The NHS breast screening programme invites women aged 50-64 for screening every 3 years. In this programme the term interval cancer is applied to a breast cancer occurring within 3 years of a screening test with negative results. Substantially higher than anticipated rates of interval cancers have already been reported from the NHS breast screening programme,1,2 and there is conflicting evidence on whether the survival rates of women with interval cancers are different from those of women with breast cancer occurring in an unscreened population.3,4 Were interval cancers to have a worse prognosis than cancers in an unscreened population, the reduction in mortality from breast cancer in the screened population might be substantially less than predicted.
To interpret survival estimates for women with interval cancers requires identification of a suitable group of unscreened women for compari- son. In the context of a national screening programme this is difficult. Women who do not respond to an invitation for screening, for example, have been shown to have a worse outcome than unscreened women and are therefore unsuitable.4 The use of historical controls may also be inappropriate because of recent advances in managing breast cancer. Fortuitously, the phased introduction of the NHS screening programme in the north west has resulted in a group of women with breast cancer who lived in areas where screening had yet to be intro- duced whose survival can be compared with that of women diagnosed with interval cancers during the same calendar period. We report for the first time survival rates for interval cancers diagnosed during 1988-91 in the NHS breast screening programme.
Subjects, methods, and results
The NHS breast screening programme in the north west started in 1988 and by 1991 was under way in 14 district health authorities. Women resident in the five remaining districts in the region were not invited for screening before 1991 and form the control population. We identified all invasive interval cancers diagnosed between 1988 and 1991, using published methods.1 We identified breast cancers presenting during this period in women aged 50-67 years in the control population from records held by the regional cancer registry. We determined date of death from data routinely notified to the registry. We calculated estimates of relative survival over five years and made comparisons using an appropriate proportional hazards regression that controlled for age.5
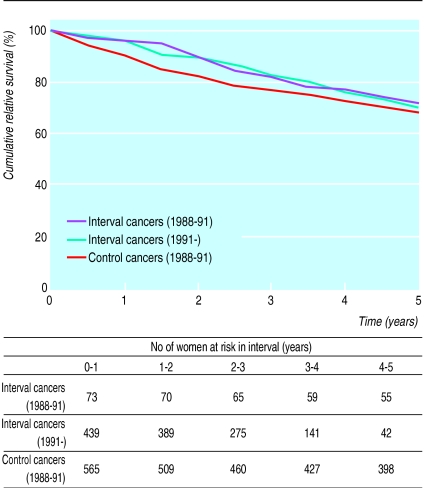
Seventy three interval cancers and 565 cancers from the control population were diagnosed during the study period. No significant difference could be shown between the relative survival rates of women from the control population with breast cancer and those of women presenting with interval cancers during the same period (hazards ratio 0.81 (95% confidence interval 0.50 to 1.31),χ=0.67, df=1, P=0.41). The robustness of this result is supported by the analysis of a further 441 interval cancers diagnosed after 1991, which showed a survival curve similar to that based on the 73 cancers (figure).
Comment
Although our results suggest no difference between the survival rates of women with interval cancers and those of women from the control population, variations in the quality of care provided for women from the two distinct areas could have invalidated this comparison. However, an analysis of survival rates for breast cancer, undertaken for the period immediately before the introduction of the screening programme in the north west, showed no significant differences when women were grouped according to their district of residence. It is reassuring that breast screening has not been detrimental to the survival of those women who presented with an interval cancer in the NHS screening programme. However, minimising the occurrence of interval cancers must remain a high priority if substantial reductions in mortality are to be achieved.
Figure.
Relative survival rates for women with interval cancers and women with breast cancer in control group
Footnotes
Funding: None.
Conflict of interest: None.
References
- 1.Woodman CBJ, Threlfall AG, Boggis CRM, Prior P. Is the three year breast screening interval too long? Occurrence of interval cancers in NHS breast screening programme’s north western region. BMJ. 1995;310:224–226. doi: 10.1136/bmj.310.6974.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Day N, McCann J, Camilleri-Ferrante C, Britton P, Hurst G, Cush S, et al. Monitoring interval cancers in breast screening programmes: the East Anglian experience. J Med Screening. 1995;2:180–185. doi: 10.1177/096914139500200402. [DOI] [PubMed] [Google Scholar]
- 3.Andersson I, Aspegren K, Janzon L, Landberg T, Lindholm K, Linell F, et al. Mammographic screening and mortality from breast cancer: the Malmo mammographic screening trial. BMJ. 1988;297:943–948. doi: 10.1136/bmj.297.6654.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tabar L, Faggerberg G, Duffy SW, Day NE, Gad A, Grontoft O. Update of the Swedish two-county program of mammographic screening for breast cancer. Radiol Clin North Am. 1992;30:187–210. [PubMed] [Google Scholar]
- 5.Hakulinen T, Abeywickrama KH. A computer program package for relative survival analysis. Computer Programs in Biomedicine. 1985;19:197–207. doi: 10.1016/0010-468x(85)90011-x. [DOI] [PubMed] [Google Scholar]