Abstract
Acidaminococcus gen. n. and the type species Acidaminococcus fermentans sp. n. were described. Amino acids, of which glutamic acid is the most important, could serve as the sole energy source for growth. Acetic and butyric acids and CO2 were produced; propionic acid and hydrogen were not produced. Amino acid media supporting growth and the amino acid and vitamin requirements were described. Glucose was frequently not fermented or was weakly catabolized. Derivative products from glucose autoclaved in media, but not glucose itself, stimulated or were required for growth in amino acid media. A wide range of polyols and carbohydrates were not attacked. Lactate, fumarate, malate, succinate, citrate, and pyruvate were not used as energy sources for growth. Pyruvate completely suppressed growth. Cytochrome oxidase and benzidine reactions were negative; catalase, indole, acetyl methyl carbinol, and H2S were not produced; nitrate and sulfonthalein indicators were not reduced; ammonia was produced; gelatin liquefaction was negative or slow and partial; vancomycin (7.5 μg/ml) was resisted. Acidaminococcus was different from Veillonella in morphology, serology, nutrition, utilization of substrates, and accumulation of products in media supporting growth; Acidaminococcus resembled Peptococcus in utilization of glutamic acid and accumulation of similar products, but the two genera differed in morphology, gram reaction, serology, guanine plus cytosine content of deoxyribonucleic acid, and nutrition.
Full text
PDF
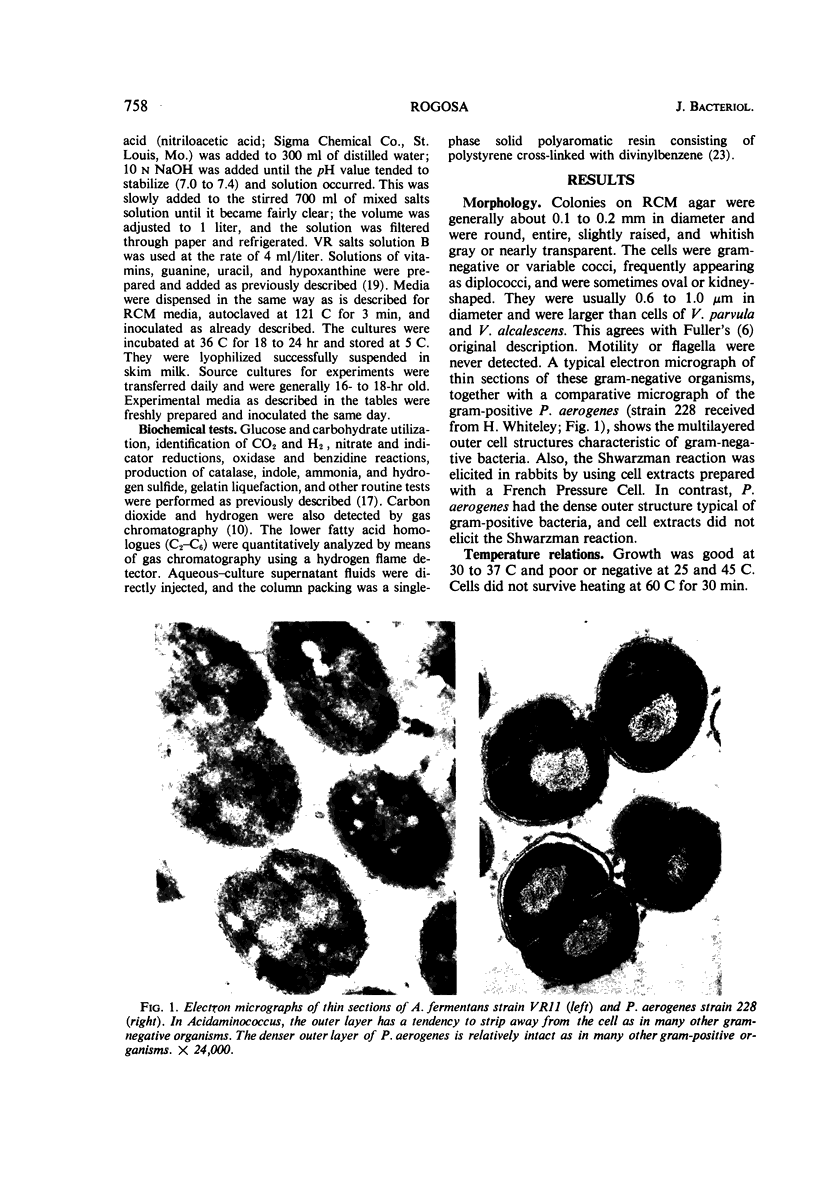

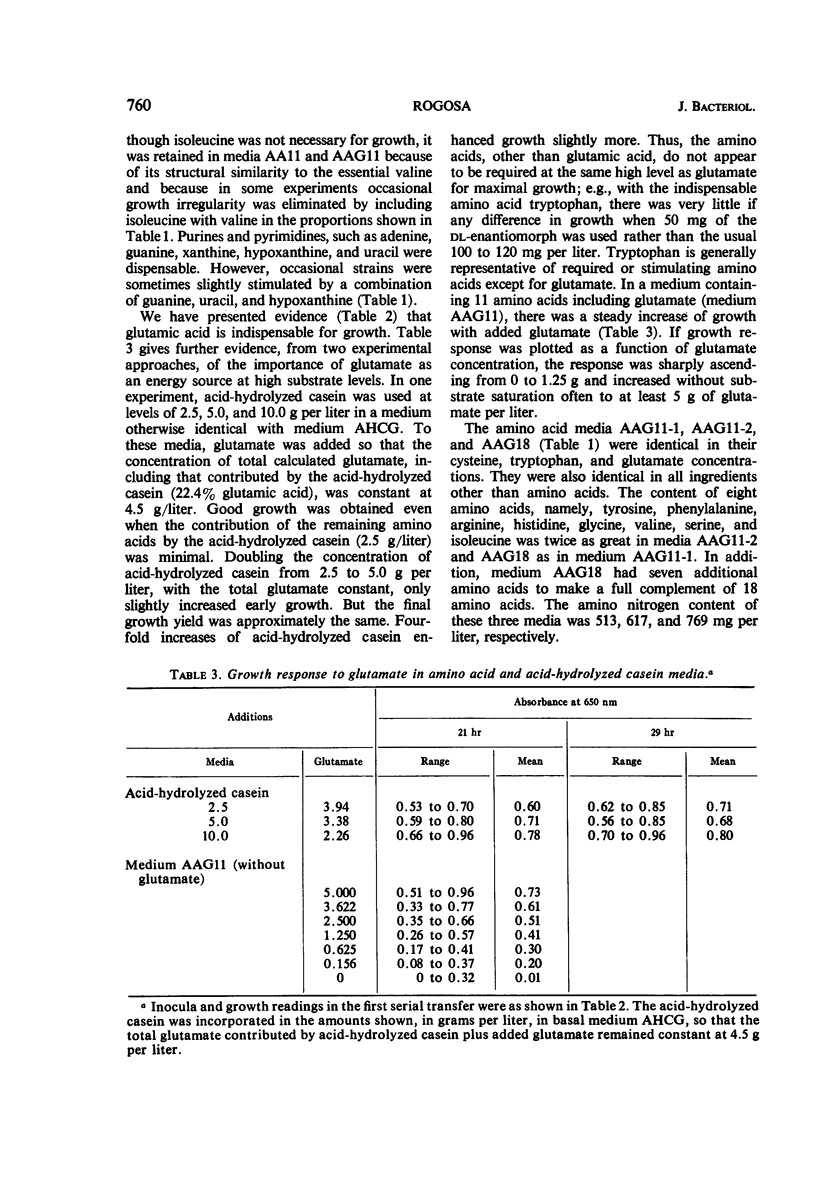






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FIELD M. F., LICHSTEIN H. C. Growth stimulating effect of autoclaved glucose media and its relationship to the CO2 requirement of propionibacteria. J Bacteriol. 1958 Nov;76(5):485–490. doi: 10.1128/jb.76.5.485-490.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foubert E. L., Douglas H. C. Studies on the Anaerobic Micrococci: I. Taxonomic Considerations. J Bacteriol. 1948 Jul;56(1):25–34. [PMC free article] [PubMed] [Google Scholar]
