Abstract
Objective
Periodontal disease is an inflammatory disorder with widespread morbidities involving both oral and systemic health. The primary goal of periodontal treatment is the regeneration of the lost or diseased periodontium. In this study, we retrospectively examined feasibility and safety of reconstructing the periodontal intrabony defects with autologous periodontal ligament progenitor (PDLP) implantation in three patients.
Materials and Methods
In this retrospective pilot study, we treated 16 teeth with at least one deep intrabony defect of probing depth (PD) ≥ 6 mm with PDLP transplantation and evaluated clinical outcome measures in terms of probing depth, gingival recession and attachment gain for a duration of 32–72 months. Furthermore, we compare PDLPs with standard PDL stem cells (PDLSCs) and confirmed that PDLPs possessed progenitor characters.
Results
Clinical examination indicated that transplantation of PDLPs may provide therapeutic benefit for the periodontal defects. All treated patients showed no adverse effects during the entire course of follow up. We also found that PDLPs were analogous to PDLSCs in terms of high proliferation, expression of mesenchymal surface molecules, multipotent differentiation, and in vivo tissue regain. However, PDLPs failed to express scleraxis, a marker of tendon, as seen in PDLSCs.
Conclusions
This study demonstrated clinical and experimental evidences supporting a potential efficacy and safety of utilizing autologous PDL cells in the treatment of human periodontitis.
Keywords: periodontal ligament progenitors, regeneration, periodontitis
Introduction
Periodontitis is a chronic infectious disease of the alveolar bone and tissue surrounding and supporting the teeth, which is a major cause for tooth loss in adults (Kinane and Marshall, 2001; Moutsopoulos and Madianos, 2006). Recent scientific studies suggest that periodontal disease plays a role in a variety of systemic diseases such as diabetes mellitus, cardiovascular disease and premature low birth weight (Kiran et al, 2005; Andriankaja et al, 2006; Geismar et al, 2006; Holmlund et al, 2006; Manau et al, 2008; Mealey and Rose, 2008). Therefore, appropriate treatment for periodontal disease will benefit oral and systemic health overall. Classically, several regenerative approaches were proposed in the treatment of periodontal disease, including guided tissue regeneration, topical application of enamel matrix derivative, and various growth factors (Venezia et al, 2004; Goncalves et al, 2006; Hoffmann et al, 2006; Kaigler et al, 2006; Needleman et al, 2006). However, research indicated that the efficacy of these treatments was lacking in that the regeneration of periodontal tissue was limited. Recently, identification of PDLSCs presented an opportunity for utilizing stem cells to regain periodontal structures in these detrimental cases (Seo et al, 2004; Liu et al, 2008). Prior to PDLSC identification, we conducted autologous cell implantation for periodontitis treatment. Therefore, there was no knowledge on progenitor cell property of these implanted cells. In this study, we characterize stem cell features of implanted cells termed (PDLPs) in comparison with PDLSCs. Then we examined the clinical outcome of three patients previously treated with autologous PDLPs in an effort to provide primary knowledge on the effectiveness of this treatment approach and preliminary clinical evidence for randomized controlled trial in the future.
Material and methods
Periodontitis patients
Three male patients, 25 (patient nos. 1 and 2) and 42 (patient no. 3) years of age, with periodontitis and pocket depth from 4.8 to 10 mm were selected for autologous PDLP implantation. There were 12 teeth involved in patient no. 1, 3 teeth in patient no. 2 and 1 tooth in patient no. 3. All patients gave informed consent to enroll in the clinical treatment from November of 1997 to December of 2001 and were followed up for 32–72 months. This clinical study was approved by the Ethics Committee of the Institutional Review Board of Tri-Service General Hospital, National Defense Medical Center Taiwan (#096-05-142).
Inclusion criteria included
No systemic diseases or pregnancy, no smoking, no regular medications for any reason and/or no use of recreational drugs. Selected patients had chronic generalized periodontitis, completion of the initial treatment including subgingival scaling and root planning, compliance with the maintenance program, presence of at least one deep intrabony defect with probing depth of ≥6 mm. All selected teeth were vital, free of radiographic signs of periapical abscesses, free of carious lesions in the region of the bony defect. Some teeth showed furcation area involvement. All three patients had third molar available for PDLP isolation. The patients attended multiple sessions of oral hygiene technique training for 3 months prior to PDLP transplantation surgery.
Culture and expansion of human PDLPs
Six third molars were extracted atraumatically from patients following standard treatment protocol and approved by Institutional Review Board (IRB). PDL tissues were separated from tooth roots and processed using tissue explantation method. Briefly, PDLP cells were passaged and cultured on 10 × 10 cm culture dishes in alpha minimum essential medium (αMEM) supplemented with 10% fetal bovine serum (FBS). Bone grafting material CALCITITE 4060-2 (40–60 mesh size; Zimmer Dental Inc., Carlsbad, CA, USA) was added on the surface of cultured PDLPs at day 3 at passage no. 1. After reaching full confluency, PDLPs were cultured in the absence of serum overnight and washed for five times using phosphate buffered-saline (PBS). The PDLP/CALCITITE 4060-2 construct was removed as a sheet of tissue for transplantation studies. Although this experiment was conducted prior to the good laboratory practice guideline for cell therapy was issued, we had IRB approval for PDL cell culture.
