Abstract
Cytokine-activated macrophages restrain the replication of intracellular parasites and disrupt the integrity of vacuolar pathogens. Here we show that NOS2 and the IRG family member Irgm3, respectively are required for the ability of in vivo primed macrophages to restrain the growth of Toxoplasma gondii and to destroy the parasite's intracellular niche. Remarkably, virulent Type I strains of T. gondii evade IRG-dependent vacuolar disruption, while remaining susceptible to iNOS-dependent restriction. The ability of virulent T. gondii to escape killing by macrophages is controlled at the level of the individual vacuole and is associated with differential permissiveness for association of the IRG proteins Irga6 (IIGP1) and Irgb6 (TGTP) to the vacuolar membrane. Surprisingly, expression of the Type I ROP-18 virulence determinant in an avirulent strain did not confer the evasive phenotype. These results pinpoint evasion of vacuolar disruption by IRG proteins as a new determinant of pathogen virulence.
Keywords: Monocytes/Macrophages, Parasitic-Protozoan, Cytotoxicity, Immunity-Related GTPases, Nitric Oxide
Introduction
Toxoplasma gondii is an obligate intracellular parasite that can invade and replicate in multiple cell types and cause infection and disease in diverse vertebrate species. Most healthy individuals infected by the parasite remain asymptomatic, because an efficient immune response can rapidly eliminate the acute tachyzoite stage of infection and provide immunosurveillance to maintain latent infection in organs where the encysted bradyzoite stage of the parasite persists. Considerable interest and effort has been focused on understanding the population structure and genetic basis of virulence in T. gondii (1, 2). Most Toxoplasma strains isolated in North America and Europe belong to three clonal lines referred to as: Type I, II and III (3, 4). Type I strains are hypervirulent and infection with very low doses of tachyzoites causes death in immunocompetent mice. Type II and III strains cause non-lethal infections, characterized by the development of chronic latent infections of the CNS and skeletal muscles. Mice inoculated with low numbers of the hypervirulent Type I RH strain succumb to overwhelming parasite outgrowth and systemic overproduction of pro-inflammatory cytokines (5) and yet, the doubling time of Type I strains is not shorter than that of avirulent Type II and Type III strains (6). Thus, other biological features of Type I strains, including an altered expression program for latency-associated genes required for bradyzoite differentiation (4), increased migratory and invasive potential (7) and an enhanced ability to modulate and subvert the host cellular and immune responses have been invoked as potential virulence determinants for Toxoplasma.
Invasive Toxoplasma tachyzoites have been shown to modulate several aspects of the host immune response. Macrophages infected with the parasite have an impaired IL-12 and TNFα cytokine response and a blockade in the translocation of NF-κB to the nucleus in response to TLR agonists (8). Remarkably, T. gondii infection of macrophages has been shown to induce phosphorylation of STAT-3, independently of the action of host cell derived IL-10 (9). Recently, a polymorphic parasite encoded serine-threonine kinase, ROP-16 from Type I and Type III strains has been shown to indirectly induce phosphorylation of STAT-3 and STAT-6 when expressed in a type II strain (10). The inability of Type II ROP-16 to induce prolonged STAT-3 phosphorylation may explain the more robust innate IL-12 response induced by type II parasites (11). T. gondii infection has also been shown to antagonize the induction of NOS2 and MHC class II by IFN-γ (12). Transcriptional profiling of the effects of T. gondii on the gene expression response to IFN-γ in fibroblasts indicated a globally repressive effect of infection, through blockade of STAT-1 function in the nucleus and interference with STAT-1 induced expression of interferon regulatory factor 1 (IRF-1), a transcriptional activator of many IFN-γ responsive genes (13). The ability of T. gondii to inhibit NF-κB and IRF-1 activation and STAT-1 function in response to immune agonists, coupled with its ROP-16 associated STAT-3/STAT-6 activating effect, would tend to favor dampening of the afferent arms of the innate and adaptive phase of the immune response. Recently, a patatin-like phospholipase of a Type II strain Toxoplasma has been shown to be essential for parasite survival and replication within IFN-γ-activated macrophages and for the suppression of macrophage NO production (14). Whether virulent and avirulent Toxoplasma strains differ in their abilities to evade and survive the onslaught of microbiostatic and microbicidal activities of already activated effector cells remains unknown. Here we report that virulent Type I strains of Toxoplasma are uniquely capable of resisting destruction of their intracellular vacuolar niche within fully armed effector macrophages that were elicited by in vivo priming.
Materials and Methods
Experimental animals, parasites and macrophages
C57BL/6 and NOS2−/− mice were obtained from the Jackson Laboratory. These mice, along with Irgm3−/− mice (16) and Irgm3/NOS2 double knockout mice (17) (both on the C57BL/6 background) were bred and maintained under specific pathogen-free conditions at the UMDNJ. The uracil auxotrophic CPS strain (29) of T. gondii was provided by D. Bzik (Dartmouth Medical School, Hanover, NH). CPS parasites were γ irradiated with 15,000 rads before use. Toxoplasma strain of GFP-PTG and GFP-RH were obtained from ATCC. T. gondii Type I strain of GT-1, Type II strain of PE and Type III strains of CTG were obtained from L. David Sibley (Washington University, MO). Type 1 allele ROP-18-transgenic CTG strain (Type III) parasites have been described (23). The highly virulent V1 clone expressing Type 1 ROP-18 in a CTG background (CTG-ROP18 V1) was compared with the L1 mutant clone, which expresses a catalytically inactive form of ROP-18 (CTG-ROP18L1), and a control transformant of CTG containing the selectable marker (CTG-Ble). DsRed-RH was a gift of MJ Gubbels (Department of Biology, Boston College). For preparation of peritoneal exudate cells (PECs) including macrophages, mice were i.p. primed on day 0 and day 4 with 1 × 106 irradiated CPS cells respectively. PECs were harvested on day 7 by washing the peritoneum with 10 ml invasion medium (RPMI 1640 with 1% fetal bovine serum) as previously described (30).
