Abstract
Objective:
No therapy is known to improve health-related quality of life (HRQL) or dyspnoea in patients with idiopathic pulmonary fibrosis (IPF). This study investigated longitudinal changes in HRQL and dyspnoea and explored the effects of bosentan on these endpoints during the BUILD-1 trial.
Methods:
In total 154 subjects received oral bosentan (n=71) or placebo (n=83). Changes in HRQL and dyspnoea from baseline to month 6 (M6) and up to month 12 (M12) were measured using the St George's Respiratory Questionnaire (SGRQ), Short-Form 36-item instrument (SF-36), Transition Dyspnoea Index, and Borg Dyspnoea Index.
Results:
Overall, minimal changes occurred in measures of HRQL and dyspnoea among placebo-treated subjects during the study. Effects of bosentan treatment on HRQL and dyspnoea in the all-treated population were minimal. However, in the subset of subjects who underwent surgical lung biopsy (SLB) for diagnosis of IPF, treatment effects were observed up to M12 in the Impacts domain of the SGRQ and the Physical functioning, General health and Role emotional domains of the SF-36.
Conclusions:
HRQL and dyspnoea changed minimally during the course of the study. Observations from exploratory analyses suggest benefits of bosentan on HRQL among patients who underwent SLB for diagnosis and merit further investigation.
Keywords: bosentan, dyspnoea, idiopathic pulmonary fibrosis, health-related quality of life
INTRODUCTION
Idiopathic pulmonary fibrosis (IPF) is a progressive, severe disease with a bleak prognosis. Median survival is very poor, estimated to be 3 years from diagnosis [1]. The predominant symptom of IPF is dyspnoea, which has a strong influence on health-related quality of life (HRQL) [2]. Limited available data suggest that no pharmacologic agent improves survival, symptoms, or HRQL in patients with IPF. According to a recent hypothesis, IPF is an ‘epithelial-fibroblastic’ disease [3], and the pathogenesis of IPF results from the convergence of several complex, profibrotic pathways, leading to aberrant and dysregulated repair of injured lung tissue. Recent therapeutic trials have targeted molecules within these profibrotic cascades.
Endothelin-1 (ET-1) is a potent mediator of fibrosis, inflammation, hypertrophy, and vasoconstriction and is implicated in the pathophysiology of lung fibrosis [4, 5]. The deleterious effects of ET-1 are mediated via two receptors, ETA and ETB. Recently, the Bosentan Use in Interstitial Lung Disease (BUILD-1) trial evaluated the oral, dual endothelin receptor antagonist bosentan for the treatment of IPF [6]. Although bosentan did not show superiority over placebo in the primary endpoint of 6-minute walk distance (6MWD), a trend favouring bosentan was observed in the combined outcome of time to disease progression or death. This effect was most pronounced in a post-hoc analysis of the predefined subset of patients who underwent surgical lung biopsy (SLB) for diagnosis of IPF.
Like patients with other life-shortening diseases, patients with IPF value the quality of their lives, in addition to the duration of their life expectancy; HRQL is therefore an important outcome requiring systematic assessment in therapeutic trials for IPF [7]. The BUILD-1 trial was one of the largest studies to assess the impact of medical therapy on HRQL and dyspnoea in IPF patients. The aim of this exploratory analysis was to examine longitudinal changes in HRQL and dyspnoea in IPF and to explore if bosentan treatment had beneficial effects on these outcomes. We hypothesized that bosentan would mitigate impairment of HRQL and dyspnoea to a greater extent than placebo, particularly among the subset of subjects who underwent SLB for diagnosis of IPF.
METHODS
Study subjects
The inclusion and exclusion criteria and the study protocol for the BUILD-1 trial have been described previously [6]. Briefly, the randomised controlled trial enrolled subjects with a well-defined diagnosis of IPF [8] and excluded subjects who had interstitial lung disease due to conditions other than IPF, very severe restrictive pulmonary physiology, obstructive lung disease, or echocardiographic evidence of severe pulmonary hypertension (systolic pulmonary pressure ≥50 mmHg) or congestive heart failure [6]. All subjects provided written, informed consent to participate in the study, which was approved by the appropriate independent ethics committees or institutional review boards. The study was conducted in accordance with the principles of the Declaration of Helsinki and local laws and guidelines for good clinical practice.