Transplantation of autologous PDLPs in periodontitis patients
During PDLP transplantation surgery, a mucoperiosteal flap was raised and inflammatory periodontal tissues were removed using routine surgery approach. After modifying the margin of alveolar bone, the PDLP/CALCITITE 4060–2 were inserted into periodontal defect areas and sutured. Only deep intrabony defects of pocket depth ≥6 mm are treated by PDLP transplantation.
Clinical evaluation
Clinical outcome measures including plaque index, sulcus bleeding index, probing depth (PD), gingival recession, and clinical attachment gain, were recorded at presurgery and post-PDLP transplantation from 3 to 72 months. Depths of the intrabony defects were measured and defect types (one-, two-, or three-walled defects) were recorded prior to the PDLP pretransplantation. All measurements were made using periodontal probe (Hu Friedy, Chicago, IL, USA) by a blinded examiner at three independent trials and the average was recorded. In patient no. 2, reentry surgery was conducted at 72 months post-PDLP implantation.
Postoperative care and re-examination
The patients were instructed to use 0.2% chlorhexidine daily and avoid brushing the surgical areas until 4 weeks postsurgery. The patients were followed up and re-examined individually as shown in the Results.
Calibration
All clinical measurements were conducted by the first author. Intra-examiner reproducibility was assessed by duplicate PPD measurements. Intra-examiner Intra Class Correlation ICC was 95%.
Samples and cell culture
For in vitro experiments, extracted teeth were obtained from donors at dental clinic of University of Southern California (USC) following the approved IRB guidelines. Periodontal ligaments were gently separated from the surface of the root and rinsed with PBS for twice. The PDL was separated into two parts, one was for PDLP isolation and another was for PDLSC isolation. PDLPs were isolated using explanation outgrowth method. Briefly, PDL tissues were minced to small fragment approximately 1 mm3, placed onto 10 cm culture dish (Costar, Cambridge, MA, USA), and incubated for 5 min at 37°C. PDLPs migrated from PDL fragments and attached on the surfaces of culture dishes. Culture media contained αMEM (Invitrogen, Carlsbad, CA, USA), 15% FBS (Equitech-Bio, Kerrville, TX, USA), 100 μM l-ascorbic acid 2-phosphate (WAKO, Tokyo, Japan), 2 mM l-glutamine, and 100 U ml−1 penicillin/100 μg ml−1 streptomycin (Biofluids Inc., Rockville, MD, USA). The primary PDLPs were cultured at 37°C with 5% carbon dioxide. After 2 weeks, PDLPs were passaged (passage no. 1) and used for surface marker analysis and proliferation analysis. PDLPs at passage nos. 3–4 were used for in vitro differentiation and in vivo tissue regeneration experiments. For PDLSC isolation and culture expansion, periodontal ligaments were separated from the surface of the root and then digested in a solution of 3 mg ml−1 collagenase type I (Worthington Biochem, Freehold, NJ, USA) and 4 mg ml−1 dispase (Boehringer Mannheim, GmbH, Germany) for 1 h at 37°C. PDL derived from different subjects were pooled together to obtain single cell suspensions by passing the cells through a 70 μm strainer (Falcon, BD Labware, Franklin Lakes, NJ, USA). Single cell suspensions (1 × 104) of PDL were seeded into 10 cm culture dishes (Costar) with α-MEM (GIBCO BRL, Grand Island, NY, USA) supplemented with 15% FBS (Equitech-Bio Inc.), 100 μM l-ascorbic acid 2-phosphate (WAKO, Tokyo, Japan), 2 mM l-glutamine, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin (Biofluids Inc.), then incubated at 37°C in 5% CO2. The cell proliferation assay from day 1 to 8 was performed using cell counting kit-8 (DOJINDO Molecular Tech., Rockville, MD, USA) according to manufacturer's instruction. The absorbance was measured at 450 nm by a color photometer.
Antibodies
For flow cytometric analysis, R-Phycoerythrin (PE) conjugated monoclonal anti-human antibodies to CD34, CD45, CD73, CD105, CD146, and CD166 were purchased from BD Bioscience (San Jose, CA, USA). SSEA4 antibody was purchased from Chemicon (Temecula, CA, USA). 3G5 and STRO-1 antibodies were from Dr. Stan Gronthos (Institute of Medical and Veterinary Science, Australia).
Flow cytometric analysis
The cells were suspended (2 × 105/100 μl) in a wash buffer containing 5% heat-inactivated FBS in PBS. For indirect immunostaining, some of the cells were incubated with 100 μl of antiserum or 1 μg of each antibody or isotype-matched immunoglobulins for 45 min on ice. After washing, samples were incubated with R-PE conjugated secondary antibody for 30 min on ice, and then washed with the wash buffer. For direct immunostaining, the cells were treated with 1 μg of R-PE conjugated antibody for 45 min on ice. After washing, all cells were analyzed using FACSCalibur flow cytometer (BD Bioscience).
Scratch assay for cell motility
PDLSCs and PDLPs (0.2 × 106) were seeded in a 6 cm dish (Costar) and cultured reach to 80% confluence. Monolayer cells were scratched using pipette tips to make cell free strip area and washed with PBS to eliminate floating cells. After 24 and 48 h, cells were fixed and stained with 2% paraformaldehyde (PFA, Sigma, St Louis, MO, USA) and 1% toluidine blue solution in PBS.