Ex vivo killing assay and GFP-PTG in vivo challenge
PECs were infected by GFP-PTG or GFP-RH in vitro at a multiplicity of infection (MOI) of 1 in invasion medium. At 0h, 13h and 26h post-infection, cells were spun onto slides followed by fixation in methanol and staining in Hemacolor (Harleco, Inc.) dye. 200 macrophages randomly selected from each slide were observed by light microscopy, and the numbers of total macrophages, infected macrophages, total vacuoles, and total parasites were counted. The replication of parasites was represented by the number of parasites per vacuole in infected macrophages at 0h, 13h and 26h post-infection. To measure relative resistance to T. gondii infection in vivo, mice were i.p. primed on day 0 with 1 × 106 irradiated CPS parasites and boosted on day 4 with 1 × 105 irradiated CPS. On day 7, 2.5 × 105 freshly prepared GFP-PTG were injected into peritoneum of mice. Percent survival mice were monitored for a total 16 days post-challenge. The number of mice used in each group is indicated in the figure legends.
Immunofluorescence and ultrastructural microscopy assays
For ex vivo infection, PECs were allowed to adhere onto coverslips for 2h, and nonadherent cells were washed away with PBS. After incubation for 15min with different T. gondii strains (as indicated in the figure legend) in invasion medium at 37°C, adherent macrophages were washed extensively with PBS to remove extracellular parasites. Infected macrophages were incubated in RPMI 1640 medium with 1% FBS at 37°C until being processed at different time points for immunofluorescent staining. Infected macrophages were washed with PBS twice before fixation with 4% formaldehyde (EMS, Hatfield, PA) for 15 min at room temperature. After permeabilization (0.2% saponin in PBS for 10 min) and blocking (3% BSA, 0.1% saponin in PBS for 1 h), cells were incubated with rabbit anti-GRA-7 antibody (gift of Isabelle Coppens, John Hopkins University), goat anti-Irgb6 (A20, Santa Cruz, CA) and mouse anti-Irga6 5D9 (31) or rabbit anti-Irga6 165 (32) for 45 min. After washing 3 times, cells were further incubated with Alexa Fluor 350–conjugated donkey anti–rabbit, Alexa Fluor 568–conjugated donkey anti–mouse or Alexa Fluor 647–conjugated donkey anti–goat antibody (Invitrogen, Carlsbad, CA) for 30 min. Phase and fluorescence images were captured by either a Leica TCS SP2 AOBS confocal laser scanning microscope (Leica Microsystems, Bannockburn, IL) or a conventional fluorescence microscope Zeiss Axiovert 200M equipped with the ApoTome module (Thornwood, NY). For ultrastructural analysis of GFP-PTG and GFP-RH's fate, T. gondii-infected macrophages were fixed in fixative (3% Glutaraldehyde in 0.1M Sodium Cacodylate Buffer with 3% Sucrose, pH 7.2) and processed and imaged at John Radcliffe Hospital, Oxford, UK.
Results
Dissociation of toxoplasmacidal and toxoplasmastatic activities of primed macrophages
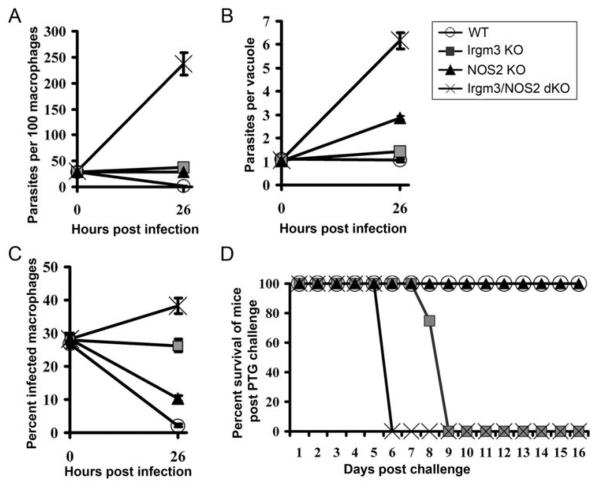
Mice lacking either Irgm3 (IGTP) or NOS2 exhibit enhanced susceptibility to T. gondii infection and their cytokine-activated macrophages have impaired resistance to Toxoplasma infection in vitro (15-17). However, the distinctive roles of Irgm3 and NOS2 in restricting parasite replication versus causing parasite death or immune elimination have not been clearly delineated. We therefore compared the toxoplasmacidal and toxoplasmastatic activities of primed macrophages derived from IGTP−/− or NOS2−/− mice to those from wildtype C57BL/6 mice. Toxoplasmastatic activity was defined as the ability of in vivo primed macrophages to restrict the replication of T. gondii tachyzoites within individual vacuoles while toxoplasmacidal activity was assayed by the ability of primed cells to eliminate vacuolar parasites. As a source of fully armed effector macrophages, we used adherent peritoneal cells harvested from mice immunized with the carbamoyl phosphate synthetase II (CPS)-deficient uracil auxotrophic strain of T. gondii and used avirulent GFP-tagged PTG strain tachyzoites for ex vivo challenge infection (17). We have previously shown that macrophages from these CPS-primed mice express very high levels of Irgm3 and NOS2 and are primed to resist T. gondii (a Type II GFP-PTG strain) infection in vivo and ex vivo. Consistent with previous reports, both NOS2−/− and Irgm3−/− cells show impaired resistance to Toxoplasma infection compared to wildtype control primed macrophages (Figure 1A), which showed a decrease in the number of parasite-infected cells over time. However, the underlying defects are mechanistically distinct. While NOS2-/- macrophages can decrease the frequency of infected cells, they were inefficient in inhibiting intracellular tachyzoite multiplication, as measured by the average number of tachyzoites per parasitophorous vacuole (Figure 1B). On the other hand, Irgm3−/− cells were clearly capable of restricting Toxoplasma replication but were ineffective in causing parasite elimination (Figure 1C). These results indicate separable roles for NOS2 in restricting parasite growth and for Irgm3 in parasite destruction by primed macrophages. As expected, a deficiency in both enzymes resulted in enhanced permissiveness of primed macrophages to ex vivo infection (Figure 1A) and rapid death of mice following in vivo infection with avirulent Type II strain T. gondii (Figure 1D). Interestingly, parasite replication in Irgm3−/−NOS2−/− cells was greater than in NOS2−/− macrophages (see Figure 1B), suggesting that Irgm3 may also exert or facilitate a toxoplasmastatic effect, in addition to its potent toxoplasmacidal function.