Study design and assessment tools
The objectives of the present exploratory analyses were to examine changes over time in HRQL and dyspnoea and to evaluate the effects of bosentan on changes from baseline in HRQL and dyspnoea during the BUILD-1 trial [6]. Eligible subjects were randomised 1:1 to receive oral bosentan 62.5 mg twice daily for 4 weeks, up-titrated to 125 mg twice daily thereafter, or matching placebo. Subjects completed HRQL and dyspnoea assessments at baseline, month 6 (M6), and month 12 (M12) or sooner if study medication was discontinued prematurely (up to M12).
Quality of Life
Assessment of HRQL was performed using two instruments: the St George's Respiratory Questionnaire (SGRQ) [9] and the Medical Outcomes Study 36-Item short-form instrument (SF-36) [10]. Questionnaires were provided to subjects in their local language, after review by native speakers and persons knowledgeable in this field, prior to and following back-translation into English.
The SGRQ is a self-administered, respiratory-specific instrument that comprises three domains: Symptoms, Activity, and Impacts. Each SGRQ domain is scored from 0–100, with higher scores indicating worse HRQL [9]. Among patients with chronic obstructive pulmonary disease, a change in the total or any SGRQ domain score of 4 points is considered a minimum important difference [11].
The SF-36 is a self-administered, generic instrument that measures health status and well-being. It comprises eight domains, scored individually from 0–100. Higher scores correspond to better HRQL [10]. The minimum important difference for SF-36 domain scores has not been clearly established; however, some investigators consider these to be in the range of 3–5 points [12]. The SGRQ and SF-36 were selected for this trial because each performed well in prior cross-sectional studies enrolling subjects with IPF [7], providing each instrument with a foundation of validity for use in this population.
Dyspnoea
Dyspnoea was assessed using two instruments: (i) the Borg dyspnoea scale [13], and (ii) the Baseline Dyspnoea Index (BDI) and Transition Dyspnoea Index (TDI) [14]. The Borg scale is a simple, self-administered visual analogue scale that scores the extent of dyspnoea from 0 (no dyspnoea) to 10 (most severe dyspnoea). Subjects were asked to gauge dyspnoea using the Borg scale immediately following measurement of 6MWD.
The BDI was performed only at baseline, at rest, and prior to assessments of 6MWD and pulmonary function. Changes in dyspnoea from baseline to M6 and up to M12 were assessed using the TDI, which was performed at rest and prior to assessments of 6MWD and pulmonary function [14]. The BDI and TDI each comprise three domains: functional impairment, magnitude of task, and magnitude of effort. The BDI evaluates the extent of dyspnoea on a scale that ranges from 0 (very severe impairment) to 4 (no impairment) for each of three domains [14]. The BDI total score is the sum of the three domain scores and ranges from 0–12. The TDI score ranges from −3 (major deterioration) to +3 (major improvement) for each domain, with baseline status (BDI scores) as the comparator. The TDI total score is the sum of the three domain scores and ranges from −9 to +9. Changes in TDI total score ≥1 were considered clinically meaningful [15].
Statistical methods
Baseline measurements of HRQL and dyspnoea, and changes from baseline in these parameters, were summarised as medians with 95% confidence intervals [CI], unless stated otherwise. The Wilcoxon two-sample test was used to examine differences between treatment groups in HRQL and dyspnoea from baseline to M6 and from baseline up to M12. Differences between responses observed in bosentan-treated versus placebo-treated patients were expressed as placebo-corrected median treatment effects.
Comparative analyses were performed in patients who had at least one valid post-baseline assessment (all-treated population), and in a subset of subjects who underwent SLB for diagnosis (SLB subset). Unless stated otherwise, results are presented for the all-treated population. Only patients with valid baseline values were included in the analysis of each parameter. Data were not replaced for patients with missing baseline values; missing data were handled in accordance with published recommendations [9, 10]. For patients with missing post-baseline scores, analyses were performed with the last observation carried forward. In cases of worsening disease or death that precluded data collection, imputed values of 100 for the SGRQ, 0 for the SF-36, 10 for the Borg dyspnoea index, and −9 for the TDI, were applied.
