Abstract
Context: The LH receptor (LHCGR) drives fetal testosterone secretion, which is vital for human masculinization. Maternal smoking is associated with defective masculinization, but the relationship between smoking, tropic hormones, testosterone, and functional LHCGR expression is poorly understood.
Objective: This study aimed to investigate developmental changes in fetal gonadotropins, human chorionic gonadotropin (hCG), and expression of fetal testicular LHCGR isoforms and the effects of maternal cigarette smoking.
Design: We conduced an observational study of the male fetus, comparing pregnancies in which the mothers did or did not smoke.
Setting: The study was conducted at the Universities of Aberdeen and Glasgow.
Patients/Participants: Testes and blood were collected from 54 morphologically normal human male fetuses of women undergoing elective termination of normal second-trimester pregnancies.
Main Outcome Measures: We measured circulating testosterone, hCG, LH, prolactin, FSH, and testicular LHCGR isoform expression.
Results: Fetal testosterone and hCG, but not LH, significantly declined between 11 and 19 wk gestation with no significant change in testicular responsiveness. The proportion of nonfunctional LHCGR transcript in fetal testes was 2.3-fold lower than in adults. Fetal hCG was reduced 38% (P = 0.021) and the ratio of inactive vs. active LHCGR isoforms lowered by smoking.
Conclusions: Falling second-trimester fetal testosterone is probably due to declining maternal hCG because Leydig cell LH/hCG responsiveness remains constant. Although maternal cigarette smoking reduces fetal hCG, the ratio of inactive LHCGR isoforms is reduced and gonadotropin drive maintains testosterone production near control levels. The lower relative abundance of inactive isoforms compared with the adult testis reflects the importance of LHCGR.
Analysis of LH, its receptor isoforms, and other hormones in the human fetus reveal a pattern unlike the adult and only modestly affected by maternal cigarette smoking.
The LH receptor (LHCGR) is fundamentally important for normal reproductive development and function in men (1). In the human, fetal LH and maternal human chorionic gonadotropin (hCG) act on testicular LHCGR to stimulate both testosterone and insulin-like-hormone-3 secretion (2,3), which drive testis development, masculinization, and testis descent. Mutations and polymorphisms reducing LHCGR activity impair normal reproductive development, leading to a hypogonadal phenotype (4,5,6). The developmental importance of the LHCGR is underscored by the phenotype of the LHCGR knockout mouse (7). In these animals the testes show little postnatal development and testosterone levels are markedly reduced. Whereas inactivation of LHCGR reduces testosterone and impairs Leydig cell development, excess LHCGR action, manifesting in prematurely elevated testosterone levels, also impairs Leydig cell differentiation in mice (8).
The finding of a primate-specific exon [6A (6)] within the LHCGR gene, and the potential generation of inactive forms of the receptor, offers the possibility of novel control mechanisms of fetal Leydig cell development. The principal isoform of LHCGR generated in the adult human testis is a truncated, inactive species terminating in exon 6A and only about 15% of isoforms encode functional receptors (6). In adult men LHCGR expression is 10% of that seen in the rodent (9) with much lower levels of associated testosterone secretion. If this relative insensitivity in the adult human is also true of the fetus, it may be particularly important with respect to endocrine disruption, either through endocrine-disrupting compounds, such as phthalates (10) or maternal cigarette smoking during gestation (11).
Testosterone production by the human fetal testis is essential for masculinization of the fetus and testosterone itself is important for fetal testis development (12). In the human fetus, testosterone levels peak at around 12–14 wk (13), driving Wolffian duct development. Despite the importance of fetal testicular androgen production, the hormonal drivers that maintain production remain uncertain. During the first trimester, testicular androgen production is dependent on maternal hCG production, which peaks at about 9 wk and then starts to decline (14). At the same time, as hCG levels are declining, the fetal hypothalamic-pituitary axis develops and fetal LH is released into the circulation (15,16,17,18). It is possible therefore that fetal LH starts to play an important role in fetal androgen production during the second trimester, but measurements of circulating fetal LH levels are currently lacking. To examine therefore the relationship between LH, hCG, and testosterone production in the second term human fetus, we have measured levels of these hormones in the fetal circulation.
We therefore aimed to determine whether fetal human testicular LHCGR gene expression patterns were different from those in the adult and to determine whether maternal cigarette smoking affected LHCGR transcript expression.
Subjects and Methods
Study design
This study was designed, specifically, to investigate the relationship between gonadotropin drive, LHCGR isoforms expression and testicular responsiveness in the fetal testis and to test the hypothesis that maternal smoking affects these parameters in utero. All eligible women (see Sample collection) were recruited prospectively, with maternal and fetal morphological data recorded during consent and sample processing. In total, 46 fetuses were used to determine circulating hormones and cotinine levels from cardiac blood. In a subset of 22 fetuses, the transcript expression levels of LHCGR isoforms transcripts (6) were determined. Cases were matched for gestational stage between smoking and control (nonsmoking) groups.
