Abstract
Multidetector CT (MDCT) with 64-slice capability continues to gain momentum for cardiovascular imaging. Beyond images of coronary arteries, it also provides reliable information on left ventricular structure and function, cardiac venous anatomy, the pulmonary venous system, and right ventricular function—all aspects important in the management of heart failure patients. Potential unique applications in heart failure include cardiac dyssynchrony evaluation, assessing cardiomyopathies, and post-transplant annual follow-up. This review details the multiple applications and limitations of MDCT in the heart failure population, including comparison with other commonly used imaging modalities such as echocardiography and MRI.
Introduction
The introduction of the current generation of multidetector CT (MDCT) scanners with improved temporal and spatial resolution has allowed noninvasive imaging of coronary arteries and provides information on other cardiac structures and their function. These include left and right ventricular function and structure, atrial anatomy, cardiac and pulmonary venous systems assessment, and the pericardium. Evaluation of these structures is important in the assessment and management of heart failure (HF) patients. With increased utilization of devices in HF such as implantable cardioverter defibrillators (ICDs) and pacemakers and subsequent limitation of the use of MRI, cardiac MDCT allows an alternative noninvasive option for assessing these patients. Over the last decade, the four-slice MDCT system technology and software innovations evolved to eight- and 16-slice to the current 64-slice MDCT system in 2004. The system refinements include increased gantry rotation speed combined with electrocardiogram-gated images, allowing cardiac phase–correlated image reconstruction retrospectively for specified images during desired phases of the cardiac cycle.
Scanning Patients With HF
Breath-hold
Via simultaneous acquisition of 64 parallel cross-sections, image acquisition time is now reduced to 10 to 15 seconds. During this time, patients hold their breath to minimize motion artifact, and this short timeframe for breath-hold makes it possible to achieve good image quality. For most patients with respiratory and cardiac comorbidities, this is feasible.
Radiation exposure
The radiation exposure of MDCT is between 5 and 20 mSv, which is comparable to several other imaging modalities, such as nuclear perfusion tests.
Contrast exposure
Iodinated contrast requirement is typically between 60 and 100 mL for coronary vessel visualization; this may be affected by patient size and scanner type. Because renal dysfunction is prevalent in HF, exposure to contrast media needs special consideration and may limit MDCT use in some patients.
Intravenous β-blockers
The administration of β-blockers intravenously is common prior to MDCT scanning; this may be problematic in some HF patients with low blood pressure. However, most HF patients are likely to be already taking these drugs orally, and lower heart rates at baseline may obviate the need for intravenous β-blockers.
Cardiovascular Evaluation in HF
Left ventricle
Evaluation of the left ventricular structure and function, specifically ejection fraction, is an integral step in determining prognosis and therapy for HF patients [1]. Common clinical practice utilizes two-dimensional echocardiography, a modality that is easily obtained and has no risks. Limitations of echocardiography, however, are inadequate image quality in some patients resulting from body habitus and the inherent geometric assumptions necessary for calculations. MRI is the gold standard for cardiac structure and function evaluation secondary to its excellent spatial resolution, accuracy, and reproducibility of measurements without any geometric assumptions. However, due to the duration and multiple prolonged breath-holds required to obtain adequate images, inability to perform the study in patients with devices, and claustrophobia in some patients, MRI is not always feasible.
Left ventricular volume calculation
Left ventricular volume measurements by MDCT are based on short-axis image reformations (similar to MRI and echocardiography). At the midventricular level, a single image is reconstructed every 5% of the R-R interval to obtain both a diastolic and systolic phase. Appropriate reconstruction windows are identified at the points of the minimum ventricular diameter (approximately 25% of the R-R interval) and the maximum ventricular diameter (approximately 85% of the R-R interval) [2•]. Left ventricular volume can be measured using two approaches. In the Simpson method (also used in MRI), left ventricular volume is calculated by adding all cross-sectional areas of equal width (obtained from contiguous, short-axis images of the left ventricle) multiplied by the section thickness. Despite cumbersome calculation, the Simpson method is quite accurate as it has no reliance on geometric assumptions [3]. The threshold-based, direct-volume measurement method uses a segmentation technique that detects density or signal intensity differences between contrast-filled cardiac chambers and the myocardium. Using a predefined attenuation threshold, the sum of contiguous voxels greater than this threshold is defined as the total chamber volume [4].
Juergens and Fischbach [4] summarized data from 18 studies. They compared MDCT (including four- to 16-slice MDCT) to cine ventriculography, two-dimensional echocardiography, and MRI, specifically evaluating left ventricular end-diastolic volumes, left ventricular end-systolic volumes, and ejection fraction. The correlation coefficients differed minimally among the various studies: left ventricular end-diastolic volume was 0.86 to 0.99, left ventricular end-systolic volume was 0.90 to 0.99, and ejection fraction was 0.80 to 0.99. In another study, 16-slice MDCT was compared with MRI for assessment of left ventricular wall function in a group of patients with high prevalence of wall motion abnormalities, including those with previous myocardial infarction, arrhythmogenic right ventricular cardiomyopathy, and dilated cardiomyopathy. Concordance coefficients for the volumes were as follows: left ventricular end-diastolic volume, pc = 0.96; left ventricular end-systolic volume, pc = 0.943; and left ventricular stroke volume, pc = 0.939. Correlation for left ventricular ejection fraction was moderate (pc = 0.835), with a mean difference and standard deviation of −2.5 ± 4.2 (P = 0.02). Wall motion abnormalities correlated well between modalities, with MDCT and MRI in agreement in 416 of 480 segments (86.7%). However, the correlation was weaker in segments with wall motion impairment; MDCT had the tendency to underestimate the degree of impairment compared with MRI [5].