Reverse transcriptase polymerase chain reaction
The specific primers used in reverse transcriptase polymerase chain reaction (RT-PCR) are follows: peroxisome proliferatoractivated receptor-γ2 (PPARγ2, GenBank accession no. XM_003059), sense; 5′-CT CCTATTGACCCAGAAAGC-3′ (nucleotides 114–133) antisense; 5′-GTAGAGCTGAGTCTTCTCAG-3′ (nucleotides 441–460); lipoprotein lipase (LPL, GenBank accession no. XM_044682), sense; 5′-ATGGAGAGCAAAGCCCTGCTC-3′ (nucleotides 175–195), antisense 5′-GTTAGGTCCAGCTGGATCGAG-3′ (nucleotides 718–738); runt-related transcription factor 2 (Runx2, GenBank accession no. L40992), sense; 5′-CAGTTCCCAAGCATTTCATCC-3′ (nucleotides 880–900), antisense; 5′-TCAATATGGTCGCCAAACAG-3′ (nucleotides 1304–1323); osteocalcin (OCN, GenBank accession no. X53698), sense; 5′-CATGAGAGCCCTCACA-3′ (nucleotides 18–33), antisense; 5′-AGAGCGACACCCTAGAC-3′ (nucleotides 316–332); scleraxis (SCX, GenBank accession no. BK000280), sense; 5′-CTGGCCTCCAGCTACATCTC-3′ (nucleotide 900–919), antisense; 5′-CTTTCTCTGGTTGCTGAGGC-3′ (1090–1109); human collagen type I (COLI, GenBank accession no. NM_007400), sense; 5′-cctccccagccacaaaga-3′ (nucleotide 86–106), antisense; 5′-cgtcatcgcacaacacct-3′ (nucleotide 326–343); glyceraldehyde 3-phosphate dehydrogenase (GAPDH, GenBank accession no. M33197), sense; 5′-AGCCGCATCTTCTTTTGCGTC-3′ (nucleotides 12–32), antisense; 5′-TCATATTTGGCAGGTTTTT CT-3′ (nucleotides 807–827). Total RNA isolation, first-strand cDNA synthesis, and PCR processes were performed as previously described (Bi et al, 2007).
Transplantation
Approximately 4 × 106 of PDLSCs or PDLPs were transplanted with 40 mg of hydroxyapatite/tricalcium phosphate ceramic particles (HA/TCP, Zimmer, Warsaw, IN, USA) subcutaneously into the dorsal surfaces of 10-week-old immunocompromised mice (Beige XIDIII nude/nude, Harlan Sprague–Dawley, Indianapolis, IN, USA) as previously described (Seo et al, 2004). All animal experiments were performed under an institutionally approved protocol for the use of animal research (USC #10941). The transplants were recovered at 8 weeks post-transplantation, fixed with 4% PFA, decalcified with buffered 5% ethylenediaminetetraacetic acid (pH7.4), and then embedded in paraffin. Sections were used for hematoxylin and eosin (H&E) and trichrome staining.
Statistical analysis
Student's t-test was used to analyze the significance between two groups. P value of less than 0.05 was considered as a significant difference.
Results
Phenotype characterization of PDLPs
As a previous study showed that PDLSCs have a therapeutic capacity in a swine model, it is critical to examine stem cell characteristics of PDLPs that were used to treat periodontitis patients in comparison with PDLSCs. Although both PDLPs and PDLSCs are derived from the PDL, their isolation methods were different (Figure 1a). The most significant disparity in the isolation method was that PDLP was not derived from single colony cluster (Figure 1a). PDLPs and PDLSCs, derived from the same PDL tissues, showed a similar proliferation rate over the first 3 days of in vitro culture (Figure 1b). However, PDLPs proliferated at an accelerated rate from day 4 to 7 in culture (Figure 1b). After 8 days of culture, PDLPs and PDLSCs resulted in the same proliferation capacity (Figure 1b). Flow cytometric analysis can be used to analyze surface molecule expression in single cell suspension via a specific antibody binding to the antigen. In this study, we utilized flow cytometry to indicate that PDLPs were analogous to PDLSCs being negative to hematopoietic markers to CD34 and CD45, but highly expressed positive mesenchymal associated markers CD73, CD166, and 3G5 (Figure 1c). In addition, PDLPs were found to express the cell surface molecules STRO-1, CD146, and SSEA4, three early mesenchymal stem cell (MSC) markers previously found to be present on bone marrow MSCs. The expression levels of CD146 (40%), SSEA4 (49%), and STRO-1 (22%) in PDLPs appeared lower than that in PDLSCs as seen by CD146 (57%), SSEA4 (80%), and STRO-1 (26%) (Figure 1c) in three tested groups. The surface marker analysis demonstrated that PDLPs were mesenchymal progenitor cells, but may not be of early lineage as PDLSCs.
Figure 1.