Sample collection
The collection of fetal material was approved by the National Health Service Grampian Research Ethics Committees (REC 04/S0802/21). [For a detailed explanation, see (13)]. Women seeking elective, medical, terminations of pregnancy were recruited with full written, informed, consent by nurses working independently at Aberdeen Pregnancy Counseling Service. There was no change in patient treatment or care and women were able to withdraw from the study at any point. Only normally progressing pregnancies from women over 16 yr of age and between 11 and 21 wk of gestation were collected. Fetuses were transported to the laboratory within 30 min of delivery, weighed, crown-rump length recorded, and sexed. The gonads were either snap frozen in liquid nitrogen and stored at −85 C or fixed overnight in neutral buffered formalin, transferred to 70% ethanol, and processed for histology.
Endocrine and cotinine assays
Testosterone, a key marker of masculinization, was determined using a DELFIA kit (PerkinElmer Life Sciences, Cambridge, UK) with a detection limit of 0.3 nmol/liter and intra- and interassay coefficients of variation 3.2 and 8.6%, respectively. Before testosterone assay, fetal plasma samples were extracted on C18 spin columns (GE Healthcare, Bucks, UK; C18 self-pack POROS 10 R2; Applied Biosystems Ltd., Warrington, UK) to avoid interference caused by hemolysis. The recovery of testosterone was 122 ± 9.7% (13). LH, FSH, prolactin (PRL), and intact hCG were measured using DELFIA kits robust for hemolysis, with detection limits and inter- and intraassay coefficients of variation of 0.05 U LH/liter, 2.4 and 4.2%, 0.5 U FSH per liter, 2.8 and 2.0%, 0.04 mU PRL per liter, 2.0 and 3.0%, and 0.5 U hCG per liter, 4.1 and 4.6% (11). There was a 0.02% cross-reaction of intact hCG in the LH assay. Cotinine, a metabolite of nicotine and a marker of smoking, was determined with a kit (Cozart P.L.C., Abingdon, Kent, UK) giving semiquantitative determination with values between 0 and 12 ng cotinine per milliliter being considered negative.
Real-time quantitative PCR
For quantification of specific mRNA species in fetal testes (see supplemental Table S1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org), real-time PCR was used after reverse transcription of the isolated RNA (detailed in Ref. 13). To allow specific mRNA levels to be expressed per testis and control for RNA extraction efficiency, RNA degradation, and the reverse transcription step, 5 ng external standard (luciferase mRNA; Promega UK, Southampton, UK) was added to each ovary at the start of the RNA extraction. Testicular RNA was extracted using TRIzol (Life Technologies, Paisley, UK) and reverse transcribed using random hexamers and Moloney murine leukemia virus reverse transcriptase (Superscript II; Life Technologies). The real-time PCR approach used the SYBR green method in a 96-well plate format using a MX3000 cycler; (Stratagene, Amsterdam, The Netherlands). Reactions contained 5 μl 2× SYBR master mix (Stratagene), primer (100 nm), and template in a total volume of 10 μl, and a melting curve analysis was carried out on the products formed. The quantity of each measured cDNA from the real-time PCR was expressed relative to the standard luciferase cDNA in the same sample. This method allows direct comparison of expression levels per testis between different samples (19). All primers were designed by Primer Express 2.0 (Applied Biosystems) using parameters previously described (20). Primers used to measure LHCGR isoforms are detailed elsewhere (6) with the exception of primers designed to measure exons 9-11, which were forward, gctgtgcttttagaaacttgccaacaa and reverse, ttcatagtcccagccactcagttcact, and terminal exon 6A, which were forward, acataaccaccatacc aggaaatgctttt and reverse, tgctctgtattcttttgattgcattaatctgag. Different primers were used for one of the LHCGR isoforms in this study (terminal exon 6A) to allow the amplicon to cross an intron-exon boundary. The efficiency of the quantitative PCR was similar to the previous primer set, which therefore allows comparison between adult (6) and fetal (present study) tissues.
Statistical analysis
Analyses were performed using JMP (version 7.0.1; Thompson Learning, London, UK). Normality of data distribution was tested with the Shapiro-Wilk test and nonnormally distributed data were log transformed and rechecked for normality before analysis. To avoid bias, care was taken to ensure that no comparison involved groups with any difference between smokers and nonsmokers in terms of stage of gestation. Because LHCGR transcripts showed changes in expression across the second trimester, two-way ANOVA was used to test the combined effects of gestational age (weeks) and maternal smoking (yes/no) on the data. Relationships between variables were explored by linear regression. For the cotinine assay, values between 0 and 50 ng/ml were expressed at the detected values, whereas all values less than 0 ng/ml were expressed as 0 ng/ml and values greater than 50 ng/ml were expressed as 51 ng/ml for the purposes of statistical analysis.
