R, Nimgaonkar VL, Deshpande SN. Correlates of hallucinations in schizophrenia: A crosscultural evaluation. Schizophr Res. 2007;92:41–49. doi: 10.1016/j.schres.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deshpande SN, Mathur MN, Das SK, Bhatia T, Sharma S, Nimgaonkar VL. A Hindi version of the Diagnostic Interview for Genetic Studies. Schizophr Bull. 1998;24:489–493. doi: 10.1093/oxfordjournals.schbul.a033343. [DOI] [PubMed] [Google Scholar]
- 39.Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. discussion 863-864. [DOI] [PubMed] [Google Scholar]
- 40.Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33:766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- 41.Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) Iowa City. Iowas: The University of Iowa; 1983. [Google Scholar]
- 42.Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) Iowa City. Iowas: The University of Iowa; 1984. [Google Scholar]
- 43.Kendler KS, Lieberman JA, Walsh D. The Structured Interview for Schizotypy (SIS): a preliminary report. Schizophr Bull. 1989;15:559–571. doi: 10.1093/schbul/15.4.559. [DOI] [PubMed] [Google Scholar]
- 44.McGuffin P, Farmer A, Harvey I. A polydiagnostic application of operational criteria in studies of psychotic illness. Development and reliability of the OPCRIT system. Arch Gen Psychiatry. 1991;48:764–770. doi: 10.1001/archpsyc.1991.01810320088015. [DOI] [PubMed] [Google Scholar]
- 45.Usall J, Ochoa S, Araya S, Márquez M. Gender differences and outcome in schizophrenia: a 2-year follow-up study in a large community sample. Eur Psychiatry. 2003;18:282–284. doi: 10.1016/j.eurpsy.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 46.Faraone SV, Chen WJ, Goldstein JM, Tsuang MT. Gender differences in age at onset of schizophrenia. Br J Psychiatry. 1994;164:625–629. doi: 10.1192/bjp.164.5.625. [DOI] [PubMed] [Google Scholar]
- 47.Häfner H, Hambrecht M, Löffler W, Munk-Jørgensen P, Riecher-Rössler A. Is schizophrenia a disorder of all ages? A comparison of first episodes and early course across the life-cycle? Psychol Med. 1998;28:351–365. doi: 10.1017/s0033291797006399. [DOI] [PubMed] [Google Scholar]
- 48.Usall J, Ochoa S, Araya S, Gost A, Busquets E Group NEDES. [Symptomatology and gender in schizophrenia] Actas Esp Psiquiatr. 2000;28:219–223. [PubMed] [Google Scholar]
- 49.Kaloumenou I, Mastorakos G, Alevizaki M, Duntas LH, Mantzou E, Ladopoulos C, et al. Thyroid autoimmunity in schoolchildren in an area with long-standing iodine sufficiency: correlation with gender, pubertal stage, and maternal thyroid autoimmunity. Thyroid. 2008;18:747–754. doi: 10.1089/thy.2007.0370. [DOI] [PubMed] [Google Scholar]
- 50.Shimizu T, Toriumi H, Sato H, Shibata M, Nagata E, Gotoh K, et al. Distribution and origin of TRPV1 receptor-containing nerve fibers in the dura mater of rat. Brain Res. 2007;1173:84–91. doi: 10.1016/j.brainres.2007.07.068. [DOI] [PubMed] [Google Scholar]
- 51.Uzunlulu M, Yorulmaz E, Oguz A. Prevalence of subclinical hypothyroidism in patients with metabolic syndrome. Endocr J. 2007;54:71–76. doi: 10.1507/endocrj.k06-124. [DOI] [PubMed] [Google Scholar]
- 52.Chu CC, Abi-Dargham A, Ackerman B, Cetingök M, Klein HE. Sex differences in schizophrenia. Int J Soc Psychiatry. 1989;35:237–244. doi: 10.1177/002076408903500304. [DOI] [PubMed] [Google Scholar]
- 53.Galdos P, van Os J. Gender, psychopathology, and development: from puberty to early adulthood. Schizophr Res. 1995;14:105–112. doi: 10.1016/0920-9964(94)00020-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.