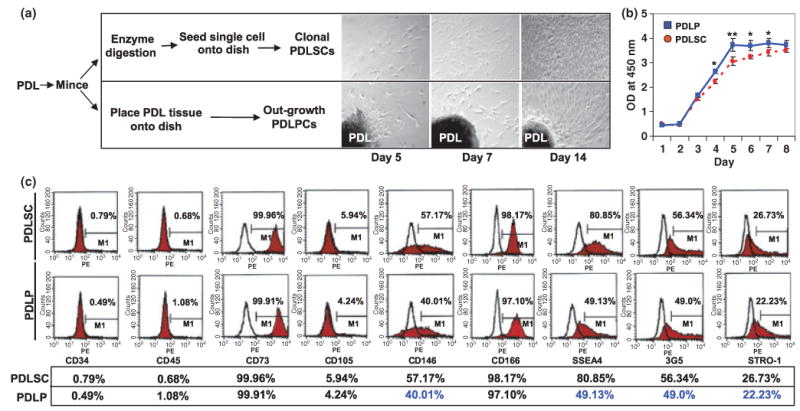
Isolation of human PDLPs. (a) Comparison procedure of isolating PDLPs and PDLSCs from periodontal tissues and morphology of culture expanded cells at 5, 7, and 14 days (magnification 100×). (b) At days 1–3, PDLPs showed similar proliferation rate as PDLSCs, but showed an elevated proliferation from day 4 to 7. After day 8, PDLPs have similar proliferation rate as PDLSCs in the culture. (c) Cytometric flow analysis indicated that PDLPs have similar surface molecule phenotype as PDLSCs derived from same donor including negative for CD34 and CD45, and positive for CD73, CD105, CD146, CD166, SSEA4, 3G5, and STRO-1 (*P < 0.05; **P < 0.01). Experiments were repeated three times
Multipotent differentiation of PDLPs
The multi-lineage differentiation potential of PDLPs as compared with PDLSCs was further examined. The potential of cementoblastic/osteoblastic differentiation of PDLPs was studied through secondary PDLP cultures supplemented with l-ascorbate-2-phosphate, dexamethasone, and inorganic phosphate to induce mineralization in vitro as previously described (Seo et al, 2004; Bi et al, 2007). Alizarin Red-positive nodules were formed in the PDLP cultures following 4 weeks of induction, indicating calcium accumulation in vitro (Figure 2a). However, compared with PDLSCs, PDLPs formed fewer mineralized nodules (Figure 2a). As expected, PDLP expressed cementoblastic/osteoblastic markers including Runx2 and OCN by RT-PCR analysis (Figure 2a). It appeared that PDLPs expressed lower levels of Runx2 and OCN than PDLSCs (Figure 2a). Further examination helped to study the differentiation potential of PDLPs into other cell lineages such as adipocytes. In this manner, PDLPs were cultured with an adipogenic inductive cocktail for 3 weeks and developed into oil red O-positive lipid-laden fat cells (Figure 2b). These results correlated with an up-regulation in the expression of two adipocyte specific transcripts, PPARγ2 and LPL, as detected using RT-PCR (Figure 2b). However, PDLPs showed lower adipogenic differentiation capacity than PDLSCs (Figure 2b). We next examined the expression level of SCX, a tendon and PDLSC specific marker, in PDLPs to study extensive differences between the cell types. Utilizing RT-PCR analysis we found that PDLPs failed to express SCX (Figure 2c), indicating that PDLPs were different from PDLSCs. Moreover, using a culture scratch assay, it was determined that PDLPs possess elevated cell migration capacity in comparison with PDLSCs (Figure 2d), suggesting that PDLPs may possess biological activities when implanted in vivo.
Figure 2.
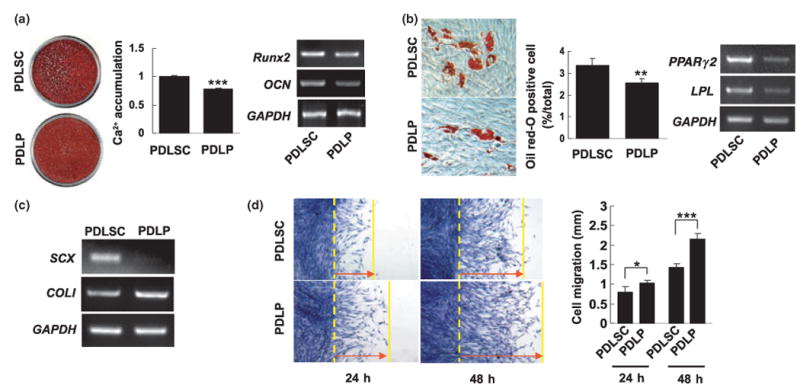
Comparison differentiation of PDLPs with PDLSCs. (a) PDLPs were cultured with L-ascorbate-2-phosphate, dexamethasone, and inorganic phosphate for 4 weeks. Alizarin red staining showed that PDLPs form mineralized nodules. In comparison with induced PDLSCs, PDLP cultures accumulated significantly less amounts of calcium (P < 0.005) than that of PDLSCs. PDLPs expressed Runx2 and OCN by RT-PCR analysis, but expression levels were lower than PDLSCs. (b) Under the adipogenic induction, PDLPs were capable of forming oil red-O positive cells, but PDLPs form significantly less oil red-O cells than PDLSCs (P < 0.01) (magnification 100×). RT-PCR analysis showed that PDLPs express lower levels of PPARγ2 and LPL when compared with PDLSCs. (c) RT-PCR analysis showed that cultured PDLPs failed to express SCX, a transcription factor specifically expressed in tendon cells and PDLSCs. But analogous to PDLSCs, PDLPs express COLI. (d) Cell Scratch assay showed that PDLPs have significantly higher cell motility than PDLSCs at 24 and 48 h (*P < 0.05; ***P < 0.005) (magnification 40×). Experiments were repeated three times
In vivo tissue regeneration of PDLPs
While PDLSCs were capable of regenerating cementum, collagen fibers, and Sharpey's fibers, it was speculated that PDLPs may possess tissue regeneration capability. To validate the tissue regeneration capacity of these cells, in vitro expanded PDLPs were transplanted into immunocompromised mice using HA/TCP as a carrier. A typical cementum-like structure was regenerated on the surface of the HA/TCP (Figure 3a). Clearly, PDLPs regenerated less cementum than that of PDLSCs (Figure 3a). At high magnification, we found that PDLPs were analogous to PDLSCs capable of forming Sharpey's fibers (Figure 3b,c) and collagen fibers (Figure 3d,e).