Results
Activity of the fetal human LH/hCG-testosterone axis
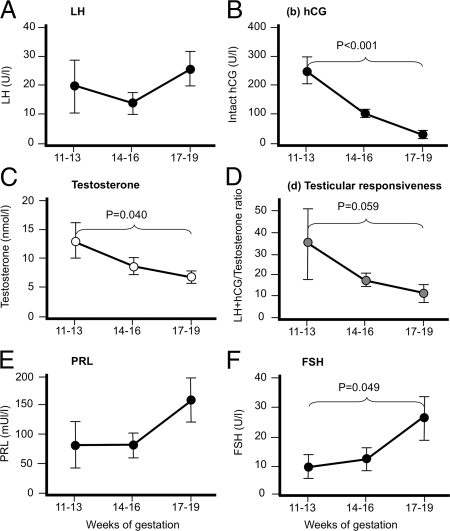
Concentrations of circulating fetal LH did not change significantly during the second trimester (Fig. 1A). In contrast, intact hCG (from the mother) fell 9.8-fold over the same period (Fig. 1, A and B) so that by wk 17–19 there were similar levels of LH and hCG in the fetal circulation (26.1 ± 5.8 U LH per liter vs. 28.1 ± 23.1 U hCG per liter, respectively). Testosterone secretion also fell significantly over the second trimester (by 1.9-fold) and testicular responsiveness to LH/hCG (LH+hCG/testosterone) was 3.1-fold higher at the end of the second trimester, although this did not quite achieve statistical significance (Fig. 1, C and D). Neither hCG nor LH correlated with testosterone (r = 0.232, P = 0.202, and r = −0.061, P = 0.744, respectively), and this did not change when LH and hCG were combined (r = 0.168, P = 0.366). We also measured FSH and PRL (Fig. 1, E and F), which increased 1.94- and 2.86-fold, respectively, across the second trimester, although only significantly in the case of FSH. Neither PRL nor FSH correlated with testosterone (r = 0.299, P = 0.147, and r = −0.239, P = 0.196, respectively).
Figure 1.
Changes in gonadotropin drive and fetal human testis response during the second trimester. Fetal LH (A) remains constant, whereas intact hCG (B) in the fetal circulation falls significantly, mirrored by a significant reduction in testosterone (C) and increase in testicular responsiveness (calculated by combining fetal LH and hCG and dividing this by testosterone): lower values indicate that less gonadotropin is required to drive testosterone output. Values are mean ± sem (n = 11–17 per stage shown).
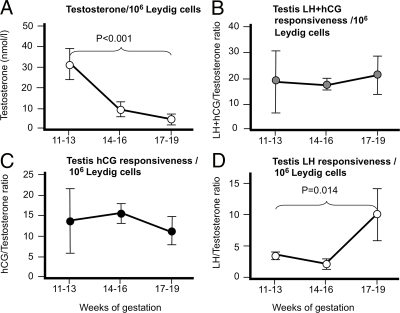
Leydig cell numbers increase over the second trimester (13), and we used these data, which were derived from the same population of fetuses as in the present study, to normalize testosterone levels. When testosterone levels were normalized in this way to Leydig cell numbers (Fig. 2, A and B), the fall in testosterone output per Leydig cell was marked (8.8-fold) and highly significant. This change in testosterone per Leydig cell was also highly correlated with LH+hCG (r = 0.490, P = 0.005) or hCG alone (r = 0.536, P = 0.002), but there was no significant relationship with fetal LH alone (r = 0.037, P = 0.843). The testicular responsiveness to hCG+LH remained unchanged through the second trimester as did responsiveness calculated in terms of hCG alone, whereas there was a significant (5.05-fold) decline in responsiveness per Leydig cell calculated relative to LH alone at 17–19 wk (Fig. 2, C and D). Taken altogether, these data suggest that maternally derived hCG is the main driver for fetal human testicular testosterone production during the second trimester despite relatively high levels of circulating fetal LH.
Figure 2.
Changes in gonadotropin drive and fetal human testis response during the second trimester normalized per million Leydig cells because Leydig cell numbers peak after 17 wk gestation (13). Calculated relative to total gonadotropin (LH+hCG) drive, testosterone output (A) remained on a significant downward trajectory, whereas testicular responsiveness (B) remained constant. When testicular responsiveness was calculated relative to hCG alone (C), it remained stable across the second trimester but, calculated relative to LH alone (D), showed significant reduction at 17–19 wk. Values are mean ± sem (n = 11–17 per stage shown).
Fetal testis LHCGR isoforms expression
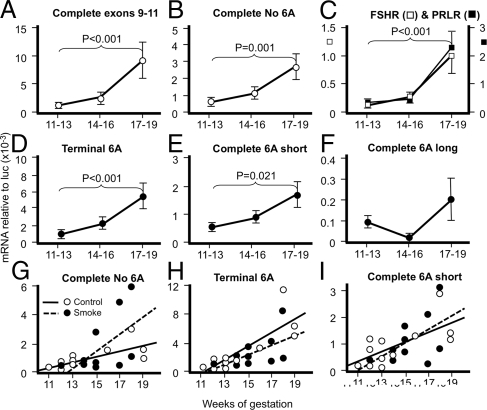
The transcript measured by another report (13) and the transcript equivalent to complete no. 6A reported by Kossack et al. (6) reflect the full, active LHCGR. Both transcripts (Fig. 3, A and B) showed significantly increasing expression across the second trimester (8.3- and 5.75-fold, respectively). Similar changes in transcript expression were seen for terminal 6A and complete 6A short isoforms (4.19- and 3.11-fold, respectively) but not for the complete 6A long isoforms (Fig. 3, D–F). Transcripts for FSH receptor (FSHR) and PRL receptor (PRLR) (Fig. 3C) also significantly increased sharply between 14–16 and 17–19 wk of gestation, as previously reported (13). All LHCGR transcripts, except for the 6A long isoform, correlated significantly with numbers of Leydig cells derived from (13) (r = 0.549–0.722, P = 0.006–0.001). The relative ratios of active to inactive LHCGR isoforms (Table 1) is different between fetal human testis (our study) and adult human testis reported previously (6). Specifically, the active isoforms constitute 27% of the fetal isoforms but only 14% of the adult isoforms. There was also a 3- to 10-fold difference in proportions of the different inactive isoforms between fetal and adult testes.