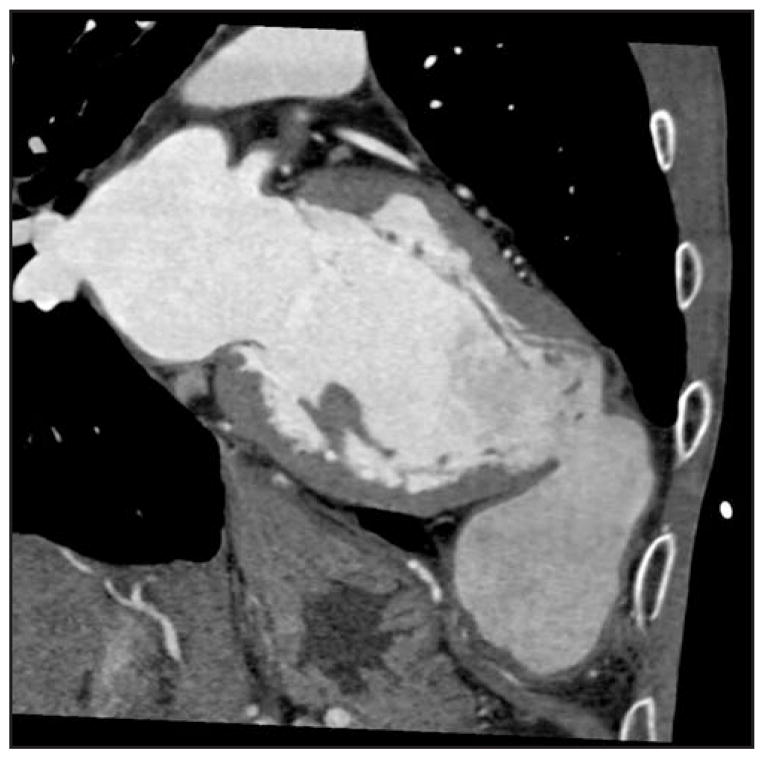
The findings of lower ejection fraction (likely contributed to by overestimation of the mean left ventricular end-diastolic volume/left ventricular end-systolic volume secondary to temporal resolution timing issues with the four- and 16-slice CT) are described in other studies [6]. One study specifically compared the evaluation of left ventricular contractile function via percent systolic wall thickening in MDCT versus wall motion index by transthoracic echocardiography. A significant inverse linear correlation was noted between regional myocardial systolic wall thickening and wall motion index by transthoracic echocardiography (r = −0.8; P < 0.001). Using receiver operating characteristic analysis, the area under the curve was 0.83 (95% CI = 0.80–0.87) to differentiate normal and dysfunctional myocardial segments (wall motion score > 1). For systolic wall thickening, 40% was the optimal cutoff resulting in sensitivity of 76% and specificity of 78%. Systolic wall thickening demonstrated patients with global left ventricular systolic dysfunction with high diagnostic accuracy, including sensitivity of 92% and specificity of 98% for identification of patients with wall motion index greater than 1.5. Even in this study with 64-slice CT, overestimation of left ventricular ejection fraction, end-diastolic volume, and end-systolic volume was seen and was statistically significant (4.8% with 95% lines of agreement ranging from −8.8% to 18.3%, P < 0.001 for all three measurements), but with overall strong correlation of r = 0.85 (P < 0.001) for end-diastolic volume and r = 0.93 (P < 0.001) for end-systolic volume [7]. Butler and colleagues [8] compared 64-slice MDCT in HF patients specifically with transthoracic echocardiography. Correlations with volumes reported were as follows: left ventricular end-diastolic volume, 0.62; left ventricular end-systolic volume, 0.67; and stroke volume, 0.60; all P values were more than 0.15. In this cohort of depressed ejection fraction (mean ejection fraction 36% ± 8% with echocardiography, versus 38% ± 12% by MDCT; r = 0.67, P = 0.28), wall thickness (r = 0.76) and regional wall motion assessment were not significantly different by the two techniques [8]. Overall, MDCT appears to correlate well with both MRI and transthoracic echocardiography for left ventricular assessment and has been specifically evaluated recently in HF patients. However, clinicians need to be aware that MDCT-derived values for volumes and ejection fraction are slightly, yet systematically, higher compared with transthoracic echocardiography–derived values. It is worth noting that MDCT is a true volumetric method; remodeling of the chambers should not influence the accuracy. Figure 1 depicts structural remodeling in a patient with dilated cardiomyopathy with a pseudoaneurysm. Although MDCT may not be a first-line modality for left ventricular assessment, it is important to know its potential for detailed left ventricular evaluation. It can be considered as an alternative in specific situations, and MDCT performed for coronary imaging can report these measures routinely.