Figure 1.
Toxoplasmacidal activity of primed macrophages is dependent on IRG protein, Irgm3 (IGTP) while toxoplasmastasis is dependent on NOS2. (A) CPS vaccine-primed macrophages from wildtype, Irgm3−/−, NOS2−/− and Irgm3−/−/NOS2−/− mice were infected with GFP-PTG tachyzoites (MOI=1.0). Total parasites per 100 macrophages were counted at 0h and 26h post a 15-minute pulse infection. Three mice per group were used. (B) The number of parasites per vacuole was scored among infected macrophages. (C) Percentage of infected macrophages among 200 macrophages was counted at 0h and 26h post a 15-minute pulse infection respectively. (D) 2.5 × 105 GFP-PTG tachyzoites were injected into peritoneum of CPS-primed wildtype and knockout mice (12 mice per group used). The percentage survival of mice was monitored over a period of 16 days.
Virulent Toxoplasma gondii strains resist primed macrophage-mediated destruction
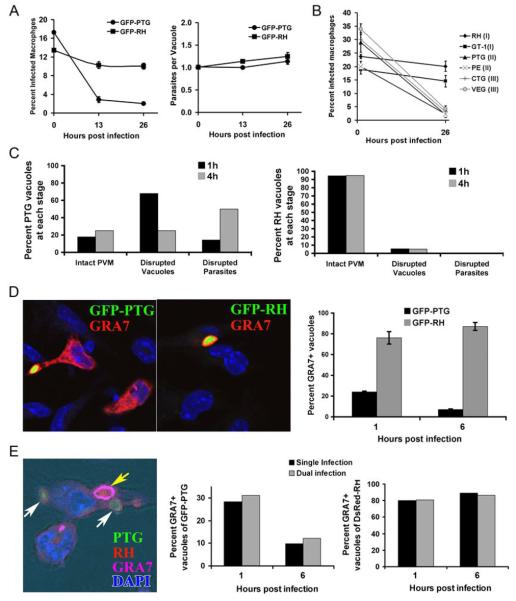
To investigate whether virulent strains of T. gondii could resist the immune mechanisms deployed by in vivo activated macrophages, we compared the fate of virulent GFP-RH (Type I) strain versus avirulent (Type II) GFP-PTG within primed wildtype macrophages that were primed in vivo by CPS-vaccination and then tested ex vivo. Virulent RH and avirulent PTG parasites were equally sensitive to the NOS2-dependent toxoplasmastatic activity of macrophages (Figure 2A, middle). Remarkably, we observed that RH parasites were completely refractory to the Irgm3-dependent elimination activity of CPS-primed macrophages (Figure 2A, left). Thus, the virulent RH strain of Toxoplasma can resist immune destruction while remaining sensitive to NO-mediated cytostasis. To further investigate whether resistance to macrophage killing is a hallmark of virulence in Toxoplasma, we extended our analysis to additional virulent Type I as well as avirulent Type II and Type III strains. As shown in Figure 2A (right panel), only virulent Type I strains, but not avirulent Type II or Type III strains exhibited the ability to resist elimination by highly primed macrophages elicited in vivo by CPS-vaccination.
Figure 2.
Virulent Type I strains of T. gondii resist macrophages-mediated parasite elimination. (A) Comparison of infection rate (left panel) and number of parasites per vacuole (right panel) between PTG (Type II) and RH (Type I) at 0h, 13h and 26h following a 15-minute pulse infection. The graphs are from one of three representative experiments and error bars correspond to standard error for three mice. (B) Comparison of infection rate between Type I strains RH and GT-1, Type II strains PTG and PE and Type III strains CTG and VEG at 1h and 26h following pulse infection. Data are representive of three independent experiments. (C) Ultrastructural microscopic analysis of PTG (left panel) and RH (right panel) at 1h and 4h post invasion in CPS-primed wildtype macrophages. About 40 parasite profiles per time point were classified as either residing in an intact PVM or in disrupted vacuoles or with the parasite membrane itself being disrupted. Nearly all RH vacuoles remained intact 4h post infection (right panel). (D) The integrity of the vacuoles formed by avirulent GFP-PTG (left panel) and virulent GFP-RH (middle panel) parasites was monitored using anti-GRA-7 (Alexa Fluor 568, shown in red) staining 1h post infection of CPS-primed macrophages. In the right-hand panel, over 100 vacuoles were counted per time point and data is representative of two independent experiments. (E) Primed peritoneal macrophages were infected with DsRed-RH first for 15 min, washed and then infected with GFP-PTG 30min later. One hr post-PTG infection cells were stained with anti-GRA-7 antibodies (Alexa Fluor 647, shown in magenta). The left most picture shows a macrophage containing a single intact GRA7+ RH vacuole (yellow arrow) and two disrupted GRA-7-negative GFP-PTG vacuoles (white arrows). The kinetics of vacuolar disruption of GFP-PTG (middle panel) and DsRed-RH (right panel) in singly and dually infected cells is shown. The percentage of GRA-7+ vacuoles in a total of 100 vacuoles per time point was scored at 1 and 6 hr post infection in either RH or PTG singly infected macrophages or in RH and PTG dually infected macrophages.