Figure 3.
Testicular transcripts for both active (A and B) and inactive (D–F) LHCGR isoforms increase significantly across the second trimester, except for complete 6A long isoforms (F). FSHR and PRLR also increased significantly at 17–19 wk (C). Complete exons 9-11 (A) reflect expression levels for the LHCGR sequence used in other reports (11,13). Values are mean ± sem (n = 5–6 per stage shown). The subtle dysregulation of LHCGR isoform expression between control fetuses (open circles) and smoke-exposed fetuses (closed circles) relative to weeks of gestation are shown in G–I. Linear regressions are shown by solid lines (control) and dotted lines (smoke exposed) with the respective (controls first) equations as follows: G, complete 6A, y = −1.760 + 0.195x, P = 0.007, y = −7.203 + 0.583x, P = 0.085; H, terminal 6A, y = −10.500 + 0.933x, P = 0.001, y = −7.668 + 0.0667x, P = 0.086; I, complete 6A short, y = −1.661 + 0.182x, P = 0.017, y = −2.713 + 0.249x, P = 0.118.
Table 1.
Fetal human testicular LHCGR isoforms transcript expression ratios differ from those in normal adult men
| Isoforms | Fetal testis | Adult testis |
|---|---|---|
| Complete 6A | 1 | 1 |
| Terminal 6A | 2 | 6 |
| Complete 6A short | 0.7 | 0.2 |
| Complete 6A long | 0.01 | 0.1 |
Data for adult men is derived from elsewhere (6), and transcript values were divided by complete 6A (active LHCGR isoforms) values to obtain the isoforms ratios for the inactive forms.
Effects of maternal cigarette smoking on LHCGR isoforms expression and endocrine activity
As can be seen in Table 2, maternal age, body mass index, fetal age, length and weight, and testis weight were very similar between smoking and control groups. Fetal cotinine levels reflected accurate assignment of fetuses to the two groups, with maternal smoking of 12 cigarettes/d reflecting heavy smoking. In terms of fetal endocrinology, intact hCG in the fetal circulation (but derived via the placenta from the maternal circulation) was significantly reduced in the smoking group. Expressing testosterone production relative to LH+hCG suggested that the testes of fetuses exposed to cigarette chemicals tended to be more sensitive to gonadotropin drive (P = 0.064). When LHCGR isoforms were compared between smoking and control groups (Table 3), there were no significant differences in transcript expression levels. Maternal smoking also had no significant effect on FSHR and PRLR expression, as previously reported (11). However, normalizing LHCGR isoforms transcript expression relative to the active form (complete, no 6A), there was a significant reduction in the relative amount of inactive LHCGR when the mother smoked cigarettes. The rate at which transcript isoform expression increased across the second trimester was subtly altered by maternal smoking. As can be seen in Table 3, whereas all except the complete 6A long isoforms showed a strong linear correlation with gestational weeks in the control group, all isoforms in the smoking group showed lower r values and failed to reach significance.
Table 2.
Morphological and endocrine data for mothers and male fetuses (mean ± sem)
| Control | Smoker | P value | |
|---|---|---|---|
| n | 19 | 27 | |
| Maternal characteristics | |||
| Age (yr) | 24 ± 1 | 24 ± 1 | 0.949 |
| Body mass index (kg/m2) | 25 ± 1 | 24 ± 1 | 0.681 |
| Cigarettes per day | 0 | 12 ± 1 | |
| Fetal characteristics | |||
| Age (wk) | 15.6 ± 0.4 | 15.6 ± 0.3 | 0.971 |
| Weight (g) | 107 ± 14 | 120 ± 12 | 0.798 |
| Crown-rump length (mm) | 110 ± 5 | 112 ± 4 | 0.766 |
| Testis weight (mg) | 20 ± 2 | 20 ± 2 | 0.987 |
| Cotinine (ng/ml) | 2 ± 2 | 45 ± 2 | <0.001 |
| Testosterone (nmol/liter) | 8.0 ± 1.6 | 7.8 ± 1.2 | 0.915 |
| LH (U/liter) | 20.1 ± 6.4 | 16.7 ± 4.7 | 0.701 |
| Intact hCG (U/liter) | 103.4 ± 15.9 | 64.2 ± 1.8 | 0.021 |
| LH+hCG to testosterone ratio | 21.8 ± 3.8 | 13.7 ± 2.8 | 0.064 |
| Normalised per million Leydig cells | |||
| Testosterone | 11.5 ± 3.1 | 10.2 ± 2.5 | 0.565 |
| LH+hCG to testosterone ratio | 23.1 ± 5.4 | 16.3 ± 4.0 | 0.198 |
Testicular responsiveness is calculated by combining the gonadotropin drive (LH+intact hCG) and dividing this by testis output (testosterone). Significantly different P values are highlighted in bold.
Table 3.