Figure 1.
Left ventricle pseudoaneurysm located adjacent to the chest wall using multidetector CT in a patient with dilated cardiomyopathy.
Myocardial infarct and viability assessment
The assessment of microvascular flow following reperfusion after an acute myocardial infarction has been shown in recent literature to help determine viability versus scar, as well as prognosis. Size of the infarct is one of the most significant predictors of clinical outcome and long-term left ventricular function [9]. The “delayed enhancement” phenomenon is the cornerstone for differentiation of scar versus viability with MRI [10]. Attenuation differences between normal and infarcted myocardium help to accurately characterize infarct size, which is correlated with cardiac enzyme release in not only nuclear modalities and MRI but recently in MDCT [10–13]. Dual-phase evaluation in MDCT helps yield a more detailed vantage of recovery in the chronic infarcted states. However, the increased radiation for obtaining both phases of the images should be noted. The advantage of CT in these settings is that it has a shorter acquisition time (10–30 seconds) and can accommodate metal devices (such as pacemakers, ICDs, and infusion pumps) [14]. MRI assessment remains the gold standard in this field; MDCT, although touted for other myocardial assessments, has not matured in this arena.
Cardiac dyssynchrony
Cardiac resynchronization therapy, a new therapy applied to patients with advanced HF via the implantation of a biventricular pacemaker, is a therapeutic option in patients with left ventricular dysfunction (≤ 35%), a wide QRS complex (≥ 120 ms), and New York Heart Association class III/IV symptoms. This therapy has been shown to improve symptoms and ventricular size and function while decreasing hospitalizations and mortality [15,16]. Tissue velocity imaging via echocardiography is the most commonly used technique to identify intraventricular dyssynchrony [17]. To improve success rates of cardiac resynchronization, not only does the amount of left ventricular dyssynchrony help predict response, but also correlating venous anatomy to the area of last ventricular activation and avoiding areas of myocardial scar to maximize cardiac resynchronization therapy efficacy. Pre-evaluation via MDCT can be used for the feasibility of a transvenous approach versus a minimally invasive surgical approach for left ventricle lead implantation. In a recent study, 64-slice MDCT was compared with invasive venography, each utilizing tissue velocity imaging. Strong correlation coefficients (r = 0.82–0.95) between MDCT and venography were observed with quantitative assessment of multiple cardiac veins [18]. Recently, Truong and colleagues [19••] evaluated 64-slice MDCT using a novel CT-based measure of left ventricular dyssynchrony, a “dyssynchrony index,” which is based on timing of changes in wall thickness. They compared patients with HF and wide QRS, HF patients with narrow QRS, and age-matched controls. The mean dyssynchrony index was significantly different for all three groups (P < 0.001). The mean dyssynchrony index was 152 ± 44 ms (HF patients with wide QRS), 121 ± 58 ms (HF patients with narrow QRS), and 65 ± 12 ms (age-matched controls), with significance in means attained with control subjects and HF patients but no significant difference between the two HF groups (P = 0.131). Compared with two-dimensional echocardiography dyssynchrony assessment (with speckle tracking longitudinal strain), good correlation was seen (r = 0.65, P = 0.012). A similar correlation was seen with three-dimensional echocardiography dyssynchrony assessment (r = 0.68, P = 0.008). The number of patients with all three modalities performed was limited in this study (n = 13), and the dyssynchrony metrics between modalities are not directly comparable; thus, these results must be cautiously interpreted. The major limitation with MDCT in dyssynchrony evaluation has been temporal resolution. Other newer modalities, such as dual-source CT (which provides improved temporal resolution below 100 ms), might be comparable to other technology, such as three-dimensional echocardiography and cardiac MRI, in evaluating dyssynchrony precisely.
Cardiac venous system
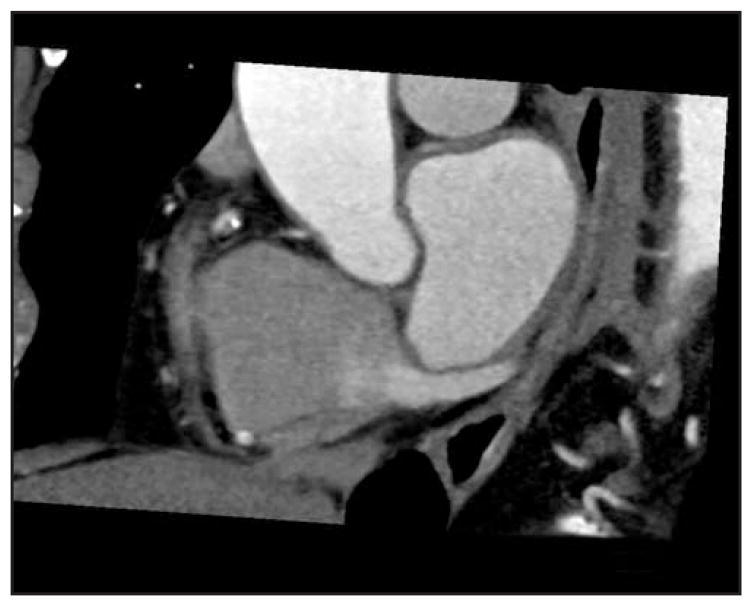
Multiple studies have investigated the utility of MDCT for assessing the coronary venous system [20,21]. Invasive procedures such as coronary sinus lead positioning for biventricular pacing and ablation procedures are not uncommon in HF. Figure 2 depicts coronary sinus vein visualization using MDCT. A dilated, asynchronous left ventricle can prove technically challenging, with tortuosity, displacement, acute angulation, and even obstruction of veins on the epicardial surface [22]. Recently, Chen and colleagues [23•] evaluated MDCT specifically in systolic HF patients. Using MDCT, they compared systolic HF patients with age-matched controls and were able to clearly visualize all coronary veins in 46 patients. Total coronary venous length was positively correlated with left ventricular volume (r = 0.65, P < 0.001). Secondary angle of the coronary sinus relative to the superior vena cava axis was more acute in systolic HF patients than normal patients (30° ± 7° and 44° ± 8°, respectively; P < 0.001). Using this modality for determining feasibility and approach may help minimize time and risk involved with invasive procedures.