RESULTS
Baseline characteristics and patient disposition
Of the 158 subjects randomised (bosentan, n=74; placebo, n=84), 154 received at least one dose of study medication, had at least one valid post-baseline value for the primary endpoint and were included in the all-treated population (bosentan, n=71; placebo, n=83). Treatment groups were generally well-matched with regards to demographics and baseline characteristics [6], including measures of HRQL (Table 1). Mean exposure to study drug was 54.0 and 56.1 weeks in the bosentan and placebo groups, respectively.
Table 1.
Baseline HRQL in the all-treated population, assessed by the SGRQ and SF-36 instruments, showing that patients in both groups were similarly impaired at baseline
| Bosentan | Placebo | |
|---|---|---|
| SGRQ, median (95% CI) | ||
| Symptoms components | 51.2a (44.7, 56.0) | 54.8b (47.9, 59.1) |
| Activity components | 59.5c (53.6, 66.1) | 59.5d (56.0, 65.6) |
| Impacts components | 32.9c (25.0, 40.3) | 30.5e (25.7, 38.4) |
| Total score | 43.9f (36.6, 52.1) | 44.3g (40.2, 50.0) |
|
| ||
| SF-36, median (95% CI) | ||
| Physical functioning | 55.0a (40.0, 65.0) | 47.5b (35.0, 55.0) |
| Role – physical | 25.0c (25.0, 50.0) | 25.0e (25.0, 50.0) |
| Bodily pain | 74.0a (64.0, 100.0) | 64.0b (61.0, 74.0) |
| Social functioning | 75.0a (75.0, 87.5) | 81.3b (62.5, 87.5) |
| Mental health | 80.0c (68.0, 84.0) | 76.0b (68.0, 80.0) |
| Role – emotional | 100.0a (66.7, 100.0) | 100.0e (66.7, 100.0) |
| Vitality | 50.0c (40.0, 55.0) | 50.0b (40.0, 55.0) |
| General health | 47.0a (37.0, 57.0) | 46.0b (40.0, 52.0) |
n=68
n=82
n=67
n=83
n=81
n=66
n=80
95% CI, 95% confidence interval of the median; HRQL, health-related quality of life; SF-36, 36-item short-form instrument; SGRQ, St George's Respiratory Questionnaire.
Quality of life
All-treated population
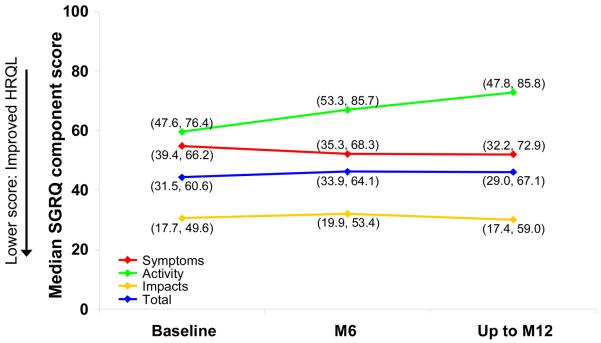
As demonstrated in previous studies and compared with general population norms, considerable impairment of HRQL was observed at baseline (Table 1). Among subjects who received placebo, minimal changes in median measures of HRQL from baseline to M6 and up to M12 were observed during the course of the study (Figures 1 and 2). At M6, a change from baseline in SGRQ Total score indicated improvement in bosentan-treated patients; however, up to M12, no differences were observed between treatment groups in any domain of the SGRQ (data not shown).
Figure 1. Median SGRQ component scores at baseline, M6 and up to M12 in placebo-treated patients in the all-treated population.
Data in brackets are interquartile ranges. HRQL, quality of life; SGRQ, St George's Respiratory Questionnaire.
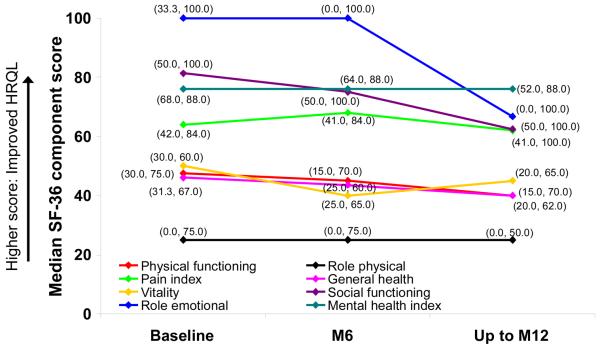
Figure 2. Median SF-36 domain scores at baseline, M6 and up to M12 in placebo-treated patients in the all-treated population.