Maternal cigarette smoking significantly alters the relative ratios of active LHCGR isoforms but not expression levels (mean ± sem)
| Isoform | Control | Smoker | P value |
|---|---|---|---|
| n | 12 | 10 | |
| Expression levels | |||
| Complete exons 9-11 (a) | 2.84 ± 1.42 | 5.64 ± 1.61 | 0.813 |
| Complete 6A (a) | 1.04 ± 0.36 | 1.84 ± 0.41 | 0.921 |
| Terminal 6A (i) | 2.87 ± 0.58 | 2.66 ± 0.66 | 0.651 |
| Complete 6A short (i) | 0.95 ± 0.21 | 1.14 ± 0.24 | 0.911 |
| Complete 6A long (i) | 0.16 ± 0.05 | 0.04 ± 0.05 | 0.775 |
| Expression ratios | |||
| Complete, 6A (a) | 1.00 ± 0.02 | 1.00 ± 0.25 | 0.078 |
| Terminal 6A (i) | 2.76 ± 0.46 | 1.45 ± 0.52 | 0.013 |
| Complete 6A short (i) | 0.91 ± 0.16 | 0.62 ± 0.18 | 0.167 |
| Complete 6A long (i) | 0.15 ± 0.04 | 0.02 ± 0.05 | 0.735 |
| Control r | P value | Smoker r | P value | |
|---|---|---|---|---|
| Relationship to gestational age (wk) | ||||
| Complete exons 9-11 (a) | 0.900 | <0.001 | 0.571 | 0.085 |
| Complete 6A (a) | 0.730 | 0.007 | 0.571 | 0.085 |
| Terminal 6A (i) | 0.825 | 0.001 | 0.568 | 0.087 |
| Complete 6A short (i) | 0.673 | 0.017 | 0.526 | 0.118 |
| Complete 6A long (i) | 0.515 | 0.087 | 0.228 | 0.524 |
Discussion
In the absence of functional LHCGR in the human, there is a marked underdevelopment of the Leydig cells and androgen-dependent structures such as the mesonephric duct derivatives fail to develop in utero (4,5,6). This highlights the essential role of the LHCGR in regulating human fetal masculinization and shows that fetal Leydig cells in the human are absolutely dependent on hormonal stimulation of the LHCGR. Human fetal Leydig cells are exposed to two different hormones, LH and hCG, which can act through the LHCGR, and in studies reported here, we examined changes in the components of the hormone-receptor drive regulating testosterone secretion by the fetal Leydig cells during the second trimester.
In this study and our other recent studies of the human fetal testis (11,13), we used fetal plasma to measure fetal hormones, not cord blood as is commonly the case. The latter, at least at term, shows a variable agreement with maternal circulating and amniotic fluid steroid concentrations (21), indicating that it is a mix of maternal and fetal blood. This is an important point because fetal plasma will reflect the degree of accumulation of maternal hormones, such as hCG, and overall levels of exposure to these hormones as well as levels of fetal hormone production.
The levels of hCG and testosterone per Leydig cell, but not LH and testosterone per Leydig cell, were strongly correlated. Together with the declining Leydig cell sensitivity expressed relative to hCG, but not LH, this suggests that fetal testosterone falls during the second trimester simply because of the decline in maternal hCG. Clearly changes in LHCGR function could modify this conclusion. Earlier data showing that in individuals lacking functional LH, there is normal masculinization in utero, which must be largely through hCG stimulation of the fetal Leydig cells (22,23,24), also support our conclusion. This clearly begs the question why fetal LH does not contribute more to the androgen secretion by the fetal testis, given that the fetal circulation at the end of the second trimester contains up to 30 U LH per liter. In contrast, circulating LH in men ranges between 1 and 7 IU per liter, far lower than in the fetal circulation (25). In presenting the data, we made no assumptions about respective gonadotropin activity. The half-lives of hCG and LH are greater than 30 h, and around 20 min, respectively, there is no apparent marked difference in their affinity for the LHCGR (26,27). Indeed, in vivo, there is disagreement as to whether hCG is more effective at stimulating testosterone secretion than recombinant human LH in men (28,29). Similarly, whereas in short-term human fetal testis cultures, 1000 IU hCG per liter appears to stimulate twice the release of testosterone, relative to constitutive output than 1000 IU LH per liter (10,30), the fact that these exposures were done in different laboratories means that this conclusion should be viewed with caution.
Other androgens are known to be produced by the fetal human testis (31), but all show a similar pattern of secretion with testosterone the highest of the C19 steroids measured. The study by Tapanainen et al. (32) did not measure levels of 5α-reduced steroids, although the fetal testis does not have high levels of 5α-reductase activity, and there is evidence that little dihydrotestosterone is produced by the male fetus (33,34). It appears likely therefore that testosterone is the major androgen produced by the human fetal testis both in quantitative terms and terms of potency. With respect to gonadotropin secretion, FSH but not LH continued to increase in concentration across the second trimester. Falling testosterone at this time would suggest decreased negative feedback and thus LH would be expected to increase. However, it is likely that LH decreases and FSH increases simply because an increasing number of fetal pituitary gonadotropes are secreting FSH indeed by 17 wk nearly 50% of gonadotropes are monohormonal FSH gonadotropes (18).