Figure 2.
Visualization of the coronary sinus with 64-slice multidetector CT.
Coronary artery imaging
In HF, various indications prompt coronary artery imaging. Evaluation of new-onset HF and patients with known HF and previous revascularizations, recurrence of angina, or escalations of HF symptoms may require coronary imaging. MDCT has been shown to have very high negative predictive value, high sensitivity, and high specificity in evaluating severe stenosis. Details of coronary artery imaging, evaluation of coronary artery bypass grafts, and in-stent restenosis with MDCT compared with coronary angiography are outside the scope of this review and are discussed elsewhere.
Pulmonary veins
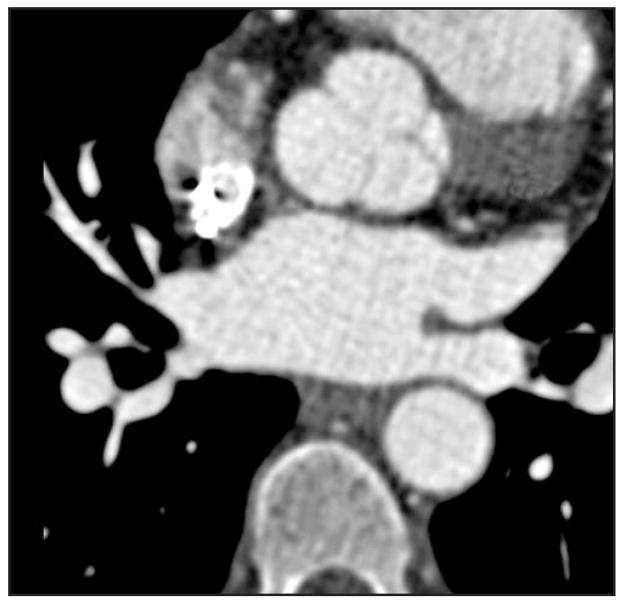
Radiofrequency catheter ablation is a newer therapy used to treat refractory atrial fibrillation. Atrial fibrillation is common in the HF population and is associated with worse outcomes. Precise mapping of the anatomic origin of these arrhythmogenic foci, the atriopulmonary venous junction, must be delineated. Pulmonary vein isolation remains the target in most current ablation strategies; however, other sites, such as the coronary sinus or left atrial appendage, may be necessary to isolate in some patients [24]. Intracardiac echocardiography and nonfluoroscopic three-dimensional mapping and navigation systems are helpful for catheter manipulation and specific ablation maneuvers; however, their limitations with operator dependability, geometric approximations, and lack of anatomic precision are well-recognized [25]. Cardiac MRI and cardiac MDCT use for three-dimensional mapping has been well-delineated in the past several years. In HF patients with metallic devices, MRI may not always be possible. The detailed 0.5-mm spatial resolution and operator independence favors MDCT use. Imaging reconstruction models involve three-dimensional volume-based images (including endoscopic images), which provide anatomic details of the pulmonary vein and left atrium from not only external vantage points but also endoscopic views as well [26]. Postprocedural complications of ablation include pulmonary vein stenosis and pleural or pericardial effusions, all of which can be detected with MDCT [27]. Figure 3 shows pulmonary vein visualization using MDCT.
Figure 3.
Pulmonary vein mapping using multidetector CT with clear delineation of surrounding structures.