Figure 3.
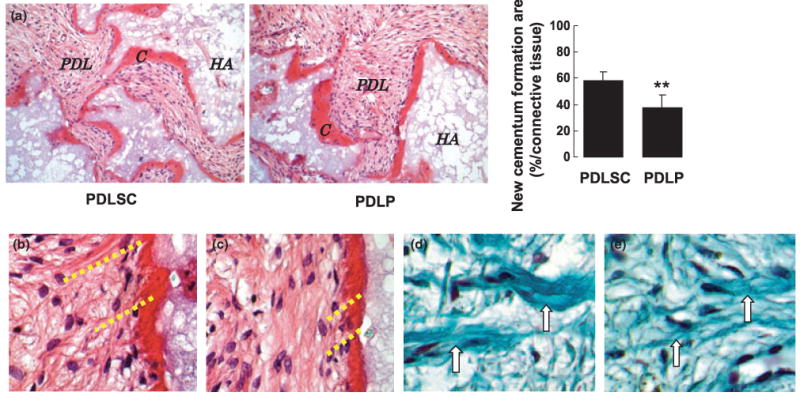
PDLPs generated cementum-like and PDL-like structures in vivo. (a) After 8 weeks of transplantation, PDLPs were able to form cementum-like structure (C) on the surfaces of (HA) carrier, PDL-like tissue (PDL) was also generated. But PDLPs generated significantly less amount of cementum than PDLSCs (**P < 0.01) (magnification 200×). (b, c) H&E staining showed that PDLPs were capable of regenerating Sharpey's fibers (b) as seen in PDLSC transplant (c) (magnification 400×). (d, e) Trichrome staining showed that PDLP transplants contain newly regenerated collagen fibers (d, arrows) same as observed in PDLSC transplants (e, arrows) (magnification 400×)
Transplantation of autologous PDLPs
PDL derived stem cells have been reported to give rise to cementum and Sharpey's fibers and capable of regenerating the periodontium in the treatment of periodontitis in swine model (Seo et al, 2004; Liu et al, 2008). Therefore, we used in vitro expanded PDLPs mixed with bone grafting material, CALCITITE 4060-2, to transplant into the intrabony defects of deep periodontal pockets of three patients following standard surgical debridement (Figure 4a,b). This approach required patients who provided written consent to participate in this study and had a third molar available for PDLP isolation. Following periodontal surgery and PDLP transplantation, the patients were monitored carefully over the course of 3, 6, 12, 26, 32, 42, and 72 months as seen in Figures 4 and 5. Clinical evaluations included probing depth, gingival recession, attachment level and attachment gain. At several follow-up intervals, 3, 6, and 42 months, there was a consistent decrease in probing depth and attachment levels as compared with those at the presurgery time as seen with patient no. 1 (Figure 4c). As expected, we observed periodontal tissue regain, specifically marked increase in gingival recession and attachment gain at 3, 6, and 42 months post-treatment (Figure 4c). In patient no. 2, we observed a similar therapeutic effect as seen with patient no. 1, including a significant decrease in probing depth and attachment level along with increased gingival recession and attachment gain at 3, 6, 9, 12, 42, and 72 months post-treatment (Figure 4d). After receiving consent from the patients, the transplanted sites, at tooth no. 30 and no. 13 from patient nos. 1 and 2 respectively, were re-evaluated through an invasive surgical procedure and data indicated a reasonable regain of healthy tissue (Figure 5a,b). X-ray evaluation indicated a periodontal tissue regain at 72 months post-PDLP implantation when compared with pretreatment conditions (Figure 5a,b). In addition, resorption kinetics of the carrier material was observed from 1 to 72 months postsurgery as shown a mineral density decrease at 4 month postoperation compared with 1 week postoperation in patient no. 1 (Figure 5a). In patient no. 3, only one tooth was treated and followed up for 3, 9, 12, 26, and 32 months. We observed a significantly improved clinical benefit specifically a decrease in tooth movement and probing depth (Table 1) along with increased gingival recession and stable improvement of attachment gain (Table 1). These results suggest that PDLP implantation may be a promising treatment in periodontitis patients. Importantly, all patients showed no inflammation in the treatment area and no systemic disorder that may have been associated with autologous PDLP transplantation, implying that this method is a safe intervention for periodontitis.
Figure 4.