Fate of parasitophorous vacuoles formed by virulent Toxoplasma
Previous studies in astrocytes and macrophages infected with avirulent Toxoplasma strains have shown that immune GTPases mediate vesiculation of the T. gondii parasitophorous vacuole membrane, resulting in the exposure of disrupted parasites to the host cytosol (17-19). In primed macrophages, large double-membraned autophagosomes were also observed that delivered parasite corpses to a pathway involving lysosomal destruction (17), although this phenomemon was not observed in macrophages activated in vitro (20). To investigate the point at which virulent RH parasites thwarted the IRG-dependent toxoplasmacidal process, we performed EM analyses of virulent RH and avirulent PTG profiles found within primed macrophages at 1 hr and 4 hrs following ex vivo infection. We classified parasite profiles into three broad categories: those with intact parasitophorous vacuole membranes (PVM), those with disrupted PVM but intact parasite plasma membrane, and those with naked and degraded parasites with breached plasma membranes. Consistent with our previous report (17), avirulent PTG parasites invade primed macrophages but were rapidly subjected to vacuolar membrane disruption, with only 20% remaining within intact vacuoles 1 hr post infection (Figure 2B, left panel). The fraction of intracellular PTG parasites exhibiting a moribund or disrupted profile, as expected, increased at 4 hrs post infection. In contrast, ultrastructural examination of invaded virulent RH strain parasites reveal that nearly all parasites established and persisted as normal parasites within intact vacuoles (Figure 2B, right panel), with no evidence of the PVM remodeling or plasma membrane disruption observed in vacuoles containing avirulent PTG parasites. Consistent with this conclusion, staining of the parasite dense granule protein GRA-7, which associates with the parasitophorous vacuole membrane demonstrated that the integrity of the PVM formed by RH parasites in primed macrophages remained persistently intact, while GRA-7 clearly “leaks-out” into the cytosol (18) of primed macrophages wherein disrupted PTG parasites were evident (Figure 2D). Together, the ultrastructural and immunofluorescence analyses indicate that the parasitophorous vacuole membrane formed by virulent RH strain parasites was able to resist or evade macrophage-instigated vesiculation and disruption, explaining the lack of RH parasite elimination.
Vacuole-autonomous mode of evasion by virulent Toxoplasma
Virulent RH parasites might escape destruction by globally inhibiting the critical functions of IRGs required for PVM disruption. Alternatively, virulent T. gondii could evade elimination by locally controlling the recruitment or activation of IRGs on the PVM. To distinguish between these alternative modes of resistance, we followed the fate of virulent RH and avirulent PTG parasites in dually infected cells. As depicted in Figure 2E (left most panel), pre-infection of RFP-expressing virulent RH parasites did not prevent or inhibit disruption of vacuoles subsequently formed by GFP-PTG parasites. Avirulent parasites underwent vacuolar disruption regardless of whether they were inside cells singly infected or co-infected with virulent parasites (Figure 2E, middle and right panels). In the same way, vacuoles containing virulent parasites remained intact in singly infected cells or in cells also infected with an avirulent parasite. Thus, the fate of each tachyzoite and its ability to resist macrophage destruction is determined vacuole-autonomously. This observation argues against a scenario where virulence factor(s) produced by RH parasites globally result in functional inactivation of the macrophage's GTPase-dependent toxoplasmacidal activity.
Differential trafficking of IRGs to the PVM formed by virulent and avirulent Toxoplasma
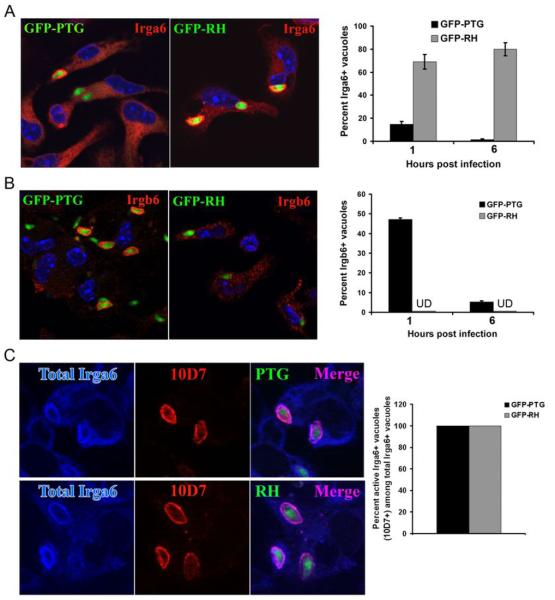
The apparent absence of vacuolar membrane damage in RH-infected macrophages raised the important question of whether IRGs were precluded from associating with the PVM. Several members of the IRG protein family, including Irgm3, Irga6 and Irgb6 are induced by Th1 cytokines (16-20) and have been shown to associate with the parasitophorous vacuole formed by T. gondii (18). Although both are essential for resistance to Toxoplasma, Irgm1 and endogenous Irgm3 are not readily detected in the PVM by immunofluorescence microscopy. In astrocytes, Irga6 and Irgb6 have been shown to be abundantly associated with the PVM prior to the PVM vesiculation and disruption (18), but the in vivo resistance phenotype of Irga6 and Irgb6 null-mice to Toxoplasma infection has not been reported. We therefore assayed for the presence of Irga6 and Irgb6 on the PVM formed by virulent RH and avirulent PTG parasites. As shown in Figure 3A, Irga6 localized to the