Post–cardiac transplant
Surveillance of the post–heart transplant patient often includes a routine assortment of invasive and noninvasive assessments, including angiography for the evaluation of coronary allograft vasculopathy, endomyocardial biopsies for graft rejection, and imaging via two-dimensional echocardiography for functional and structural assessments. Coronary allograft vasculopathy, an aggressive form of coronary disease in the post–cardiac transplant patient known to limit survival, develops in 10% of recipients each year. A distinct entity from traditional coronary disease, coronary allograft vasculopathy is a diffuse process that often initiates in distal vessels with eventual involvement of the entire intramyocardial and epicardial arteries of the allograft. Pathologically, concentric intimal hyperplasia is found involving cells (T lymphocytes and macrophages) and smooth muscle proliferation. Aggressive intimal proliferation and persistence of an intact internal elastic lamina and media, with rare calcium, are hallmark histologic features of coronary allograft vasculopathy [28]. Over a decade ago it was found that routine angiography, limited with its outline of the coronary lumen via contrast, underestimated early lesions with less than 25% luminal narrowing [29,30]. Intravascular ultrasound, a catheter with sonography capabilities, has proven to be a much more sensitive modality to detect intimal changes and has been used to predict morbidity and mortality in post-transplant patients [31,32]. In a study comparing 64-slice MDCT with coronary angiography with intravascular ultrasound, investigators demonstrated 37% coronary allograft vasculopathy detection in MDCT (compared to 46% with intravascular ultrasound). In all segments, MDCT compared with intravascular ultrasound showed 70% sensitivity, 92% specificity, and negative predictive value 77%, with an accuracy of 82%. Excellent correlation of vessel diameter measurements was found with invasive quantitative coronary angiography (r = 0.89). Non-evaluable segments (17%) by MDCT were located mainly in the distal coronary arteries, which limits evaluation in early and small-vessel involvement of coronary allograft vasculopathy [33]. General limitations of MDCT in vessel evaluation may have higher hurdles in the transplant patient. Higher baseline heart rates with the denervated transplanted hearts can be problematic. With frequent coronary allograft vasculopathy evaluation in this population, the increased radiation dose compared with standard angiography could cumulatively prove to be substantial in the post-transplant years.
MDCT has been compared with two-dimensional echocardiography for left ventricular structure and function in post-transplant patients. Ferencik and colleagues [34] used 64-slice MDCT in heart transplant recipients to compare cardiac dimensions, mass, and function with two-dimensional echocardiography. Although MDCT trended to have decreased values of ejection fraction compared with echocardiography, this difference was not statistically significant (mean difference −2% ± 9%, P = 0.29). Left ventricular mass, left ventricular end-diastolic volume, left ventricular end-systolic volume, and left atrial diameter were significantly different between the two modalities; volume and diameter measurements tended to be higher using MDCT. These differences may be explained by the inherent geometric assumptions and limitations that are forced with two-dimensional echocardiography compared with three-dimensional modalities (eg, MDCT and MRI) [34].
Right ventricle
Prognosis in HF patients is strongly associated with right ventricular function. As previously discussed, MDCT does not make geometric assumptions like echocardiography. With image reconstruction during any point in the cardiac cycle with flexible orientation, visualization and assessment of the complex right ventricular shape can be performed [35]. Plumhans and colleagues [36•] evaluated right ventricle assessment via electrocardiogram-gated 64-slice MDCT compared with MRI and found that the mean right ventricular ejection fraction (± standard deviation) was 51% ± 7.8% (51.4% ± 7.3% via MRI), with excellent correlations for ejection fraction (r = 0.97), right ventricular end-diastolic volume (r = 0.99), end-systolic volume (r = 0.98), and stroke volume (r = 0.98). Similar to left ventricular volumetric assessment, MDCT was noted to have a slight overestimation.
Cardiomyopathies
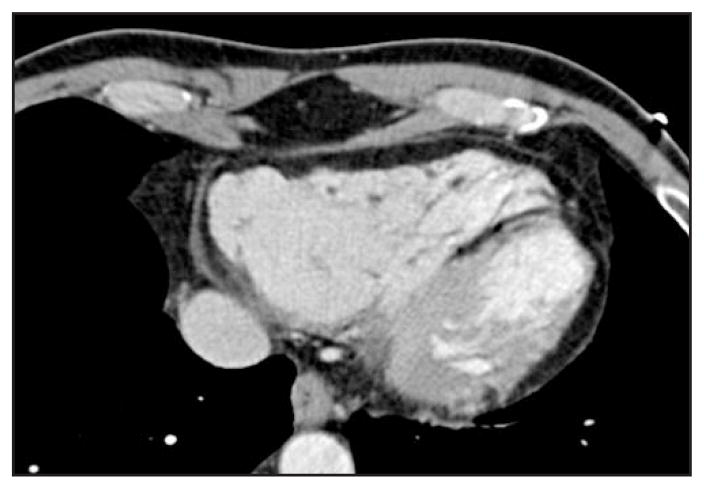
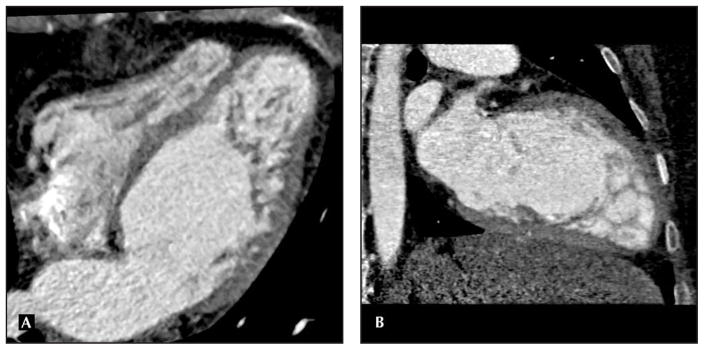