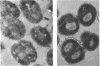
- Fuller R. Some morphological and physiological characteristics of gram negative anaerobic bacteria isolated from the alimentary tract of the pig. J Appl Bacteriol. 1966 Aug;29(2):375–379. doi: 10.1111/j.1365-2672.1966.tb03486.x. [DOI] [PubMed] [Google Scholar]
- Horler D. F., McConnell W. B., Westlake D. W. Glutaconic acid, a product of the fermentation of glutamic acid by Peptococcus aerogenes. Can J Microbiol. 1966 Dec;12(6):1247–1252. doi: 10.1139/m66-166. [DOI] [PubMed] [Google Scholar]
- Horler D. F., Westlake D. W., McConnell W. B. Conversion of glutamic acid to volatile acids by Micrococcus aerogenes. Can J Microbiol. 1966 Feb;12(1):47–53. doi: 10.1139/m66-008. [DOI] [PubMed] [Google Scholar]
- KRICHEVSKY M. I., ROGOSA M., BISHOP F. S. GAS CHROMATOGRAPHIC ANALYSIS OF HYDROGEN-CARBON DIOXIDE MIXTURES. Anal Biochem. 1964 Mar;7:350–356. doi: 10.1016/0003-2697(64)90142-3. [DOI] [PubMed] [Google Scholar]
- LANKFORD C. E., RAMSEY H. H. Stimulation of growth initiation by heat degradation products of glucose. J Bacteriol. 1956 Oct;72(4):511–518. doi: 10.1128/jb.72.4.511-518.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGOSA M., BISHOP F. S. THE GENUS VEILLONELLA . II. NUTRITIONAL STUDIES. J Bacteriol. 1964 Mar;87:574–580. doi: 10.1128/jb.87.3.574-580.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGOSA M., FITZGERALD R. J., MACKINTOSH M. E., BEAMAN A. J. Improved medium for selective isolation of Veillonella. J Bacteriol. 1958 Oct;76(4):455–456. doi: 10.1128/jb.76.4.455-456.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGOSA M., FRANKLIN J. G., PERRY K. D. Correlation of the vitamin requirements with cultural and biochemical characters of Lactobacillus spp. J Gen Microbiol. 1961 Jul;25:473–482. doi: 10.1099/00221287-25-3-473. [DOI] [PubMed] [Google Scholar]