PVM of both PTG and RH parasites. Interestingly, while the percentage of PTG parasites showing vacuolar Irga6 decreased with time (Figure 3A, right panel), the percentage of RH parasites with Irga6+ vacuoles remained elevated, likely a reflection of the fact that vacuoles formed remained intact and continually accumulate Irga6 (evident as an increase in Irga6 staining intensity, data not shown). In contrast to the pattern of Irga6 staining, no Irgb6 was seen to localize to RH vacuoles, while it readily associated with the PVM formed by avirulent PTG parasites (Figure 3B). These results demonstrate that the lack of PVM disruption in the RH vacuole is not due to a wholesale exclusion of IRG proteins. Instead, the picture that emerges is that selective exclusion of distinct IRG family members from the PVM could be responsible for the lack of vesiculation and disruption of the RH vacuolar membrane. To probe the status of the Irga6 recruited to the RH-vacuole formed in primed macrophages, we used the 10D7 MAb, which recognizes a conformational epitope of Irga6 when it is GTP-bound (21). As shown in Figure 3C, all Irga6+ RH and PTG vacuoles stained with a rabbit anti-Irga6 Ab were similarly positive for the 10D7 epitope. Thus, RH resistance is not associated with a failure of Irga6 to adopt a GTP-bound conformation at the PVM formed by virulent Toxoplasma. The recruitment of GTP-bound Irga6 to the RH PVM coupled with the apparent exclusion of Irgb6 from the PVM favors the view that virulent RH parasites passively evade vacuolar disruption, rather than actively inhibiting a critical yet unknown step in the IRG-mediated vacuolar disruption mechanism. The fact that Irgb6 and several murine guanylate-binding proteins (mGBPs) (22) fail to associate with the PVM formed by virulent, but not avirulent Toxoplasma is consistent with this view.
Figure 3.
IRG proteins show differential association with the PVM formed by virulent RH and avirulent PTG strains of T. gondii. (A) Images of Irga6 staining of vacuoles in GFP-PTG and GFP-RH infected macrophages are shown in the left panel. Percentage of Irga6+ vacuoles formed by GFP-PTG or GFP-RH at 1h and 6h post-infection are shown in the right panel. Data shown are mean values of two independent experiments. (B) Images of Irgb6 staining of vacuoles in GFP-PTG and GFP-RH infected macrophages are shown in the left panel. Percentage of Irgb6+ vacuoles formed by GFP-PTG or GFP-RH at 1h and 6h post-invasion are shown in the right panel. Irgb6 staining was undetectable (UD) on PVMs formed by RH parasites. Data shown are mean values of two independent experiments. (C) Staining for total Irga6+ vacuoles (rabbit anti-Irga6 165, shown in blue) and GTP-bound Irga6+ vacuoles (mAb 10D7, shown in red) in GFP-PTG (left, upper panel) and GFP-RH (left, lower panel) infected macrophages. In the right panel, percent GTP-bound Irga6+ vacuoles among total Irga6+ vacuoles and comparison between PTG and RH is shown. A total of 60 vacuoles for each time point per strain was scored.
Evasion of IRG-mediated vacuolar disruption as a new virulence determinant
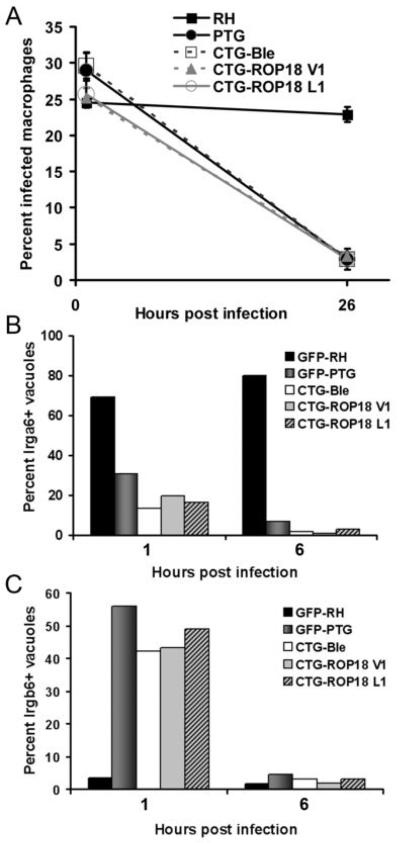
Evasion of vacuolar disruption might represent a new virulence mechanism of Type I strains of Toxoplasma. The genetic and molecular basis for the ability of virulent parasites to evade IRG-mediated destruction is currently not known. Using forward genetic analysis of progeny between a Type I (GT-1) and a Type III parental strain, the polymorphic serine threonine kinase ROP-18 encoded by GT-1 parasites was identified as the major virulence factor for Type I strains of Toxoplasma (23, 24). Transgenic expression of Type I ROP-18 in a Type III CTG strain resulted in dramatic increases in mouse virulence (measured by mortality assays) and an enhancement of parasite growth and motility phenotypes. To ascertain whether the same ROP-18 allele accounts for the different outcomes of Type I versus Type III infection in primed macrophages, we followed the fate of a Type III (CTG) strain parasite lines expressing either wildtype ROP-18 (CTG-ROP18V1) or mutated ROP-18 lacking catalytic activity (CTG-ROP18L1) in CPS-primed effector macrophages. As shown in Figure 4, Type III CTG strain parasites were eliminated (Figure 4A), underwent vacuolar disruption with time (Figure 4B and 4C) and did not exclude Irgb6 from the PVM (Figure 4C), regardless of whether they were expressing wildtype or mutated Type I ROP-18 allele. This result suggested that another polymorphic parasite protein, distinct from ROP-18, could mediate the evasive maneuver of Type I parasites. Preliminary linkage analysis of F1 progeny clones from a genetic cross between Type I and Type III parental strains (data not shown) supports ROP-18-independence of the evasive phenotype, consistent with the multi-locus control of virulence in Toxoplasma.