Intrinsic cardiomyopathies often have distinct clinical and structural attributes that allow for possible diagnosis via imaging modalities. Hypertrophic cardiomyopathy is characterized by extreme hypertrophy of the left ventricle often involving the interventricular septum and left ventricular outflow tract. Percutaneous transluminal septal myocardial ablation is a procedure in which an ethanol injection into septal branches of the localized hypertrophic myocardium induces an “intentional” myocardial necrosis leading to decreased left ventricular outflow gradients, thinning of hypertrophic wall, and improvement in clinical symptoms. MDCT pre-procedure has been used for simultaneous delineation of the septal branches with their relationship to the coronary anatomy and myocardium, thus increasing precision [37]. Other modalities, such as transthoracic echocardiography or coronary angiography, only provide coronary anatomy or structural details, but not both. Arrhythmogenic right ventricular dysplasia is a genetic cardiomyopathy in which there is a replacement of right ventricular musculature by fibrofatty tissue, leading to ventricular arrhythmias and right ventricular failure. Hallmark morphologic features of arrhythmogenic right ventricular dysplasia include disruption of epicardial fat and underlying myocardium demarcation, focal right ventricle wall thinning (secondary to apoptotic myocyte loss), right ventricle wall thickening, trabecular disarray, and right ventricle outflow tract enlargement (Fig. 4) MRI and MDCT were recently shown to be excellent modalities for diagnosing arrhythmogenic right ventricular dysplasia. Tandri and colleagues [38] studied 13 prospectively diagnosed patients with arrhythmogenic right ventricular dysplasia. They observed good correlation between right ventricular end-diastolic volume and right ventricular inflow diameter, with only one of the 13 patients showing left ventricular involvement with wall thinning and fat infiltration. However, with the improved temporal resolution and good fat discrimination of 64-slice MDCT, it will continue to have a more involved role in these patients [39••]. Noncompaction cardiomyopathy is a congenital abnormality arising from intrauterine failure of normal compaction of endomyocardial trabeculations that form during the first trimester. It leads to development of a thin epicardial layer and thickened, trabeculated spongiform-like endocardial layer. Echocardiography is the current standard imaging modality, with characteristic findings of an increased ratio of 2 or more for noncompacted to compacted areas at end-systole with two-layered left ventricular wall and segmental thickening, with localization often in apical, mid-lateral, and mid-inferior regions of the left ventricle (Fig. 5). Case studies have evaluated this entity using MDCT, with clear delineation of the affected epicardial border [40,41].
Figure 4.
Arrhythmogenic right ventricular dysplasia—note the trabecular disarray in the affected myocardium and focal right ventricular wall thickening.
Figure 5.
A and B, Left ventricular noncompaction illustrated in two views. Note the increased ratio of ≥ 2 between noncompacted to compacted myocardium.
MDCT Limitations
MDCT is evolving with increasing applicability to the HF population; however, clinicians must recognize some inherent limitations in HF patients beyond the general issues with MDCT. Slower and regular heart rates are important for adequate image acquisition. Beat-to-beat variability at low heart rates and premature atrial/ventricular beats can generate image artifact, particularly for visualization of finer structures (ie, coronary vessels). Also, up to a third of HF patients may have atrial fibrillation. Breath-hold can be an issue for HF patients with dyspnea. Contraindications such as contrast dye allergies and moderate to severe renal insufficiency can preclude the use of MDCT. Repetitive use of MDCT may pose an issue in regard to radiation exposure.
Conclusions
MDCT provides an accurate and detailed evaluation of cardiac structure and function that may aid HF management. Further studies will continue to define and refine its role in this population.
Footnotes
Disclosure
No potential conflicts of interest relevant to this article were reported.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 2•.Orakzai SH, Orakzai RH, Nasir K, Budoff MJ. Assessment of cardiac function using multidetector row computed tomography. J Comput Assist Tomogr. 2006;30:555–563. doi: 10.1097/00004728-200607000-00001. This comprehensive article details cardiac function assessment using MDCT, with comparison of other modalities with MDCT. [DOI] [PubMed] [Google Scholar]
- 3.Juergens KU, Grude M, Maintz D, et al. Multi-detector row CT of left ventricular function with dedicated analysis software versus MR imaging: initial experience. Radiology. 2004;230:403–410. doi: 10.1148/radiol.2302030042. [DOI] [PubMed] [Google Scholar]
- 4.Juergens KU, Fischbach R. Left ventricular function studied with MDCT. Eur Radiol. 2006;16:342–357. doi: 10.1007/s00330-005-2888-5. [DOI] [PubMed] [Google Scholar]
- 5.Fischbach R, Juergens KU, Ozgun M, et al. Assessment of regional left ventricular function with multidetector-row computed tomography versus magnetic resonance imaging. Eur Radiol. 2007;17:1009–1017. doi: 10.1007/s00330-006-0438-4. [DOI] [PubMed] [Google Scholar]