In the human LHCGR, there is an additional exon, which permits further levels of control of functional LHCGR in the Leydig cells (6). The higher relative expression of functional LHCGR in the fetal testis compared with the adult testis is interesting, although it should be viewed with caution because the data come from different studies. Nevertheless, it suggests that correct transduction of the LH/hCG signal is important in the fetus. It has long been recognized that LHCGR in the neonatal rodent testis, and the second trimester human testis, do not down-regulate on prolonged exposure to LH/CG (35,36). Therefore, if the fetal testis has a higher ratio of active LHCGR isoforms and is exposed to much higher levels of LHCGR stimulus (LH+hCG) than the adult testis, it would be expected that it would respond strongly to stimulation in terms of testosterone output. This is not the case because the LH/testosterone ratio in men is in the region of 0.02–1.08 (25), dramatically different from the LH+hCG to testosterone ratio of 14–22, we observed in the second trimester human fetus (present study). It appears therefore that the fetal human testis is actually relatively insensitive to stimulation of LHCGR, at least in terms of testosterone secretion. Reports that cultured human fetal testes (24 h cultures) showed only a 50% increase above constitutive testosterone secretion in response to 10 IU of highly purified human LH, and at 1000 IU, testosterone reached 200–250% of basal output (30) supports this suggestion.
Our understanding of the relative drives to the testis is complicated by the changing gonadotropin titers during the second trimester, together with the increase in Leydig cell numbers across the same period. At the start of the second trimester, there is 11.2-fold more hCG than LH in the fetal circulation, and this falls to only 1.1-fold more hCG than LH at 17–19 wk. Therefore, up to 16 wk, maternal hCG must be the primary drive to the Leydig cells, with fetal LH making a more important contribution at 17–19 wk. Despite gonadotropin titers in excess of the adult male human, the fetal Leydig cells nevertheless secrete progressively less testosterone per cell as the second trimester continues. Teasing out the relative roles of these parameters in dictating circulating testosterone in the human fetus will require further research.
In many studies maternal cigarette smoking is associated with disturbed reproductive development and subsequently reduced fertility in the male offspring (37,38,39). Furthermore, we demonstrated that maternal cigarette smoking halves expression of the desert hedgehog (DHH) gene, a key component in testicular hedgehog signaling (11). Cigarette smoke contains many potentially bioactive chemicals, including metals, nicotine, and polycyclic aromatic hydrocarbons, among others, which reach the fetus [e.g. (11)]. Some components of cigarette smoke such as arsenic can reduce androgen receptor activity (40), which might be expected to affect circulating LH. Instead, we observed reduced hCG, which could be due to either lowered maternal hCG or reduced transplacental hCG transport when the mother continued to smoke cigarettes. Interestingly, this drop in hCG was not matched by a decline in testosterone in fetuses whose mothers smoked during pregnancy (as seen in Table 2 and Ref. 11). The mechanism involved in maintaining testosterone secretion in these fetuses is unclear, particularly because fetal testosterone production appears to be regulated primarily by hCG. One obvious possibility is that levels of hCG remain sufficiently high to drive testosterone release, but if this was the case, it is unclear why testosterone output per Leydig cell falls so sharply during the second trimester. A second possibility therefore is that the LHCGR is one potential locus of adaptation to reduced drive. Whereas LHCGR transcript expression levels did not differ between control and smoking groups (Table 3), the relative proportion of total inactive vs. active LHCGR isoforms were 3.82:1 in controls compared with 2.09:1 in smokers. This represents a 1.83-fold decease in inactive LHCGR isoforms expression if the mother smoked and may be part of an adaptive mechanism maintaining the vital output of testosterone in these fetuses. Either way, the reduced hCG in smoking mothers may leave the male fetus more susceptible to other perturbations, which might affect Leydig cell function and subsequent development of reproductive health.
We conclude that maternal hCG reaching the fetal circulation is the main driver of testosterone secretion, despite levels of circulating LH more than twice those seen in men. The fetal testis appears relatively insensitive to LH/hCG with a testosterone to hCG+LH ratio 23-fold higher than in men, even though the fetal testis expresses a 2-fold higher ratio of active LHCGR isoforms than the adult. Maternal cigarette smoking has subtle effects on fetal testicular LHCGR isoforms and testosterone output remains similar despite a significant lowering in maternal hCG reaching the fetal circulation.
Supplementary Material
Acknowledgments
The impartial help of the staff of the Pregnancy Counseling Service was essential for the collection of the fetuses. We are grateful to Ms. Margaret Fraser and Gillian Moir for their expert technical assistance.
Footnotes
This work was supported by grants from the Chief Scientist Office (Scottish Executive, CZG/1/109), the Wellcome Trust (063552), and the German Research Foundation (DFG-GR 1547/6-1).
Disclosure Summary: All authors have nothing to declare.
First Published Online October 16, 2009
Abbreviations: FSHR, FSH receptor; hCG, human chorionic gonadotropin; LHCGR, LH receptor; PRL, prolactin; PRLR, PRL receptor.