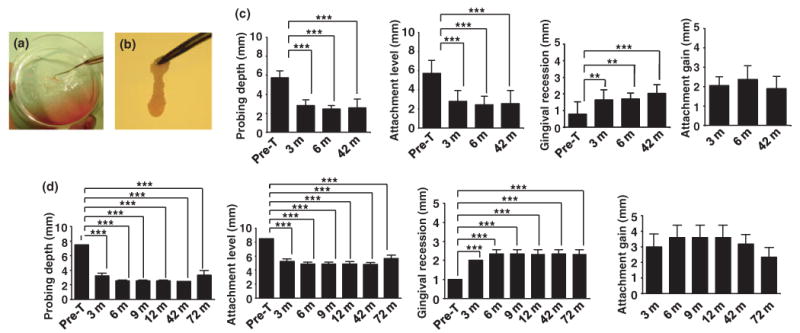
Autologous PDLP-mediated treatment to periodontitis. (a, b) Procedures of generating PDLPs/bone grafting material CALCITITE 4060-2 complex. Autologous PDLPs were cultured with bone grafting material CALCITITE 4060-2 and reach confluence. After washing with PBS, PDLP/bone grafting material CALCITITE 4060-2 complex was removed (a) and delivered to periodontal defect area in which inflammatory tissues were surgically removed (b). (c) In patient no. 1, 12 teeth were treated and followed up for 3, 6, and 42 months. From 3 to 42 month post-treatment, there was significantly decreased probing depths and attachment losses compared with the pretreatment level (Pre-T) (***P < 0.001). Although there was significantly increased gingival recession compared with the pretreatment (Pre-T) (***P < 0.001, **P < 0.005), attachment gain also marked increased after treatment. Moreover, there was no significant change of attachment gain on 42 months after treatment compared with that of on 3 months. (d) In patient no. 2, three teeth were treated and followed up for 3, 6, 9, 12, 42 and 72 months. From 3 to 72 month post-treatment, there was significantly decreased probing depths and attachment losses compared with the pretreatment level (Pre-T) (***P < 0.001). There was significantly increased gingival recession compared with the pretreatment (Pre-T) (***P < 0.001), however, attachment gain also marked increased after treatment. There was no significant change of attachment gain among the different time points after treatment
Figure 5.
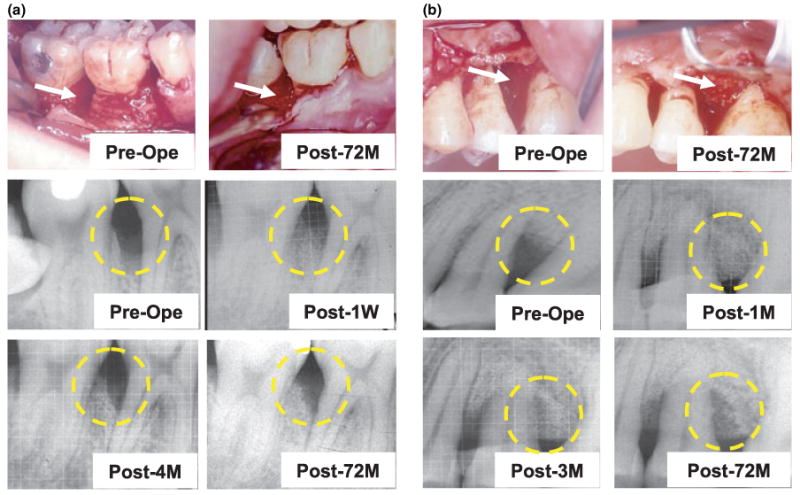
Clinical evaluation of autologous PDLP-mediated treatment to periodontitis. (a) Patient no. 1 showed periodontal tissue destruction at preoperation caused by periodontitis in tooth no. 30 (arrow in Pre-Ope). After 72 months autologous PDLP implantation, surgical reentry to the implanted area showed periodontal tissue regeneration (arrows in Post-72M). X-ray analysis showed bone resorption at preoperation (yellow circle in Pre-Ope) and PDLP/carrier material implantation at 1 week postoperation (yellow circle in Post-1W), 4 months postoperation (yellow circle in Post-4M), and 72 months postoperation (yellow circle in Post-72M). (b) Patient no. 2 showed periodontal tissue destruction at preoperation caused by periodontitis in tooth no. 13 (arrow in Pre-Ope). After 72 months autologous PDLP implantation, surgical reentry to the implanted area showed periodontal tissue regeneration (arrows in Post-72M). X-ray analysis showed bone resorption at preoperation (yellow circle in Pre-Ope) and PDLP/carrier material implantation at 1 month postoperation (yellow circle in Post-1M), 3 months postoperation (yellow circle in Post-3M), and 72 months postoperation (yellow circle in Post-72M)
Table 1.
Clinical evaluation of autologous PDLP-mediated treatment in patient no. 3
| Probing depth (mm) | Gingival recession (mm) | Attachment gain (mm) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tooth mobility | DB | B | MB | DL | L | ML | DB | B | MB | DL | L | ML | DB | B | MB | DL | L | ML | |
| Baseline | II | 4 | 5 | 9 | 8 | 9 | 10 | 2 | 1 | 2 | 2 | 1 | 2 | ||||||
| Presurgery | II | 4 | 5 | 8 | 8 | 8 | 10 | 2 | 1 | 2 | 2 | 1 | 2 | 0 | 0 | 1 | 0 | 1 | 0 |
| Post-3M | I | 3 | 3 | 4 | 3 | 4 | 4 | 3 | 2 | 3 | 3 | 3 | 3 | 0 | 1 | 3 | 4 | 3 | 5 |
| 9M | I | 3 | 3 | 3 | 3 | 3 | 4 | 3 | 2 | 3 | 3 | 3 | 3 | 0 | 1 | 4 | 4 | 4 | 5 |
| 12M | I | 3 | 3 | 3 | 3 | 2 | 3 | 3 | 2 | 3 | 3 | 3 | 3 | 0 | 0 | 4 | 4 | 4 | 6 |
| 26M | 0 | 3 | 2 | 3 | 3 | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 0 | 0 | 4 | 4 | 4 | 6 |
| 32M | 0 | 3 | 2 | 3 | 3 | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 0 | 0 | 4 | 4 | 4 | 6 |
DB, distal buccal; B, buccal; MB, mesial buccal; DL, distal lingual; L, lingual; ML, mesial lingual.