Figure 4.
Evasion of macrophage-mediated parasite elimination is independent of the Type I ROP-18 virulence determinant. (A) CPS-primed peritoneal macrophages were infected with either RH (Type I), PTG (Type II), CTG (Type III) expressing a control vector (CTG-Ble), CTG expressing a wildtype Type I ROP18 transgene (CTG-ROP18 V1), or CTG expressing a mutated ROP18 (CTG-ROP18 L1). Percentages of infected macrophages were monitored 1h and 26h post infection. Data represents two individual experiments and are expressed as means ± standard error. The percentage of lrga6+ (B) and lrgb6+ (C) vacuoles was determined 1hr and 6 hr post infection of CPS-primed peritoneal macrophages with the parental Type I, Type II, and transgenic Type III strains outlined in (A).
Discussion
Our data demonstrate that the microbiostatic and microbiocidal activities of primed macrophages are mediated by distinct gene products induced by the principal Th1 cytokine, IFN-γ. NOS2 generated NO radicals may target metabolic enzymes involved in DNA replication and in mitochondrial respiration resulting in inhibition of parasite multiplication. On the other hand, members of the IRG protein family traffic to the Toxoplasma parasitophorous vacuole membrane, and are thought to effect vesiculation and physical disruption of the protective membrane formed by the parasite (18). In this report, we demonstrate that virulent Type I strains of T. gondii remarkably resist IRG-mediated disruption of their vacuoles and, ultimately, escape autophagic elimination, while simultaneously remaining vulnerable to NOS2-mediated growth restriction. The resistance phenotype of virulent Toxoplasma has also recently been found to be operative within IFN-γ-activated mouse fibroblasts (Zhao YO et al. 2008, Mem Inst Oswaldo Cruz, In press). Resistance to killing by armed effector macrophages and cytokine-activated parenchymal cells may explain, in part, how virulent Toxoplasma strains invariably kill immunocompetent mice, even in the face of a full-blown Th1 response (5). The ability to forestall elimination could widen the parasite's window of opportunity for deactivating the infected macrophage by suppressing de novo gene induction by IFN-γ. The molecular basis for how virulent T. gondii escapes destruction within the macrophage awaits further investigations, but our current results showing vacuole-autonomous control and the differential permissiveness for IRG protein recruitment are consistent with either passive or active models of evasive action. A polymorphic parasite molecule encoded by virulent Toxoplasma and present on the PVM may present a non-permissive interface for the proper trafficking and assembly of the complement of IRGs required for effecting vacuole membrane vesiculation and disruption. Presumably, the same PVM associated protein from avirulent strains allow for recruitment of critical GTPases and subsequent vacuolar membrane remodeling events. Alternatively, the PVM-localized virulent determinant could actively prevent the proper targeting of a subset of essential IRGs to the PVM by modifying a key host factor required for this process. Identification of the strain-variant Toxoplasma molecule(s) responsible for this evasive phenotype will not only further our understanding of parasite virulence mechanisms, but may also elucidate a key step(s) in the assembly and function of the IRG protein machinery responsible for vacuolar membrane disruption.
Microbial interference with the trafficking, assembly and function of IRGs may prove to be a convergent theme underlying not only virulence characteristics, but also the host range and restriction of multiple pathogens. Exposure of mouse cells to IFN-γ induces the transcription and biosynthesis of a large group of GTPases, including many 47 kDa IRGs and 10 mGBPs (22, 25, 26). The antimicrobial activities of IRGs have been implicated in resistance to different viruses, bacteria and eukaryotic pathogens (27). With regard to host-species restriction of pathogens, it is interesting to note that the human pathogen Chlamydia trachomatis is effectively restricted, while the mouse pathogen Chlamydia muridarum is refractory to inhibition by cytokine-activated murine fibroblasts (28). Inclusion bodies containing C. trachomatis efficiently recruit multiple IRG proteins, while C. muridarum inclusion bodies remain negative. Although inefficient, C. muridarum can prevent recruitment of IRG proteins to the C. trachomatis vacuole in doubly infected mouse cells, suggesting that the chlamydial factor may be injected into the host cytosol and traffic more extensively between vacuoles in the same infected cell. On the other hand, the vacuole-autonomous control of the virulent Toxoplasma-evasion phenotype suggests the putative Toxoplasma evasive determinant may be highly restricted to the PVM. In this regard, the localization of the principal Toxoplasma virulence determinant ROP-18 to the PVM made it an obvious candidate evasion molecule. Nevertheless, transgenic expression of the Type I allele of ROP-18 in an avirulent Type III parasite strain did not confer an evasive phenotype and was not sufficient to overcome the disruptive function of in vivo primed effector macrophages, suggesting involvement of another polymorphic rhoptry protein encoded by virulent Toxoplasma. It is interesting to note that in primates, including humans, only two IRG gene homologues, neither of which is interferon-inducible, are present (26). Further, the existence of a pathway for vesiculation and disruption of the Toxoplasma PVM has not yet been documented in human cells. Thus, Toxoplasma may have to deploy a diverse set of evasive and resistance tactics, in anticipatory response to the variability of growth restrictive and parasite killing mechanisms used by different host cell types and species. In the case of Type I strain infection of laboratory mice, the evolution of a mechanism for evasion of vacuolar disruption by IRG proteins is seemingly maladaptive, resulting in acute virulence and premature death of both parasite and host. It may be that the same Type 1 polymorphism giving rise to virulence in laboratory mice conferred avirulence and a co-adaptive advantage in distantly-related rodent intermediate hosts, which possess a different genetic profile for immunity-related GTPases and which play a more significant role in the ecology of the parasite.