- 6.Grude M, Juergens KU, Wichter T, et al. Evaluation of global left ventricular myocardial function with electrocardiogram-gated multidetector computed tomography: comparison with magnetic resonance imaging. Invest Radiol. 2003;38:653–661. doi: 10.1097/01.rli.0000077070.40713.76. [DOI] [PubMed] [Google Scholar]
- 7.Kristensen TS, Kofoed KF, Moller DV, et al. Quantitative assessment of left ventricular systolic wall thickening using multidetector computed tomography. Eur J Radiol. 2008 Aug 5; doi: 10.1016/j.ejrad.2008.06.028. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 8.Butler J, Shapiro MD, Jassal DS, et al. Comparison of multidetector computed tomography and two-dimensional transthoracic echocardiography for left ventricular assessment in patients with heart failure. Am J Cardiol. 2007;99:247–249. doi: 10.1016/j.amjcard.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Burns RJ, Gibbons RJ, Yi Q, et al. The relationships of left ventricular ejection fraction, end-systolic volume index and infarct size to six-month mortality after hospital discharge following myocardial infarction treated by thrombolysis. J Am Coll Cardiol. 2002;39:30–36. doi: 10.1016/s0735-1097(01)01711-9. [DOI] [PubMed] [Google Scholar]
- 10.Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 11.Baks T, Cademartiri F, Moelker AD, et al. Assessment of acute reperfused myocardial infarction with delayed enhancement 64-MDCT. AJR Am J Roentgenol. 2007;188:W135–W137. doi: 10.2214/AJR.05.1176. [DOI] [PubMed] [Google Scholar]
- 12.Sato A, Hiroe M, Nozato T, et al. Early validation study of 64-slice multidetector computed tomography for the assessment of myocardial viability and the prediction of left ventricular remodelling after acute myocardial infarction. Eur Heart J. 2008;29:490–498. doi: 10.1093/eurheartj/ehm630. [DOI] [PubMed] [Google Scholar]
- 13.Cury RC, Nieman K, Shapiro MD, et al. Comprehensive assessment of myocardial perfusion defects, regional wall motion, and left ventricular function by using 64-section multidetector CT. Radiology. 2008;248:466–475. doi: 10.1148/radiol.2482071478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koyama Y, Mochizuki T, Higaki J. Computed tomography assessment of myocardial perfusion, viability, and function. J Magn Reson Imaging. 2004;19:800–815. doi: 10.1002/jmri.20067. [DOI] [PubMed] [Google Scholar]
- 15.Cazeau S, Leclercq C, Lavergne T, et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001;344:873–880. doi: 10.1056/NEJM200103223441202. [DOI] [PubMed] [Google Scholar]
- 16.Swedberg K, Cleland J, Dargie H, et al. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): The Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26:1115–1140. doi: 10.1093/eurheartj/ehi204. [DOI] [PubMed] [Google Scholar]
- 17.Bank AJ, Kelly AS. Tissue Doppler imaging and left ventricular dyssynchrony in heart failure. J Card Fail. 2006;12:154–162. doi: 10.1016/j.cardfail.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Van de Veire NR, Marsan NA, Schuijf JD, et al. Noninvasive imaging of cardiac venous anatomy with 64-slice multi-slice computed tomography and noninvasive assessment of left ventricular dyssynchrony by 3-dimensional tissue synchronization imaging in patients with heart failure scheduled for cardiac resynchronization therapy. Am J Cardiol. 2008;101:1023–1029. doi: 10.1016/j.amjcard.2007.11.052. [DOI] [PubMed] [Google Scholar]
- 19••.Truong QA, Singh JP, Cannon CP, et al. Quantitative analysis of intraventricular dyssynchrony using wall thickness by multidetector computed tomography. JACC Cardiovasc Imaging. 2008;1:772–781. doi: 10.1016/j.jcmg.2008.07.014. This study utilizes a new dyssynchrony index using MDCT for identification of dyssynchrony, compared with two-dimensional echocardiography. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jongbloed MR, Lamb HJ, Bax JJ, et al. Noninvasive visualization of the cardiac venous system using multislice computed tomography. J Am Coll Cardiol. 2005;45:749–753. doi: 10.1016/j.jacc.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 21.Van de Veire NR, Schuijf JD, Sutter J, et al. Non-invasive visualization of the cardiac venous system in coronary artery disease patients using 64-slice computed tomography. J Am Coll Cardiol. 2006;48:1832–1838. doi: 10.1016/j.jacc.2006.07.042. [DOI] [PubMed] [Google Scholar]
- 22.Leon AR, Delurgio DB, Mera F. Practical approach to implanting left ventricular pacing leads for cardiac resynchronization. J Cardiovasc Electrophysiol. 2005;16:100–105. doi: 10.1046/j.1540-8167.2005.04600.x. [DOI] [PubMed] [Google Scholar]
- 23•.Chen JJ, Lee WJ, Wang YC, et al. Morphologic and topologic characteristics of coronary venous system delineated by noninvasive multidetector computed tomography in chronic systolic heart failure patients. J Card Fail. 2007;13:482–488. doi: 10.1016/j.cardfail.2007.02.007. This study provides a detailed evaluation of the coronary venous system in patients with systolic HF. [DOI] [PubMed] [Google Scholar]