Discussion
Studies suggest that the human PDL contains a population of multipotent postnatal stem cells that can be expanded ex vivo, providing a unique reservoir of stem cells (Seo et al, 2004). Interestingly, these PDLSCs were capable of offering optimal treatment for periodontitis in conjunction with a standard surgical procedure in a swine model (Liu et al, 2008). Presently, it is extremely important to examine whether a stem cell-based intervention is an appropriate approach for treating periodontitis in human. PDLPs were used to treat periodontal disease several years ago, prior to the identification of PDLSCs. Therefore, in this study, we used in vitro and in vivo approaches to examine the nature of PDLPs in comparison with well-characterized PDLSCs. It is interesting to find that PDLPs contain progenitor cells. These data provided rational for us to evaluate PDLP implanted patients and encourage further clinical studies to investigate efficacy of stem cell-based therapy for periodontal diseases.
In this study, we examined sixteen periodontitis teeth from only three patients who received autologous PDLP transplantation. All patients showed clinical benefits during a follow up from 3 to 72 months post-PDLP transplantation as compared with the presurgery conditions. Because of the feasibility of isolating PDLPs in a clinical setting, we explore whether PDLPs can serve as the cell source in cell-based periodontal treatment and whether this early clinical study using a tissue outgrowth approach to collect PDLPs (Nishimura and Terranova, 1996; D'Errico et al, 1999) can provide a practical and more clinic-friendly biological intervention without the need for further cell characterization as in PDLSCs (Seo et al, 2004). Therefore, we examined whether PDLPs differ from PDLSCs that derived from the same PDL tissues in terms of their stem cell properties. The data presented here indicate that PDLPs showed stem cell properties including expression of extensive MSC surface molecules, multi-lineage differentiation, high proliferation rate, and more importantly, in vivo cementum-collagen-Sharpey's fiber regeneration. However, PDLPs resulted in a lower level of MSC markers STRO-1, CD146, and SSEA4 when compared with PDLSCs. Moreover, PDLPs showed decreased capacities of osteo- and adipogenic differentiation and expression levels of SCX as compared with PDLSCs. By contrast, PDLPs acquired an elevated cell migration in the culture dishes. This data suggest that PDLPs are a progenitor cell population that is capable of expressing stem cell markers and repairing periodontal tissues in vivo. However, PDLPs represent a later lineage cell population compared with PDLSCs. Therefore, this pilot study has provided promising preliminary evidences in terms of efficacy and safety of utilizing PDL progenitor cells for clinical therapies in the future. Although it was reported that bone marrow MSCs derived from older people maintain osteogenic capacity and intrinsic alterations with aging may contribute to the process of skeletal aging (Zhou et al, 2008), there is no experimental evidence showing differences between PDL cells derived from young and older people. In real life when a young person needs cell-based periodontal treatment, it is highly likely that they will develop more problems with age and will need treatment for more teeth later in life. Therefore, we have developed methods to preserve PDL tissue or PDLSCs for future's usage (Seo et al, 2005). PDLPs used for bench-top experiments in comparison with PDLSCs are not the exact cell source used in clinical therapy.
As inclusion criteria for the clinical study, we enrolled only patients who underwent third molar extraction and who would avail their samples for PDLP isolation. This limited the number of patients enrolled in this study. Our clinical outcome measures of periodontal regeneration were evaluated in terms of long-term efficacy and safety. There are several advantages to this treatment method that can benefit the dental field to make stem cell-based periodontal disease treatment a reality. First, periodontal disease is not only a local disorder; it can become a systemic problem as related to diabetes mellitus, cardiovascular disease and premature low birth weight (Kiran et al, 2005; Andriankaja et al, 2006; Geismar et al, 2006; Holmlund et al, 2006; Manau et al, 2008; Mealey and Rose, 2008). The development of more advanced therapeutic interventions for periodontal disease is an urgent clinical necessity. Secondly, the availability of high quality periodontal stem progenitor cells makes stem cell-based periodontal therapy a feasible reality. Thirdly, the direct delivery of stem cells into periodontal defect sites is an easy approach in conjunction with well-established surgical procedures to ensure the removal of infectious tissues. Finally, stem cells will mediate tissue regeneration and re-establish a healthy microenvironment at the post-treatment stage, which ensures a long-term favorable therapeutic effect. Because the baseline 0 was used as a reference for attachment gain, we ca not statistically analyze the attachment again between pre- and post-treatments. We compared attachment gain among the different time points after treatments and found no significant change, suggesting that the therapeutic effects were stable. Therefore, the attachment gains after treatment were considered as stable improvement.
In this study, we provided experimental evidences and early clinical data supporting potential efficacy and safety of utilizing autologous PDLPs as a cell-based surgical intervention in the treatment of periodontitis in human. It is speculated that PDLSCs can serve as an abundant cell source, easily accessible from extracted tooth and will provide excellent therapeutic effect in promoting periodontal regeneration. This is a preliminary pilot study with three patients. It is necessary to conduct well-designed clinical trial with reasonable number of patients in treatment and control groups to elucidate the role of PDLSCs in periodontal disease therapies.