References
- Huhtaniemi I 1995 Molecular aspects of the ontogeny of the pituitary-gonadal axis. Reprod Fertil Dev 7:1025–1035 [DOI] [PubMed] [Google Scholar]
- Huhtaniemi IT, Korenbrot CC, Jaffe RB 1977 HCG binding and stimulation of testosterone biosynthesis in the human fetal testis. J Clin Endocrinol Metab 44:963–967 [DOI] [PubMed] [Google Scholar]
- Toppari J, Kaleva M, Virtanen HE, Main KM, Skakkebaek NE 2007 Luteinizing hormone in testicular descent. Mol Cell Endocrinol 269:34–37 [DOI] [PubMed] [Google Scholar]
- Gromoll J, Schulz A, Borta H, Gudermann T, Teerds KJ, Greschniok A, Nieschlag E, Seif FJ 2002 Homozygous mutation within the conserved ala-phe-asn-glu-thr motif of exon 7 of the LH receptor causes male pseudohermaphroditism. Eur J Endocrinol 147:597–608 [DOI] [PubMed] [Google Scholar]
- Bruysters M, Christin-Maitre S, Verhoef-Post M, Sultan C, Auger J, Faugeron I, Larue L, Lumbroso S, Themmen AP, Bouchard P 2008 A new LH receptor splice mutation responsible for male hypogonadism with subnormal sperm production in the propositus, and infertility with regular cycles in an affected sister. Hum Reprod 23:1917–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossack N, Simoni M, Richter-Unruh A, Themmen AP, Gromoll J 2008 Mutations in a novel, cryptic exon of the luteinizing hormone/chorionic gonadotropin receptor gene cause male pseudohermaphroditism. PLoS Med 5:e88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang FP, Poutanen M, Wilbertz J, Huhtaniemi I 2001 Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Mol Endocrinol 15:172–183 [DOI] [PubMed] [Google Scholar]
- Coonce MM, Rabideau AC, McGee S, Smith K, Narayan P 2009 Impact of a constitutively active luteinizing hormone receptor on testicular gene expression and postnatal Leydig cell development. Mol Cell Endocrinol 298:33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhtaniemi I, Bolton N, Leinonen P, Kontturi M, Vihko R 1982 Testicular luteinizing hormone receptor content and in vitro stimulation of cyclic adenosine 3′,5′-monophosphate and steroid production: a comparison between man and rat. J Clin Endocrinol Metab 55:882–889 [DOI] [PubMed] [Google Scholar]
- Hallmark N, Walker M, McKinnell C, Mahood IK, Scott H, Bayne R, Coutts S, Anderson RA, Greig I, Morris K, Sharpe RM 2007 Effects of monobutyl and di(n-butyl) phthalate in vitro on steroidogenesis and leydig cell aggregation in fetal testis explants from the rat: comparison with effects in vivo in the fetal rat and neonatal marmoset and in vitro in the human. Environ Health Perspect 115:390–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler PA, Cassie S, Rhind SM, Brewer MJ, Collinson JM, Lea RG, Baker PJ, Bhattacharya S, O'Shaughnessy PJ 2008 Maternal smoking during pregnancy specifically reduces human fetal desert hedgehog gene expression during testis development. J Clin Endocrinol Metab 93:619–626 [DOI] [PubMed] [Google Scholar]
- Hannema SE, Scott IS, Rajpert-De Meyts E, Skakkebaek NE, Coleman N, Hughes IA 2006 Testicular development in the complete androgen insensitivity syndrome. J Pathol 208:518–527 [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy PJ, Baker PJ, Monteiro A, Cassie S, Bhattacharya S, Fowler PA 2007 Developmental changes in human fetal testicular cell numbers and messenger ribonucleic acid levels during the second trimester. J Clin Endocrinol Metab 92:4792–4801 [DOI] [PubMed] [Google Scholar]
- Fowler PA, Evans LW, Groome NP, Templeton A, Knight PG 1998 A longitudinal study of maternal serum inhibin-A, inhibin-B, activin-A, activin-AB, pro-αC and follistatin during pregnancy. Hum Reprod 13:3530–3536 [DOI] [PubMed] [Google Scholar]
- Gilmore DP, Dobbie HG, McNeilly AS, Mortimer CH 1978 Presence and activity of LH-RH in the mid-term human fetus. J Reprod Fertil 52:355–359 [DOI] [PubMed] [Google Scholar]
- Castillo RH, Matteri RL, Dumesic DA 1992 Luteinizing hormone synthesis in cultured fetal human pituitary cells exposed to gonadotropin-releasing hormone. J Clin Endocrinol Metab 75:318–322 [DOI] [PubMed] [Google Scholar]
- Hagen C, McNeilly AS 1975 Identification of human luteinizing hormone, follicle-stimulating hormone, luteinizing hormone β-subunit and gonadotrophin α-subunit in foetal and adult pituitary glands. J Endocrinol 67:49–57 [DOI] [PubMed] [Google Scholar]
- Pope C, McNeilly JR, Coutts S, Millar M, Anderson RA, McNeilly AS 2006 Gonadotrope and thyrotrope development in the human and mouse anterior pituitary gland. Dev Biol 297:172–181 [DOI] [PubMed] [Google Scholar]
- Baker PJ, O'Shaughnessy PJ 2001 Expression of prostaglandin D synthetase during development in the mouse testis. Reproduction 122:553–559 [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy PJ, Willerton L, Baker PJ 2002 Changes in Leydig cell gene expression during development in the mouse. Biol Reprod 66:966–975 [DOI] [PubMed] [Google Scholar]