Acknowledgements
We thank Mike Ling for his initial contributions to the project and are grateful to our many colleagues (Isabelle Coppens, Jens Zerrahn, Marc Jan Gubbels and Greg Taylor) for invaluable parasite, mouse and antibody reagents, discussions and suggestions for improving the manuscript. We would like to thank Utz Herbig (NJMS) for use of his fluorescent microscope.
3Abreviations used in this paper
- CPS
carbamoyl phosphate synthetase II
- EM
electron microscopy
- NOS2
inducible nitric oxide synthase
- IRF
interferon regulator factor
- IRG
immunity-related GTPase
- MOI
multiplicity of infection
- PECs
peritoneal exudate cells
- PVM
parasitophorous vacuole membrane
- WT
wild-type
Footnotes
Disclosures
The authors have no financial conflicts of interest.
This work is supported by an R21 exploratory grant from the National Institutes of Health (AI074914 to G.S.Y) and funds from UMDNJ-New Jersey Medical School. L.D.S. was supported by National Institutes of Health Grant AI036629.
References
- 1.Sibley LD, Ajioka JW. Population structure of Toxoplasma gondii: clonal expansion driven by infrequent recombination and selective sweeps. Annu Rev Microbiol. 2008;62:329–351. doi: 10.1146/annurev.micro.62.081307.162925. [DOI] [PubMed] [Google Scholar]
- 2.Boothroyd JC, Grigg ME. Population biology of Toxoplasma gondii and its relevance to human infection: do different strains cause different disease? Curr Opin Microbiol. 2002;5:438–442. doi: 10.1016/s1369-5274(02)00349-1. [DOI] [PubMed] [Google Scholar]
- 3.Howe DK, Sibley LD. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis. 1995;172:1561–1566. doi: 10.1093/infdis/172.6.1561. [DOI] [PubMed] [Google Scholar]
- 4.Saeij JP, Boyle JP, Boothroyd JC. Differences among the three major strains of Toxoplasma gondii and their specific interactions with the infected host. Trends Parasitol. 2005;21:476–481. doi: 10.1016/j.pt.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Mordue DG, Monroy F, La Regina M, Dinarello CA, Sibley LD. Acute toxoplasmosis leads to lethal overproduction of Th1 cytokines. J Immunol. 2001;167:4574–4584. doi: 10.4049/jimmunol.167.8.4574. [DOI] [PubMed] [Google Scholar]
- 6.Radke JR, Striepen B, Guerini MN, Jerome ME, Roos DS, White MW. Defining the cell cycle for the tachyzoite stage of Toxoplasma gondii. Mol Biochem Parasitol. 2001;115:165–175. doi: 10.1016/s0166-6851(01)00284-5. [DOI] [PubMed] [Google Scholar]
- 7.Barragan A, Sibley LD. Migration of Toxoplasma gondii across biological barriers. Trends Microbiol. 2003;11:426–430. doi: 10.1016/s0966-842x(03)00205-1. [DOI] [PubMed] [Google Scholar]
- 8.Butcher BA, Kim L, Johnson PF, Denkers EY. Toxoplasma gondii tachyzoites inhibit proinflammatory cytokine induction in infected macrophages by preventing nuclear translocation of the transcription factor NF-kappa B. J Immunol. 2001;167:2193–2201. doi: 10.4049/jimmunol.167.4.2193. [DOI] [PubMed] [Google Scholar]
- 9.Butcher BA, Kim L, Panopoulos AD, Watowich SS, Murray PJ, Denkers EY. IL-10-independent STAT3 activation by Toxoplasma gondii mediates suppression of IL-12 and TNF-alpha in host macrophages. J Immunol. 2005;174:3148–3152. doi: 10.4049/jimmunol.174.6.3148. [DOI] [PubMed] [Google Scholar]
- 10.Saeij JP, Coller S, Boyle JP, Jerome ME, White MW, Boothroyd JC. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature. 2007;445:324–327. doi: 10.1038/nature05395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robben PM, Mordue DG, Truscott SM, Takeda K, Akira S, Sibley LD. Production of IL-12 by macrophages infected with Toxoplasma gondii depends on the parasite genotype. J Immunol. 2004;172:3686–3624. doi: 10.4049/jimmunol.172.6.3686. [DOI] [PubMed] [Google Scholar]
- 12.Luder CG, Algner M, Lang C, Bleicher N, Gross U. Reduced expression of the inducible nitric oxide synthase after infection with Toxoplasma gondii facilitates parasite replication in activated murine macrophages. Int J Parasitol. 2003;33:833–844. doi: 10.1016/s0020-7519(03)00092-4. [DOI] [PubMed] [Google Scholar]
- 13.Kim SK, Fouts AE, Boothroyd JC. Toxoplasma gondii dysregulates IFN-gamma-inducible gene expression in human fibroblasts: insights from a genome-wide transcriptional profiling. J Immunol. 2007;178:5154–5165. doi: 10.4049/jimmunol.178.8.5154. [DOI] [PubMed] [Google Scholar]