Acknowledgments
The authors thank Dr. Stan Gronthos for providing antibodies for this study. This work was supported by the grant from California Institute for Regenerative Medicine (RN1-00572 for S.S.) and the grant R21 DE017632 from the National Institute of Dental and Craniofacial Research, National Institutes of Health, for cell characterization. This study was partially supported by the grant from Forward Dentist Group,Taiwan (FDG860504A).
Funding sources: This work was supported by the grant from California Institute for Regenerative Medicine (RN1-00572 for S.S.) and Grant from Forward Dentist Group, Taiwan (FDG860504A).
References
- Andriankaja OM, Genco RJ, Dorn J, et al. The use of different measurements and definitions of periodontal disease in the study of the association between periodontal disease and risk of myocardial infarction. J Periodontol. 2006;77:1067–1073. doi: 10.1902/jop.2006.050276. [DOI] [PubMed] [Google Scholar]
- Bi Y, Ehirchiou D, Kilts TM, et al. Identification of tendon stem/progenitor cells and their extracellular matrix-rich niche. Nat Med. 2007;13:1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- D'Errico JA, Ouyang H, Berry JE, et al. Immortalized cementoblasts and periodontal ligament cells in culture. Bone. 1999;25:39–47. doi: 10.1016/s8756-3282(99)00096-4. [DOI] [PubMed] [Google Scholar]
- Geismar K, Stoltze K, Sigurd B, et al. Periodontal disease and coronary heart disease. J Periodontol. 2006;77:1547–1554. doi: 10.1902/jop.2006.050405. [DOI] [PubMed] [Google Scholar]
- Goncalves PF, Gurgel BC, Pimentel SP, et al. Effect of two different approaches for root decontamination on new cementum formation following guided tissue regeneration: a histomorphometric study in dogs. J Periodontal Res. 2006;41:535–540. doi: 10.1111/j.1600-0765.2006.00902.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann T, Richter S, Meyle J, et al. A randomized clinical multicentre trial comparing enamel matrix derivative and membrane treatment of buccal class II furcation involvement in mandibular molars. Part III: patient factors and treatment outcome. J Clin Periodontol. 2006;33:575–583. doi: 10.1111/j.1600-051X.2006.00947.x. [DOI] [PubMed] [Google Scholar]
- Holmlund A, Holm G, Lind L. Severity of periodontal disease and number of remaining teeth are related to the prevalence of myocardial infarction and hypertension in a study based on 4,254 subjects. J Periodontol. 2006;77:1173–1178. doi: 10.1902/jop.2006.050233. [DOI] [PubMed] [Google Scholar]
- Kaigler D, Cirelli JA, Giannobile WV. Growth factor delivery for oral and periodontal tissue engineering. Expert Opin Drug Deliv. 2006;3:647–662. doi: 10.1517/17425247.3.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinane DF, Marshall GJ. Periodontal manifestations of systemic disease. Aust Dent J. 2001;46:2–12. doi: 10.1111/j.1834-7819.2001.tb00267.x. [DOI] [PubMed] [Google Scholar]
- Kiran M, Arpak N, Unsal E, et al. The effect of improved periodontal health on metabolic control in type 2 diabetes mellitus. J Clin Periodontol. 2005;32:266–272. doi: 10.1111/j.1600-051X.2005.00658.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zheng Y, Ding G, et al. Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem Cells. 2008;26:1065–1073. doi: 10.1634/stemcells.2007-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manau C, Echeverria A, Agueda A, et al. Periodontal disease definition may determine the association between periodontitis and pregnancy outcomes. J Clin Periodontol. 2008;35:385–397. doi: 10.1111/j.1600-051X.2008.01222.x. [DOI] [PubMed] [Google Scholar]
- Mealey BL, Rose LF. Diabetes mellitus and inflammatory periodontal diseases. Curr Opin Endocrinol Diabetes Obes. 2008;15:135–141. doi: 10.1097/MED.0b013e3282f824b7. [DOI] [PubMed] [Google Scholar]
- Moutsopoulos NM, Madianos PN. Low-grade inflammation in chronic infectious diseases: paradigm of periodontal infections. Ann N Y Acad Sci. 2006;1088:251–264. doi: 10.1196/annals.1366.032. [DOI] [PubMed] [Google Scholar]
- Needleman IG, Worthington HV, Giedrys-Leeper E, et al. Guided tissue regeneration for periodontal infra-bony defects. Cochrane Database Syst Rev. 2006;19:CD001724. doi: 10.1002/14651858.CD001724.pub2. [DOI] [PubMed] [Google Scholar]
- Nishimura F, Terranova VP. Comparative study of the chemotactic responses of periodontal ligament cells and gingival fibroblasts to polypeptide growth factors. J Dent Res. 1996;75:986–992. doi: 10.1177/00220345960750041401. [DOI] [PubMed] [Google Scholar]
- Seo BM, Miura M, Gronthos S, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- Seo BM, Miura M, Sonoyama W, et al. Recovery of functional postnatal stem cells from cryopreserved human periodontal ligament. J Dent Res. 2005;84:907–912. doi: 10.1177/154405910508401007. [DOI] [PubMed] [Google Scholar]
- Venezia E, Goldstein M, Boyan BD, et al. The use of enamel matrix derivative in the treatment of periodontal defects: a literature review and meta-analysis. Crit Rev Oral Biol Med. 2004;15:382–402. doi: 10.1177/154411130401500605. [DOI] [PubMed] [Google Scholar]
- Zhou S, Greenberger JS, Epperly MW, et al. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008;7:335–343. doi: 10.1111/j.1474-9726.2008.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