Data in brackets are interquartile ranges. HRQL, quality of life; SF-36, 36-item short-form instrument.
When QoL was assessed using the SF-36, no differences were observed between treatment groups in any domain at M6. Up to month 12, a change from baseline in the Role Emotional domain suggested improvement in bosentan-treated patients (data not shown).
SLB subset
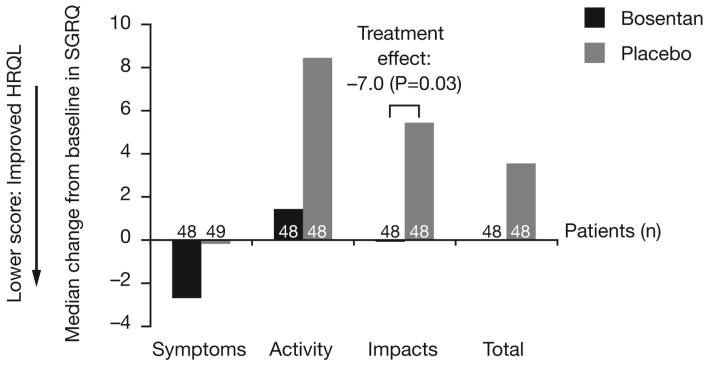
From baseline to M6, improvements were observed in the Impacts domain and Total score of the SGRQ among patients who underwent SLB for diagnosis and received bosentan treatment (data not shown). Changes in SGRQ scores from baseline up to M12 are illustrated in Figure 3A.
Figure 3A. Median changes from baseline in component and total SGRQ scores in the SLB subset up to month 12.
Treatment effects are illustrated where P<0.05; HRQL, quality of life; SGRQ, St George's Respiratory Questionnaire.
There were no differences between treatment groups in SF-36 change scores at M6 (data not shown). Changes in SF-36 scores from baseline up to M12 are illustrated in Figure 3B.
Dyspnoea
All-treated population
Minimal changes were observed during the course of the study among placebo-treated subjects who completed assessments of dyspnoea at baseline, M6, and up to M12. Median Borg scores for these patients were 2.0 (2.0, 3.0) at baseline, 3.0 (2.0, 3.0) at M6, and 3.0 (3.0, 3.0) up to M12 (n=83). Median changes in dyspnoea measured using the TDI at M6 and up to M12 were −1.0 (−2.0, 0.0) and −3.0 (−3.0, 0.0), respectively (n=82).
No differences between treatment groups were observed in Borg scores either at baseline or in changes from baseline to M6 or up to M12 (data not shown). At M6, TDI scores declined from baseline in the placebo group but not in the bosentan group (data not shown). The treatment effect of bosentan at M6 was an increase in TDI of 1.1 (95% CI, 0.0 to 2.8; P=0.02). Up to month 12, the median TDI declined in both groups (data not shown). At M6, the number of subjects with improved dyspnoea identified by a TDI ≥1 – the reported minimum important difference for this instrument – was 18 (26.9%) in the bosentan group and 10 (12.2%) in the placebo group, corresponding to a relative risk of 2.20 (95% CI, 1.09 to 4.45). This effect was not apparent up to M12 (relative risk, 0.98; 95% CI, 0.48 to 2.01).
SLB subset
There were no differences in baseline Borg scores between treatment groups and at M6, the median Borg score was 0.0 (95% CI, 0.0 to 0.0) in each group. Up to M12, the Borg score was unchanged in the bosentan group and had changed to 1.0 (95% CI, 0.0 to 1.0) in the placebo group, yielding a treatment effect of bosentan of −0.7 (95% CI, −1.4 to 0.0; P=0.03).
A treatment effect of bosentan of 1.3 (95% CI, 0.0 to 2.9; P=0.01) was observed in the TDI at M6. Up to M12, the median TDI declined in both treatment groups (data not shown). At M6, the number of subjects in the SLB subset with improved dyspnoea identified by a TDI ≥1 was 12 (25.0%) in the bosentan group and 4 (8.2%) in the placebo group, corresponding to a relative risk of 3.06 (95% CI, 1.06 to 8.83). This effect was not apparent up to M12 (relative risk, 0.92; 95% CI, 0.41 to 2.06).