- van de Beek C, Thijssen JH, Cohen-Kettenis PT, van Goozen SH, Buitelaar JK 2004 Relationships between sex hormones assessed in amniotic fluid, and maternal and umbilical cord serum: what is the best source of information to investigate the effects of fetal hormonal exposure? Horm Behav 46:663–669 [DOI] [PubMed] [Google Scholar]
- Weiss J, Axelrod L, Whitcomb RW, Harris PE, Crowley WF, Jameson JL 1992 Hypogonadism caused by a single amino acid substitution in the β subunit of luteinizing hormone. N Engl J Med 326:179–183 [DOI] [PubMed] [Google Scholar]
- Valdes-Socin H, Salvi R, Daly AF, Gaillard RC, Quatresooz P, Tebeu PM, Pralong FP, Beckers A 2004 Hypogonadism in a patient with a mutation in the luteinizing hormone β-subunit gene. N Engl J Med 351:2619–2625 [DOI] [PubMed] [Google Scholar]
- Lofrano-Porto A, Barra GB, Giacomini LA, Nascimento PP, Latronico AC, Casulari LA, da Rocha Neves Fde A 2007 Luteinizing hormone β mutation and hypogonadism in men and women. N Engl J Med 357:897–904 [DOI] [PubMed] [Google Scholar]
- Boyce MJ, Baisley KJ, Clark EV, Warrington SJ 2004 Are published normal ranges of serum testosterone too high? Results of a cross-sectional survey of serum testosterone and luteinizing hormone in healthy men. BJU Int 94:881–885 [DOI] [PubMed] [Google Scholar]
- Saal W, Glowania HJ, Hengst W, Happ J 1991 Pharmacodynamics and pharmacokinetics after subcutaneous and intramuscular injection of human chorionic gonadotropin. Fertil Steril 56:225–229 [PubMed] [Google Scholar]
- Damewood MD, Shen W, Zacur HA, Schlaff WD, Rock JA, Wallach EE 1989 Disappearance of exogenously administered human chorionic gonadotropin. Fertil Steril 52:398–400 [DOI] [PubMed] [Google Scholar]
- Handelsman DJ, Goebel C, Idan A, Jimenez M, Trout G, Kazlauskas R2008 Effects of recombinant human luteinizing hormone and human chorionic gonadotropin on serum and urine LH and androgens in men. Clin Endocrinol (Oxf) 71:417–428 [DOI] [PubMed] [Google Scholar]
- Cailleux-Bounacer A, Reznik Y, Cauliez B, Menard JF, Duparc C, Kuhn JM 2008 Evaluation of endocrine testing of Leydig cell function using extractive and recombinant human chorionic gonadotropin and different doses of recombinant human LH in normal men. Eur J Endocrinol 159:171–178 [DOI] [PubMed] [Google Scholar]
- Fowler PA, Abramovich DR, Haites NE, Cash P, Groome NP, Al-Qahtani A, Murray TJ, Lea RG 2007 Human fetal testis leydig cell disruption by exposure to the pesticide dieldrin at low concentrations. Hum Reprod 22:2919–2927 [DOI] [PubMed] [Google Scholar]
- Tapanainen J, Kellokumpu-Lehtinen P, Pelliniemi L, Huhtaniemi I 1981 Age-related changes in endogenous steroids of human fetal testis during early and midpregnancy. J Clin Endocrinol Metab 52:98–102 [DOI] [PubMed] [Google Scholar]
- Azoury R, Eyal F, Springer C 1982 5α-Reductase activity in human fetal testis. Int J Biochem 14:577–580 [DOI] [PubMed] [Google Scholar]
- Dawood MY, Saxena BB 1977 Testosterone and dihydrotestosterone in maternal and cord blood and in amniotic fluid. Am J Obstet Gynecol 129:37–42 [DOI] [PubMed] [Google Scholar]
- Wudy SA, Dörr HG, Solleder C, Djalali M, Homoki J 1999 Profiling steroid hormones in amniotic fluid of midpregnancy by routine stable isotope dilution/gas chromatography-mass spectrometry: reference values and concentrations in fetuses at risk for 21-hydroxylase deficiency. J Clin Endocrinol Metab 84:2724–2728 [DOI] [PubMed] [Google Scholar]
- Pakarinen P, Vihko KK, Voutilainen R, Huhtaniemi I 1990 Differential response of luteinizing hormone receptor and steroidogenic enzyme gene expression to human chorionic gonadotropin stimulation in the neonatal and adult rat testis. Endocrinology 127:2469–2474 [DOI] [PubMed] [Google Scholar]
- Leinonen PJ, Jaffe RB 1985 Leydig cell desensitization by human chorionic gonadotropin does not occur in the human fetal testis. J Clin Endocrinol Metab 61:234–238 [DOI] [PubMed] [Google Scholar]
- Jensen MS, Toft G, Thulstrup AM, Bonde JP, Olsen J 2007 Cryptorchidism according to maternal gestational smoking. Epidemiology 18:220–225 [DOI] [PubMed] [Google Scholar]
- Ramlau-Hansen CH, Thulstrup AM, Storgaard L, Toft G, Olsen J, Bonde JP 2007 Is prenatal exposure to tobacco smoking a cause of poor semen quality? A follow-up study. Am J Epidemiol 165:1372–1379 [DOI] [PubMed] [Google Scholar]
- Werler M 2007 Maternal smoking and undescended testes: reaching a tipping point. Epidemiology 18:197–198 [DOI] [PubMed] [Google Scholar]
- Rosenblatt AE, Burnstein KL 2009 Inhibition of androgen receptor transcriptional activity as a novel mechanism of action of arsenic. Mol Endocrinol 23:412–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.