- 24.Haissaguerre M, Sanders P, Hocini M, et al. Catheter ablation of long-lasting persistent atrial fibrillation: critical structures for termination. J Cardiovasc Electrophysiol. 2005;16:1125–1137. doi: 10.1111/j.1540-8167.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 25.Hsu LF. Image integration for catheter ablation: searching for the perfect match. Heart Rhythm. 2008;5:536–537. doi: 10.1016/j.hrthm.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 26.Niinuma H, George RT, Arbab-Zadeh A, et al. Imaging of pulmonary veins during catheter ablation for atrial fibrillation: the role of multi-slice computed tomography. Europace. 2008;10(Suppl 3):iii14–iii21. doi: 10.1093/europace/eun230. [DOI] [PubMed] [Google Scholar]
- 27.Lacomis JM, Wigginton W, Fuhrman C, et al. Multi-detector row CT of the left atrium and pulmonary veins before radiofrequency catheter ablation for atrial fibrillation. Radiographics. 2003;23:S35–S48. doi: 10.1148/rg.23si035508. discussion S48–S50. [DOI] [PubMed] [Google Scholar]
- 28.Aranda JM, Jr, Hill J. Cardiac transplant vasculopathy. Chest. 2000;118:1792–1800. doi: 10.1378/chest.118.6.1792. [DOI] [PubMed] [Google Scholar]
- 29.Johnson DE, Alderman EL, Schroeder JS, et al. Transplant coronary artery disease: histopathologic correlations with angiographic morphology. J Am Coll Cardiol. 1991;17:449–457. doi: 10.1016/s0735-1097(10)80114-7. [DOI] [PubMed] [Google Scholar]
- 30.St Goar FG, Pinto FJ, Alderman EL, et al. Intracoronary ultrasound in cardiac transplant recipients. In vivo evidence of “angiographically silent” intimal thickening. Circulation. 1992;85:979–987. doi: 10.1161/01.cir.85.3.979. [DOI] [PubMed] [Google Scholar]
- 31.Kobashigawa JA, Tobis JM, Starling RC, et al. Multicenter intravascular ultrasound validation study among heart transplant recipients: outcomes after five years. J Am Coll Cardiol. 2005;45:1532–1537. doi: 10.1016/j.jacc.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 32.Tuzcu EM, Kapadia SR, Sachar R, et al. Intravascular ultrasound evidence of angiographically silent progression in coronary atherosclerosis predicts long-term morbidity and mortality after cardiac transplantation. J Am Coll Cardiol. 2005;45:1538–1542. doi: 10.1016/j.jacc.2004.12.076. [DOI] [PubMed] [Google Scholar]
- 33.Gregory SA, Ferencik M, Achenbach S, et al. Comparison of sixty-four-slice multidetector computed tomographic coronary angiography to coronary angiography with intravascular ultrasound for the detection of transplant vasculopathy. Am J Cardiol. 2006;98:877–884. doi: 10.1016/j.amjcard.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 34.Ferencik M, Gregory SA, Butler J, et al. Analysis of cardiac dimensions, mass and function in heart transplant recipients using 64-slice multi-detector computed tomography. J Heart Lung Transplant. 2007;26:478–484. doi: 10.1016/j.healun.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 35.Raman SV, Shah M, McCarthy B, et al. Multi-detector row cardiac computed tomography accurately quantifies right and left ventricular size and function compared with cardiac magnetic resonance. Am Heart J. 2006;151:736–744. doi: 10.1016/j.ahj.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 36•.Plumhans C, Muhlenbruch G, Rapaee A, et al. Assessment of global right ventricular function on 64-MDCT compared with MRI. AJR Am J Roentgenol. 2008;190:1358–1361. doi: 10.2214/AJR.07.3022. This is a comparison of MRI with MDCT for evaluation of right ventricular function. [DOI] [PubMed] [Google Scholar]
- 37.Mitsutake R, Miura S, Sako H, et al. Usefulness of multi-detector row computed tomography for the management of percutaneous transluminal septal myocardial ablation in patients with hypertrophic obstructive cardiomyopathy. Int J Cardiol. 2008;129:e61–e63. doi: 10.1016/j.ijcard.2007.06.132. [DOI] [PubMed] [Google Scholar]
- 38.Tandri H, Bomma C, Calkins H, Bluemke DA. Magnetic resonance and computed tomography imaging of arrhythmogenic right ventricular dysplasia. J Magn Reson Imaging. 2004;19:848–858. doi: 10.1002/jmri.20078. [DOI] [PubMed] [Google Scholar]
- 39••.Sparrow P, Merchant N, Provost Y, et al. Cardiac MRI and CT features of inheritable and congenital conditions associated with sudden cardiac death. Eur Radiol. 2009;19:259–270. doi: 10.1007/s00330-008-1169-5. This is a comprehensive review of congenital and inheritable cardiomyopathies; it outlines multiple imaging modalities for accurate diagnosis of the various cardiomyopathies. [DOI] [PubMed] [Google Scholar]
- 40.Ito H, Dajani KA. A case with noncompaction of the left ventricular myocardium detected by 64-slice multidetector computed tomography. J Thorac Imaging. 2009;24:38–40. doi: 10.1097/RTI.0b013e31818965f1. [DOI] [PubMed] [Google Scholar]
- 41.Jenni R, Oechslin E, Schneider J, et al. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: a step towards classification as a distinct cardiomyopathy. Heart. 2001;86:666–671. doi: 10.1136/heart.86.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]