Safety
The safety profile of bosentan in the BUILD-1 trial has been reported previously [6]. The observed incidences of pulmonary adverse events were generally less frequent in the bosentan group versus the placebo group, including cough (17.6% vs. 27.4%), worsening of IPF (16.2% vs. 23.8%), and exacerbation of dyspnoea (13.5% vs. 19.0%). The observed incidence of nasopharyngitis and events related to increased liver aminotransferases in bosentan-treated patients (13.5% and 13.5%, respectively) were greater than those observed in placebo-treated patients (4.8% and 1.2%, respectively).
Data and instrument issues
Domains from both the SGRQ and SF-36 exhibited floor and ceiling effects, i.e. a proportion of subjects in the all-treated population exhibited domain scores at either the lowest or highest possible values for these instruments. The proportion of patients who exhibited floor and ceiling effects in the SGRQ was ≤3.0% for all domains except Activity, in which 10.6% of bosentan-treated patients and 11.3% of placebo-treated patients exhibited the worst possible scores. The proportion of patients who exhibited floor and ceiling effects in the SF-36 was consistently low for the domains of Physical functioning, General health, Vitality and Mental health. In the remaining domains, between 18.8 and 58.2% of patients in each treatment group exhibited the best possible scores. In the Role physical and Role emotional domains of the SF-36, between 20.9 and 46.3% of patients in each treatment group exhibited the worst possible scores.
Within the proportion of patients who dropped out of the study, baseline HRQL scores were similar in each treatment arm (data not shown). A number of subjects had at least one missing post-baseline value for any domain of any instrument at M6 and up to M12 (Table 2). For these patients, analyses were performed with the last observation carried forward, or with imputed values following worsening of disease or death, in accordance with the protocol.
Table 2.
The number of subjects for whom analyses were performed with the last observation carried forward or with imputed values in the all-treated population
| Bosentan n=71 |
Placebo n=83 |
|||
|---|---|---|---|---|
| Assessment time | M6 | Up to M12 | M6 | Up to M12 |
| Last observation carried forward, n | ||||
| SGRQ | 5 | 7 | 5 | 6 |
| SF-36 | 5 | 4 | 3 | 6 |
| Borg dyspnoea index | 7 | 9 | 3 | 4 |
| TDI | 4 | 3 | 1 | 1 |
|
| ||||
| Imputed values, n | ||||
| SGRQ | 4 | 5 | 3 | 6 |
| SF-36 | 4 | 5 | 3 | 6 |
| Borg dyspnoea index | 4 | 5 | 3 | 8 |
| TDI | 3 | 4 | 3 | 6 |
M6, month 6; M12, month 12; SF-36, 36-item short-form instrument; SGRQ, St George's Respiratory Questionnaire; TDI, transition dyspnoea index.
DISCUSSION
HRQL and dyspnoea are important components of well-being in patients with IPF. In this study, we used data from the BUILD-1 trial to assess changes in these two patient-reported outcomes over 12 months. Overall, there was relatively little change in either outcome. Among subjects who underwent SLB for diagnosis of IPF (by identification of the histological pattern of usual interstitial pneumonia), bosentan treatment appeared to have beneficial effects on both HRQL and dyspnoea.
To date, there have been few large, placebo-controlled trials of therapeutic agents for IPF [6, 16, 17]. Neither interferon-gamma 1b [17] nor pirfenidone [16] were found to have beneficial effects on HRQL or dyspnoea. To our knowledge, bosentan is the first agent suggested to show benefit on these important outcomes in patients with IPF.
Often, the absence of ‘honeycombing’ on high resolution computed tomography (HRCT) makes a clinical diagnosis of IPF more difficult. Consequently, current guidelines recommend performing SLB in patients with suspected IPF who do not exhibit significant ‘honeycombing’ on HRCT [8]. One hypothesis generated from the BUILD-1 trial was that the beneficial effects of bosentan would be greater in subjects with less ‘honeycombing’ on HRCT. To investigate this hypothesis, we also performed analyses using data only from subjects whose diagnosis was ascertained by the presence of usual interstitial pneumonia on histological examination of SLB specimens – the SLB subset. Indeed, beneficial effects of bosentan on HRQL and dyspnoea were suggested by analyses in this subset.