- ROGOSA M. THE GENUS VEILLONELLA. I. GENERAL CULTURAL, ECOLOGICAL, AND BIOCHEMICAL CONSIDERATIONS. J Bacteriol. 1964 Jan;87:162–170. doi: 10.1128/jb.87.1.162-170.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGOSA M., WISEMAN R. F., MITCHELL J. A., DISRAELY M. N., BEAMAN A. J. Species differentiation of oral lactobacilli from man including description of Lactobacillus salivarius nov spec and lactobacillus Cellobiosus nov spec. J Bacteriol. 1953 Jun;65(6):681–699. doi: 10.1128/jb.65.6.681-699.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogosa M., Krichevsky M. I., Bishop F. S. Truncated Glycolytic System in Veillonella. J Bacteriol. 1965 Jul;90(1):164–171. doi: 10.1128/jb.90.1.164-171.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogosa M., Love L. L. Direct quantitative gas chromatographic separation of C2-C6 fatty acids, methanol, and ethyl alcohol in aqueous microbial fermentation media. Appl Microbiol. 1968 Feb;16(2):285–290. doi: 10.1128/am.16.2.285-290.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogosa M. The Genus Veillonella IV. Serological Groupings, and Genus and Species Emendations. J Bacteriol. 1965 Sep;90(3):704–709. doi: 10.1128/jb.90.3.704-709.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Smiley K. L., Niven C. F., Sherman J. M. The Nutrition of Streptococcus salivarius. J Bacteriol. 1943 May;45(5):445–454. doi: 10.1128/jb.45.5.445-454.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITELEY H. R. Fermentation of amino acids by Micrococcus aerogenes. J Bacteriol. 1957 Sep;74(3):324–330. doi: 10.1128/jb.74.3.324-330.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITELEY H. R., ORDAL E. J. Fermentation of alpha keto acids by Micrococcus aerogenes and Micrococcus lactilyticus. J Bacteriol. 1957 Sep;74(3):331–336. doi: 10.1128/jb.74.3.331-336.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlake D. W., Horler D. F., McConnell W. B. The effect of sodium on the fermentation of glutamic acid by Peptococcus aerogenes. Biochem Biophys Res Commun. 1967 Feb 21;26(4):461–465. doi: 10.1016/0006-291x(67)90569-4. [DOI] [PubMed] [Google Scholar]