- 14.Mordue DG, Scott-Weathers CF, Tobin CM, Knoll LJ. A patatin-like protein protects Toxoplasma gondii from degradation in activated macrophages. Mol Microbiol. 2007;63:482–496. doi: 10.1111/j.1365-2958.2006.05538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scharton-Kersten TM, Yap G, Magram J, Sher A. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med. 1997;185:1261–1273. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor GA, Collazo CM, Yap GS, Nguyen K, Gregorio TA, Taylor LS, Eagleson B, Secrest L, Southon EA, Reid SW, Tessarollo L, Bray M, McVicar DW, Komschlies KL, Young HA, Biron CA, Sher A, Vande Woude GF. Pathogen-specific loss of host resistance in mice lacking the IFN-gamma-inducible gene IGTP. Proc Natl Acad Sci U S A. 2000;97:751–755. doi: 10.1073/pnas.97.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ling YM, Shaw MH, Ayala C, Coppens I, Taylor GA, Ferguson DJ, Yap GS. Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages. J Exp Med. 2006;203:2063–2071. doi: 10.1084/jem.20061318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martens S, Parvanova I, Zerrahn J, Griffiths G, Schell G, Reichmann G, Howard JC. Disruption of Toxoplasma gondii Parasitophorous Vacuoles by the Mouse p47-Resistance GTPases. PLoS Pathog. 2005;1:e24. doi: 10.1371/journal.ppat.0010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melzer T, Duffy A, Weiss LM, Halonen SK. The gamma interferon (IFN-gamma)-inducible GTP-binding protein IGTP is necessary for toxoplasma vacuolar disruption and induces parasite egression in IFN-gamma-stimulated astrocytes. Infect Immun. 2008;76:4883–4894. doi: 10.1128/IAI.01288-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Z, Fux B, Goodwin M, Dunay IR, Strong D, Miller BC, Cadwell K, Delgado MA, Ponpuak M, Green KG, Schmidt RE, Mizushima N, Deretic V, Sibley LD, Virgin HW. Autophagosome-independent essential function for the autophagy protein Atg5 in cellular immunity to intracellular pathogens. Cell Host Microbe. 2008;4:458–469. doi: 10.1016/j.chom.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papic N, Hunn JP, Pawlowski N, Zerrahn J, Howard JC. Inactive and Active States of the Interferon-inducible Resistance GTPase, Irga6, in Vivo. J Biol Chem. 2008;283:32143–32151. doi: 10.1074/jbc.M804846200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Degrandi D, Konermann C, Beuter-Gunia C, Kresse A, Wurthner J, Kurig S, Beer S, Pfeffer K. Extensive characterization of IFN-induced GTPases mGBP1 to mGBP10 involved in host defense. J Immunol. 2007;179:7729–7740. doi: 10.4049/jimmunol.179.11.7729. [DOI] [PubMed] [Google Scholar]
- 23.Taylor S, Barragan A, Su C, Fux B, Fentress SJ, Tang K, Beatty WL, Hajj HE, Jerome M, Behnke MS, White M, Wootton JC, Sibley LD. A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii. Science. 2006;314:1776–1780. doi: 10.1126/science.1133643. [DOI] [PubMed] [Google Scholar]
- 24.El Hajj H, Lebrun M, Arold ST, Vial H, Labesse G, Dubremetz JF. ROP18 is a rhoptry kinase controlling the intracellular proliferation of Toxoplasma gondii. PLoS Pathog. 2007;3:e14. doi: 10.1371/journal.ppat.0030014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 26.Bekpen C, Hunn JP, Rohde C, Parvanova I, Guethlein L, Dunn DM, Glowalla E, Leptin M, Howard JC. The interferon-inducible p47 (IRG) GTPases in vertebrates: loss of the cell autonomous resistance mechanism in the human lineage. Genome Biol. 2005;6:R92. doi: 10.1186/gb-2005-6-11-r92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor GA, Feng CG, Sher A. Control of IFN-gamma-mediated host resistance to intracellular pathogens by immunity-related GTPases (p47 GTPases) Microbes Infect. 2007;9:1644–1651. doi: 10.1016/j.micinf.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Coers J, Bernstein-Hanley I, Grotsky D, Parvanova I, Howard JC, Taylor GA, Dietrich WF, Starnbach MN. Chlamydia muridarum evades growth restriction by the IFN-gamma-inducible host resistance factor Irgb10. J Immunol. 2008;180:6237–6245. doi: 10.4049/jimmunol.180.9.6237. [DOI] [PubMed] [Google Scholar]
- 29.Fox BA, Bzik DJ. De novo pyrimidine biosynthesis is required for virulence of Toxoplasma gondii. Nature. 2002;415:926–929. doi: 10.1038/415926a. [DOI] [PubMed] [Google Scholar]
- 30.Zhao Y, Wilson D, Matthews S, Yap GS. Rapid elimination of Toxoplasma gondii by gamma interferon-primed mouse macrophages is independent of CD40 signaling. Infect Immun. 2007;75:4799–4803. doi: 10.1128/IAI.00738-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zerrahn J, Schaible UE, Brinkmann V, Guhlich U, Kaufmann SH. The IFN-inducible Golgi- and endoplasmic reticulum- associated 47-kDa GTPase IIGP is transiently expressed during listeriosis. J Immunol. 2002;168:3428–3436. doi: 10.4049/jimmunol.168.7.3428. [DOI] [PubMed] [Google Scholar]
- 32.Martens S, Sabel K, Lange R, Uthaiah R, Wolf E, Howard JC. Mechanisms regulating the positioning of mouse p47 resistance GTPases LRG-47 and IIGP1 on cellular membranes: retargeting to plasma membrane induced by phagocytosis. J Immunol. 2004;173:2594–2606. doi: 10.4049/jimmunol.173.4.2594. [DOI] [PubMed] [Google Scholar]