The mechanisms underlying the apparent beneficial effects of bosentan on HRQL and dyspnoea in the patient subgroup with biopsy proven IPF observed in this study are unclear. Treatment with bosentan may delay disease progression and physiological decline, hence influencing HRQL and dyspnoea. Another consideration is that bosentan may have improved pulmonary vascular parameters, which led to the suggested benefits in patient-centred outcomes. The latter factor seems unlikely since patients with echocardiographic evidence of significant pulmonary hypertension were excluded from this study. We consider results from the current study to stem from, and mirror, trends observed in a post-hoc analysis of the BUILD-1 trial favouring bosentan [6]. Beneficial trends in physiologic parameters and mortality were particularly evident in the subset of subjects with SLB-proven IPF [6]. We hypothesise that these observations arose because these subjects had less extensive fibrosis, made apparent by a lack of significant ‘honeycombing’ on HRCT.
This study has a few limitations. The BUILD-1 trial was not powered for evaluation of the HRQL or dyspnoea outcomes that were used as exploratory endpoints; therefore, the sample size may not have been sufficient to detect differences in certain instrument or domain scores. Response data were obtained using the last observation carried forward or imputation during some assessments of HRQL and dyspnoea in up to 13 subjects. Also, the between-group differences in change scores were relatively small.
An analysis of subjects who completed the study versus those who discontinued was not performed; however, the proportion of patients who discontinued was similar in both treatment arms. Therefore, substitution rules were applied approximately equally. Although, several observations were considered to differ between treatment groups, the confidence intervals in some results covered the published minimum important differences for these instruments. As such, these observations may not carry strong clinical significance. However, the minimum important differences for the SGRQ, SF-36, BDI, and TDI, have not been established in IPF. It is therefore possible that the differences observed here possess clinical importance. Furthermore, the exploratory nature of these analyses should be viewed as hypothesis-generating and should not be considered definitive evidence that bosentan has beneficial effects on these important outcomes.
Certain domains from the SGRQ and SF-36 exhibited floor and ceiling effects, which may have influenced results in either direction. For example, such effects may have restricted detection of the true underlying improvement in the 33% of bosentan-treated subjects whose baseline scores from the SF-36 Role physical domain were already at 100 –its highest value. Likewise, a floor effect could have occurred if the 36% of placebo-treated subjects with the lowest baseline score on the Role emotional domain actually declined further, as their scores could not decrease further despite perception of decline. Finally, the responsiveness of the instruments used in this study is unknown and needs to be determined in future studies.
In conclusion, HRQL and dyspnoea changed minimally over 12 months. Potential beneficial effects of bosentan on important patient-centred outcomes were identified in the subset of subjects who underwent SLB as part of their diagnostic evaluation. These observations will be investigated further in the ongoing BUILD-3 trial.
Figure 3B. Median changes from baseline in domains of the SF-36 in the SLB subset up to month 12.
Data in brackets are interquartile ranges. Treatment effects are illustrated where P<0.05; HRQL, quality of life; SF-36, 36-item short-form instrument.
ACKNOWLEDGEMENTS
The authors would like to gratefully acknowledge all of the Investigators involved in this study: Ishaar Ben-Dov, Charles Chan, Jean-Francois Cordier, James Dauber, Joao De Andrade, Adaani Frost, Thomas Geiser, Marilyn Glassberg, Jeffrey Golden, Gary Hunninghake, Sanjay Kalra, Lisa Lancaster, Robert Levy, Fernando Martinez, Keith Meyer, Joachim Mueller-Quernheim, Paul Noble, Christophe Pison, Charles Poirier, Milton Rossman, Paola Rottoli, Gerd Staehler, Dominique Valeyre, Athol Wells, Gordon Yung and David Zisman. The authors are indebted to the coordinators and patients involved with this study. Additionally, the authors acknowledge the editorial assistance of Andrew Gray, funded by Actelion Pharmaceuticals Ltd, during the preparation of this manuscript.
Role of the funding source: This study was supported by a research grant from Actelion Pharmaceuticals Limited for logistical support, monitoring, project management, data management, statistical analysis and reporting.
Footnotes
ClinicalTrials.gov identifier: NCT00071461
REFERENCES
- 1.Bjoraker JA, Ryu JH, Edwin MK, Myers JL, Tazelaar HD, Schroeder DR, Offord KP. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;157:199–203. doi: 10.1164/ajrccm.157.1.9704130. [DOI] [PubMed] [Google Scholar]
- 2.Nishiyama O, Taniguchi H, Kondoh Y, Kimura T, Ogawa T, Watanabe F, Nishimura K. Health-related quality of life in patients with idiopathic pulmonary fibrosis. What is the main contributing factor? Respir Med. 2005;99:408–414. doi: 10.1016/j.rmed.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Selman M, King TE, Pardo A, American Thoracic Society. European Respiratory Society. American College of Chest Physicians Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 4.Park SH, Saleh D, Giaid A, Michel RP. Increased endothelin-1 in bleomycin-induced pulmonary fibrosis and the effect of an endothelin receptor antagonist. Am J Respir Crit Care Med. 1997;156:600–608. doi: 10.1164/ajrccm.156.2.9607123. [DOI] [PubMed] [Google Scholar]
- 5.Shi-Wen X, Denton CP, Dashwood MR, Holmes AM, Bou-Gharios G, Pearson JD, Black CM, Abraham DJ. Fibroblast matrix gene expression and connective tissue remodeling: role of endothelin-1. J Invest Dermatol. 2001;116:417–425. doi: 10.1046/j.1523-1747.2001.01256.x. [DOI] [PubMed] [Google Scholar]
- 6.King TE, Jr, Behr J, Brown KK, du Bois RM, Lancaster L, de Andrade JA, Stähler G, Leconte I, Roux S, Raghu G. BUILD-1: a randomized, placebo-controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177:75–81. doi: 10.1164/rccm.200705-732OC. [DOI] [PubMed] [Google Scholar]
- 7.Tomioka H, Imanaka K, Hashimoto K, Iwasaki H. Health-related quality of life in patients with idiopathic pulmonary fibrosis--cross-sectional and longitudinal study. Intern Med. 2007;46:1533–1542. doi: 10.2169/internalmedicine.46.6218. [DOI] [PubMed] [Google Scholar]
- 8.American Thoracic Society Idiopathic pulmonary fibrosis: Diagnosis and treatment. Am J Respir Crit Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 9.Jones P, Quirk F, Baveystock C. The St. George's Respiratory Questionnaire. Respir Med. 1991;85:25–31. doi: 10.1016/s0954-6111(06)80166-6. [DOI] [PubMed] [Google Scholar]
- 10.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 11.Schunemann HJ, Griffith L, Jaeschke R, Goldstein R, Stubbing D, Guyatt GH. Evaluation of the minimal important difference for the feeling thermometer and the St. George's Respiratory Questionnaire in patients with chronic airflow obstruction. J Clin Epidemiol. 2003;56:1170–1176. doi: 10.1016/s0895-4356(03)00115-x. [DOI] [PubMed] [Google Scholar]
- 12.Hays RD, Morales LS. The RAND-36 measure of health-related quality of life. Ann Med. 2001;33:350–357. doi: 10.3109/07853890109002089. [DOI] [PubMed] [Google Scholar]
- 13.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 14.Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest. 1984;85:751–758. doi: 10.1378/chest.85.6.751. [DOI] [PubMed] [Google Scholar]
- 15.Witek TJ, Jr, Mahler DA. Minimal important difference of the transition dyspnoea index in a multinational clinical trial. Eur Respir J. 2003;21:267–272. doi: 10.1183/09031936.03.00068503a. [DOI] [PubMed] [Google Scholar]
- 16.Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K, Taguchi Y, Nagai S, Itoh H, Ohi M, Sato A, Kudoh S. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171:1040–1047. doi: 10.1164/rccm.200404-571OC. [DOI] [PubMed] [Google Scholar]
- 17.Raghu G, Brown KK, Bradford WZ, Starko K, Noble PW, Schwartz DA, King TE, Jr, Idiopathic Pulmonary Fibrosis Study Group A placebo-controlled trial of interferon gamma-1b in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2004;350:125–133. doi: 10.1056/NEJMoa030511. [DOI] [PubMed] [Google Scholar]